Abstract
Activity-dependent dendritic translation in CNS neurons is important for the synapse-specific provision of proteins that may be necessary for strengthening of synaptic connections. A major rate-limiting factor during protein synthesis is the availability of eukaryotic translation initiation factor 4E (eIF4E), an mRNA 5'-cap-binding protein. In this study we show by fluorescence in situ hybridization (FISH) that the mRNA for eIF4E is present in the dendrites of cultured rat hippocampal neurons. Under basal culture conditions, 58.7 ± 11.6% of the eIF4E mRNA clusters localize with or immediately adjacent to PSD-95 clusters. Neuronal activation with KCl (60 mM, 10 min) very significantly increases the number of eIF4E mRNA clusters in dendrites by 50.1 and 74.5% at 2 and 6 h after treatment, respectively. In addition, the proportion of eIF4E mRNA clusters that localize with PSD-95 increases to 74.4 ± 7.7% and 77.8 ± 7.6% of the eIF4E clusters at 2 and 6 h after KCl treatment, respectively. Our results demonstrate the presence of eIF4E mRNA in dendrites and an activity-dependent increase of these clusters at synaptic sites. This provides a potential mechanism by which protein translation at synapses may be enhanced in response to synaptic stimulation.
Keywords: dendrites; eukaryotic initiation factor-4E; immunohistochemistry; in situ hybridization, fluorescence; potassium chloride; protein biosynthesis; synapses
Introduction
Neuronal dendrites are complex structures decorated with synapses that are dynamically regulated both morphologically and in their strength of connectivity. The dynamic changes in synapses may be partially maintained by local protein synthesis in dendrites. Specific mRNAs are transported to neuronal dendrites to serve as substrates for translation of proteins in specific compartments (Sutton and Schuman, 2006; reviewed in Sossin and DesGroseillers, 2006). A requirement for dendritic mRNA localization and subsequent local protein translation has been demonstrated in several forms of behavioral learning paradigms such as associative learning (Ashraf et al., 2006), spatial learning, and contextual conditioning (Miller et al., 2002). An essential role for local translation in synaptic plasticity was demonstrated by protein synthesis-dependent potentiation of synaptic transmission in response to brain-derived neurotrophic factor (BDNF) in hippocampal slices in which the CA1 dendrites were surgically isolated from their cell bodies (Kang and Schuman, 1996). Similarly, induction of some forms of LTP in isolated hippocampal dendritic fields is dependent on protein synthesis (Cracco et al., 2005; Huang and Kandel, 2005; Vickers et al., 2005).
Eukaryotic initiation factor 4E (eIF4E) plays a central role in the control of post-transcriptional gene expression. In eukaryotic cells, the rate of translation is primarily regulated at the initiation phase (Mathews et al., 2000; von der Haar et al., 2004), in which the small ribosomal subunit is recruited to the 5'-terminal mRNA cap, the methylated guanine moiety m7GpppN (where N is the first transcribed nucleotide). eIF4E has cap-binding activity (Browning, 1996; von der Haar et al., 2004), and binding of eIF4E and another initiation factor, eIF4G, to the cap via the activity of eIF4E is essential for translation both in vivo and in vitro (Gross et al., 2003; von der Haar et al., 2004). Thus eIF4E can control initiation of protein translation.
eIF4E is present in dendrites and can redistribute to synaptic sites. Electron microscopy studies revealed that eIF4E is localized to microvesicle-like structures underneath the postsynaptic membrane near the postsynaptic density (PSD) (Asaki et al., 2003). Immunohistochemical studies in cultured neurons demonstrated that BDNF, which induces a form of synaptic potentiation in the hippocampus that depends on local protein synthesis (Kang and Schuman, 1996; Ying et al., 2002), facilitates translocation of eIF4E into dendritic spines (Smart et al., 2003). Despite a potentially critical role of eIF4E in the control of dendritic translation, we have little information about eIF4E mRNA in dendrites. In this study we carried out fluorescence in situ hybridization (FISH) and show that eIF4E mRNA clusters are present in dendrites. KCl treatment upregulated the expression of the eIF4E gene and increased the density of its RNA clusters in dendrites. Furthermore, by combining FISH with immunocytochemistry, we show that KCl treatment increases the proportion of eIF4E mRNA granules clustered near synaptic sites.
Results
The eIF4E mRNA is localized in dendrites of rat hippocampal neurons in culture
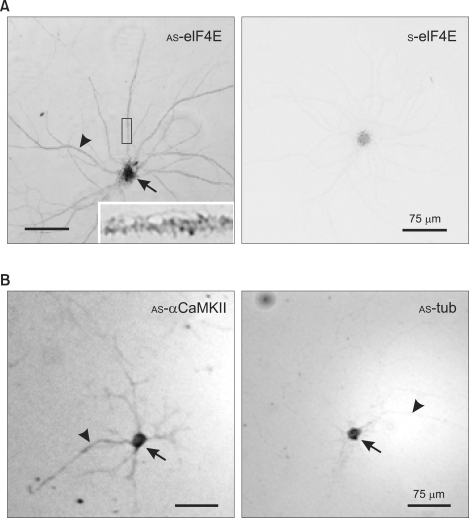
We first tested whether eIF4E mRNA is localized in dendrites by in situ hybridization (ISH) of cultured rat hippocampal neurons. After PFA/MeOH fixation, cells were hybridized in situ with DIG-labeled sense (S)- or antisense (AS)-eIF4E riboprobes, and ISH signals were visualized with biotin-conjugated anti-digoxin antibody and alkaline phosphatase-conjugated streptavidin as detailed in Methods. As shown in the bright field light micrographs (Figure 1), the AS-riboprobe revealed significant ISH signals for eIF4E mRNA in dendrites (Figure 1A, arrowhead), although the strongest signals were associated with the soma (Figure 1A, arrow). The magnified image of a portion of dendrite (Figure 1A, inset) showed that the eIF4E ISH signals form clusters in the dendrites. In contrast, the sense probe did not reveal any significant signal above background (Figure 1A, S-eIF4E). We performed control ISH for mRNA of the α-subunit of the type II Ca2+/calmodulin-dependent protein kinase (αCaMKII), which is localized in both neuronal somas and dendrites (Burgin et al., 1990; Paradies and Steward, 1997; Tian et al., 1999), and for β-tubulin mRNA, which is restricted to the soma (Kleiman et al., 1994; Paradies and Steward, 1997; Tian et al., 1999). As expected, AS-αCaMKII riboprobes revealed CaMKII mRNA in dendrites as well as in the soma (Figure 1B, AS-αCaMKII arrowhead and arrow, respectively), while the AS-β-tubulin ISH signal was restricted to the soma (Figure 1B, AS-tub, arrow) and the signal in dendrites was very weak (arrowhead). These results indicate that the eIF4E mRNA is localized to dendrites.
Figure 1.
In situ hybridization showing dendritic localization of eIF4E mRNA. (A) eIF4E. DIV18 dissociated rat hippocampal neurons were hybridized with DIG-labeled anti-sense (AS-eIF4E) or sense (S-eIF4E) cRNA probes prepared by in vitro transcription. The boxed area is shown enlarged in the inset. (B) Controls. Hippocampal neurons were hybridized with DIG-labeled anti-sense αCaMKII (AS-αCaMKII) or β-tubulin (AS-tub). The soma and dendrites are marked by arrows and arrowheads, respectively. Scale bars; 75 µm.
Some eIF4E mRNA clusters are positioned at or near postsynaptic sites in dendrites of unstimulated hippocampal neurons
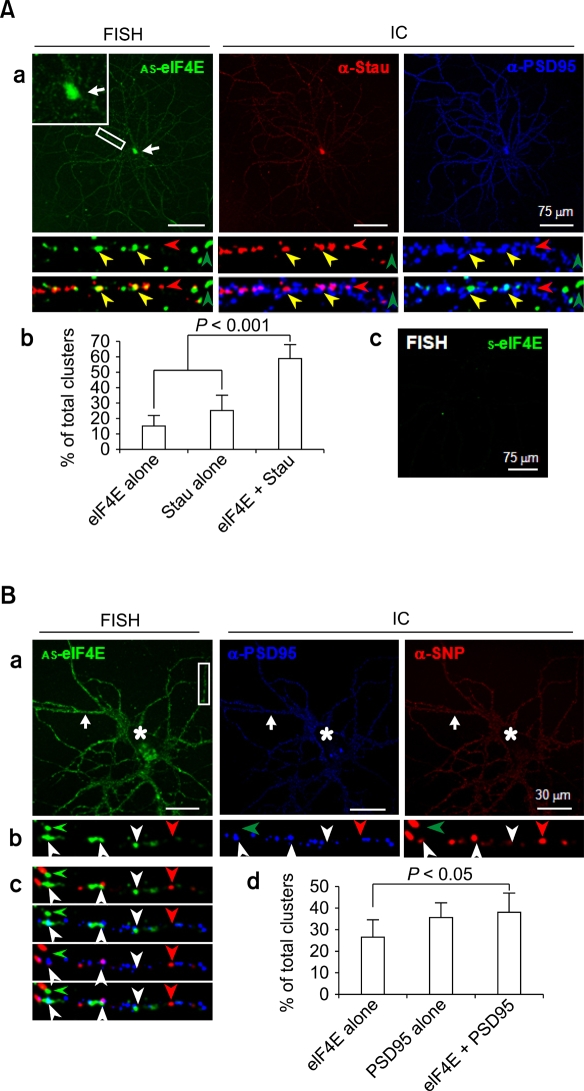
Neuronal dendrites have a complex morphology. Therefore, the relative position of eIF4E may be strategically important for tight regulation of local translation. We recently designed a sequential PFA/MeOH fixation procedure for combined FISH and immunocytochemistry that allows high resolution analysis of the location of both mRNAs and proteins (Moon et al., 2007). With the use of this new protocol, we carried out FISH combined with immunocytochemistry to find the relative position of eIF4E mRNA in dendrites. We first verified that the FISH signals are at RNA granules by showing colocalization of staufen protein, a marker protein for RNA granules, with eIF4E mRNA. PFA/MeOH-fixed hippocampal neurons were subjected to FISH with DIG-labeled eIF4E riboprobes, followed by immunocytochemistry with antibodies against staufen and PSD-95 (Figure 2). Consistent with our earlier results, the FISH signals for eIF4E mRNA were present in clusters along dendrites (Figure 2A, a, AS-eIF4E), while there was no significant signal for sense riboprobes (Figure 2A, c, S-eIF4E). The immunocytochemistry signals of staufen were also present as clusters along dendritic shafts (Figure 2A, a, α-Stau), a distribution profile similar to previous reports (Kiebler et al., 1999; Kohrmann et al., 1999; Macchi et al., 2003; Mallardo et al., 2003; Kim et al., 2005). The merged images of staufen immunocytochemistry and eIF4E FISH exhibited many overlapping clusters (Figure 2A, a, bottom panels, yellow arrowheads), although clusters labeled for only staufen or eIF4E are also present (red and green arrowheads, respectively, in Figure 2A, a, lower panels). Some of the clusters labeled for both staufen and eIF4E mRNA were in close proximity to or overlap with those of PSD-95 (Figure 2A, a, bottom panels). Statistical analyses showed that majority of the clusters (59.1 ± 9.2% of the combined total number of eIF4E FISH and staufen immunocytochemistry clusters; n = 213) overlapped with each other (Figure 2A, b). This proportion was very significantly (P < 0.001) higher than the clusters labeled for only staufen or eIF4E mRNA (25.3 ± 10.0 and 15.7 ± 6.0%, respectively), strongly indicating that the FISH clusters represent eIF4E mRNA in granules. The eIF4E mRNA and staufen clusters were rather centrally located (Figure 2A, a, bottom; green and red, respectively), while those of PSD-95 were positioned towards the periphery of dendrites (Figure 2A, a, bottom; blue), further supporting the identity of eIF4E FISH clusters as RNA granules.
Figure 2.
Confocal micrographs of combined FISH and immunocytochemistry (IC) images showing the relative position of eIF4E mRNA clusters in dendrites of rat hippocampal neurons. (A) Colocalization of FISH signals with staufen immunopuncta. (a) Combined FISH and IC. Rat hippocampal cultures (DIV18) were hybridized with DIG-labeled anti-sense eIF4E (AS-eIF4E) cRNA probes (FISH) followed by immunocytochemistry with antibodies against staufen (α-Stau) and PSD-95 (α-PSD95). The soma is marked by an arrow and shown enlarged in the inset of the FISH image. Images corresponding to the boxed area are shown enlarged at the bottom in single or merge colors. The eIF4E mRNA clusters colocalizing with staufen immunopuncta are marked by yellow arrowheads, while single eIF4E mRNA and staufen clusters are marked by green and red arrowheads, respectively. (b) Statistics. Clusters per 30 µm were counted and presented in percents of total clusters. Statistical significance was assessed by Mann-Whitney U-test. (c) Control FISH. Hippocampal neurons were hybridized with DIG-labeled sense-eIF4E cRNA probes (S-eIF4E). Scale bars; 75 µm. (B) Relative position of eIF4E mRNA clusters in dendrites. (a-c) Combined FISH and IC. Rat hippocampal cultures (DIV18) were hybridized with DIG-labeled anti-sense eIF4E (AS-eIF4E) cRNA probes (FISH) followed by immunocytochemistry with antibodies against PSD-95 (α-PSD95) and synaptophysin (α-SNP) (panel a). The soma and dendrites are marked by arrows and arrowheads, respectively. The boxed area is shown enlarged in single (panel b) and merged colors (panel c). Marks are same as in A. (d) Statistics. Clusters per 30 µm were counted and presented in percents of total. Scale bars; 75 µm.
To understand the localization of eIF4E mRNA granules relative to spines or synapses, we carried out eIF4E mRNA FISH combined with immunocytochemistry using antibodies against PSD-95 and synaptophysin, postsynaptic and presynaptic markers, respectively (Figure 2B, a). A boxed region is shown enlarged in single-labeled or various combinations of merged images (Figure 2B, b and c, respectively). Robust PSD-95 and synaptophysin immunopuncta were distributed along the plasma membranes of soma and dendrites (Figure 2B, a, asterisks and arrows, respectively). This distribution profile is consistent with the clustering of PSD-95 in postsynaptic spine heads (Cho et al., 1992) and synaptophysin in presynaptic axon terminals. In addition, the alignment of the presynaptic and postsynaptic sites is such that the two clusters juxtapose or partially overlap. Statistical analysis indicates that clusters of PSD-95 and eIF4E mRNA that overlap with or are immediately adjacent to each other are a significant portion (38.1 ± 9.1%; n = 187) of the combined total number of clusters of PSD-95 and eIF4E (Figure 2B, d). This proportion was very significantly (P = 0.007) higher than the clusters labeled for only eIF4E mRNA (26.6 ± 8.1%). When only eIF4E mRNA clusters (n = 121) are considered, more than half (58.7 ± 11.6%) overlap with or are immediately adjacent to PSD-95 clusters. This result indicates that a significant portion of eIF4E mRNA clusters are near spines in basal unstimulated culture conditions.
KCl increases the density of eIF4E mRNA clusters and their localization with synaptic markers
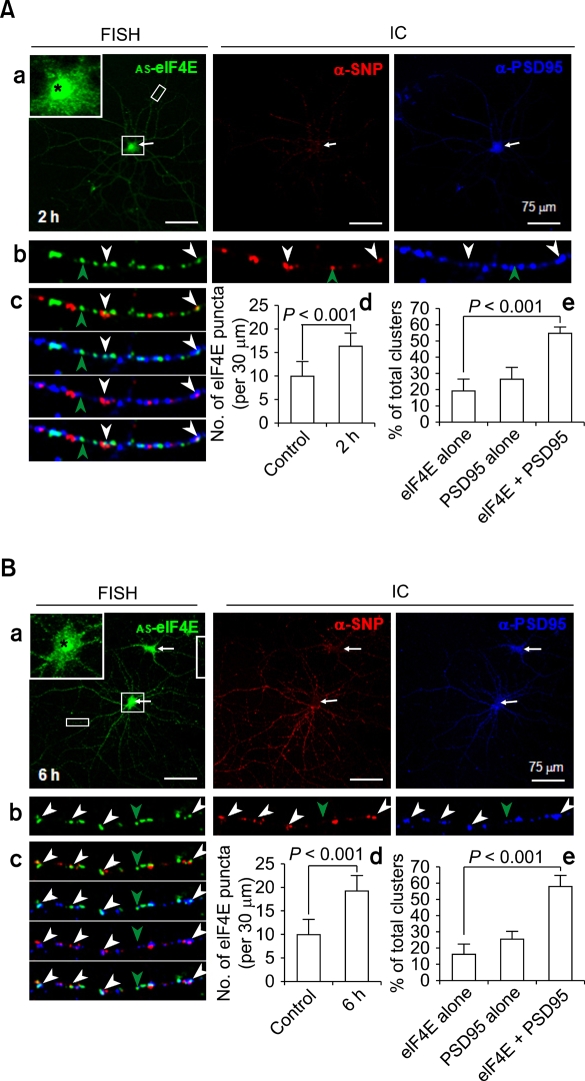
To investigate whether the localization of eIF4E mRNA clusters in dendrites is regulated by neuronal activity, we added 60 mM KCl to the culture medium for 10 min to depolarize the neurons. Neurons were fixed 2 or 6 h later and processed for FISH combined with immunocytochemistry. When examined 2 h after KCl treatment, the numbers of eIF4E mRNA clusters were noticeably increased in both the soma and dendrites (Figure 3A, a, AS-eIF4E). As shown in the inset, in which the soma (marked by an arrow) is shown enlarged, a highly elevated eIF4E FISH signal at the nucleus (asterisk) and abundant RNA clusters in the perikaryon were evident. Statistical analyses revealed that the density of eIF4E mRNA clusters in dendrites increased by 65% (from 10.1 ± 3.3 to 16.6 ± 2.7 clusters per 30 µm). An example of dendritic eIF4E mRNA clusters is shown in single-labeled or in various combinations of merged images of synaptophysin or PSD-95 immunopuncta (Figure 3A, b and c, respectively). Statistical analyses indicated that a large fraction (54.2 ± 4.2%) of the combined number of eIF4E and PSD-95 clusters (n = 226) overlapped with or were immediately adjacent to each other (Figure 3B, d). This is a statistically very significant (P < 0.001) increase from unstimulated control. When only eIF4E mRNA clusters are considered, an even larger fraction (74.4 ± 7.7%) of eIF4E clusters (n = 166) overlapped with or were immediately adjacent to PSD-95 clusters. These results indicate that neuronal activation by KCl treatment increases both the density of eIF4E mRNA clusters and their colocalization with PSD-95 in dendrites.
Figure 3.
Upregulation and redistribution of eIF4E mRNA clusters in dendrites of rat hippocampal neurons by KCl treatment. (A) 2 h post-KCl exposure. a-c, Combined FISH and IC. Rat hippocampal cultures (DIV18) were treated with KCl (60 mM, 10 min). After 2 h, cells were hybridized with DIG-labeled anti-sense eIF4E (AS-eIF4E) cRNA probes (FISH) followed by immunocytochemistry with antibodies against PSD-95 (α-PSD95) and synaptophysin (α-SNP) (panel a). The soma is marked by an arrow and shown enlarged in the inset of the FISH image. Images corresponding to the boxed dendritic area is shown enlarged at the bottom in single (panel b) or merged colors (panel c). The eIF4E mRNA clusters colocalizing with PSD-95 immunopuncta are marked by white arrowheads, while single eIF4E mRNA FISH clusters are marked by green ones. d & e, Statistics. Increase in the densitity of eIF4E clusters per 30 µm was shown (d). Co-localizing (eIF4E+PSD95) clusters along with non-colocalizing (eIF4E alone, PSD95 alone) ones were presented in percents of total (e). Statistical significance was assessed by Mann-Whitney U-test. Scale bars; 75 µm. (B) 6 h post-KCl exposure. Presentation and markings are same as in A. Scale bars; 75 µm. Note strong upregulation of eIF4E mRNA in the nuclei and perikaryon (insets) at 2 and 6 hr post-KCl, and in dendrites at 6 h post-KCl exposure.
A typical image of eIF4E FISH after 6 hr of KCl treatment is shown in Figure 3B along with immunocytochemistry images of synaptophysin (α-SNP) and PSD-95 (α-PSD95). FISH signals against eIF4E in the soma and dendrites are still increased 6 h after stimulation compared to unstimulated neurons (Figure 3B, a, AS-eIF4E). Statistical analyses showed that, compared to the untreated control, the density for eIF4E mRNA clusters (n = 192) in dendrites increased by 90% (from 10.1 ± 3.3 to 19.2 ± 3.4 clusters per 30 µm). Although this was still an increase by 16% from 2 h after KCl treatment, it was not statistically significant (P = 0.068). There was a slight increase in the fraction of eIF4E mRNA clusters that overlap with or are immediately adjacent to PSD-95 immunopuncta from 2 h post-KCl (from 74.4 ± 7.7% at 2 h to 77.8 ± 7.6% at 6 h). This difference was not statistically significant (P = 0.255). These results indicate that neurons in culture maintain elevated transcription and dendritic trafficking of eIF4E mRNA at least for 6 hrs after KCl treatment.
Discussion
Dendritic trafficking and strategic positioning of mRNAs at or near synapses are critical for local activity-dependent synapse-specific protein synthesis. In addition, strategic positioning of translation factors and their mRNAs at or near synaptic sites may be important for tight regulation of local dendritic translation. In this report we provided evidence for the presence of eIF4E mRNA granules in the dendrites of cultured rat hippocampal neurons. In addition, the density of these granules in dendrites is increased and becomes more closely associated with synaptic markers after neuronal activation by KCl.
Our ISH data indicate that eIF4E mRNAs are localized to dendrites. When viewed at high resolution by FISH, they are distributed in clusters similar to those for other known dendritic mRNAs such as αCaMKII (Mallardo et al., 2003; Kanai et al., 2004), β-actin (Tiruchinapalli et al., 2003), or AMPA-type glutamate receptor subunits 1 and 2 (GluR1 and GluR2) (Grooms et al., 2006). Combined eIF4E FISH with staufen immunocytochemistry images showed that eIF4E mRNA clusters are centrally located in dendritic cytoplasm. It is known that RNA granules are transported to the dendritic regions by kinesin, a microtubule-dependent molecular motor (Knowles et al., 1996; Brendza et al., 2000; Krichevsky and Kosik, 2001; Kanai et al., 2004; Anderson and Kedersha, 2006; Hirokawa, 2006). Therefore, the spatial distribution of eIF4E mRNA clusters in dendritic shafts further augments their identity as RNA granules. However, eIF4E mRNA is not included in the lists of dendritic mRNAs from recent reports in which gene chip analyses were carried out using either PSD fraction-associated mRNAs (Suzuki et al., 2007) or mRNAs isolated from the dendrites in the stratum radiatum of the CA1 region of rat hippocampus (Zhong et al., 2006). eIF4E mRNA may not have been detected in those studies due to low abundance of eIF4E mRNA in dendrites under basal conditions. The eIF4E protein, however, is present in dendrites (Smart et al., 2003), especially at postsynaptic sites in association with microvesicle-like structures underneath the postsynaptic membrane in the spine, some of which were localized in close proximity to PSD (Asaki et al., 2003). Although the dendritically localized eIF4E protein could have been transported from soma (Smart et al., 2003), our data suggest that it is also translated locally in dendrites.
Our results indicate that eIF4E mRNA clusters are upregulated by KCl-induced depolarization. When neurons were treated with KCl (60 mM, 10 min), highly elevated eIF4E FISH signal at the nucleus were evident, indicating that the transcription of eIF4E mRNA is upregulated. Dendritic eIF4E mRNA clusters were also noticeably increased, which indicates that the upregulated eIF4E mRNA is transported into dendrites. Upregulation of dendritic mRNA by neural activity and various signaling molecules was not unprecedented. When RNA clusters were visualized in cultured rat cortical neurons with the use of the dye SYTO 14, which labels poly-ribosome complexes, Knowles and Kosik (1997) observed enhanced labeling in dendrites after neurotrophin-3 treatment, indicating increased trafficking of RNAs in general into dendrites. An increase of specific RNA species in dendrites in response to stimulation has also been reported. Increased numbers of mRNA clusters were observed for β-actin (Tiruchinapalli et al., 2003), eukaryotic elongation factor 1A (eEF1A) (Moon et al., 2008), fragile X mental retardation 1 (Fmr1) (Antar et al., 2004), and TrkB (Tongiorgi et al., 1997; Righi et al., 2000) in dendrites of cultured hippocampal neurons when they were depolarized by KCl. Transport of eIF4E mRNA into the dendritic domain as we show in the current study may be required for the enhanced translation of any mRNAs that move into dendrites.
Our results further indicate that neuronal activation results in spatial redistribution of eIF4E mRNA clusters toward increased association with synaptic sites. Neuronal dendrites are decorated with spines and filopodia that can be rapidly modified structurally and functionally. Therefore, the relative position of eIF4E in dendrites may be important for tight regulation of local translation. Our data revealed that the eIF4E mRNA clusters are not randomly distributed but strategically positioned at or near synaptic sites in dendrites. Even in basal unstimulated culture conditions, more than half (58.7 ± 11.6%) of the total eIF4E mRNA clusters overlap with or are immediately adjacent to PSD-95 clusters. Interestingly, the density of eIF4E mRNA clusters and their degree of synaptic localization are very significantly increased by KCl treatment for at least 6 h after KCl treatment. The increased fraction of PSD-95-associated eIF4E mRNA clusters after KCl-induced depolarization is similar to the spatial redistribution observed for zipcode binding protein 1 (ZBP1) and β-actin mRNA (Tiruchinapalli et al., 2003) and Fmr1 (Antar et al., 2004), eEF1A (Moon et al., 2008) mRNA clusters. Using fluorescence microscopy and digital imaging of EGFP-ZBP1 granules in live cultured hippocampal neurons, Tiruchinapalli et al. (2003) visualized dynamic movements of ZBP1 in response to KCl-induced depolarization at high spatial and temporal resolution. They observed that, in addition to rapid, directed movement, EGFP-ZBP granules can stably localize at the base of extending filopodia and at the neck and head of spines. In addition, new granules can sometimes appear at the base of dendritic spines. Since β-actin mRNA FISH and ZBP1 immunocytochemistry signals often colocalize (Tiruchinapalli et al., 2003), their results indicate that neuronal activation can re-localize β-actin mRNA/ZBP1 granules to filopodia and spines. Therefore, accumulating data suggest that at least some kinds of dendritic mRNAs are redistributed toward postsynaptic sites.
Why eIF4E is upregulated and spatially recruited into postsynaptic sites? Increases in the postsynaptic eIF4E (Smart et al., 2003) and its mRNA (present study) suggest that elevated translation takes place at or near spines with increased neural activity. Previous reports suggest enhanced synaptic translation at active synapses. Through microarray analyses, Matsumoto et al. (2007) found that neural activity caused by an electroconvulsive shock triggered a redistribution of the dendritic transcriptome towards the area assumed to be the necks of spines, and speculated that the redistribution may accompany some changes in the ability to translate the transcriptome in response to synaptic input (Matsumoto et al., 2007). Kanhema et al. (2006) reported that BDNF-induced LTP led to rapid, transient phosphorylation of both eIF4E and elongation factor-2 in dentate gyrus homogenates, and that phospho-eIF4E and total eIF4E were enhanced in dentate granule cells. Although somatic eIF4E can be transported into dendrites and spines (Smart et al., 2003), the localization of the eIF4E mRNA in dendrites and its movement into postsynaptic sites as shown in the present study strongly suggests tight regulation of on-site protein synthesis upon neuronal activity. Strengthening this tight on-site translational regulation, Li et al. (2004) observed that electrically stimulated regions of the dendrite of an individual neuron in a hippocampal slice first caused accumulation of mitochondria in the dendrite followed by a movement of mitochondria into the spines, indicating a movement of an energy source into the active sites. Taken together, these phenomena imply that both dendritic mRNA and the machinery necessary for local translation are dynamically upregulated and recruited to postsynaptic sites upon neuronal activation.
Methods
Animals
All studies were conducted with a protocol approved by the University of Connecticut Animal Care and Use Committee in compliance with NIH guidelines for the care and use of experimental animals.
Primary culture and fixation of rat hippocampal neurons
Dissociated hippocampal cells from Sprague-Dawley rat pups on embryonic day 18 (E18) or E19 were plated onto 12-mm diameter polylysine/laminin-coated glass coverslips at a density of ~150 neurons/mm2 as described (Brewer et al., 1993) and grown in astrocyte-conditioned neurobasal media as described (Goslin, 1998; Moon et al., 2007). Cells were fixed by a sequential PFA/MeOH fixation procedure [incubation in 4% paraformaldehyde in PBS (20 mM sodium phosphate buffer, pH 7.4, 0.9% NaCl) at room temperature (RT) for 10 min followed by incubation in methanol at -20℃ for 20 min] (Moon et al., 2007).
In vitro transcription
A 672 bp DNA fragment encoding the mouse eIF4E gene (from -21 in 5'-UTR to termination) was amplified from a cDNA library (Marathon-Ready™ cDNA, Clontech, Mountain View, CA) by PCR with the primers: 5'-ccAGATCTgtgcgatcagatcgatc-3'/5'-gcGGTACCaacaacaaacctatttttagt-3'. Capital letters indicate sequences of restriction sites (BglII and KpnI, respectively) added for cloning purposes. The mouse and rat eIF4E genes have 97.5% homology with one gap at the nucleic acid level. The PCR product was cloned into the BglII and KpnI sites of a pCRII-TOPO transcription vector (Invitrogen). The digoxigenin (DIG)-labeled antisense and sense riboprobes were prepared by in vitro transcription with SP6 or T7 RNA polymerase using DIG-RNA Labeling mix according to manufacturer's instruction (Roche Applied Science, Indianapolis, IN).
In situ hybridization (ISH)
The PFA/MeOH fixed cells were incubated overnight with DIG-labeled riboprobes (200 ng/ml) at 53℃ in 400 µl of the NorthernMax Prehybridization/Hybridization buffer (Ambion, Austin, TX) and washed finally in 0.1× SSC at 53℃ for 15 min. The riboprobes were visualized by color development using monoclonal biotin-conjugated anti-digoxin antibodies (Clone DI-22, Sigma, St. Louis, MO), 1-Step NBT/BCIP substrate (Pierce, Rockford, IL), and alkaline phosphatase-conjugated streptavidin (diluted 1:1,000; Jackson ImmunoResearch Laboratories, West Grove, PA) as described (Moon et al., 2007).
Combined fluorescence in situ hybridization (FISH) and immunocytochemistry
Combined FISH and immunocytochemistry were performed with primary [biotin-conjugated mouse monoclonal anti-digoxin (1:500; Sigma), rabbit anti-synaptophysin (SNP) (1:500; Chemicon, Temecula, CA), chicken anti-PSD-95 (1:1,000; antiserum UCT-C1, Murphy et al., 2006), or rabbit anti-staufen (1:100; Chemicon)] and secondary antibodies [Alexa Fluor 488-conjugated Streptavidin, Alexa Fluor 568-conjugated goat anti-rabbit and Alexa Fluor 647-conjugated goat anti-chicken IgG (each diluted 1:1,000 in blocking buffer; Invitrogen)] as described (Moon et al., 2007).
Light and laser-scanning confocal microscopy
A Leica Research Microscope DM IRE2 (Leica Microsystems AG, Wetzlar, Germany) was used for light microscopy of color-developed ISH samples. Images were acquired with a high-resolution CoolSNAP™ CCD camera (Photometrics Inc., Germany) under the control of a computer equipped with Leica FW4000 software. Exposure times were set so that pixel brightness was not saturated and were held constant during acquisition of all images (1388 × 1039 pixels) for each pair of sense- and antisense-probed cells. Confocal images of combined FISH and immunocytochemistry (1024 × 1024 pixels) were acquired using 100× oil-immersion lens on the Leica TCS SP2 confocal system with laser lines at 488, 543, and 633 nm and processed with the use of Adobe Systems Photoshop 5.0 software.
Analysis
The number of puncta from FISH or immunocytochemistry per 30 µm dendrites (n = 10~15) of typical pyramidal neurons (n = 2~4) from two independent experiments were counted, and expressed in % of total (mean ± SD). Statistical significance was assessed by Mann-Whitney U-test. The P values less than 0.05 and 0.01 were considered to be significant and very significant, respectively.
Acknowledgments
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD) (KRF-2006-311-E00143).
Abbreviations
- eIF4E
eukaryotic translation initiation factor 4E
- FISH
fluorescence in situ hybridization
- αCaMKII
α-subunit of the type II Ca2+/calmodulin-dependent protein kinase
References
- 1.Anderson P, Kedersha N. RNA granules. J Cell Biol. 2006;172:803–808. doi: 10.1083/jcb.200512082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antar LN, Afroz R, Dictenberg JB, Carroll RC, Bassell GJ. Metabotropic glutamate receptor activation regulates fragile x mental retardation protein and FMR1 mRNA localization differentially in dendrites and at synapses. J Neurosci. 2004;24:2648–2655. doi: 10.1523/JNEUROSCI.0099-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asaki C, Usuda N, Nakazawa A, Kametani K, Suzuki T. Localization of translational components at the ultramicroscopic level at postsynaptic sites of the rat brain. Brain Res. 2003;972:168–176. doi: 10.1016/s0006-8993(03)02523-x. [DOI] [PubMed] [Google Scholar]
- 4.Ashraf SI, McLoon AL, Sclarsic SM, Kunes S. Synaptic protein synthesis associated with memory is regulated by the RISC pathway in Drosophila. Cell. 2006;124:191–205. doi: 10.1016/j.cell.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 5.Brendza RP, Serbus LR, Duffy JB, Saxton WM. A function for kinesin I in the posterior transport of oskar mRNA and Staufen protein. Science. 2000;289:2120–2122. doi: 10.1126/science.289.5487.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- 7.Browning KS. The plant translational apparatus. Plant Mol Biol. 1996;32:107–144. doi: 10.1007/BF00039380. [DOI] [PubMed] [Google Scholar]
- 8.Burgin KE, Waxham MN, Rickling S, Westgate SA, Mobley WC, Kelly PT. In situ hyridization histochemistry of Ca2+/calmodulin-dependent protein kinase in developing rat brain. J Neurosci. 1990;10:1788–1798. doi: 10.1523/JNEUROSCI.10-06-01788.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho KO, Hunt CA, Kennedy MB. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron. 1992;9:929–942. doi: 10.1016/0896-6273(92)90245-9. [DOI] [PubMed] [Google Scholar]
- 10.Cracco JB, Serrano P, Moskowitz SI, Bergold PJ, Sacktor TC. Protein synthesis-dependent LTP in isolated dendrites of CA1 pyramidal cells. Hippocampus. 2005;15:551–556. doi: 10.1002/hipo.20078. [DOI] [PubMed] [Google Scholar]
- 11.Goslin K, Assmussen H, Banker G. Rat hippocampal neurons in low density culture. In: Banker G, Goslin K, editors. Culturing Nerve Cells. 2nd Ed. Cambridge, MA: MIT Press; 1998. pp. 339–370. [Google Scholar]
- 12.Grooms SY, Noh KM, Regis R, Bassell GJ, Bryan MK, Carroll RC, Zukin RS. Activity bidirectionally regulates AMPA receptor mRNA abundance in dendrites of hippocampal neurons. J Neurosci. 2006;26:8339–8351. doi: 10.1523/JNEUROSCI.0472-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gross JD, Moerke NJ, von der Haar T, Lugovskoy AA, Sachs AB, McCarthy JE, Wagner G. Ribosome loading onto the mRNA cap is driven by conformational coupling between eIF4G and eIF4E. Cell. 2003;115:739–750. doi: 10.1016/s0092-8674(03)00975-9. [DOI] [PubMed] [Google Scholar]
- 14.Hirokawa N. mRNA Transport in Dendrites: RNA Granules, Motors, and Tracks. J Neurosci. 2006;26:7139–7142. doi: 10.1523/JNEUROSCI.1821-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang YY, Kandel ER. Theta frequency stimulation induces a local form of late phase LTP in the CA1 region of the hippocampus. Learn Mem. 2005;12:587–593. doi: 10.1101/lm.98905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanai Y, Dohmae N, Hirokawa N. Kinesin transports RNA: isolation and characterization of an RNA-transporting granule. Neuron. 2004;43:513–525. doi: 10.1016/j.neuron.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 17.Kang H, Schuman EM. A requirement for local protein synthesis in neurotrophin-induced synaptic plasticity. Science. 1996;273:1402–1406. doi: 10.1126/science.273.5280.1402. [DOI] [PubMed] [Google Scholar]
- 18.Kanhema T, Dagestad G, Panja D, Tiron A, Messaoudi E, Havik B, Ying SW, Nairn AC, Sonenberg N, Bramham CR. Dual regulation of translation initiation and peptide chain elongation during BDNF-induced LTP in vivo: evidence for compartment-specific translation control. J Neurochem. 2006;99:1328–1337. doi: 10.1111/j.1471-4159.2006.04158.x. [DOI] [PubMed] [Google Scholar]
- 19.Kiebler MA, Hemraj I, Verkade P, Kohrmann M, Fortes P, Marion RM, Ortin J, Dotti CG. The mammalian staufen protein localizes to the somatodendritic domain of cultured hippocampal neurons: implications for its involvement in mRNA transport. J Neurosci. 1999;19:288–297. doi: 10.1523/JNEUROSCI.19-01-00288.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HK, Kim YB, Kim EG, Schuman E. Measurement of dendritic mRNA transport using ribosomal markers. Biochem Biophys Res Commun. 2005;328:895–900. doi: 10.1016/j.bbrc.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 21.Kleiman R, Banker G, Steward O. Development of subcellular mRNA compartmentation in hippocampal neurons in culture. J Neurosci. 1994;14:1130–1140. doi: 10.1523/JNEUROSCI.14-03-01130.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knowles RB, Kosik KS. Neurotrophin-3 signals redistribute RNA in neurons. Proc Natl Acad Sci USA. 1997;94:14804–14808. doi: 10.1073/pnas.94.26.14804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knowles RB, Sabry JH, Martone ME, Deerinck TJ, Ellisman MH, Bassell GH, Kosik KS. Translocation of RNA granules in living neurons. J Neurosci. 1996;16:7812–7820. doi: 10.1523/JNEUROSCI.16-24-07812.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohrmann M, Luo M, Kaether C, DesGroseillers L, Dotti CG, Kiebler MA. Microtubule-dependent recruitment of Staufen-green fluorescent protein into large RNA-containing granules and subsequent dendritic transport in living hippocampal neurons. Mol Biol Cell. 1999;10:2945–2953. doi: 10.1091/mbc.10.9.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krichevsky AM, Kosik KS. Neuronal RNA granules: a link between RNA localization and stimulation-dependent translation. Neuron. 2001;32:683–696. doi: 10.1016/s0896-6273(01)00508-6. [DOI] [PubMed] [Google Scholar]
- 26.Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Macchi P, Kroening S, Palacios IM, Baldassa S, Grunewald B, Ambrosino C, Goetze B, Lupas A, St Johnston D, Kiebler M. Barentsz, a new component of the Staufen-containing ribonucleoprotein particles in mammalian cells, interacts with Staufen in an RNA-dependent manner. J Neurosci. 2003;23:5778–5788. doi: 10.1523/JNEUROSCI.23-13-05778.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mallardo M, Deitinghoff A, Muller J, Goetze B, Macchi P, Peters C, Kiebler MA. Isolation and characterization of Staufen-containing ribonucleoprotein particles from rat brain. Proc Natl Acad Sci USA. 2003;100:2100–2105. doi: 10.1073/pnas.0334355100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathews MB, Sonenberg N, Hershey JWB. Origins and principles of translational control. In: Sonenberg N, Hershey JWB, Mathews MB, editors. Translational Control in Gene Expression. New York, NY: Cold Spring Harbor Laboratory Press; 2000. pp. 1–32. [Google Scholar]
- 30.Matsumoto M, Setou M, Inokuchi K. Transcriptome analysis reveals the population of dendritic RNAs and their redistribution by neural activity. Neurosci Res. 2007;57:411–423. doi: 10.1016/j.neures.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 31.Miller S, Yasuda M, Coats JK, Jones Y, Martone ME, Mayford M. Disruption of dendritic translation of CaMKIIalpha impairs stabilization of synaptic plasticity and memory consolidation. Neuron. 2002;36:507–519. doi: 10.1016/s0896-6273(02)00978-9. [DOI] [PubMed] [Google Scholar]
- 32.Moon IS, Cho SJ, Jin I, Walikonis R. A simple method for combined fluorescence in situ hybridization and immunocytochemistry. Mol Cells. 2007;24:76–82. [PubMed] [Google Scholar]
- 33.Moon IS, Cho SJ, Lee H, Seog DH, Jung YW, Jin I, Walikonis R. Upregulation by KCl treatment of eukaryotic translation elongation factor 1A (eEF1A) mRNA in the dendrites of cultured rat hippocampal neurons. Mol Cells. 2008;25:538–544. [PubMed] [Google Scholar]
- 34.Murphy JA, Jensen ON, Walikonis RS. BRAG1, a Sec7 domain-containing protein, is a component of the postsynaptic density of excitatory synapses. Brain Res. 2007;1120:35–45. doi: 10.1016/j.brainres.2006.08.096. [DOI] [PubMed] [Google Scholar]
- 35.Paradies MA, Steward O. Multiple subcellular mRNA distribution patterns in neurons: a nonisotopic in situ hybridization analysis. J Neurobiol. 1997;33:473–493. doi: 10.1002/(sici)1097-4695(199710)33:4<473::aid-neu10>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 36.Righi M, Tongiorgi E, Cattaneo A. Brain-derived neurotrophic factor (BDNF) induces dendritic targeting of BDNF and tyrosine kinase B mRNAs in hippocampal neurons through a phosphatidylinositol-3 kinase-dependent pathway. J Neurosci. 2000;20:3165–3174. doi: 10.1523/JNEUROSCI.20-09-03165.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smart FM, Edelman GM, Vanderklish PW. BDNF induces translocation of initiation factor 4E to mRNA granules: evidence for a role of synaptic microfilaments and integrins. Proc Natl Acad Sci USA. 2003;100:14403–14408. doi: 10.1073/pnas.2436349100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sossin WS, DesGroseillers L. Intracellular trafficking of RNA in neurons. Traffic. 2006;7:1581–1589. doi: 10.1111/j.1600-0854.2006.00500.x. [DOI] [PubMed] [Google Scholar]
- 39.Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127:49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki T, Tian QB, Kuromitsu J, Kawai T, Endo S. Characterization of mRNA species that are associated with postsynaptic density fraction by gene chip microarray analysis. Neurosci Res. 2007;57:61–85. doi: 10.1016/j.neures.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 41.Tian QB, Nakayama K, Okano A, Suzuki T. Identification of mRNAs localizing in the postsynaptic region. Brain Res Mol Brain Res. 1999;72:147–157. doi: 10.1016/s0169-328x(99)00214-4. [DOI] [PubMed] [Google Scholar]
- 42.Tiruchinapalli DM, Oleynikov Y, Kelic S, Shenoy SM, Hartley A, Stanton PK, Singer RH, Bassell GJ. Activity-dependent trafficking and dynamic localization of zipcode binding protein 1 and beta-actin mRNA in dendrites and spines of hippocampal neurons. J Neurosci. 2003;23:3251–3261. doi: 10.1523/JNEUROSCI.23-08-03251.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tongiorgi E, Righi M, Cattaneo A. Activity-dependent dendritic targeting of BDNF and TrkB mRNAs in hippocampal neurons. J Neurosci. 1997;17:9492–9505. doi: 10.1523/JNEUROSCI.17-24-09492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vickers CA, Dickson KS, Wyllie DJ. Induction and maintenance of late-phase long-term potentiation in isolated dendrites of rat hippocampal CA1 pyramidal neurons. J Physiol. 2005;568:803–813. doi: 10.1113/jphysiol.2005.092924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.von der Haar T, Gross JD, Wagner G, McCarthy JE. The mRNA cap-binding protein eIF4E in post-transcriptional gene expression. Nat Struct Mol Biol. 2004;11:503–511. doi: 10.1038/nsmb779. [DOI] [PubMed] [Google Scholar]
- 46.Ying SW, Futter M, Rosenblum K, Webber MJ, Hunt SP, Bliss TV, Bramham CR. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J Neurosci. 2002;22:1532–1540. doi: 10.1523/JNEUROSCI.22-05-01532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhong J, Zhang T, Bloch LM. Dendritic mRNAs encode diversified functionalities in hippocampal pyramidal neurons. BMC Neurosci. 2006;7:17. doi: 10.1186/1471-2202-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]