Abstract
This study has assessed the relative proportions of type I and II collagens and IIA procollagen in full depth biopsies of repair tissue in a large sample of patients treated with autologous chondrocyte implantation (ACI). Sixty five full depth biopsies were obtained from knees of 58 patients 8–60 months after treatment by ACI alone (n = 55) or in combination with mosaicplasty (n = 10). In addition articular cartilage was examined from eight individuals (aged 10–50) as controls. Morphology and semi-quantitative immunohistochemistry for collagen types I and II and procollagen IIA in the repair tissue were studied. Repair cartilage thickness was 2.89 ± 1.5 mm and there was good basal integration between the repair cartilage, calcified cartilage and subchondral bone. Sixty five percent of the biopsies were predominantly fibrocartilage (mostly type I collagen and IIA procollagen), 15% were hyaline cartilage (mostly type II collagen), 17% were of mixed morphology and 3% were fibrous tissue (mostly type I collagen). Type II collagen and IIA procollagen were usually found in the lower regions near the bone and most type II collagen was present 30–60 months after treatment. The presence of type IIA procollagen in the repair tissue supports our hypothesis that this is indicative of a developing cartilage, with the ratio of type II collagen:procollagen IIA increasing from < 2% in the first two years post-treatment to 30% three to five years after treatment. This suggests that cartilage repair tissue produced following ACI treatment, is likely to take some years to mature.
Keywords: Collagen types, Hyaline cartilage, Fibrocartilage, ACI, Morphology
1. Introduction
Damaged articular cartilage is known to be less capable of repair than most other tissues in the body due to the absence of a vascular system, the immobility of chondrocytes and the limited ability of mature chondrocytes to proliferate and regenerate new cartilage [1,2]. However, various surgical techniques have been developed to stimulate biological repair, such as microfracture, subchondral drilling, osteochondral autologous transplantation (OATS or mosaicplasty) or autologous chondrocyte implantation (ACI [3]). The latter is the most commonly used cell-based therapy for the treatment of cartilage defects in the young human [4,5]. The objective of these procedures is to stimulate autologous cells to synthesise components of articular cartilage and to organize them in a manner resembling that seen in normal articular cartilage.
Type II collagen makes up more than 90% of the collagen found in adult articular (hyaline) cartilage [6] and it occurs to a smaller extent in fibrocartilage tissue such as the intervertebral disc and the meniscus. In contrast, type I collagen is either present in very small amounts or is absent from hyaline cartilage but is abundant in fibrocartilage [7–9]. These fibrillar collagens are synthesized from larger, precursor procollagen molecules. Type II procollagen has been shown to exist in two forms generated by alternative splicing of the precursor mRNAs: type IIA procollagen, which contains a region of a cysteine-rich NH2 propeptide consisting of 69 amino acids, and type IIB procollagen in which this extra propeptide is absent [10,11]. Type IIB procollagen is synthesized by mature, fully differentiated chondrocytes whilst type IIA is thought to be produced by chondroprogenitor cells (e.g. chondrogenic mesenchymal and perichondrium cells) [12]. The extra 69 amino acid domain in the procollagen type IIA propeptide has been shown to bind with bone morphogenetic growth factors including BMP-2 and TGF-β. This has led to a suggested role for procollagen IIA of binding growth factors in the developing chondrogenic matrix [12].
Investigators have found that different surgical methods result in varying types of repair tissue. For example, fibrocartilage often forms after subchondral drilling and microfracture [13,14], whereas following ACI a mixture of hyaline and fibrocartilage occurs, with a greater presence of type II collagen reported [15], particularly in the deep zone [16,17]. There is some evidence that the repair tissue may mature with time after ACI treatment [18], with 45% of patients demonstrating hyaline cartilage more than 18 months post-ACI compared to 24% at earlier time points [19]. The presence of hyaline tissue containing type II collagen in post-ACI repair correlated with good to excellent clinical results [15,19,20]. Therefore, it has been assumed that the presence of type II collagen is the ideal aim for healthy hyaline cartilage [9]. Whilst type II collagen has been reported in repair tissue [21], this alone is not an indication of hyaline cartilage as it can be common in fibrocartilage, such as the intervertebral disc (where it can constitute 80% of the total collagen) [7]. Hence, the presence of type II collagen alone is not an adequate marker of hyaline cartilage repair.
Studies to date have been carried out on either relatively small samples or with limited histological information [3,9,15–18,21–28]. The aim of the present study was to carry out a comprehensive histological study of the repair tissue formed in a large sample of patients treated with ACI for chondral defects or ACI and mosaicplasty for osteochondral damage and attempt to determine the proportion of collagen types I and II. In addition, type IIA procollagen was investigated, to test the hypothesis that the repair tissue would resemble developing cartilage found in young, immature articular cartilage.
2. Materials and methods
Sixty five full depth biopsies were obtained from 58 patients (at an average age of 34 ± 9.8 years) who had been treated with ACI for cartilage defects according to the method of Brittberg et al. [3]; 10 of these had osteochondral defects with damage to the bone of more than 3 mm in depth and thus received ACI in combination with mosaicplasty (carried out as one procedure). Arthroscopic examination and biopsy of the graft were performed at approximately 12 months (mean 15.7 ± 10.7, range 8–60 months), as approved by the local Ethical Committee. Biopsies of 1.8 mm in diameter were taken (using a juvenile bone marrow biopsy needle) from the centre of the treated region and included both the repair cartilage tissue and, where possible, the subchondral bone, as detailed previously [17]. A mapping system is used at all stages of ACI in our centre, ensuring accurate sampling location of the biopsy [29]. Thirty-five biopsies were taken from the left knee and 30 from the right. Forty five biopsies were taken from the medial femoral condyle which was the most common site of treatment, 16 biopsies from the lateral femoral condyle, three from the trochlea and one from the patella. For comparison, ‘normal’ articular cartilage was examined from individuals aged 19 and 33 years from femoral heads obtained at autopsy, from the medial femoral condyles of the knee from individuals aged 22, 30, 40 and 50 years obtained at autopsy and from the ankle joints of individuals aged 10 and 13 years undergoing corrective surgery.
The biopsy samples were either snap frozen and stored in liquid nitrogen, or fixed in formalin, decalcified and paraffin embedded until processing for histology and immunohistochemistry. Frozen sections (7 µ thick) or wax sections (4 µ thick) were cut and stained with haematoxylin and eosin (H&E) or toluidine blue to assess morphology and the degree of metachromatic staining (as a crude assessment of glycosaminoglycan content), respectively. If any samples appeared to have mineralization within the repair cartilage on H&E stained sections, this was confirmed by von Kossa staining on frozen sections [30]. Sections were viewed under normal bright field illumination and polarized light, as recommended by the International Cartilage Repair Society [31]. Other histological parameters were assessed and scored, the sum of them giving an overall semi-quantitative ‘OsScore’, which we have used previously [17]. A maximum score of 10 corresponded to normal adult articular cartilage. These parameters included the integrity of the articular surface, integration with the underlying bone, the presence of ectopic mineralization or vascularisation within the cartilage, the degree of cell clustering, metachromasia and the morphology or type of repair cartilage present. Tissue morphology was classified as predominantly hyaline, predominantly fibrocartilage, a mixture of the two (mixed) or fibrous tissue with no true cartilage present, as described previously [17,28]. Hyaline cartilage was clearly differentiated from fibrocartilage by the homogenous appearance of the matrix, particularly when viewed under polarized light, and the round or oval shape of the cells, often surrounded by lacunae. Fibrocartilage, in contrast, had obvious bundles of collagen fibres lying in a random, irregular manner and often more elliptical shaped cells.
Tissue sections were fixed on a slide if frozen or de-waxed if they were paraffin embedded, followed by treatment with hyaluronidase (Sigma; 4800 U/ml in 0.025 M NaCl 0.05 M sodium acetate pH 5.0, 2 h at RT for frozen sections) or hyaluronidase and trypsin (Sigma; 0.1% hyaluronidase and 0.2% trypsin 1 h 37 °C for wax sections) to unmask the collagen antigens [17]. Sections were then incubated for 1 h at room temperature with primary antibodies against type I collagen (monoclonal antihuman, clone no 1-8H5; ICN) and against type II collagen (CIICI; Developmental Studies Hybridoma Bank, Iowa, USA) or overnight with a polyclonal antibody raised in rabbits against type IIA procollagen [32]. Type IIA procollagen immunostaining was only performed on frozen sections, whilst either frozen or wax sections were used for immunostaining of type I and II collagens. Endogenous peroxidase was blocked with 0.3% hydrogen peroxide in methanol before sections were incubated with the biotinylated secondary antibody, either anti-mouse (for collagen types I and II primary antibody) or anti-rabbit (for type IIA procollagen). The signal was amplified using an avidin–biotin–peroxidase reagent (Vectastain Elite ABC kit; Vector Laboratories, Peterborough, UK) and labelling was visualized with diaminobenzidine as substrate. Sections were then washed, dehydrated, and mounted in pertex. ‘Control’ sections of biopsy samples were treated either with normal mouse IgG, normal rabbit serum or phosphate buffered saline alone, in place of the primary antibodies and then treatment continued in the same way as all the other slides.
The degree of immunohistochemical labelling was assessed semi-quantitatively by measuring the areas of the repair cartilage, on adjacent sections where possible, which were immunostained for collagen types I and II and type IIA procollagen. Images were captured using an Optivision Image Capture system. The area of positive staining was delineated and this was calculated as a percentage of the total area of uncalcified cartilage. Calculations were performed using an image analysis system (Aequitas 1a, Dynamic Data Links, Cambridge, UK). The coefficient of variance for these measurements was 1.1%.
Non-parametric statistical analyses (Mann–Whitney U test and Spearman rank (rs) correlations) were carried out using a software programme (Analyse-it Software Ltd, Leeds, UK). Means are quoted ± standard deviation.
3. Results
The repair cartilage was on average 2.89 mm (± 1.5, range 0.2–7.8) in thickness (compared to 1.8 mm ± 0.6 for the control cartilage). Morphological analysis of all the biopsy samples revealed that 63% (41/65) of the biopsies were predominantly fibrocartilage, 23% (15/65) mixed (fibrocartilage and hyaline cartilage) morphology, 10.8% (7/65) predominantly hyaline cartilage and 3.1% (2/65) fibrous tissue. There was no apparent difference between locations treated. Of the 11 biopsies from patients treated with mosaicplasty in combination with ACI, a predominance of hyaline cartilage was seen in two, fibrocartilage in five and mixed morphology in four. This was in comparison to the samples of ‘normal’ articular cartilage (both cadaveric and surgical), which were all hyaline cartilage throughout, with no variation between locations.
The surface integrity and smoothness was normal or near normal in 75% of samples and abnormal in 25%. Eighty two percent of the biopsies included the underlying bone and in all cases there was good basal integration between the repair cartilage, calcified cartilage and subchondral bone (Fig. 1a). A minority of sections showed vascularisation and mineralization (Fig. 1b–c) in the repair tissue, occurring in 12% of the biopsies for both parameters. When mineralization was present it was usually in very discrete, small areas. The overall OsScore for all repair biopsies was 6.6 ± 1.7 (n = 65, range 2.5–10), whilst the ‘normal’ samples scored 9.7 ± 0.4 (range 9.2–10).
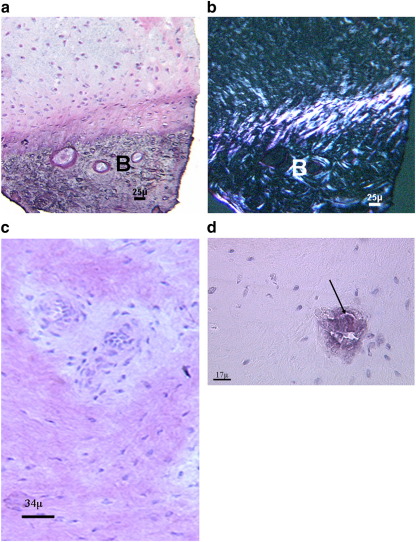
Fig. 1.
(a) Good integration of the repair tissue with the underlying bone (B) was a very common finding. This is clearly visible when viewed with polarized light (b) (a and b: 40 year old male; 13 months post-ACI). Vascularisation (arrow) was an infrequent occurrence (c; 39 year old male, 16 months post-ACI treatment), as was mineralization (d; arrow; 25 year old male, 21 months post-ACI treatment). H&E stained sections.
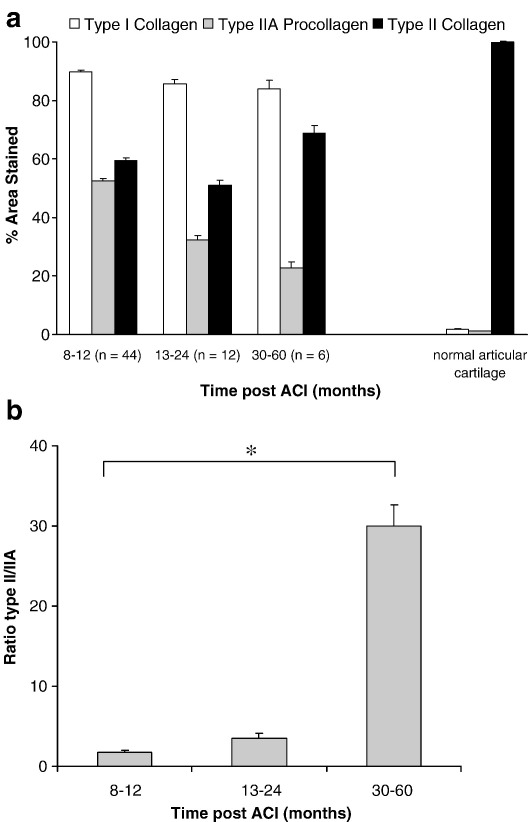
Immunohistochemistry results demonstrated some immunopositivity for type I and II collagens to varying extents in all patient samples, whilst all but two stained for procollagen IIA. The percentage of the area stained for each antibody is shown in Table 1; treatment with ACI and mosaicplasty combined appeared to have little effect on the results, apart from having slightly more type II collagen (probably from the implanted core). The pattern of immunostaining for type IIA procollagen was typically in the lower part of the core, close to the calcified cartilage and bone; sometimes it was throughout the matrix (weakly), but it was more commonly cell associated (Fig. 2). ‘Normal’ articular cartilage samples were immunopositive for type II collagen with no staining (or at most a thin strip at the surface) for type I collagen. Procollagen IIA, in contrast, showed an apparent age-related difference in distribution in the ‘normals’. The cartilage from younger individuals (13 years of age and less) was weakly positive throughout the matrix with stronger pericellular staining around cells in the deeper zones. A similar but weaker pattern was seen in cartilage from the 19 year old but there was little staining in any samples from older individuals.
Table 1.
Percentage area of repair tissue stained immunopositively for antibodies against collagen types I, II or procollagen IIA.
| Collagen | All samples | ACI alone | ACI + mosaicplasty | ‘Control’ cartilage |
|---|---|---|---|---|
| I | 90.9 ± 22.3 (65) | 90.9 ± 23.9 (55) | 89.8 ± 23.3 (10) | 1.7 ± 0.8 (8) |
| II | 60.0 ± 31.3 (65) | 56.8 ± 33.8 (55) | 72.8 ± 24.0 (10) | 100 ± 0.1 (8) |
| Procollagen IIA | 45.5 ± 35.5 (54) | 44.4 ± 37.1 (46) | 47.0 ± 27.5 (8) | 1.2 ± 0.4 (5) |
Results are means ± standard deviation with number of samples analysed in parentheses.
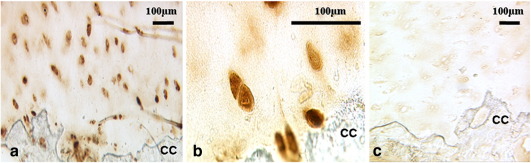
Fig. 2.
Immunostaining for type IIA procollagen was frequently strong in the cells closest to the calcified cartilage (CC) and bone. (a,b) Type IIA procollagen and (c) normal rabbit control; 46 year old female 14 months post-ACI.
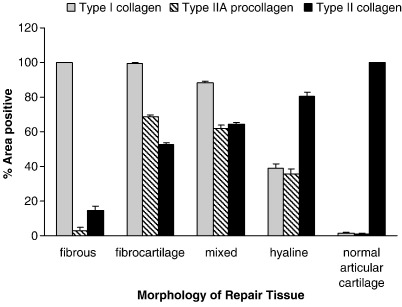
The percentage of areas of normal articular cartilage immunopositively stained for collagen type I was 1.7% and for type II collagen was 100%. This is in accordance with biochemical measures of 0.2% of the total collagen in articular cartilage being type I collagen and 96% being type II [33]. The predominant type of collagen in the repair tissue in our study varied significantly with different cartilage morphologies and these are displayed in Fig. 3. The greatest area of immunostaining for type I collagen was seen in fibrous tissue and fibrocartilage and least in hyaline cartilage (p < 0.0001), whereas the greatest area of immunostaining for type II collagen was in the hyaline cartilage repair tissue and least in fibrous and fibrocartilaginous repair (p < 0.001 between hyaline and fibrocartilage). Whilst 63% ± 44, (n = 29) of the area of fibrocartilage and 48% ± 32, (n = 5) of hyaline cores stained for type IIA procollagen, the difference was not significant. There was, however, some correlation in the areas that immunostained for type II collagen and type IIA procollagen (rs = 0.62; p = < 0.0001), as expected, partly due to the presence of the epitope recognized by the type II antibody in the procollagen.
Fig. 3.
The percentage of the sections of repair cartilage which was immunopositive for the collagens I, II or procollagen IIA varied according to morphology, in comparison to normal articular cartilage. Bars represent standard error of the mean.
There was a difference in the areas, which were immunopositive for the collagen types in cores taken at different intervals after treatment (Fig. 4). The percentage of areas of sections staining positively for type I collagen or type IIA procollagen were the least at three or more years post-treatment whilst for type II collagen it was greater three years or more post-treatment (Fig. 5a). The ratio of type II/IIA was significantly greater (at 30.1% ± 31.4; p = 0.005) at 3 years post-ACI than at 12 months when it was 1.8% ± 2.7 (Fig. 5b). Seventy-five percent (± 21, n = 5) of the area of biopsies was immunopositive for type II collagen in those taken more than 36 months after treatment whereas it was 52% ± 42 (n = 9) of the area in those taken less than 12 months after.
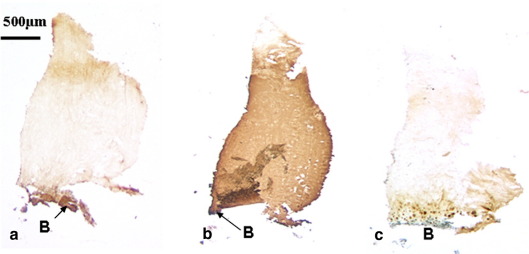
Fig. 4.
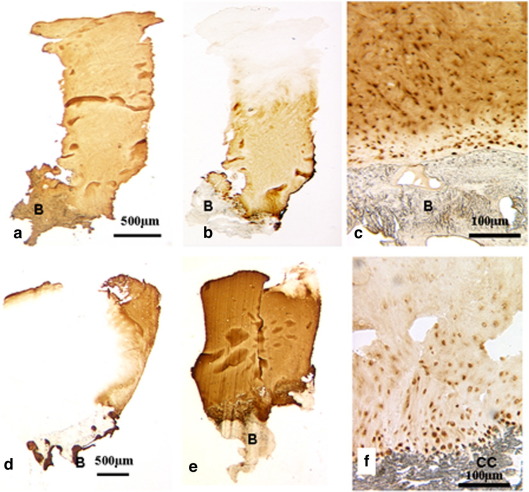
A biopsy obtained from a 32 year old, 60 months post-ACI treatment (a–c) showed little staining for either types I (a) or IIA procollagen (c), whereas there was immunopositivity for type II collagen throughout, apart from at the surface (b). B: Bone.
Fig. 5.
(a) The percentage of sections of repair cartilage immunopositive for collagens I, II or procollagen IIA at different time intervals post-treatment. (b) The ratio of areas immunostained with type II:type IIA procollagen increased significantly with time after treatment (⁎p = 0.005). Bars represent standard error of the mean.
In some samples the repair tissue was completely immunopositive for all three antibodies used. For others, particularly where fibrocartilage predominated, staining was present throughout the core for type I collagen (Fig. 6a) whilst collagen type II and procollagen IIA staining was restricted to the lower regions (Fig. 6b and c). In contrast, other samples, particularly those that were mostly hyaline cartilage, had little staining for type I collagen compared to types II or IIA (Fig. 6d–f). If staining for type II collagen and type IIA procollagen did not occur throughout the complete core, then it was most frequently found towards the lower region of the core in the cartilage nearest the bone. There was often a great deal of overlap of regions positive for collagen types I, II and IIA procollagen.
Fig. 6.
Most samples which were fibrocartilaginous or mixed morphology (as viewed with polarized light) were immunopositive for type I throughout (a) with some type II in the lower region (b). In contrast tissue of hyaline morphology was strongly stained for type II collagen (e) in the matrix with little type I (d). Procollagen IIA was seen both in the cells and the pericellular matrix in the deep zone (c,f). (a, b 25 year old male 20 months post-ACI; c 58 year old male, 14 months post-ACI; d–f 42 year old female, 11 months post-treatment). B: Bone; CC: calcified cartilage.
4. Discussion
This study provides the most extensive histological examination of a large series of biopsies from ACI-treated patients to date. In addition, the complete area from articulating surface to the bone, has been assessed semi-quantitatively for the make-up of the fibrillar collagens, types I and II, in addition to procollagen IIA.
All samples showed good basal integration with the underlying bone; this is the trend with human ACI repair and unlike the situation in animal models in which there is often poor and incomplete integration with the host tissue [34,35]. This species difference may depend to some extent on post-operative loading, since the type of motion applied to cartilage repair systems has been shown to be very important in vitro [36]. The relevance of mineralization and vascularisation in the repair tissue is not known. Certainly neither is a feature of normal articular cartilage. These parameters were sought and included in the histological score, OsScore (as negative features), specifically because the periosteum, which is used in traditional ACI, is a potentially active tissue and can lead to calcification or have blood vessels within it. One might expect that vascularisation and/or mineralization of the repair tissue could lead to its premature degradation. However, there is no evidence from this or other studies to date of the deleterious effect of either.
The proportion of samples which were hyaline or a mixture of hyaline and fibrocartilage morphology in this study (35%) is not so dissimilar to that reported by Bartlett et al. [27]: 44% in ACI and 36% in matrix autologous chondrocyte implantation (MACI) or 48% of ACI with hyaluronan [19]. Parameters used in our centre to classify morphology are fairly stringent in comparison to many other studies. Tissue, which under normal bright light microscopy may appear hyaline (i.e. with oval chondrocyte-like cells in lacunae, sparsely populating the matrix), can often be clearly demonstrated to be fibrocartilage if viewed with polarized light. In addition, in our centre we obtain and assess the full depth of repair tissue, which is not the case in all reports [28,31]. It might be expected that biopsies from patients treated with the combination of mosaicplasty and ACI would show predominantly hyaline cartilage. However, of the ten biopsies from patients who underwent this combined treatment, only two were classified as hyaline. This may have arisen because biopsies were taken from areas between the transplanted cores, a situation which occurred for two likely reasons: (1) defects treated in this manner had fewer transplanted cores (covering approximately 50% of the area) than if treated with mosaicplasty alone, and (2) the ‘intercore’ area was targeted for biopsy to some extent because it was a region of particular interest to monitor post-treatment events. Unfortunately we were not able to assess the lateral integration of the repair and adjacent tissue microscopically in this study, as the biopsy was taken from the central region as recommended by the ICRS [29].
Those samples that were hyaline in nature had more immunopositivity for type II collagen than type I or type IIA procollagen and, as we have found previously, the hyaline tissue occurred in the lower zones nearer the bone. In addition, as expected, the predominantly fibrous and fibrocartilage cores stained throughout for type I collagen, whilst areas of staining for types II and IIA were smaller and restricted to the lower zones of these samples. This study highlights the important fact that repair cartilage which is of fibrocartilage morphology contains a significant proportion of type II collagen; hence type II collagen alone is not a sufficient indication of hyaline cartilage regeneration. Development of type II collagen production appeared to increase with time after ACI repair (59% and 69% of areas were immunopositive at < 12 and 30–60 months, respectively), but this was not significant. The presence of a large amount of immunostaining for type I collagen in what is classified as hyaline cartilage may be surprising and it is certainly more than would be expected for mature adult human articular cartilage.
Type IIA procollagen is important in cartilage development [37,38], particularly in the early stages [39]. Type IIA procollagen, together with the mRNA transcript, is re-expressed by adult articular chondrocytes in the mid zone of osteoarthritic cartilage, whilst neither is found in normal adult articular cartilage (over the age of 40 years) [11]. It is not currently clear whether the procollagen of osteoarthritic cartilage has a role in repair but it is comparable to the foetal chondroprogenitor phenotype observed in embryonic development [11] and suggests that early phases of repair involve re-expression of a developmental sequence by chondrocytes [40]. During chondrogenesis the expression of type IIA procollagen by chondroprogenitor cells precedes the expression of type IIB procollagen (‘mature’ type II collagen) by chondrocytes and chondroblasts. Hence the correlation between the area of cores immunopositive for type II collagen and type IIA procollagen is not surprising. The fact that the ratio of collagen II:IIA is so much higher at 3–5 years post-treatment than at 12 months suggests that IIA procollagen here really is indicative of chondrogenesis rather than a marker of osteoarthritis as is sometimes suggested [41]. The distribution of staining for type IIA in the repair biopsies, i.e. often being pericellular, particularly in the lower zones, is similar to the distribution seen in the younger samples of ‘normal’ cartilage examined here and also to the only other report of type IIA expression in cartilage repair tissue [21]. The presence of type IIA procollagen in 96% of our biopsies of ACI-treated sites is thus indicative of a developing cartilage with a high degree of cellular activity. This supports other studies on similar samples where other markers demonstrated an active remodelling process [18] and tissue maturation [19] on-going in the repair site [33]. Since type IIA procollagen contains a chordin-like repeat which binds growth factors including TGFβ and BMP-2, its presence could potentially facilitate a speedier production of matrix molecules and in-filling of the defect. In addition, BMP-2 is capable of reversing the de-differentiation of chondrocytes, such that it potentiates the expression of the mature type IIB procollagen by chondrocytes [41].
This study has limitations in that we have used immunohistochemistry to assess the amount of different collagen types within cartilage repair tissue. This is not a truly quantitative method [9] as uniform thickness of tissue in the sections cannot be guaranteed, nor the stoichiometry of the antibody labelling and staining procedure. In addition, the biopsy specimens are necessarily small and from a very discrete part of the treated region — no more than 1% of its total area. Hence one could question how representative and useful such a small area is. Nonetheless, this method does provide much information about the distribution of the components of the repair tissue and an indication of the degree of staining for them.
In summary, results from our study demonstrate replacement of chondral defects, which have been treated with ACI, with cartilage tissue in 97% of cases. The immunostaining of the deeper zones of biopsied material was generally typical of hyaline cartilage, being positive here for type II collagen in 96% of samples. In contrast, the presence of type II collagen was less in repair tissue of fibrocartilage morphology; here, and in fibrous tissue, type I collagen immunostaining was most common. Type IIA procollagen was present in both hyaline and fibrocartilage tissue, indicating a high degree of activity in the process of cartilage repair. Thus this supports our hypothesis that the repair tissue does indeed resemble young, immature cartilage and has chondrogenic potential. We would suggest that the same sequence of events which happens in the developing skeleton, i.e. mesenchymal cells switching expression from type I collagen to IIA and to IIB procollagen (i.e. mature type II collagen) [39], is happening with time in this repair cartilage. If the formation of true hyaline cartilage with predominantly type II collagen is important to function, then this may explain the good long term clinical results reported by Peterson et al. [15] for ACI at 9 years post-treatment.
5. Conflict of interest
There are no known conflicts of interest of any of the authors for this manuscript.
Acknowledgement
We are grateful to the Arthritis Research Campaign, UK (RO590).
References
- 1.Hunter W. The classic: of the structure and disease of articulating cartilages. Clin Orthop Relat Res. 1995;317:2–6. [PubMed] [Google Scholar]
- 2.Newman A.P. Articular cartilage repair. Am J Sport Med. 1998;26:309–324. doi: 10.1177/03635465980260022701. [DOI] [PubMed] [Google Scholar]
- 3.Brittberg M., Lindahl A., Nilsson A., Ohlsson C., Isaksson O., Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. New Eng J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 4.Bentley G., Minas T. Treating joint damage in young people. BMJ. 2000;320:1585–1588. doi: 10.1136/bmj.320.7249.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minas T., Nehrer S. Current concepts in the treatment of articular cartilage defects. Orthopaedics. 1997;20:525–538. doi: 10.3928/0147-7447-19970601-08. [DOI] [PubMed] [Google Scholar]
- 6.Eyre D. Collagen of articular cartilage. Arthritis Res. 2002;4:30–35. doi: 10.1186/ar380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eyre D.R., Muir H. Quantitative analysis of types I and II collagens in human intervertebral discs at various ages. Biochim Biophys Acta. 1977;492:29–42. doi: 10.1016/0005-2795(77)90211-2. [DOI] [PubMed] [Google Scholar]
- 8.Dudhia J., McAlinden A., Muir P., Bayliss M. The meniscus—structure, composition, and pathology. In: Hazleman B., Riley G., Speed C., editors. Soft tissue rheumatology part 1 the science of soft tissue disorders. Oxford University Press; 2004. pp. 80–96. [Google Scholar]
- 9.Hollander A.P., Dickinson S.C., Sims T.J., Soranzo C., Pavesio A. Tissue engineering of cartilage and bone. 2003. Quantitative analysis of repair tissue biopsies following chondrocyte implantation; pp. 218–233. [PubMed] [Google Scholar]
- 10.Ryan M.C., Sandell L.J. Differential expression of a cysteine-rich domain in the amino-terminal propeptide of type II (cartilage) procollagen by alternative splicing of mRNA. J Biol Chem. 1990;265:10334–10339. [PubMed] [Google Scholar]
- 11.Aigner T., Zhu Y., Chansky H.H., Matsen F.A., Maloney W.J., Sandell L.J. Reexpression of type IIA procollagen by adult articular chondrocytes in osteoarthritic cartilage. Arthritis Rheum. 1999;42:1443–1450. doi: 10.1002/1529-0131(199907)42:7<1443::AID-ANR18>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 12.Zhu Y., Oganesian A., Keene D.R., Sandell L.J. Type IIA procollagen containing the cysteine-rich amino propeptide is deposited in the extracellular matrix of prechondrogenic tissue and binds to TGF-β1 and BMP-2. J Cell Biol. 1999;144:1069–1080. doi: 10.1083/jcb.144.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furukawa T., Eyre D.R., Koide S., Glimcher M.J. Biochemical studies on repair cartilage resurfacing experimental defects in the rabbit knee. J Bone Jt Surg. 1980;62-A:79–89. [PubMed] [Google Scholar]
- 14.Marlovits S., Hombauer M., Truppe M., Vècsei V., Schlegel W. Changes in the ratio of type-I and type-II collagen expression during monolayer culture of human chondrocytes. J Bone Jt Surg. 2004;86-B:286–295. doi: 10.1302/0301-620x.86b2.14918. [DOI] [PubMed] [Google Scholar]
- 15.Peterson L., Minas T., Brittberg M., Nilsson A., Sjogren-Jansson E., Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;374:212–234. doi: 10.1097/00003086-200005000-00020. [DOI] [PubMed] [Google Scholar]
- 16.Richardson J.B., Caterson B., Evans E.H., Ashton B.A., Roberts S. Repair of human articular cartilage after implantation of autologous chondrocytes. J Bone Jt Surg. 1999;81-B:1064–1068. doi: 10.1302/0301-620x.81b6.9343. [DOI] [PubMed] [Google Scholar]
- 17.Roberts S., McCall I.W., Darby A.J., Menage J., Evans E.H., Harrison P.E. Autologous chondrocyte implantation for cartilage repair: monitoring its success by magnetic resonance imaging and histology. Arthritis Res Ther. 2003;5:R60–R73. doi: 10.1186/ar613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts S., Hollander A.P., Caterson B., Menage J., Richardson J.B. Matrix turnover in human cartilage repair tissue in autologous chondrocyte implantation. Arthritis Rheum. 2001;44:2586–2598. doi: 10.1002/1529-0131(200111)44:11<2586::aid-art439>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 19.Brun P., Dickinson S.C., van B., Cortivo R., Hollander A.P., Abatangelo G. Characteristics of repair tissue in second-look and third-look biopsies from patients treated with engineered cartilage: relationship to symptomatology and time after implantation. Arthitis Res Ther. 2008;R132:1–8. doi: 10.1186/ar2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knutsen G., Drogset J.O., Engebretsen L., Grontvedt T., Isaksen V., Ludvigsen T.C. A randomized trial comparing autologous chondrocyte implantation with microfracture. J Bone Jt Surg. 2007;89:2105–2112. doi: 10.2106/JBJS.G.00003. [DOI] [PubMed] [Google Scholar]
- 21.Briggs T.W.R., Mahroof S., David L.A., Flannelly J., Pringle J., Bayliss M. Histological evaluation of chondral defects after autologous chondrocyte implantation of the knee. J Bone Jt Surg. 2003;85-B:1077–1083. doi: 10.1302/0301-620x.85b7.13672. [DOI] [PubMed] [Google Scholar]
- 22.Peterson L., Brittberg M., Kiviranta I., Akerlund E.L., Lindahl A. Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sport Med. 2002;30:2–12. doi: 10.1177/03635465020300011601. [DOI] [PubMed] [Google Scholar]
- 23.Horas U., Pelinkovic D., Herr G., Aigner T., Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Jt Surg Am. 2003;85-A:185–192. doi: 10.2106/00004623-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Henderson I., Tuy B., Oakes B. Reoperation after autologous chondrocyte implantation. Indications and findings. J Bone Jt Surg. 2004;86-B:205–211. doi: 10.1302/0301-620x.86b2.14324. [DOI] [PubMed] [Google Scholar]
- 25.Henderson I.J.P., Tuy B., Connell D., Oakes B., Hettwer W.H. Prospective clinical study of autologous chondrocyte implantation and correlation with MRI at three and 12 months. J Bone Jt Surg. 2003;85-B:1060–1066. doi: 10.1302/0301-620x.85b7.13782. [DOI] [PubMed] [Google Scholar]
- 26.Bentley G., Biant L.C., Carrington R.W.J., Akmal M., Goldberg A., Williams A.M. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85-B:223–230. doi: 10.1302/0301-620x.85b2.13543. [DOI] [PubMed] [Google Scholar]
- 27.Bartlett W., Skinner J.A., Gooding C.R., Carrington R.W.J., Flanagan A.M., Briggs T.W.R. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee. J Bone Joint Surg Br. 2005;87-B:640–645. doi: 10.1302/0301-620X.87B5.15905. [DOI] [PubMed] [Google Scholar]
- 28.Knutsen G., Engebretsen L., Ludvigsen T.C., Drogset J.O., Grontvedt T., Solheim E. Autologous chondrocyte implantation compared with microfracture in the knee. J Bone Jt Surg. 2004;86-A:455–464. doi: 10.2106/00004623-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Talkhani I.S., Richardson J.B. Knee diagram for the documentation of arthroscopic findings of the knee-cadaveric study. Knee. 1999;6:95–101. [Google Scholar]
- 30.Roberts S., Menage J. Microscopic methods for the analysis of engineered tissues. In: Hollander A.P., Hatton P.V., editors. Methods in molecular biology. vol. 238. Humana Press Inc.; Totowa: 2004. pp. 171–195. (Biopolymer Methods in Tissue Engineering). [DOI] [PubMed] [Google Scholar]
- 31.Mainil-Varlet P., Aigner T., Brittberg M., Bullough P., Hollander A.P., Hunziker E. Histological assessment of cartilage repair. A report by the histology endpoint committee of the International Cartilage Repair Society (ICRS) J Bone Jt Surg. 2003;85-A:45–57. [PubMed] [Google Scholar]
- 32.Oganesian A., Zhu Y., Sandell L.J. Type IIA procollagen amino propeptide is localized in human embryonic tissues. J Histochem Cytochem. 1997;45:1469–1480. doi: 10.1177/002215549704501104. [DOI] [PubMed] [Google Scholar]
- 33.Dickinson S.C., Sims T.J., Pittarello L., Soranzo C., Pavesio A., Hollander A.P. Quantitative outcome measures of cartilage repair in patients treated by tissue engineering. Tissue Eng. 2005;11:277–287. doi: 10.1089/ten.2005.11.277. [DOI] [PubMed] [Google Scholar]
- 34.Grande D.A., Pitman M.I., Peterson L., Menche D., Klein M. The repair of experimentally produced defects in rabbit articular cartilage by autologous chondrocyte transplantation. J Orthop Res. 1989;7:208–218. doi: 10.1002/jor.1100070208. [DOI] [PubMed] [Google Scholar]
- 35.Breinan H.A., Minas T., Barone L., Tubo R., Hsu H.P., Shortkroff S. Histological evaluation of the course of healing of canine articular cartilage defects treated with cultured autologous chondrocytes. Tissue Eng. 1998;4:101–114. [Google Scholar]
- 36.Grad S., Lee C.R., Gorna K., Gogolewski S., Wimmer M.A., Alini M. Surface motion upregulates superficial zone protein and hyaluronan production in chondrocyte-seeded three-dimensional scaffolds. Tissue Eng. 2005;11:249–256. doi: 10.1089/ten.2005.11.249. [DOI] [PubMed] [Google Scholar]
- 37.Sandell L.J., Morris N., Robbins J.R., Goldring M.B. Alternatively spliced type II procollagen mRNAs define distinct populations of cells during vertebral development: differential expression of the amino-propeptide. J Cell Biol. 1991;114:1307–1319. doi: 10.1083/jcb.114.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aszodi A., Hunziker E.B., Olsen B.R., Fassler R. The role of collagen II and cartilage fibril-associated molecules in skeletal development. Osteoarthr Cartil. 2001;9:S150–S159. [PubMed] [Google Scholar]
- 39.Sandell L.J., Nalin A.M., Reife R.A. Alternative splice form of type II procollagen mRNA (IIA) is predominant in skeletal precursors and non-cartilaginous tissues during early mouse development. Dev Dyn. 1994;199:129–140. doi: 10.1002/aja.1001990206. [DOI] [PubMed] [Google Scholar]
- 40.Lekfoe P., Nalin A.M., Clark J.M., Reife R.A., Sugai J., Sandell L.J. Gene expression of collagen types IIA and IX correlates with ultrastructural events in early osteoarthritis: new applications of the rabbit meniscectomy model. J Rheumatol. 1997;24:1155–1163. [PubMed] [Google Scholar]
- 41.Valcourt U., Gouttenoire J., Aubert-Foucher E., Herbage D., Mallein-Gerin F. Alternative splicing of type II procollagen pre-mRNA in chondrocytes is oppositely regulated by BMP-2 and TGF-β1. FEBS Lett. 2003;545:115–119. doi: 10.1016/s0014-5793(03)00510-6. [DOI] [PubMed] [Google Scholar]