Abstract
AIM: To search the organelle based changes in hepatocytes after taurine treatment in experimental liver fibrosis induced by CCl4 administration.
METHODS: Thirty rats were divided into two groups. Group 1 (n = 15) was injected with CCl4 plus taurine and Group 2 (n = 15) with CCl4 plus saline for 12 wk. At the end of 12th wk, mitochondria, rough and smooth endoplasmic reticulum, and nuclei of hepatocytes were evaluated using a scoring system. The results were compared with histopathological findings, as well.
RESULTS: Taurine treatment reduced fibrosis scores significantly as compared to placebo. Organelle injury scores decreased significantly with taurine treatment. Ultrastructural and histopathological scores in both groups were in strong correlation (r = 0.931 for CCl4 plus taurine and r = 0.899 for CCl4 plus saline group).
CONCLUSION: Organelle based transmission electron microscopy findings can reflect successfully histological results as well as tissue healing in hepatocytes from hepatotoxin-induced liver fibrosis.
Keywords: Taurine, Liver fibrosis, Hepatocyte, Ultrastructure
INTRODUCTION
Liver cirrhosis is the terminal stage of various chronic liver diseases. Even mild but continuous injury in the liver soon results in excessive production of extracellular matrix components[1]. Deposition in the space of Disse causes capillarization of sinusoids and alterations in liver functions. This fibrotic stage ultimately progresses to cirrhosis, which is characterized by nodule formation and corruption of liver architecture.
Overproduction and accumulation of extracellular matrix proteins in the liver start usually after chronic hepatocyte injury that initiates a series of complicated cell-to-cell and cell-to-matrix interactions, eventually leading to activation of hepatic stellate cells, which are the main producers of excessive collagen during cirrhosis process[2]. Since hepatocyte injury seems to be the first and the main fibrogenic stimulus in the liver, healing of these cells could be a desirable goal in preventing progression of fibrosis.
Taurine, 2-aminoethanesulphonic acid, is an essential β-amino acid. It is present at high concentrations in many tissues. It plays important roles in numerous physiological functions including conjugation with bile acids, modulation of calcium levels, and maintenance of osmolarity, antioxidation and stabilization of membranes[3]. It was reported to have beneficial effects in various physiological and pathological conditions[4-7] by mainly diminishing production of reactive oxygen species (ROS). It also can prevent DNA damage at physiological concentrations[8]. Taurine has also hepatoprotective effects such as inhibition of extracellular matrix accumulation in experimental liver fibrosis[9,10] and improvement of liver function tests in fatty liver disease of children[11]. Hepatoprotective feature of taurine is attributed to its inhibitory activity on generation of ROS, which are known to play an important role in hepatic injury both in vitro and in vivo[12,13].
The effects of acute oxidative stress on the ultras-tructure of sinusoidal endothelium, space of Disse, hepatocytes and Kuppfer cells in perfused rat liver have been studied previously by Cogger et al[14]. They successfully demonstrated the alterations in the mitochondria of injured hepatocytes. Recently, we have shown beneficial effects of taurine on histopathology and oxidative stress parameters in a rat model of CCl4-induced liver fibrosis[15,16] where remarkable histopathological improvement in taurine treated animals subjected to hepatotoxin was observed, and this was associated with oxidative stress reduction and hepatocellular apoptosis. In this work we report on the changes in the chronic setting, with more focusing on the multiple organelle based alterations after administration of CCl4 that causes hepatic injury primarily via increasing ROS production in the liver[17]. We also studied the correlation of ultrastructural changes with histopathological findings.
MATERIALS AND METHODS
The study was approved by the Institutional Animal Use and Care Committee of the Gulhane School of Medicine, Ankara, Turkey, and was performed in accordance with the National Institute of Health guidelines for the care and handling of animals. The animals were fed with free access to standard rat food and water, and housed in metabolic cages one in one at controlled temperature and 12-h light/dark cycles before and during the experiment. Electron microscopic examinations were performed at the Department of Anatomy, Hacettepe University Medical Faculty, Ankara.
Animals and treatment strategies
Thirty male Sprague Dawley rats weighing 250-400 g were randomly divided into two groups. Group 1 (n = 15) was injected with CCl4 (0.2 mL/100 g twice weekly; S.C) plus taurine (1000 mg/kg per day; I.P), and Group 2 (n = 15) with CCl4 plus saline for 12 wk. At the end of 12th wk all rats were killed under anesthesia and the livers were excised. Adequate numbers of specimens from right and left lobes of each liver were collected for transmission electron microscopy and histopathological examination.
Light microscopy analysis
For light microscopy, tissue sections were fixed in 10% neutral buffered formalin and embedded in paraffin. Paraffin sections were stained with hematoxylin-eosin, examined and scored by two pathologists who were blinded to the treatment protocol. Degree of necrosis, inflammation, fat accumulation, and fibrosis was scored as 0: Absent; 1: Slight; 2: Moderate; and 3: Severe as described elsewhere, with small modifications[18]. Histopathological evaluation was performed twice in four sections per slide from all animals in each group.
Ultrastructural analysis
The specimens were fixed in 2.5% glutaraldehyde for 24 h and subsequently washed in phosphate buffer (pH 7.4), post-fixed in 1% osmium tetroxide in phosphate buffer (pH 7.4) and dehydrated in increasing concentrations of alcohol. Afterwards, the tissues were washed with propylene oxide and embedded in epoxy-resin embedding media. Semi-thin sections about 3 mm in thickness were cut with a glass knife on a LKB Nova ultramicrotome (Sweden). These sections were stained with methylene blue and examined by a Nikon Optiphot (Japan) light microscope. Ultrathin sections were collected on copper grids, stained with uranyl acetate and lead citrate, and finally examined under a Jeol JEM 1200 Ex (Japan) transmission electron microscope. Twenty cells from each specimen were examined. Mitochondria, nuclei, rough endoplasmic reticulum (rER) and smooth endoplasmic reticulum (sER) of hepatocytes were evaluated by using a previously described scoring system (Table 1)[19-21]. Twenty nuclei, 50 mitochondria, 20 rERs and 20 sERs were examined for each animal.
Table 1.
| Ultrastructure | Assessment | Score |
| Mitochondria | Normal | 0 |
| Prominent cristae | 1 | |
| Edematous mitochondrion | 2 | |
| Collection of amorphous material | 3 | |
| rER | Normal | 0 |
| Dilatation | 1 | |
| Irregular lamellar organization | 2 | |
| Presence of focal breaks | 3 | |
| sER | Normal | 0 |
| Dilatation | 1 | |
| Vacuolization | 2 | |
| Presence of large degenerated areas, myelin figures | 3 | |
| Nucleus | Normal | 0 |
| Irregular chromatin distribution (margination, clumping) | 1 | |
| Increased heterochromatin | 2 | |
| Degenerated nucleus | 3 |
rER: Rough endoplasmic reticulum; sER: Smooth endoplasmic reticulum.
Statistical analysis
Results are expressed as mean ± SEM. Mann-Whitney U test for histopathologic scores and Student’s t-test for ultrastructure scores were used to analyze significance of differences between groups. Correlation of histopa-thological and ultrastructural scores was assessed with Pearson correlation procedure. The differences were accepted as statistically significant when P < 0.05.
RESULTS
Two animals in Group 1 and four in Group 2 died before the end of experiment.
Light microscopy
CCl4 treatment produced hepatic necrosis, inflammation, fatty accumulation, and fibrosis by the 12th wk. Light microscopy evaluation of liver sections from animals treated with CCl4 and taurine are shown in Figure 1, and Table 2. Necrosis, inflammation, fatty accumulation and fibrosis were significantly lower in Group 1 when compared with Group 2 (Figure 1B).
Figure 1.
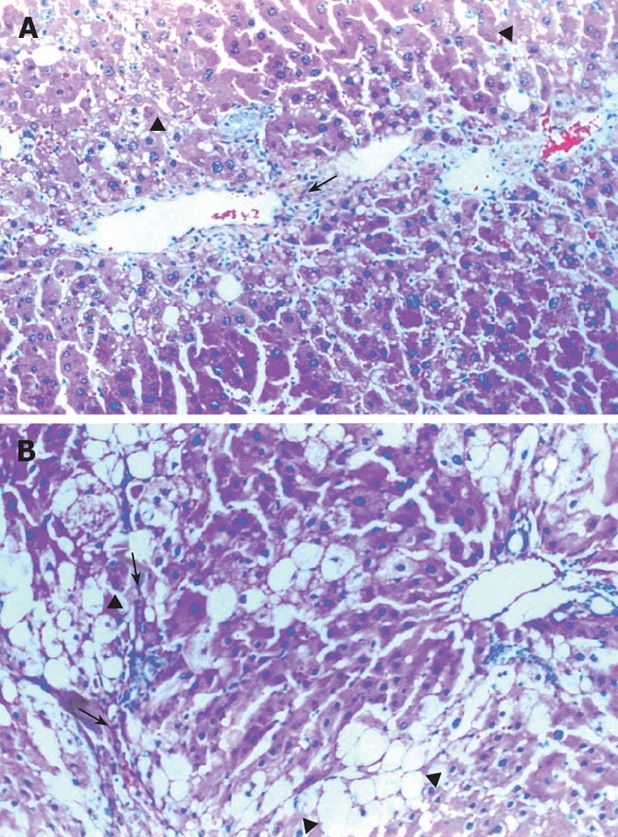
A: Mild bile duct proliferation (arrow) and microvesicular changes (arrowheads) in Taurine treated animals (HE, × 200); B: Severe macro and microvesicular fatty accumulation (arrowheads) and fibrosis (arrows) in Taurine untreated group (HE, × 50).
Table 2.
Histopathologic scoring and organelle injury scoresof Group 1 and Group 2 (mean ± SD)
| Group 1 | Group 2 | P | |
| Histopathologic scoring | |||
| Necrosis | 1.20 ± 0.15 | 2.27 ± 0.18 | < 0.0011 |
| Inflammation | 1.40 ± 0.13 | 2.00 ± 0.17 | < 0.031 |
| Fat accumulation | 1.27 ± 0.12 | 2.07 ± 0.21 | < 0.011 |
| Fibrosis | 1.40 ± 0.16 | 2.27 ± 0.18 | < 0.0051 |
| Total | 5.20 ± 0.38 | 8.60 ± 0.29 | < 0.0011 |
| Organelle injury scores | |||
| Mitochondrion | 45.7 ± 1.3 | 98.9 ± 2.1 | < 0.0012 |
| Rough ER | 22.1 ± 0.8 | 41.6 ± 2.1 | < 0.0012 |
| Smooth ER | 20.4 ± 0.8 | 43.3 ± 1.6 | < 0.0012 |
| Nucleus | 17.9 ± 1.2 | 33.3 ± 0.9 | < 0.0012 |
| Total | 106 ± 4 | 217 ± 6 | < 0.0012 |
Mann-Whitney U test;
Student’s t-test. ER: Endoplasmic reticulum.
Transmission electron microscopy
Ultrastructural analysis of the liver sections revealed significantly lower organelle injury scores in CCl4 plus taurine treated group when compared with CCl4 plus saline treatment (Table 2). Mitochondrial edema was seen in both groups but it was more extensive in saline treated animals. Additionally, mitochondrial cristae were much more visible in taurine treated animals (Figure 2A). While there were irregular lamellar organization and large dilatations with focal breaks in rERs of hepatocytes in CCl4 plus saline treated group in many areas (Figure 2B), only some focal slight dilatations were observed in taurine treated animals. Dilatations in taurine treated animals (Figure 2A) but vacuolization and myelin figures in saline treated group were sER findings (Figure 2C). Although there was irregular chromatin distribution in some areas, nuclei were almost normal in appearance with taurine treatment (Figure 2A). However, chromatin distribution was irregular and nuclei showed extensive margination and clumping in saline treated group. Other prominent findings were large vacuolization of hepatocyte cytoplasm in taurine treated animals and presence of active fibroblasts in some focal areas in saline treated group. Though the model was hepatotoxin-based, disruption of membranes was rarely seen; therefore, we did not modify the scoring table regarding the changes in membranes.
Figure 2.
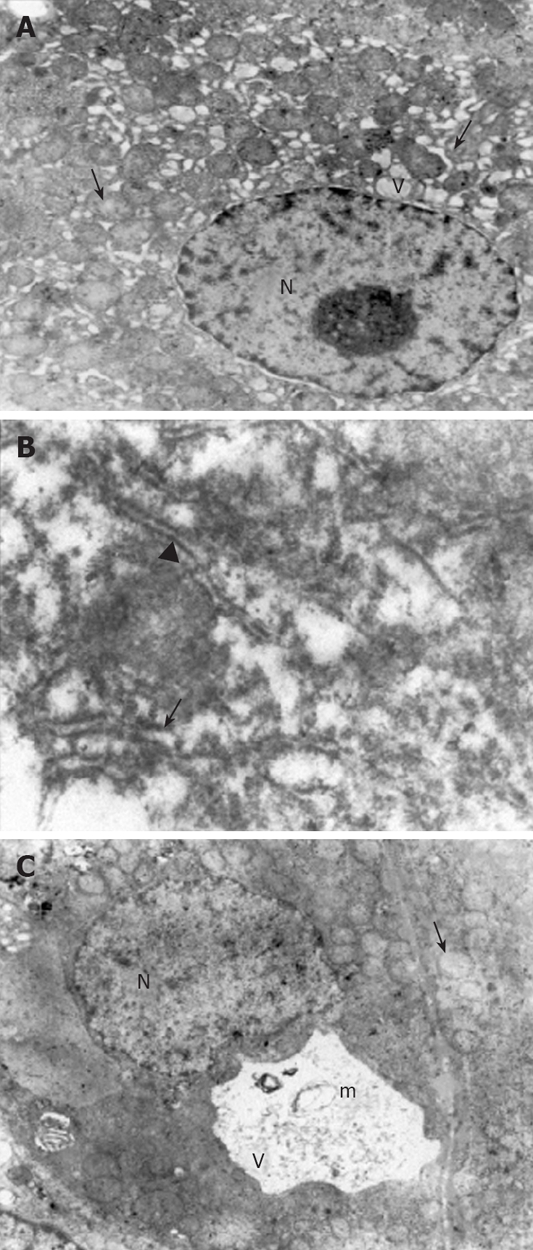
Electron micrographs. A: The dilatations in the rER (arrow) and sER (arrowhead), large vacuoles in the hepatocytes (V), a normal nucleus (N) and mitochondria with a prominent edema (small arrow) in CCl4 plus taurine treated group (× 4000); B: Large dilatations (arrow) and focal breaks (arrowhead) in the rER in CCl4 plus saline treated group (× 30 000); C: The vacuolizations (V) and myelin figures (m) in the sER, nucleus (N) and mitochondria with prominent edema (arrow) in CCl4 plus saline treated group (× 4000).
Ultrastructural scores in both groups highly correlated with the histopathologic scores (r = 0.931 for Group 1, r = 0.899 for Group 2) (Figure 3).
Figure 3.
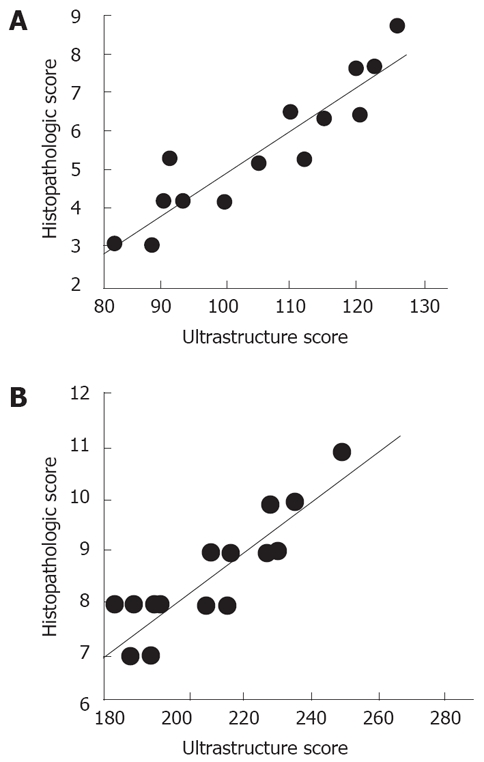
Correlation of histopathologic and ultrastructure scores in CCl4. A: Plus Taurine treated group (r = 0.931); B: Plus Saline treated group (r = 0.899).
DISCUSSION
The present study addresses the ultrastructural findings in hepatocytes and their correlation with histopathological scores after taurine treatment in CCl4 induced experimental liver fibrosis. As was shown previously in several studies, taurine treatment inhibits production of excess extracellular matrix in experimental liver fibrosis[8,10]. Although we did not measure tissue content of collagen in liver tissues, we found visible evidence under light microscopy that taurine decreased the degree of necrosis, inflammation, fatty accumulation and fibrosis. Antifibrotic activity of this endogenous amino acid in the liver was confirmed once more in the present study. Since CCl4 results in hepatotoxicity through its enzymatic conversion to reactive trichloromethyl free radicals[17], antioxidant treatment is essential to be effective in reducing the degree of injury.
We analyzed mitochondria, rER, sER and nuclei according to a previously described ultrastructural scoring table[19-21] that was successfully used in experimental pancreatitis to demonstrate the level and characteristics of injury in parenchymal cells. Such a scoring system may be useful for the researchers in following the changes in ultrastructure of hepatocytes under pathogenic stress or medications.
The most significant ultrastructural recovery with taurine treatment occurred in mitochondria and sER. Mitochondria are the energy source of the cell, as was specifically demonstrated in hepatocytes, with two membranes, one of which limits the organelle and the other of which is inside the organelle and is thrown into folds that project inward in a tubular nature called cristae mitochondria[22]. The molecules in the electron transport chain, which play the central role in ATP synthesis, are found in the cristae. It is also well known that mitochondria are both a major source of endogenous production of ROS[23]. The major mitochondrial pathology in our work was edema, the major ultrastructural sign of cellular injury, extensively invading the matrix resulting in swelling of the organelle and structural damage to cristae. Though edema was seen in both groups, it was much less in taurine treated animals. In addition, there was minimal disruption in cristae with taurine treatment and cristae integrity was preserved quite well. Swelling and loss of regular cristae structure with rupture of outer membrane, which are characteristics of deteriorated function of mitochondria[22,24,25], decreased with taurine in our study.
Endoplasmic reticulum is normally composed of tubules, vesicles and sacs forming lamel-like shape[26]. Crucial pathways that are related to carbohydrate metabolism, biotransformation, steroid metabolism and protein processing function in the ER with integration to other organelles[27]. rER carries ribosomes on its surface different from sER, which is the place of action and storage of important cellular enzymes[28]. The proteins synthesized in rER are stored in cristae. Glycogen synthesis and detoxification of drugs are performed in sER in the liver. Severely dilated ER is representative of severely damaged hepatocytes[26]. When we examined ERs of the rats treated with saline, we found serious damage as irregular lamellar organization, large dilatations and focal breaks in rERs, and vacuolizations and myelin figures in sERs in many areas. However, only some focal and slight dilatations were observed in rERs and sERs of taurine treated animals. Besides absence of dilation that is known to be reversible when the toxic state is properly opposed or ceases[29], intact lamellar organization and absence of vacuolization seem to be required for normal functioning of ER.
Nuclei, the hallmark of eukaryotic cells, contain DNA with its associated protein called chromatin[30]. The density of chromatin varies throughout the nucleus. Dense regions inside the nucleus are called heterochromatin and are found in parts of the chromosome where there are few or no genes. It lies next to inner side of the nuclear envelope. The genes in heterochromatin are generally inactive, that is, not transcribed. Euchromatin is found in parts of the chromosome that contain many active genes. We found alterations in chromatin distribution in the nuclei of hepatocytes from saline treated animals. Although we could not detect abundant heterochromatin, disorganization of nuclear content as margination and clumping of chromatin in saline treated animals may be morphological evidence of injury in the nucleus. However, severely degenerated nuclei were rarely detected. Nuclear content was almost normal in appearance and organization with taurine treatment, which was previously reported to prevent DNA damage[8]. These results confirm the basic knowledge that nucleoplasmic constituents represent the structural counterpart of transcription and processing of messenger and ribosomal RNAs, and therefore constitute fine and highly sensitive indicators of cellular activity.
Electron microscope findings in hepatocytes after hepatotoxin have not been defined systematically to date. Moreover, which changes in each organelle are reversible or not is not clear. Dincer et al, previously reported partly the ultrastructural changes in hepatocytes after taurine treatment[31]. However, the present study not only defines the organelle changes in more detail; it also provides a better assessment and measurement of ultrastructural injury.
The change in ultrastructural scores between the two groups was nearly the same when compared with histopathological scores. This indicates that the current histopathological scoring system used to describe tissue injury in the present study successfully reflects organelle based ultrastructural changes in hepatocytes. On the other hand, the results obtained it this study should not be overwhelmed, because taurine’s most significant action is directly counteracting the effect of CCl4. Protective effects of this antioxidant in clinical conditions related to other injury mechanisms in the liver might not be so strongly evident.
In conclusion, this study brought us direct view evidence of changes in morphology of hepatocyte organelles after induction of a certain hepatotoxin. Taurine preserves morphology of major organelles of hepatocytes and delays the development of fibrosis. Structural changes in hepatocyte organelles we observed in this study are likely the cause of significant histological improvements. Since transmission electron microscopy is the highest magnification tool at present, modeling new ultrastructural scoring systems including more organelles and parameters can be useful in estimating the degree of injury and outcome of alternative treatment strategies in management of chronic liver diseases.
COMMENTS
Background
Liver fibrosis and cirrhosis are untreatable conditions at present. Antioxidant medications including taurine have been reported to possess antifibrotic efficacy in experimental liver fibrosis. Taurine is one of the main components of energy drinks, which are widely consumed by healthy people around the world. The present study addresses organelle based changes in hepatocytes after taurine treatment in rat liver fibrosis.
Research frontiers
Preventive effect of taurine in continuing liver injury was tested. The study design does not include treatment after establishment of liver fibrosis. Antioxidants may be taken into consideration not only for their efficacy on established liver fibrosis but also for their hepatoprotective efficacy in the long term.
Innovations and breakthroughs
Organelle based effects of taurine in hepatocytes is to be shown for the first time on animals.
Applications
Taurine administration was previously shown be effective in delaying the development of fibrosis in experimental conditions. The present findings support the idea that conduction of large scale clinical studies on the efficacy of taurine in human liver fibrosis or cirrhosis should be encouraged.
Terminology
Liver fibrosis refers to invasion of normal liver by collagen deposits in chronic liver injury leading to destruction of normal tissue architecture and functional insufficiency. Taurine is a potent antioxidant not present in the market as a drug but is included in the majority of energy drinks.
Peer review
This paper is an experimental study in rats demonstrating that taurine protects the liver at the ultrastructural level after the administration of carbon tetrachloride. The paper is novel, well written and well organized, particularly the discussion which is comprehensive and easy to read.
Footnotes
Supported by Grants from the Gulhane Medical Research Council, No. AR-99/01
Peer reviewer: Paolo Del Poggio, PhD, Hepatology Unit, Department of Internal Medicine, Treviglio Hospital, Piazza Ospedale 1, Treviglio Bg 24047, Italy
S- Editor Li DL L- Editor Li M E- Editor Zhang WB
References
- 1.Gressner AM. The cell biology of liver fibrogenesis - an imbalance of proliferation, growth arrest and apoptosis of myofibroblasts. Cell Tissue Res. 1998;292:447–452. doi: 10.1007/s004410051073. [DOI] [PubMed] [Google Scholar]
- 2.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 3.Huxtable RJ. Physiological actions of taurine. Physiol Rev. 1992;72:101–163. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- 4.Ahn BO, Kim KH, Lee G, Lee HS, Kim CD, Kim YS, Son MW, Kim WB, Oh TY, Hyun JH. Effects of taurine on cerulein-induced acute pancreatitis in the rat. Pharmacology. 2001;63:1–7. doi: 10.1159/000056106. [DOI] [PubMed] [Google Scholar]
- 5.Chiba Y, Ando K, Fujita T. The protective effects of taurine against renal damage by salt loading in Dahl salt-sensitive rats. J Hypertens. 2002;20:2269–2274. doi: 10.1097/00004872-200211000-00027. [DOI] [PubMed] [Google Scholar]
- 6.Ozturk M, Mas MR, Yasar M, Akay C, Aydogan H, Deveci S, Comert B, Simsek I, Mas N, Kocar IH. The role of inducible nitric oxide synthase inhibitor, meropenem, and taurine in experimental acute necrotizing pancreatitis. Pancreas. 2003;26:357–362. doi: 10.1097/00006676-200305000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Park T, Lee K. Dietary taurine supplementation reduces plasma and liver cholesterol and triglyceride levels in rats fed a high-cholesterol or a cholesterol-free diet. Adv Exp Med Biol. 1998;442:319–325. doi: 10.1007/978-1-4899-0117-0_40. [DOI] [PubMed] [Google Scholar]
- 8.Messina SA, Dawson R Jr. Attenuation of oxidative damage to DNA by taurine and taurine analogs. Adv Exp Med Biol. 2000;483:355–367. doi: 10.1007/0-306-46838-7_40. [DOI] [PubMed] [Google Scholar]
- 9.Balkan J, Dogru-Abbasoglu S, Kanbagli O, Cevikbas U, Aykac-Toker G, Uysal M. Taurine has a protective effect against thioacetamide-induced liver cirrhosis by decreasing oxidative stress. Hum Exp Toxicol. 2001;20:251–254. doi: 10.1191/096032701678227758. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Li S, Zhang X. [Taurine inhibits deposition of extracellular matrix in experimental liver fibrosis in rats] Zhonghua Ganzangbing Zazhi. 1999;7:165–167. [PubMed] [Google Scholar]
- 11.Obinata K, Maruyama T, Hayashi M, Watanabe T, Nittono H. Effect of taurine on the fatty liver of children with simple obesity. Adv Exp Med Biol. 1996;403:607–613. doi: 10.1007/978-1-4899-0182-8_67. [DOI] [PubMed] [Google Scholar]
- 12.Pietrangelo A. Metals, oxidative stress, and hepatic fibrogenesis. Semin Liver Dis. 1996;16:13–30. doi: 10.1055/s-2007-1007215. [DOI] [PubMed] [Google Scholar]
- 13.Svegliati Baroni G, D'Ambrosio L, Ferretti G, Casini A, Di Sario A, Salzano R, Ridolfi F, Saccomanno S, Jezequel AM, Benedetti A. Fibrogenic effect of oxidative stress on rat hepatic stellate cells. Hepatology. 1998;27:720–726. doi: 10.1002/hep.510270313. [DOI] [PubMed] [Google Scholar]
- 14.Cogger VC, Mross PE, Hosie MJ, Ansselin AD, McLean AJ, Le Couteur DG. The effect of acute oxidative stress on the ultrastructure of the perfused rat liver. Pharmacol Toxicol. 2001;89:306–311. doi: 10.1034/j.1600-0773.2001.d01-165.x. [DOI] [PubMed] [Google Scholar]
- 15.Refik Mas M, Comert B, Oncu K, Vural SA, Akay C, Tasci I, Ozkomur E, Serdar M, Mas N, Alcigir G, et al. The effect of taurine treatment on oxidative stress in experimental liver fibrosis. Hepatol Res. 2004;28:207–215. doi: 10.1016/j.hepres.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Tasci I, Mas MR, Vural SA, Deveci S, Comert B, Alcigir G, Mas N, Akay C, Bozdayi M, Yurdaydin C, et al. Pegylated interferon-alpha plus taurine in treatment of rat liver fibrosis. World J Gastroenterol. 2007;13:3237–3244. doi: 10.3748/wjg.v13.i23.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slater TF. Free-radical mechanisms in tissue injury. Biochem J. 1984;222:1–15. doi: 10.1042/bj2220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez-Munoz R, Diaz-Munoz M, Lopez V, Lopez-Barrera F, Yanez L, Vidrio S, Aranda-Fraustro A, Chagoya de Sanchez V. Balance between oxidative damage and proliferative potential in an experimental rat model of CCl4-induced cirrhosis: protective role of adenosine administration. Hepatology. 1997;26:1100–1110. doi: 10.1002/hep.510260503. [DOI] [PubMed] [Google Scholar]
- 19.Ates Y, Mas MR, Mas N, Tasci I, Comert B, Isik AT, Yener N. Acinar cell ultrastructure after taurine treatment in rat acute necrotizing pancreatitis. Saudi Med J. 2006;27:446–452. [PubMed] [Google Scholar]
- 20.Mas N, Isik AT, Mas MR, Comert B, Tasci I, Deveci S, Ozyurt M, Ates Y, Yamanel L, Doruk H, et al. Hyperbaric oxygen-induced changes in bacterial translocation and acinar ultrastructure in rat acute necrotizing pancreatitis. J Gastroenterol. 2005;40:980–986. doi: 10.1007/s00535-005-1653-5. [DOI] [PubMed] [Google Scholar]
- 21.Mas N, Tasci I, Comert B, Ocal R, Mas MR. Ursodeoxycholic acid treatment improves hepatocyte ultrastructure in rat liver fibrosis. World J Gastroenterol. 2008;14:1108–1111. doi: 10.3748/wjg.14.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ernster L, Schatz G. Mitochondria: a historical review. J Cell Biol. 1981;91:227s–255s. doi: 10.1083/jcb.91.3.227s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Szeto HH. Cell-permeable, mitochondrial-targeted, peptide antioxidants. AAPS J. 2006;8:E277–E283. doi: 10.1007/BF02854898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vanhorebeek I, De Vos R, Mesotten D, Wouters PJ, De Wolf-Peeters C, Van den Berghe G. Protection of hepatocyte mitochondrial ultrastructure and function by strict blood glucose control with insulin in critically ill patients. Lancet. 2005;365:53–59. doi: 10.1016/S0140-6736(04)17665-4. [DOI] [PubMed] [Google Scholar]
- 26.Gartner LP, Hiatt JL. Cytoplasm. In: Color Textbook of Histology., editor. 2nd edition, WB: Saunders Company; 2001. pp. 11–49. [Google Scholar]
- 27.Csala M, Banhegyi G, Benedetti A. Endoplasmic reticulum: a metabolic compartment. FEBS Lett. 2006;580:2160–2165. doi: 10.1016/j.febslet.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 28.Sato S, Dai W, Liu XL, Asano G. The protective effect of hepatocyte growth-promoting factor (pHGF) against carbon tetrachloride-induced acute liver injury in rats: an ultrastructural study. Med Electron Microsc. 1999;32:184–192. doi: 10.1007/s007950050026. [DOI] [PubMed] [Google Scholar]
- 29.Kumar V, Abbas AK, Fausto N. Cellular Adaptations, Cell Injury and Cell Death. Vol. 32. In: Pathologic Basis of Disease, 7th ed, India: WB Saunders Company; 2005. pp. 3–46. [Google Scholar]
- 30.Gartner LP, Hiatt JL. Nucleus. In: Color Textbook of Histology., editor. 2nd edition, WB: Saunders Company; 2001. pp. 51–70. [Google Scholar]
- 31.Dincer S, Ozenirler S, Oz E, Akyol G, Ozogul C. The protective effect of taurine pretreatment on carbon tetrachloride-induced hepatic damage--a light and electron microscopic study. Amino Acids. 2002;22:417–426. doi: 10.1007/s007260200025. [DOI] [PubMed] [Google Scholar]


