Abstract
AIM: To investigate the relation of expression transformation of claudin-1 with invasiveness and metastasis of gastric carcinoma.
METHODS: By using immunohistochemistry, expression of claudin-1 in mucosa and invasive front of 136 gastric adenocarcinoma cases and proliferative index (Ki-67) were detected and analyzed.
RESULTS: In mucosa, the claudin-1 over-expression rate of mucinous adenocarcinomas (including signet-ring cell carcinomas) was the highest. It was negatively related with the differentiation but positively related with the invasiveness and metastasis of gastric cancer. In invasive front, the claudin-1 over-expression rate was positively related with the differentiation, invasiveness and metastasis of gastric carcinoma. The expression transformation of claudin-1 was found in gastric carcinoma. The expression of claudin-1 in invasive front was transformed in 28/136 gastric carcinoma cases. The transformation rate in highly differentiated tubular adenocarcinomas was the highest (51.5%, 17/33). The deeper was the invasiveness, the higher was the transformation rate. The claudin-1 expression transformation rate in serosa and omenta was significantly higher (92.9%) than in tunica muscularis of invasive gastric cancer cases, as well as in patients with lymph node metastasis than in those without lymph node metastasis.
CONCLUSION: Up-regulation of claudin-1 expression and its transformation in invasive and metastatic gastric carcinoma suggest that claudin-1 participates in the transformation of biological behaviors in neoplasms. Further study is needed to elucidate the precise mechanism and the relation of claudin-1 expression with the neoplasm progress.
Keywords: Gastric carcinoma, Claudin-1, Expression transformation, Invasiveness, Metastasis, Immunohistochemistry
INTRODUCTION
Gastric carcinoma is one of the most frequent malignant tumors in the world. Gastric carcinoma cells with a fibroblastic pattern in which intracellular adhesion is decreased show active mobility and invasiveness, indicating that epithelial mesenchymal transition (EMT) has occurred[1]. Tight junction (TJ) proteins participate in EMT of tumors[2]. The four-time transmembrane proteins of claudin family are essential components of TJ[3], but the role of TJ proteins in the development of malignant tumor is not clear. By using immunohistochemistry, claudin-1 expression in gastric carcinoma was investigated and its relation with biological behaviors of gastric carcinoma was discussed in this study.
MATERIALS AND METHODS
Patients
A total of 136 patients (106 males and 30 females) with primary gastric carcinoma, who underwent surgery between January and December 2007 in the First Affiliated Hospital of Fujian Medical University, were enrolled in this study. Their median age was 64 years, ranging 28-80 years. All patients did not receive radiation therapy or chemotherapy prior to operation. The histological findings, lymph node metastasis and TNM stage were evaluated based on World Health Organization Classification of Tumors[4].
Immunohistochemistry
Specimens were fixed in formalin, embedded in paraffin wax, cut into 4 μm thick sections and stained with hematoxylin and eosin.
The sections were immunostained with anti-rabbit polyclonal antibodies for claudin-1 (1:100, ZYMED) and Ki-67 (MB67, Ready, NeoMarkers) with the EnVision method. The sections were deparaffinized and heated in a microwave oven for 10 min to retrieve the antigens. After immersed in 3% hydrogen peroxide of 100% methanol for 10 min to block the endogenous peroxidase activity, the sections were incubated with primary antibodies for 60 min at room temperature, with EnVisionTM for 20 min, and then immersed into a DAB solution. The sections were counterstained with haematoxylin, dehydrated and mounted. Between steps, the sections were washed three times with phosphate-buffered saline (PBS). As a negative control, PBS was used instead of primary antibody. Two independent observers without knowledge of the clinical outcomes evaluated the immunohistochemical staining of sections till a complete agreement on the classification.
Immunohistochemical analysis of claudin-1 and Ki-67 labeling index
Claudin-1 was expressed in the cell membrane and/or cytoplasm (Figure 1). The intensity of staining in cell membrane and cytoplasm and the percentage of immunoreactive cells over the total tumor cells were evaluated as previously described[5]. The intensity of staining was graded as 0 when staining was not greater than negative control, 1 as light staining, and 2 as heavy staining. Immunoreactivity was scored according to the percentage of immunoreactive cells over the total tumor cells counted as 0 if < 5% cells were stained, 1 if 5%-25% cells were immunoreactive, 2 if 26%-50% cells were immunoreactive, and 3 if > 50% cells were immunoreactive. The expression of claudin-1 was finally defined according to the score obtained from the grade of intensity multiplied by the score of cell immunoreactivity, i.e. negative (-, scored 0-1), positive (+, scored 2-3), and strongly positive (++, scored 4 or above). Positive expression of Ki-67 staining was found in nuclei of carcinoma cells. Ki-67 labeling index was defined as the ratio of immunoreactive cells over 1000 tumor cells.
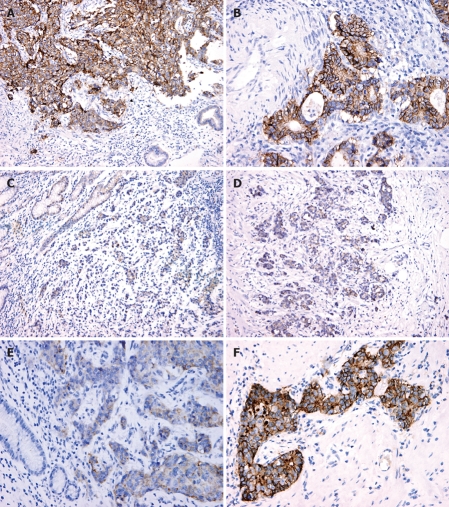
Figure 1.
Immunohisto-chemical staining (× 200) showing over-expression of claudin-1 in mucosa (A) and invasive front (B) of gastric carcinoma, low-expression in mucosa (C) and invasive front (D), and transformation of claudin-1 low-expression in mucosa (E) and over-expression in invasive front of gastric carcinoma (F).
Statistical analysis
Chi square test was used for univariable categorical analysis. All statistical analyses were performed with SPSS 10.0. P < 0.05 was considered statistically significant.
RESULTS
Relation between claudin-1 expression and clinicopathological parameters of gastric carcinoma
Claudin-1 was mainly expressed in the cell membrane and/or cytoplasm of gastric carcinoma cells. The expression of claudin-1 was related with the histological type, degree of invasiveness and lymph node metastasis of gastric cancer (P < 0.05). However, the expression of claudin-1 was not significantly related with the sex and age of gastric cancer patients (Table 1).
Table 1.
Claudin-1 expression and clinicopathologic characteristics of gastric carcinoma n (%)
| n |
Expression in mucosa |
Expression in invasive front |
|||||
| Low | Over | P | Low | Over | P | ||
| Sex | |||||||
| Male | 106 | 80 (75.5) | 26 (24.5) | 0.144 | 58 (54.7) | 48 (45.3) | 0.198 |
| Female | 30 | 27 (90) | 3 (10) | 21 (70) | 9 (30) | ||
| Age | |||||||
| ≤ 50 | 20 | 17 (85) | 3 (15) | 0.491 | 13 (65) | 7 (35) | 0.665 |
| > 51 | 116 | 90 (77.6) | 26 (22.4) | 66 (56.9) | 50 (43.1) | ||
| Histological type | |||||||
| Well-moderately differentiated | 46 | 33 (71.7) | 13 (28.3) | 0.046 | 16 (34.8) | 30 (65.2) | 0.000 |
| Poorly differentiated | 65 | 57 (87.7) | 8 (12.3) | 48 (73.8) | 17 (26.2) | ||
| Mucinous | 25 | 17 (68) | 8 (32) | 15 (60) | 10 (40) | ||
| Depth of invasion | |||||||
| Lamina propria or submucosa | 18 | 17 (94.4) | 1 (5.6) | 0.099 | 16 (88.9) | 2 (11.1) | 0.007 |
| Muscularis propria | 28 | 19 (67.9) | 9 (32.1) | 18 (64.3) | 10 (35.7) | ||
| Visceral peritoneum | 90 | 71 (78.9) | 19 (21.1) | 45 (50) | 45 (50) | ||
| Lymph node metastasis | |||||||
| Negative | 52 | 46 (88.5) | 6 (11.5) | 0.048 | 38 (82.6) | 14 (17.4) | 0.009 |
| Positive | 84 | 61 (72.6) | 23 (27.4) | 41 (48.8) | 43 (51.2) | ||
| Ki-67 index | |||||||
| I | 20 | 17 (85) | 3 (15) | 0.529 | 14 (70) | 6 (30) | 0.389 |
| II | 57 | 43 (75.4) | 14 (24.6) | 33 (57.9) | 24 (42.1) | ||
| III | 54 | 42 (77.8) | 12 (22.2) | 28 (51.9) | 26 (48.1) | ||
| IV | 5 | 5 (100) | 0 | 4 (80) | 1 (20) | ||
The claudin-1 over-expression rate was the highest in mucinous adenocarcinomas, and lower in poorly differentiated carcinomas than in well-moderately differentiated carcinomas. It was significantly higher in mucosa of patients with their tumors invaded muscularis propria and visceral peritoneum, or with lymph node metastasis than in mucosa of patients with their tumors only invaded lamina propria or submucosa, or without lymph node metastasis.
The claudin-1 expression in invasive front was different from that in the mucosa. The claudin-1 over-expression was the highest in well-moderately differentiated carcinomas and the lowest in poorly differentiated carcinomas. The deeper was the invasive depth, the higher was the claudin-1 over-expression rate. The incidence of claudin-1 over-expression rate was 50% in invasive front with tumors invaded visceral peritoneum and significantly higher in patients with lymph node metastasis. The expression of claudin-1 was not related with the proliferation index of gastric carcinoma cells.
Relation between expression transformation of claudin-1 and biological behaviors of gastric carcinoma
The expression of claudin-1 was transformed in mucosa and invasive front of gastric carcinoma patients, which was 26.2% (28/107) in mucosa and 49.1% (28/57) in invasive front (Table 2). The expression transformation rate of claudin-1 was 51.5% (17/33) in well-moderately differentiated carcinoma patients, 16.0% (9/57) in poorly differentiated carcinoma patients, and 11.8% (2/17) in mucinous carcinoma patients, respectively (Table 3).
Table 2.
Expression transformation of claudin-1 in gastric carcinomas
| Expression in mucosa | Expression in invasive front | n |
| Low-expression | Low-expression (expression invariably) | 79 |
| Low-expression | Over-expression (expression variably) | 28 |
| Over-expression | Low-expression (expression variably) | 0 |
| Over-expression | Over-expression (expression invariably) | 29 |
Table 3.
Relation between the expression transformation of claudin-1 and biological behaviors of gastric carcinomas
| n | Low-expression in mucosa | Over-expression in invasive front | |
| Histological type | |||
| Well-moderately differentiated | 17 | 33 | 30 |
| Poorly differentiated | 9 | 57 | 17 |
| Mucinous | 2 | 17 | 10 |
| Depth of invasion | |||
| Lamina propria or submucosa | 1 | 17 | 2 |
| Muscularis propria | 1 | 19 | 10 |
| Visceral peritoneum | 26 | 71 | 45 |
| Lymph node metastasis | |||
| Negative | 8 | 46 | 14 |
| Positive | 20 | 61 | 43 |
The deeper was the invasiveness, the higher was the transformation rate of claudin-1 expression. The claudin-1 expression transformation was significantly higher in patients (26/28) with their tumors invaded visceral peritoneum than in those with their tumors only invaded muscularis propria (P < 0.05), and in patients (20/61) with lymph node metastasis than in those (8/46) with no lymph node metastasis (P < 0.05).
DISCUSSION
TJs, adherent junctions and desmosomes form the apical junctional complex in epithelial cellular sheets. Adherent junctions and desmosomes are responsible for the mechanical adhesion between adjacent cells, while TJs play a mainly role in the tight sealing of cellular sheets, thus controlling the paracellular ion flux and maintaining tissue homeostasis[6]. By forming a fence that prevents lateral diffusion of membrane proteins and lipids, TJs also play a crucial role in the maintenance of cell polarity. TJs also participate in the regulation of cell proliferation and differentiation, or other cellular functions[7].
TJs are consisted of three major integral membrane proteins: claudins, occludin and junctional adhesion molecules. The role of these proteins has not been completely elucidated. However, it is presumed that claudins form the principal chain of TJ strands. The claudin protein family is consisted of 24 members of closely correlated transmembrane proteins. A number of tissues express multiple claudin proteins and form TJ strands by interacting through homotypic and/or heterotypic fashion, though the expression pattern of claudins is tissue specific.
The histological grade of carcinomas is a significant prognostic parameter and depends on the differentiated degree of glandular epithelium and cellular polarity. The invasiveness in high-grade and poorly-differentiated carcinoma is stronger than that in low-grade and well-differentiated carcinoma. One of the key determiners controlling cellular adhesion and polarity is the TJs[6]. Carcinoma cells frequently show deficiencies in structure and function of the TJs[8]. It was supposed that the TJs play a critical role in the progress of neoplasm through acting as a connector of extracellular environment affecting the intercellular signal pathway and cellular skeleton[9]. The changes of permeability in TJs may also permit the diffusion increase in nutrient substances and other factors for growth and survival of tumors. Otherwise, the loss of integration of TJs in the development of metastatic phenotype is also an important step.
The expression of claudin proteins may change in carcinoma cells. The expression of claudin-1 and claudin-4 in ovarian and prostatic carcinomas is increased[10-12], claudin-4 is over-expressed in pancreatic carcinomas[13,14], while claudin-1 expression is down-regulated in breast and colon carcinomas[15-17].
The three dimensional cultures of breast cancer cells showed that the reexpression of claudin-1 may increase apoptosis of cancer cells[18]. It was reported that the expression of claudin-1 in stage II colon carcinomas is related with a poor prognosis[17]. Another study showed that the expression of claudin-1 is up-regulated in colon carcinomas[19], and the expression level of claudin-1 is negative related with the histological grade of tumors[17].
However, investigations on the role of claudin-1 expression in the progression of gastric carcinomas are relatively few and only two studies on the expression of claudin-1 in gastric carcinomas can be found in PubMed so far. Through tissue microarray, Resnick et al[20] found that claudin-1, -3, -4 and ZO-1 are expressed in non-tumor mucosa, tumor mucosa and invasive front of gastric carcinomas, the expression of caldudin-1 is higher in intestinal subtype than in diffuse subtype of adenocarcinomas. Soini et al[21] found that the expression of claudin-1 is significantly higher in intestinal-type gastric than in diffuse-type gastric carcinomas, indicating that claudin-1 expression is the determiner of diffuse phenotype of gastric carcinoma. Our results show that claudin-1 over-expression occurred in mucinous gastric adenocarcinomas, and was negatively related with the differentiation degree of adenocarcinomas, but positively related with the invasiveness and metastasis of adenocarcinomas in mucosa. However, the expression of claudin-1 in invasive front was different from that in mucosa of gastric carcinoma. The claudin-1 over-expression in invasive front was positively related with the differentiated degree and the invasiveness and metastasis of gastric adenocarcinomas.
The transformation of claudin-1 expression companied the progression of gastric carcinomas. The expression of claudin-1 in invasive front of gastric carcinomas was transformed. The claudin-1 expression transformation percentage of well-differentiated adenocarcinomas was the highest (51.5%, 17/33). The deeper the invasiveness of gastric carcinomas was, the higher the transformation rate was. The transformation was significantly higher in patients with tumors invaded visceral peritoneum than in those with tumors only invaded muscularis propria and in patients with lymph node metastasis than in those with no lymph node metastasis. These results suggest that transformation of claudin-1 expression participates in the progression of gastric carcinomas.
The role of TJ proteins in the development of cancer is not clear. Carcinoma cells, especially those exhibiting a higher potentiality of metastasis, frequently show loss of functional TJs, such as ZO-1,-2 and occludin is decreased in tumor and its metastasis[22,23]. The exact action of claudin on cancers is not clear. It was reported that the expression of claudin-1 is decreased in invasive duct carcinoma of breast[24] while the expression of claudin-3 and -4 is increased in some other carcinomas[25,26]. The expression of claudin-1 may promote the activation of pro-MMP-9[26-28], suggesting that the expression of claudin-1 may involve the invasiveness and metastasis of adenocarcinoma. Caludin-1 is regarded as a target site of β-catenin/Tcf signals, which supports that claudin-1 down-regulates the formation of colorectal carcinomas[29]. The expression of claudin-1 mRNA is decreased in breast carcinomas[15] while the expression of claudin-23 is down-regulated in intestinal subtype of gastric carcinomas[30]. It was reported that the claudin-1 expression is frequently up-regulated in tumor tissues and its expression level is equal to or higher than in consecutive normal colon mucous membrane, suggesting that the expression of claudin-1 is related with poorly-differentiated adenocarcinoma. The loss of claudin-1 expression is a strongly predictive parameter for tumor recurrence and survival of patients. It was reported that expression of claudin-1 and -4 is increased in ovarian carcinomas and prostatic carcinomas[26,31] and over-expression of claudin-4 in pancreatic carcinomas and precancerous lesion[32] are the causative action of claudin on cellular transformation and progression of invasiveness[33].
Usually, a low expression level of claudin may result in functional damage to TJs. However, how the over-expression of claudin promotes tumor progression remains unclear[26,31,32]. One possible mechanism is that up-regulation or abnormal expression of some claudins may facilitate tumor formation by directly altering the function of TJ. Furuse et al[34] reported that up-regulation of claudin-2 expression in renal cells of Madin-Darby dogs decreases the function of TJ, and Tan et al[35] showed that the expression and distribution of claudin-1 are related with cell dissociation in pancreatic carcinoma by activating the mitogen-activated protein kinase-2. Up-regulation of claudins may also affect cell signaling pathways by binding domains to ZO-1[36]. ZO-1 interacts with several signaling proteins related with the neoplastic process, such as ras substrate AF-6[37], G-protein and connexin 43[38].
In conclusion, up-regulation and transformation of claudin expression in the invasive process of gastric carcinomas are involved in the biological behavior transformation of tumors. The exact role of claudin protein in the development of malignant tumors and their prognosis remains unclear and should be further studied.
COMMENTS
Background
Tight junction (TJ) protein participates in the processes of epithelial mesenchymal transformation (EMT) of carcinomas. Claudin proteins are the major members of the TJ family. In this study, the relation between the expression transformation of claudin-1 and the invasiveness and metastasis of gastric carcinoma was evaluated.
Research frontiers
The expression of claudin-1 was found to be significantly related with the biological behavior of gastric cancers, indicating that TJ plays a role in the development of neoplasms. The mechanism of TJ underlying the progress of cancer remains to be further studied.
Innovations and breakthroughs
This study evaluated the relation between the expression of claudin-1 and the invasiveness and metastasis of gastric carcinoma.
Applications
The importance of TJ in tumor development has not been extensively studied. The role of the claudin family in the invasiveness and metastasis of cancers is controversial. The relation between the expression transformation of TJ proteins and MET in cancer was clarified in the present study, which contributes to the exploitation of its mechanism underlying the development and progress of gastric carcinoma.
Terminology
TJs, adherent junctions and desmosomes form the apical junctional complex in epithelial cellular sheets. The claudin protein family is consisted of 24 members of the transmembrane proteins, such claudins1-4.
Peer review
This study investigated the relation between the expression transformation of claudin-1 and the invasiveness and metastasis of gastric carcinomas. The results suggest that claudin-1 participates in the transformation of the biological behaviors of gastric carcinomas. The study was well designed and the findings may be valuable for the further study on mechanism of claudin-1 underlying the development and progress of gastric carcinoma.
Footnotes
Supported by The Science Foundation of Putian City, Fujian Province, China, No. 2006D01
Peer reviewers: Toru Hiyama, PhD, Department of Health Service Center, Hiroshima University, 1-7-1 Kagamiyama, Hiagshihiroshima 739-8521, Japan; Wei Tang, MD, EngD, Assistant Professor, H-B-P Surgery Division, Artificial Organ and Transplantation Division, Department of surgery, Graduate School of Medicine, the University of Tokyo, Tokyo 113-8655, Japan; Bernd Sido, PhD, Department of General and Abdominal Surgery, Teaching Hospital of the University of Regensburg, Hospital Barmherzige Brüder, Prüfeninger Strasse 86, Regensburg D-93049, Germany
S- Editor Li DL L- Editor Wang XL E- Editor Ma WH
References
- 1.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 2.Ikenouchi J, Matsuda M, Furuse M, Tsukita S. Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. J Cell Sci. 2003;116:1959–1967. doi: 10.1242/jcs.00389. [DOI] [PubMed] [Google Scholar]
- 3.Morin PJ. Claudin proteins in human cancer: promising new targets for diagnosis and therapy. Cancer Res. 2005;65:9603–9606. doi: 10.1158/0008-5472.CAN-05-2782. [DOI] [PubMed] [Google Scholar]
- 4.Fenoglio-Preiser C, Munoz N, Carneiro F, Powell SM, Correa P, Rugge M, Guiford P, Sasako M, Lambert R, Stolte M, et al. Gastric Carcinoma [R] In: World Health Organization classification of tumors., editor. Pathology and genetics of tumours of the digestive system. Lyon: IARC Press; 2000. pp. 39–52. [Google Scholar]
- 5.Zhang ZZ, Zhang S, Lin JY, Huang PS, Chen YP. [Correlation between the expression of angiopoietins and their receptor and angiogenesis in gastric cancers] Zhonghua Zhongliu Zazhi. 2006;28:280–284. [PubMed] [Google Scholar]
- 6.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 7.Mitic LL, Anderson JM. Molecular architecture of tight junctions. Annu Rev Physiol. 1998;60:121–142. doi: 10.1146/annurev.physiol.60.1.121. [DOI] [PubMed] [Google Scholar]
- 8.Soler AP, Miller RD, Laughlin KV, Carp NZ, Klurfeld DM, Mullin JM. Increased tight junctional permeability is associated with the development of colon cancer. Carcinogenesis. 1999;20:1425–1431. doi: 10.1093/carcin/20.8.1425. [DOI] [PubMed] [Google Scholar]
- 9.Balda MS, Matter K. The tight junction protein ZO-1 and an interacting transcription factor regulate ErbB-2 expression. EMBO J. 2000;19:2024–2033. doi: 10.1093/emboj/19.9.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nunbhakdi-Craig V, Machleidt T, Ogris E, Bellotto D, White CL 3rd, Sontag E. Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. J Cell Biol. 2002;158:967–978. doi: 10.1083/jcb.200206114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishizaki T, Chiba H, Kojima T, Fujibe M, Soma T, Miyajima H, Nagasawa K, Wada I, Sawada N. Cyclic AMP induces phosphorylation of claudin-5 immunoprecipitates and expression of claudin-5 gene in blood-brain-barrier endothelial cells via protein kinase A-dependent and -independent pathways. Exp Cell Res. 2003;290:275–288. doi: 10.1016/s0014-4827(03)00354-9. [DOI] [PubMed] [Google Scholar]
- 12.Soma T, Chiba H, Kato-Mori Y, Wada T, Yamashita T, Kojima T, Sawada N. Thr(207) of claudin-5 is involved in size-selective loosening of the endothelial barrier by cyclic AMP. Exp Cell Res. 2004;300:202–212. doi: 10.1016/j.yexcr.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Yamauchi K, Rai T, Kobayashi K, Sohara E, Suzuki T, Itoh T, Suda S, Hayama A, Sasaki S, Uchida S. Disease-causing mutant WNK4 increases paracellular chloride permeability and phosphorylates claudins. Proc Natl Acad Sci USA. 2004;101:4690–4694. doi: 10.1073/pnas.0306924101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Souza T, Agarwal R, Morin PJ. Phosphorylation of claudin-3 at threonine 192 by cAMP-dependent protein kinase regulates tight junction barrier function in ovarian cancer cells. J Biol Chem. 2005;280:26233–26240. doi: 10.1074/jbc.M502003200. [DOI] [PubMed] [Google Scholar]
- 15.Kramer F, White K, Kubbies M, Swisshelm K, Weber BH. Genomic organization of claudin-1 and its assessment in hereditary and sporadic breast cancer. Hum Genet. 2000;107:249–256. doi: 10.1007/s004390000375. [DOI] [PubMed] [Google Scholar]
- 16.Tokes AM, Kulka J, Paku S, Szik A, Paska C, Novak PK, Szilak L, Kiss A, Bogi K, Schaff Z. Claudin-1, -3 and -4 proteins and mRNA expression in benign and malignant breast lesions: a research study. Breast Cancer Res. 2005;7:R296–R305. doi: 10.1186/bcr983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Resnick MB, Konkin T, Routhier J, Sabo E, Pricolo VE. Claudin-1 is a strong prognostic indicator in stage II colonic cancer: a tissue microarray study. Mod Pathol. 2005;18:511–518. doi: 10.1038/modpathol.3800301. [DOI] [PubMed] [Google Scholar]
- 18.Hoevel T, Macek R, Swisshelm K, Kubbies M. Reexpression of the TJ protein CLDN1 induces apoptosis in breast tumor spheroids. Int J Cancer. 2004;108:374–383. doi: 10.1002/ijc.11571. [DOI] [PubMed] [Google Scholar]
- 19.Hough CD, Sherman-Baust CA, Pizer ES, Montz FJ, Im DD, Rosenshein NB, Cho KR, Riggins GJ, Morin PJ. Large-scale serial analysis of gene expression reveals genes differentially expressed in ovarian cancer. Cancer Res. 2000;60:6281–6287. [PubMed] [Google Scholar]
- 20.Resnick MB, Gavilanez M, Newton E, Konkin T, Bhattacharya B, Britt DE, Sabo E, Moss SF. Claudin expression in gastric adenocarcinomas: a tissue microarray study with prognostic correlation. Hum Pathol. 2005;36:886–892. doi: 10.1016/j.humpath.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 21.Soini Y, Tommola S, Helin H, Martikainen P. Claudins 1, 3, 4 and 5 in gastric carcinoma, loss of claudin expression associates with the diffuse subtype. Virchows Arch. 2006;448:52–58. doi: 10.1007/s00428-005-0011-6. [DOI] [PubMed] [Google Scholar]
- 22.Kaihara T, Kusaka T, Nishi M, Kawamata H, Imura J, Kitajima K, Itoh-Minami R, Aoyama N, Kasuga M, Oda Y, et al. Dedifferentiation and decreased expression of adhesion molecules, E-cadherin and ZO-1, in colorectal cancer are closely related to liver metastasis. J Exp Clin Cancer Res. 2003;22:117–123. [PubMed] [Google Scholar]
- 23.Tobioka H, Isomura H, Kokai Y, Tokunaga Y, Yamaguchi J, Sawada N. Occludin expression decreases with the progression of human endometrial carcinoma. Hum Pathol. 2004;35:159–164. doi: 10.1016/j.humpath.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 24.Kominsky SL, Argani P, Korz D, Evron E, Raman V, Garrett E, Rein A, Sauter G, Kallioniemi OP, Sukumar S. Loss of the tight junction protein claudin-7 correlates with histological grade in both ductal carcinoma in situ and invasive ductal carcinoma of the breast. Oncogene. 2003;22:2021–2033. doi: 10.1038/sj.onc.1206199. [DOI] [PubMed] [Google Scholar]
- 25.Michl P, Barth C, Buchholz M, Lerch MM, Rolke M, Holzmann KH, Menke A, Fensterer H, Giehl K, Lohr M, et al. Claudin-4 expression decreases invasiveness and metastatic potential of pancreatic cancer. Cancer Res. 2003;63:6265–6271. [PubMed] [Google Scholar]
- 26.Rangel LB, Agarwal R, D’Souza T, Pizer ES, Ale PL, Lancaster WD, Gregoire L, Schwartz DR, Cho KR, Morin PJ. Tight junction proteins claudin-3 and claudin-4 are frequently overexpressed in ovarian cancer but not in ovarian cystadenomas. Clin Cancer Res. 2003;9:2567–2575. [PubMed] [Google Scholar]
- 27.Ichiyasu H, McCormack JM, McCarthy KM, Dombkowski D, Preffer FI, Schneeberger EE. Matrix metalloproteinase-9-deficient dendritic cells have impaired migration through tracheal epithelial tight junctions. Am J Respir Cell Mol Biol. 2004;30:761–770. doi: 10.1165/rcmb.2003-0370OC. [DOI] [PubMed] [Google Scholar]
- 28.Miyamori H, Takino T, Kobayashi Y, Tokai H, Itoh Y, Seiki M, Sato H. Claudin promotes activation of pro-matrix metalloproteinase-2 mediated by membrane-type matrix metalloproteinases. J Biol Chem. 2001;276:28204–28211. doi: 10.1074/jbc.M103083200. [DOI] [PubMed] [Google Scholar]
- 29.Miwa N, Furuse M, Tsukita S, Niikawa N, Nakamura Y, Furukawa Y. Involvement of claudin-1 in the beta-catenin/Tcf signaling pathway and its frequent upregulation in human colorectal cancers. Oncol Res. 2001;12:469–476. doi: 10.3727/096504001108747477. [DOI] [PubMed] [Google Scholar]
- 30.Katoh M, Katoh M. CLDN23 gene, frequently down-regulated in intestinal-type gastric cancer, is a novel member of CLAUDIN gene family. Int J Mol Med. 2003;11:683–689. [PubMed] [Google Scholar]
- 31.Long H, Crean CD, Lee WH, Cummings OW, Gabig TG. Expression of Clostridium perfringens enterotoxin receptors claudin-3 and claudin-4 in prostate cancer epithelium. Cancer Res. 2001;61:7878–7881. [PubMed] [Google Scholar]
- 32.Nichols LS, Ashfaq R, Iacobuzio-Donahue CA. Claudin 4 protein expression in primary and metastatic pancreatic cancer: support for use as a therapeutic target. Am J Clin Pathol. 2004;121:226–230. doi: 10.1309/K144-PHVD-DUPD-D401. [DOI] [PubMed] [Google Scholar]
- 33.Dhawan P, Singh AB, Deane NG, No Y, Shiou SR, Schmidt C, Neff J, Washington MK, Beauchamp RD. Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J Clin Invest. 2005;115:1765–1776. doi: 10.1172/JCI24543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J Cell Biol. 2001;153:263–272. doi: 10.1083/jcb.153.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan X, Egami H, Ishikawa S, Kurizaki T, Nakagawa M, Hirota M, Ogawa M. Arrangement of expression and distribution of tight junction protein claudin-1 in cell dissociation of pancreatic cancer cells. Int J Oncol. 2004;25:1567–1574. [PubMed] [Google Scholar]
- 36.Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto T, Harada N, Kawano Y, Taya S, Kaibuchi K. In vivo interaction of AF-6 with activated Ras and ZO-1. Biochem Biophys Res Commun. 1999;259:103–107. doi: 10.1006/bbrc.1999.0731. [DOI] [PubMed] [Google Scholar]
- 38.Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, Tada M. Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. J Biol Chem. 1998;273:12725–12731. doi: 10.1074/jbc.273.21.12725. [DOI] [PubMed] [Google Scholar]