Abstract
Transcription factor NF-κB is regulated by a family of inhibitors, IκBs, as well as the NF-κB1 and NF-κB2 precursor proteins, p105 and p100. Although the different NF-κB inhibitors can all inhibit NF-κB in vitro, their physiological functions are incompletely understood. Here we demonstrate that p105 plays an important role in the regulation of T-cell homeostasis and prevention of chronic inflammation. Mice lacking p105, but expressing the mature NF-κB1 p50, spontaneously develop intestinal inflammation with features of human inflammatory bowel disease. This inflammatory disorder occurs under specific pathogen-free conditions and critically involves T cells. Consistently, the p105-deficient mice have reduced frequency of naive T cells and increased frequency of memory/effector T cells in the peripheral lymphoid organs. Although p105 is dispensable for the production of immunosuppressive regulatory T cells (Tregs), p105 deficiency renders CD4 T cells more resistant to Treg-mediated inhibition. We further show that the loss of p105 results in hyperproduction of Th17 subset of inflammatory T cells. Together, these findings suggest a critical role for NF-κB1 p105 in the regulation of T-cell homeostasis and differentiation and the control of chronic inflammation.
Keywords: NF-κB, NF-κB1, p105, Th17, Treg, IL-6, intestinal inflammation
NF-κB family of transcription factors has diverse biological functions, including regulation of immune and inflammatory responses (1, 2). In mammalian cells, the NF-κB family is composed of five members, NF-κB1, NF-κB2, RelA, RelB, and c-Rel, which function as various hetero- and homo-dimers (3). NF-κB1 and NF-κB2 are produced as precursor proteins, p105 and p100, which undergo proteolytic processing to generate the mature NF-κB1 p50 and NF-κB2 p52, respectively (4). The mature NF-κB proteins are normally sequestered in the cytoplasmic environment through physical association with a family of inhibitory proteins, IκBs. In response to various stimuli, NF-κB dimers translocate to the nucleus as a result of IκB degradation. The presence of different IκB members, with distinct properties in signal response, creates a level of complexity in NF-κB regulation.
In addition to the typical IκBs, NF-κB inhibitors also include the NF-κB1 and NF-κB2 precursor proteins, p105 and p100, respectively. The C-terminal portion of these precursor proteins share structural homology with IκBs, characterized by the presence of ankyrin repeats. Like IκBs, p105 and p100 bind to NF-κB members and prevent the nuclear translocation of NF-κB. Thus, in addition to producing the mature NF-κB1 (p50) and NF-κB2 (p52) proteins, p105 and p100 have important roles in regulating the biological function of NF-κB. Recent studies demonstrate that p100 regulates a so-called noncanonical NF-κB signaling pathway that mediates activation of the RelB/p52 NF-κB complex and specific adaptive immune functions, including B-cell maturation and lymphoid organogenesis (5). However, the physiological function of p105 remains obscure. Unlike the signal-regulated processing of p100 (6), the processing of p105 occurs constitutively and cotranslationally (7), yielding high steady levels of both p105 and p50 in most cell types. In response to various stimuli, p105 undergoes degradation without generating p50, which may contribute to the activation of p105-sequestered NF-κB members (4). At least in macrophages, p105 also has a role in regulating MAP kinase (MAPK) signaling downstream of toll-like receptors (TLRs). p105 controls the stability and activity of Tpl2 (8, 9), a MAP kinase kinase kinase (MAP3K) mediating activation of the MAPK kinase1 (MEK1) and its downstream target extracellular signal-regulated kinase (ERK) (10). Thus, loss of p105 causes dramatic reduction in the steady level of Tpl2, resulting in attenuated activation of MEK/ERK by the TLR signals (8, 9).
The in vivo function of p105 in NF-κB regulation has been investigated using a knockin approach, which involved the insertion of a stop codon in the processing site of p105, allowing the direct production of p50 without generating p105 (11). In various cell types, these p105-deficient (p105−/−) animals have aberrant activation of NF-κB, particularly the p50-containing complexes. Probably due to the repressor function of p50 homodimers, the p105–deficient immune cells display hypo-activation of proinflammatory cytokines (11). Surprisingly, however, the p105−/−animals display inflammatory and autoimmune phenotypes characterized by immune cell infiltration into the lung and liver. To date, the mechanism by which p105 regulates autoimmunity and inflammation has not been defined.
Autoimmunity and chronic inflammation often result from aberrant T-cell responses, which in turn can be caused by impaired production or function of regulatory T cells (Tregs) (12, 13). Tregs suppress the activation and differentiation of naive T cells as well as the effector functions of differentiated T cells (13). Defect in Treg function breaks peripheral tolerance, causing spontaneous activation of naïve T cells and multi-organ inflammation. Autoimmunity and inflammation can also result from imbalanced differentiation of naïve CD4 T cells to effector T cells, including T helper (Th)1, Th2, and Th17 cells (14–16). In particular, Th17 cells are involved in various autoimmune and inflammatory diseases (17), such as experimental autoimmune encephalitis (EAE) (18, 19), rheumatoid arthritis (RA) (20–22), and inflammatory bowel disease (IBD) (23–26). These inflammatory T cells produce IL-17 family of cytokines, which mediate inflammation by recruiting innate immune cells and inducing proinflammatory cytokines (15, 18, 27, 28). Thus, characterization of the factors that regulate T-cell responses and differentiation is important for understanding the molecular mechanisms underlying autoimmue and inflammatory diseases.
In the present study, we show that the IκB-like protein p105 plays a critical role in regulating T-cell homeostasis and differentiation and preventing T-cell mediated inflammation. In addition to the previously identified autoimmune symptoms, the p105-deficient mice spontaneously develop intestinal inflammation with characteristics of human IBD. By crossing p105−/− mice to the Rag1−/− background and performing T-cell adoptive transfer, we show that T cells play an important role in mediating the autoimmune and inflammatory disorders of the p105−/− mice. Interestingly, loss of p105 causes reduction in the naïve T-cell population and concurrent increase in the memory/effector T-cell population, with a particular expansion of Th17 cells. Although p105 is dispensable for Treg development, the p105-deficient CD4 T cells are less sensitive to Treg-medaited suppression. We further show that p105 deficiency in macrophages promotes TLR-mediated induction of IL-6, a key cytokine that stimulates the differentiation of naïve CD4 T cells to Th17 cells (17). These findings establish p105 as a critical factor that maintains naïve T-cell phenotype and prevents abnormal induction of inflammatory T cells.
Materials and Methods
Mice
The nfκb1 knockout mice (in C57BL6/129 genetic background), which are deficient in both p105 and p50 (29), were purchased from Jackson Laboratories. The p105−/− mice (in C57BL6/129 genetic background), lacking p105 expression and competent in p50 expression, were provided by Bristol-Myers Squibb (11)). Rag1−/− mice (in C57BL6 genetic background) were purchased from Taconic. All mice were housed in specific pathogen-free cages and monitored periodically for the lack of common pathogens. Animal experiments were in accordance with protocols approved by the Institutional Animal Care and Use Committee of the Pennsylvania State University College of Medicine and the University of Texas MD Anderson Cancer Center.
Antibodies and reagents
Functional grade anti-mCD3ε(145-2C11), anti-mCD28 (37.51), and anti-mCD40, as well as the blocking antibodies for mIFN-γ(XMG1.2) and mIL-4 (11B11), were from eBioscience. Fluorescence-labeled anti-mCD4 (L3T4), anti-mCD25 (PC61.5), anti-mCD62L (MEL-14), anti-CD44 (IM7), anti-mIL-17A (eBio17B7), and anti-mIFN-γ(XMG1.2) were also purchased from eBioscience. The recombinant mIL-6 and hTGF-βwere purchased from PeproTech. Phorbol 12-myristate 13-acetate (PMA) and ionomycin were from Sigma, and monensin was from eBioScience.
Histology
Colons were removed from sacrificed mice and flushed with PBS. Distal and proximal halves of the colons were opened longitudinally, fixed in 10% neutral buffered formalin, embedded in paraffin, and sectioned for hematoxylin-eosin staining. Slides were analyzed blindly and scored for the degree of inflammation (0–40 scale) as described (30). Liver and lung sections were prepared similarly, and pictures were taken from typical sections.
Flow cytometry and cell sorting
Splenic and lymph node cell suspensions were prepared by gentle homogenization using a tissue homogenizer. Mononuclear cells were isolated by centrifugation over lymphocyte separation media (Cellgro) and subjected to flow cytometry analyses as previously described (31). For isolating CD4 T-cell subsets, CD4+ T cells were first enriched using CD4 microbeads (Miltenyi), and the effecter (CD4+CD25−), Treg (CD4+CD25+), naïve (CD4+CD25−CD44loCD62Lhi) and memory (CD4+CD25−CD44hiCD62Llo) CD4 T cells were isolated by flow cytometry sorting using Moflo (Dako) or FACSAria (BD Bioscience). Purity of isolated population was verified to be greater than 98%.
Intracellular cytokine staining (ICS)
T cells isolated from spleen and mLN or from in-vitro cultures were stimulated for 5 h with PMA (50 ng/ml) and Ionomycin (500 ng/ml) in the presence of monensin (2 µM). After the stimulation period, cells were fixed in 2% paraformaldehyde and permeablized in 0.5% saponin prior to staining for relevant cytokines. The stained cells were analyzed by flow cytometry.
In vitro T-cell activation and differentiation
Purified naive CD4+ T cells were stimulated in replicate wells of 96-well plates (1 × 105 cells/well) with plate-bound anti-CD3 and anti-CD28. After the indicated times of stimulation, the cells were labeled for 6 hr with 3H-thymidine for proliferation assays based on thymidine incorporation. For in vitro Th17 cell differentiation, naïve CD4 T cells were stimulated with anti-CD3 and anti-CD28 in the presence of anti-IFN-γ(10 µg/ml), anti-IL-4 (10 µg/ml), IL-6 (20 ng/ml), and TGF-β(5 ng/ml). After the indicated times, the cells were subjected to ICS and real-time PCR analyses.
Treg assays
5 × 104/well effector CD4 T cells were stimulated with soluble anti-CD3 (1 µg/ml) and 1× 105/well irradiated (3000 RAD) antigen-presenting cells (splenocytes) either in the presence or absence of Tregs. The proliferation of the effector T cells was analyzed as described above.
Preparation and stimulation of bone marrow derived macrophages (BMDM)
BMDM cells were prepared from the indicated age-matched mice using a procedure described previously (32). Before stimulation, the cells were seeded onto 100-mm petridishes and starved for 12–16 hr in DMEM supplemented with 0.5% FBS. The cells were then stimulated with the indicated inducers and collected for total RNA preparation.
Real-time quantitative RT-PCR
Total RNA was isolated from colonic tissues, T cells, or macrophages using TRI reagent (Molecular Research Center, Inc.) and subjected to cDNA synthesis using RNase H-reverse transcriptase (Invitrogen) and oligo (dT) primers. Real-time quantitative PCR was performed using iCycler Sequence Detection System (Bio-Rad) and iQ™ SYBR Green Supermix (Bio-Rad). The expression of individual genes was calculated by a standard curve method and normalized to the expression of actin. The gene-specific primer sets (all for murine genes) were: IL-17a, 5’-AGCGATGGTGGATGGCTCATGGTTAG-3’ and 5’-AGCTTTCCCTCCGCATTGACACAG-3’; IL-17f, 5’-CCCATGGGATTACAACATCACTC-3’ and 5’-CACTGGGCCTCAGCGATC-3’; IL-21, 5’-ATCCTGAACTTCTATCAGCTCCAC-3' and 5’-GCATTTAGCTATGTGCTTCTGTTTC-3’; IL-22, 5’- TCC GAG GAG TCA GTG CTA AA-3’ and 5’-AGA ACG TCT TCC AGG GTG AA-3’; IL-23R, 5’-GCCAAGAAGAC CATTCCCGA-3’ and 5’-TCAGTGCTACAATCTTCTTCAGAGGACA-3’; RORγt, 5’-CAAGTCATCTGGGATCCACTAC-3’ and 5’-TGCAGGAGTAGGCCACATTACA-3’; IL-1b, 5’-GCTCTCCACCTCAATGGACAG-3’ and 5’-GAAGACAGGCTTGTGCTCTGC-3’; IL-6, 5’-CACAGAGGATACCACTCCCAACA-3’ and 5’-TCCACGATTTCCCAGAGAACA-3’; IL-12 p35, 5’-ACTAGAGAGACTTCTTCCACAACAAGAG-3’ and 5’-GCACAGGGTCATCATCAAAGAC-3’; TNF-α, 5’-CATCTTCTCAAAATTCGAGTGACAA-3’ and 5’-CCAGCTGCTCCTCCACTTG-3’; Actin, 5’-CGTGAAAAGATGACCCAGATCA-3’ and 5’-CACAGCCTGGATGGCTACGT-3’.
T-cell adoptive transfer
Lymphocytes were isolated from mesenteric lymph nodes of Wt and p105−/− mice (6–8 wk old) and the total T-cell populations were isolated by Thy-1.2 microbeads (Miltenyi). 7 × 106 Thy1.2+ T-cells were injected into 4–6 week old RAG1−/− recipients via the tail-vein. At 6 weeks post adoptive transfer, colons were collected from sacrificed recipient mice and subjected to histology and real-time PCR analyses.
Electrophoresis mobility shift assay (EMSA)
EMSA was performed as previously described (33). Briefly, nuclear extracts were prepared and subjected to EMSA using a 32P-radiolabeled κB oligonucleotide probe (CAA CGG CAG GGG AAT TCC CCT CTC CTT) or a control probe bound by the constitutive transcription factor NF-Y (AAG AGA TTA ACC AAT CAC GTA CGG TCT).
Results
Genetic deficiency of p105 causes colonic inflammation
To investigate the in vivo function of p105 in regulating inflammation, we examined the histological phenotypes of various organs isolated from the p105-deficient (p105−/−) mice. Even when housed under stringent pathogen-free conditions, the p105−/− mice displayed leukocyte infiltration into the lung and liver (data not shown). More strikingly, these mutant animals had intestinal inflammation with prominent features of IBD (Fig. 1). The colons of p105−/− mice were often devoid of solid feces and evidently shorter and more rigid (Fig. 1A), typical macroscopic features of IBD and experimental colitis (34, 35). Large leukocyte follicles (colonic patches) were frequently detected in the colons of p105−/− mice at different ages but were not found in the control colons (Fig. 1B and data not shown). Other histological features of the p105−/− colons included crypt damages, sporadic leukocyte infiltrations, and thickening of mucosal layer (Fig. 1B). Microscopic analyses of multiple histology slides also revealed higher inflammation scores in both the proximal and distal colons of the p105−/− mice (Fig. 1C). Consistent with the histology results, the colonic tissue of p105−/− mice expressed various proinflammatory genes, including IL-1β, TNF-α, IL-6, and IL-12 (Fig. 1D).
FIGURE 1. Spontaneous development of colonic inflammation in p50 KI mice.
A, Picture to compare the colons of WT and p105−/− mice, showing the loss of fecal matters and reduced length of the p105−/− colon. Data are representative of multiple mice. B, Hematoxylin-eosin staining of tissue sections of the distal portion of the colon from 8 week old control WT and p105−/− mice. An inflammatory cell follicle in the colonic mucosa (colonic patch) is indicated by an arrowhead. Original magnification, x20 and x40. C, Histological scores of mucosal inflammation in WT and p105−/− mice. Data were from three wildtype and three p105−/− mice (8 week of age), each with two colon sections. D, Real-time PCR showing the constitutive expression of several proinflammatory genes in the colon of p105−/− mice but not the WT mice.
T cells are involved in the inflammation of p105−/− mice
Chronic inflammation is often mediated by aberrant responses of T cells (36). To examine the role of T cells in mediating the inflammatory disorders of p105−/− mice, we crossed the p105−/− mice with Rag1−/− mice to produce p105−/−Rag1−/− mice. In contrast to the prominent colonic inflammation of p105−/− mice, no obvious colonic inflammation was detected in the lymphocyte-free p105−/−Rag1−/− mice (Fig. 2A). Similarly, the inflammatory phenotype in the lung was also lost after the p105−/− mice had been crossed to the Rag1−/− background (Fig. 2A). Real-time PCR assays revealed that the constitutive production of proinflammatory cytokines in the colons of p105−/− mice was also lost in the p105−/−Rag1−/− mice (data not shown). Thus, lymphocytes play an important role in mediating the chronic inflammations in p105−/− mice.
FIGURE 2. T cells are involved in the development of autoimmune and inflammatory disorders in p105−/− mice.
A, Colons and Lungs from p105−/− mice on Rag1+/+ (p105−/−/Rag1+/+) or Rag1 knockout (p105−/−/Rag1−/−) background were subjected to histology analyses. Colonic patches (upper) and lung infiltrations (lower) are indicated by arrowheads, which were detected in p105−/−/Rag1+/+ mice but not in p105−/−/Rag1KO mice. Original magnification, x20. B, Induction of inflammatory cell infiltration by adoptively transferred p105−/− T cells. Rag1−/− mice (4–6 weeks old) were intravenously injected with T cells isolated from the mesenteric lymph nodes of WT or p105−/− mice. After 6 weeks, the recipient mice were sacrificed for histology analyses of lymphocyte infiltration in the colon. An arrowhead indicates a colonic patch detected in a recipient of p105−/− T cells. Data are representative of three mice per group. Original magnification, x20. C, RNA samples were isolated from colons of the adoptively transferred mice described in B and subjected to real-time PCR analyses to detect the relative mRNA expression of the indicated genes.
To investigate whether p105−/− T cells were sufficient for inducing inflammation in recipient mice, we adoptively transferred T cells derived from p105−/− or WT mice into Rag1−/− mice. Interestingly, transfer of p105−/− T cells into Rag1−/− mice was sufficient to cause colonic inflammation within 6 weeks, as demonstrated by both colonic patch formation (Fig. 2B) and expression of proinflammatory cytokines (Fig. 2C). In contrast, colonic patches were rarely found in Rag1−/− mice that had received WT T cells (Fig. 2B),and these control mice also did not show aberrant expression of proinflammatory cytokines in the colonic tissue (Fig. 2C). Thus, T cells play an important role in the development of colitis in p105−/− mice.
P105 deficiency causes abnormal activation and homeostasis of T cells
Because of the involvement of T cells in the inflammatory phenotype of p105−/− mice, we examined the effect of p105 deficiency on the activation of T cells. Compared to wildtype CD4 naïve T cells, the p105−/− CD4 naïve T cells displayed a low, but significant, increase in the proliferation potential upon stimulation with anti-CD3 and anti-CD28 (Fig. 3A). This phenotype was in sharp contrast to the hypoproliferation of the nfκb1−/− T cells that lack both p105 and p50. Parallel DNA binding assays revealed that loss of p105 caused a marked activation of NF-κB in CD4 T cells, and this phenotype was dependent on p50 since the NF-κB activation was attenuated in nfκb1−/− T cells (Fig. 3B). To assess the role of p105 in regulating the homeostasis of T cells in vivo, we analyzed the naïve and memory T-cell populations based on their surface markers. In wildtype mice, the majority of T cells in the spleen and lymph nodes displayed a naïve phenotype, characterized by the expression of low levels of CD44 and high levels of CD62 ligand (CD62L) (Fig. 3C). Remarkably, the CD44loCD62Lhi population of naïve T cells was substantially reduced in the p105−/− mice, with a concurrent increase in the population of CD4 T cells displaying memory or effector markers (CD44hiCD62Llo) (Fig. 3C). This abnormality in T-cell homeostasis was not seen in nfκb1−/− mice lacking the mature NF-κB1 protein p50 (Fig. 3C), thus suggesting that the IκB-like molecule p105 has a critical role in maintaining the naïve T-cell homeostasis.
FIGURE 3. Activation and homeostasis of p105-deficient CD4 T cells.
A, WT, nfκb1−/−, and p105−/− naïve CD4 T cells were either not treated (NT) or stimulated in triplicate wells with the indicated amounts of plate-bound anti-CD3 plus soluble anti-CD28. After 48 hr, cell proliferation was measured by thymidine incorporation. Data are presented as mean ± s.d. of three replicates. B, CD4 T cells were either not treated (NT) or stimulated for 24 h with anti-CD3 and anti-CD28. Nuclear extracts were subjected to EMSA using a 32P-radiolabeled κB probe (upper) or a probe that binds the constitutive nuclear protein nuclear factor Y (NF-Y). C, Mesenteric lymph node cells and splenocytes of the indicated mice were subjected to flow cytometry analyses to measure the percentage of naïve (CD44hiCD62Llo) and memory/effector (CD44loCD62Lhi) CD4 T cells within the CD4 T-cell population.
Loss of p105 does not inhibit Treg development but renders naive T cells more resistant to Treg-mediated suppression
Spontaneous activation of naïve T cells and development of autoimmune and inflammatory disorders are often caused by defect in the production of Tregs, since these cells suppress the activation of CD4 T cells by self-antigens (12, 13). The fact that p105−/− naive T cells exhibit memory/effector phenotype in vivo prompted us to examine whether the loss of p105 blocked the production of Tregs. As expected, WT mice had a population of CD4+CD25+FoxP3+ Tregs in the thymus, spleen, and mesentery lymph nodes (mLN) (Fig. 4A, left panels). Surprisingly, despite their inflammatory phenotype, the p105−/− mice produced even a higher frequency of Tregs in both the thymus and peripheral lymphoid organs (Fig. 4A, right panels). This phenotype was not seen in the nfκb1−/− mice, lacking both p105 and p50, suggesting the involvement of deregulated activation of p50-containing NF-κB complexes (Fig. 4A, central panels). To examine whether p105 is required for the effector function of Tregs, we activated effector CD4 T cells in vitro in the presence of either wildtype or p105-deficient Tregs. Both the wildtype and p105−/− Tregs potently inhibited the proliferation of the effector CD4 T cells (Fig. 4B). Thus, p105 is dispensable for both the production and effector function of Tregs.
FIGURE 4. The p105 deficiency does not inhibit the development and effector function of Tregs but renders CD4 effector T cells insensitive to Treg-mediated suppression.
A, Thymocytes, splenocytes, and mLN cells were subjected to flow cytometry, and the frequency of CD4+CD25+FoxP+ Tregs were presented as percentage of total CD4 T cells. B, WT effector CD4 T cells were activated in the presence of the indicated ratios of either WT or p105−/− Tregs, and the proliferation of effector T cells was analyzed based on 3H-thymidine incorportation. The level of Treg suppression was calculated as percentage of suppression based on the proliferation of effector T cells in the absence of Tregs. C, WT and p105−/− naïve CD4 T cells were stimulated either in the absence or presence of the indicated ratios of WT Tregs, and the proliferation of the effector CD4 T cells was analyzed based on 3H-tritium incorporation. Suppression was calculated as described in in B.
We next examined whether p105 regulates the sensitivity of CD4 effector T cells to Treg-mediated inhibition. Effector CD4 T cells derived from WT or p105−/− mice were activated in the presence or absence of WT Tregs and subjected to proliferation assays. The WT effector CD4 T cells were efficiently suppressed by Tregs even at a high effector:Treg ratio (12:1, Fig. 4C). In sharp contrast, the p105-deficient effector CD4 T cells were remarkably resistant to Treg-mediated inhibition (Fig. 4C). Even at low effector:Treg ratios, the proliferation of these mutant cells was only partially inhibited. Together, these results suggest that although p105 is not required for the production and function of Treg cells, it regulates the responsiveness of effector T cells to Treg-mediated inhibition. These findings provide insight into the spontaneous activation of T cells in the p105−/− mice.
Loss of p105 results in aberrant production of Th17 inflammatory T cells
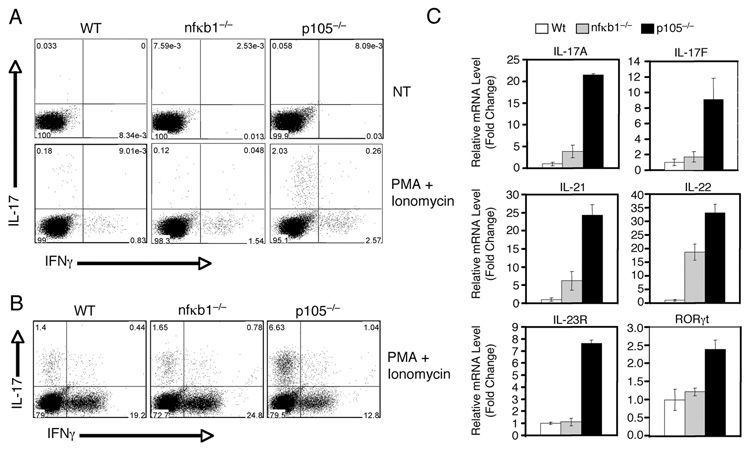
Inflammatory T cells play an important role in the induction of chronic inflammations, including IBD (26, 36). We thus examined whether the p105 deficiency affected the production of Th1 and Th17 cells, both being implicated in IBD pathogenesis (26, 37). T cells isolated from the spleen of WT, nfκb1−/−, or p105−/− mice were either untreated or stimulated briefly with mitogens to induce the production of cytokines by the effector T cells. ICS analysis of the WT CD4 T cells revealed a low percentage (0.31%) of Th17 cells and a higher percentage of Th1 cells (7.13%), based on their production of IL-17 and IFN-γ, respectively (Fig. 5A, lower left panel). Interestingly, an over 10 times higher frequency of Th17 cells was detected in the p105−/− mice (Fig. 5A, lower right panel). The p105−/− mice also displayed a moderate increase in the frequency of Th1 cells (Fig. 5A, lower right panel). In contrast, the overproduction of Th17 cells was not seen in the nfκb1−/− mice (Fig. 5A, lower central panel). In fact, these mutant mice produced reduced frequency of Th17 cells.
FIGURE 5. Loss of p105 causes aberrant production of Th17 cells.
A, Th17 frequency in total T cells. T cells were isolated from the spleen of WT or p105−/− mice and either left non-treated (NT) or stimulated with PMA (50 ng/ml) plus ionomycin (500 ng/ml) for 5 h. The IL-17 producing Th17 cells and the IFN-γ producing Th1 cells were quantified by intracellular cytokine staining and flow cytometry. Numbers represent percentage of CD4+ gated cells. B, Th17 frequency in sorted memory CD4+ T cells (CD62Llow, CD44hi, CD25−, CD4+), determined as in a. C, Expression of Th17 marker genes in the colons of p105−/− mice. Real-time PCR was performed to analyze the relative RNA concentration of the indicated genes in the colons of WT and p105−/− mice.
Since p105−/− mice had a higher number of memory T cells, we analyzed the frequency of Th1 and Th17 cells within sorted CD4+ memory T-cell population (CD4+CD25−CD62LloCD44hi). This analysis further demonstrated the aberrant production of Th17 T cells by the p105−/− mice (Fig. 5B). However, the frequency of Th1 cells within the memory T cell population was not enhanced in the p105−/− mice (Fig. 5B). Thus, the p105 deficiency causes overproduction of inflammatory T cells, particularly the Th17 cells.
Th17 cells are known to infiltrate into the colonic mucosa and mediate inflammation (38). To assess the presence of Th17 cells in the inflamed colons of p105−/− mice, we analyzed the expression of Th17 marker genes, including those encoding IL-17A, IL-17F, IL-21, IL-22, IL-23R, and the orphan nuclear hormone receptor RORγt. Real-time PCR analyses detected a low level of expression of these genes in the colonic tissue of WT mice (Fig. 5C). In sharp contrast, the expression level of all of these Th17 marker genes was strikingly elevated in the colons of p105−/− mice (Fig. 5C). Furthermore, the aberrant expression of Th17 marker genes was dependent on p50, since it was not detected in the nfκb1−/− colons (Fig. 5C). These results indicate that the p105 deficiency causes enhanced frequency of Th17 cells both in the lymphoid organs and at the site of inflammation.
p105 has no T-cell intrinsic role in Th17 differentiation but controls TLR-mediated induction of IL-6 in macrophages
The hyper production of Th17 cells in p105−/− mice could be due to enhanced response of naïve CD4 T cells to Th17 differentiation signals or deregulated cytokine production by innate immune cells. To examine whether p105 had a T-cell intrinsic role in the regulation of Th17 differentiation, we analyzed the in vitro differentiation ability of naïve CD4+ T cells derived from WT, nfκb1−/−, or p105−/− mice. As expected, WT CD4+ T cells differentiated into Th17 cells when stimulated under Th17 differentiation conditions (with recombinant IL-6 and TGF-β and blocking antibodies for IFN-γ and IL-4) (Fig. 6A). However, the Th17 differentiation efficiency was only slightly enhanced in the p105−/− T cells, thus suggesting that p105 might not play an important T-cell intrinsic role in regulating Th17 differentiation. Consistently, the loss of p50 in nfκb1−/− T cells only moderately affected the Th17 differentiation (Fig. 6A).
FIGURE 6. p105 deficiency has no effect on Th17 differentiation from naïve CD4 T cells in vitro but causes hyperproduction of IL-6 in macrophages.
A, In vitro differentiation of Th17 cells. Purified native CD4+ T cells from WT, nfκb1−/−, and p105−/− mice were stimulated for 4 days with anti-CD3 and anti-CD28 either in the absence (no cytokine) or presence of IL-6 plus TGF-β. Th17 and Th1 cells were analyzed by ICS and flow cytometry. B, Macrophages were prepared from the bone marrow cells of the indicated genotypes of mice. The cells were either not treated (NT) or stimulated for 4 hr with LPS (1 µg/ml), CpG (1 µM), or an agonistic antibody against murine CD40 (10 µg/ml). Real time RT-PCR was performed to measure the relative levels of IL-6 induction.
The results described above indicate the involvement of an indirect mechanism by which p105 regulates Th17 cell differentiation. In this regard, a striking finding in our gene expression analysis was the high level expression of IL-6 in the colons of p105−/− mice (Fig. 1D). Since IL-6 is a key proinflammatory cytokine that stimulates Th17 cell differentiation, we examined the effect of p105 deficiency on IL-6 gene induction in macrophages. In WT macrophages, IL-6 gene expression was stimulated by the TLR ligands LPS and CpG and weakly induced by the CD40 agonistic antibody (anti-CD40) (Fig. 6B). Interestingly, the IL-6 gene induction was dramatically promoted in the p105-deficient macrophages (Fig. 6B). This effect was not seen in p105−/− bone marrow-derived dendritic cells (data not shown), suggesting cell-type specificity. Since p105 has an important role in regulating Tpl2/ERK signaling in macrophages (8, 9), we performed parallel studies using the nfκb1−/− macrophages, since these cells had a similar Tpl2/ERK signaling defect due to the lack of p105. In contrast to the p105−/− cells, the nfκb1−/− cells did not show hyper induction of IL-6 (Fig. 6B). Thus, the aberrant induction of IL-6 gene in p105−/− macrophages is unlikely due to the defect in Tpl2/ERK signaling but rather the deregulated activation of p50-containing NF-κB complexes.
Discussion
NF-κB1 is cotranslationally produced as two proteins, p105 and p50, both being abundantly expressed in various cell types (7). Strong evidence suggests that p105 functions as an IκB-like molecule that inhibits the nuclear translocation of NF-κB members (4). Like IκBs, p105 undergoes phosphorylation and degradation in response to immune stimuli, which is thought to mediate nuclear translocation of NF-κB (4). However, it has been unclear what physiological functions p105 plays, particularly in the regulation of immune responses. Although extensive studies have been performed with nfκb1−/− mice, these animals are not particularly useful for the study of p105 function since they lack not only p105 but also the mature NF-κB1 p50. The p105−/− mice, on the other hand, serve as a powerful model for the study of p105 function because they are specifically deficient in p105 (11). It was previously shown that the p105−/− mice show autoimmune symptoms, characterized by immune cell infiltration into the liver and lung, although the underlying mechanism was not resolved. In the present study, we demonstrate that p105 has an important role in regulating the homeostasis of naive CD4 T cells. Loss of p105 causes a dramatic reduction in the naive CD4 T-cell population with concurrent increase in the population of CD4 T cells with memory or effector markers. We have obtained strong evidence that T cells play a critical role in mediating the autoimmune phenotype of p105−/− mice. Interestingly, in addition to lung and liver infiltrations, the p105−/− mice spontaneously develop intestinal inflammation with features of human IBD. Since the animals were housed in the specific pathogen free facility, such an inflammatory disorder must be autoimmune in nature, probably due to the deregulated T-cell responses. Indeed, the intestinal inflammation, as well as the liver and lung infiltration, was prevented when the p105−/− mice were bred to the Rag1−/− background lacking lymphocytes. Furthermore, adoptive transfer of p105−/− T cells was sufficient for inducing the autoimmune and inflammatory phenotypes.
Peripheral T cells are maintained predominantly in their naive state, which is important for normal function of the adaptive immune system (39). The loss of naive T cells can be caused by various conditions, such as defect in thymocyte development, naive T-cell apoptosis, and activation and differentiation of naive T cells by low-affinity self-antigens (40). The development of thymocytes does not require p105, although the peripheral T-cell number is moderately reduced in the p105−/− mice (data not shown and ref. (11)). Since the reduction in naïve T cells in p105−/− mice is associated with increase in memory/effector T cells, it is likely that loss of p105 causes abnormal activation of the naive T cells. The p105−/− naive CD4 T cells show moderate increase in the in vitro response to anti-CD3 stimulation. More significantly, these cells are resistant to Treg-mediated inhibition. Although how p105 regulates this novel function remains to be further investigated, deregulated activation of p50-containing NF-κB complexes appears to be responsible for the resistance of CD4 T cells to Tregs, since the nfκb1−/− CD4 effector T cells (lacking both p105 and p50) are sensitive to Tregs (data not shown). In line with these findings, a previous study, using fibroblasts, suggests that NF-κB inhibits the signaling induced by TGF-β(41), which is known as a major effector molecule of Tregs (42). Future studies will examine whether p105 regulates TGF-b-mediated T-cell suppression.
Our data suggest that the intestinal inflammation of p105−/− mice is also associated with increased frequency of Th17 cells. In peripheral lymphoid organs, the p105−/− mice contained more than ten folds of Th17 cells compared to their WT controls. The percentage of Th17 cells within the memory/effector population of CD4 T cells is also dramatically increased in the p105−/− mice. Given the important role of Th17 cells in mediating autoimmunity and inflammation (23–25), the increase in this subset of CD4 effector T cells may contribute to the development of colitis and other autoimmune disorders in the p105−/− mice. Consistent with this idea, our real-time RT-PCR analyses revealed high levels of expression of Th17-specific genes in the colons of the p105−/− mice. The hyper-production of Th17 cells in p105−/− mice involves activation of p50-containing NF-κB complexes, since this phenotype is not seen in the nfκb1−/− mice lacking both p105 and p50. In fact, the nfκb1−/− mice have reduced numbers of Th17 cells, a finding that is consistent with a previous report that the nfκb1−/− mice are refractory to the induction of EAE and RA (43, 44).
The roles of p105 in regulating T-cell homeostasis and Th17 differentiation may involve different mechanisms. p105 has a T-cell intrinsic role in regulating the response of CD4 T cells to Tregs. On the other hand, regulation of Th17 cell differentiation appears to involve an indirect mechanism, since the loss of p105 in T cells has little effect on their commitment to the Th17 lineage. More significantly, p105 negatively regulates the induction of IL-6 gene by TLR and CD40 signals in innate immune cells. Hyper expression of IL-6 is readily detected in both in vitro activated p105−/− macrophages and colonic tissues derived from p105−/− mice. Biochemical and genetic evidence suggests that IL-6 is a critical cytokine that stimulates Th17 cell differentiation (17). Notably, IL-6 not only participates in the induction of Th17 cell differentiation from naïve CD4 T cells but also reprograms Tregs to become Th17 cells (45). Since the p105−/− mice have increased frequency of Tregs, it suggests the intriguing possibility that the aberrantly expressed IL-6 in p105−/− mice may promote Th17 production from both naïve T cells and Tregs. Future studies will examine this possibility.
In macrophages, p105 is required for TLR-stimulated Tpl2/ERK signaling in addition to the regulation of NF-κB. Since p105-deficient macrophages have a defect in ERK activation, the functional phenotypes of the p105−/− mice may be contributed by deregulated activation of NF-κB or attenuated activation of ERK. The former possibility is strongly suggested by our parallel studies using the nfκb1−/− mice, which lack both p105 and the mature NF-κB1 protein p50. Like the p105−/− mice, the nfκb1−/− mice have ERK signaling defect due to the lack of p105 (9). However, the loss of p50 in nfκb1−/− mice is sufficient to correct the phenotypes of the p105−/− mice in T-cell homeostasis and autoimmune/inflammatory disorders. The nfκb1−/− macrophages also do not display the aberrant induction of IL-6. Taken together, these results suggest that p105 has a critical role in controlling the activation of p50-containing NF-κB complexes, the deregulation of which may contribute to abnormal T-cell response and differentiation as well as development of autoimmune and inflammatory disorders.
Acknowledgement
We thank Kang Li, Nate Sheaffer and David Stanford of the Pennsylvania State College of Medicine Core facilities for assistance with tissue sections and flow cytometry. We thank Bristol-Myers Squib for the p105−/− mice and members of Chen Dong’s lab for suggestions and technical help.
Footnotes
This study was supported by grants from National Institutes of Health (AI064639, GM084459, and AI057555) and start-up fund from the University of Texas MD Anderson Cancer Center.
Abbreviations used in this papre: MAPK, MAP kinase; TLR, toll-like receptor; MAP3K,MAP kinase kinase kinase; MEK1, MAPK kinase1; ERK, extracellular signal-regulated kinase; Treg, regulatory T cell; EAE, experimental autoimmune encephalitis; RA,rheumatoid arthritis; PMA, phorbol 12-myristate 13-acetate; ICS, Intracellular cytokine staining; EMSA, Electrophoresis mobility shift assay; CD62L, CD62 ligand; mLN,mesentery lymph nodes.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Silverman N, Maniatis T. NF-κB signaling pathways in mammalian and insect innate immunity. Genes & Dev. 2001;15:2321–2342. doi: 10.1101/gad.909001. [DOI] [PubMed] [Google Scholar]
- 2.Hayden MS, West AP, Ghosh S. NF-kappaB and the immune response. Oncogene. 2006;25:6758–6780. doi: 10.1038/sj.onc.1209943. [DOI] [PubMed] [Google Scholar]
- 3.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 4.Beinke S, Ley SC. Functions of NF-kappaB1 and NF-kappaB2 in immune cell biology. Biochem. J. 2004;382:393–409. doi: 10.1042/BJ20040544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Xiao G, Harhaj EW, Sun SC. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol. Cell. 2001;7:401–409. doi: 10.1016/s1097-2765(01)00187-3. [DOI] [PubMed] [Google Scholar]
- 7.Lin L, DeMartino GN, Greene WC. Cotranslational biogenesis of NF-kappaB p50 by the 26S proteasome. Cell. 1998;92:819–828. doi: 10.1016/s0092-8674(00)81409-9. [DOI] [PubMed] [Google Scholar]
- 8.Beinke S, Deka J, Lang V, Belich MP, Walker PA, Howell S, Smerdon SJ, Gamblin SJ, Ley SC. NF-kappaB1 p105 negatively regulates TPL-2 MEK kinase activity. Mol. Cell. Biol. 2003;23:4739–4752. doi: 10.1128/MCB.23.14.4739-4752.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waterfield M, Zhang M, Norman LP, Sun SC. NF-kappaB1/p105 Regulates Lipopolysaccharide-Stimulated MAP Kinase Signaling by Governing the Stability and Function of the Tpl2 Kinase. Mol. Cell. 2003;11:685–694. doi: 10.1016/s1097-2765(03)00070-4. [DOI] [PubMed] [Google Scholar]
- 10.Dumitru CD, Ceci JD, Tsatsanis C, Kontoyiannis D, Stamatakis K, Lin JH, Patriotis C, Jenkins NA, Copeland NG, Kollias G, Tsichlis PN. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell. 2000;103:1071–1083. doi: 10.1016/s0092-8674(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa H, Claudio E, Dambach D, Raventos-Suarez C, Ryan C, Bravo R. Chronic inflammation and susceptibility to bacterial infections in mice lacking the polypeptide (p)105 precursor (NF-κB1) but expressing p50. J. Exp. Med. 1998;187:985–996. doi: 10.1084/jem.187.7.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat. Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu. Rev. Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 15.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat. Rev. Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 17.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat. Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 18.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 20.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J. Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 21.Röhn TA, Jennings GT, Hernandez M, Grest P, Beck M, Zou Y, Kopf M, Bachmann MF. Vaccination against IL-17 suppresses autoimmune arthritis and encephalomyelitis. Eur. J. Immunol. 2006;36:2857–2867. doi: 10.1002/eji.200636658. [DOI] [PubMed] [Google Scholar]
- 22.Toh ML, Miossec P. The role of T cells in rheumatoid arthritis: new subsets and new targets. Curr. Opin. Rheumatol. 2007;19:284–288. doi: 10.1097/BOR.0b013e32805e87e0. [DOI] [PubMed] [Google Scholar]
- 23.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, Mckenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J. Clinic. Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kullberg MC, Jankovic D, Feng CG, Hue S, Gorelick PL, McKenzie BS, Cua DJ, Powrie F, Cheever AW, Maloy KJ, Sher A. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J. Exp. Med. 2006;203:2485–2494. doi: 10.1084/jem.20061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Z, Zheng M, Bindas J, Schwarzenberger P, Kolls JK. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm. Bowel Dis. 2006;12:382–388. doi: 10.1097/01.MIB.0000218764.06959.91. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Hinrichs DJ, Lu H, Chen H, Zhong W, Kolls JK. After interleukin-12p40, are interleukin-23 and interleukin-17 the next therapeutic targets for inflammatory bowel disease? Int. Immunopharmacol. 2007;7:409–416. doi: 10.1016/j.intimp.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 27.Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 28.Dong C. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat. Rev. Immunol. 2006;6:329–333. doi: 10.1038/nri1807. [DOI] [PubMed] [Google Scholar]
- 29.Sha WC, Liou HC, Tuomanen EI, Baltimore D. Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell. 1995;80:321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- 30.Williams KL, Fuller CR, Dieleman LA, DaCosta CM, Haldeman KM, Sartor RB, Lund PK. Enhanced survival and mucosal repair after dextran sodium sulfate-induced colitis in transgenic mice that overexpress growth hormone. Gastroenterology. 2001;120:925–937. doi: 10.1053/gast.2001.22470. [DOI] [PubMed] [Google Scholar]
- 31.Reiley WW, Zhang M, Jin W, Losiewicz M, Donohue KB, Norbury CC, Sun SC. Regulation of T cell development by the deubiquitinating enzyme CYLD. Nat. Immunol. 2006;7:411–417. doi: 10.1038/ni1315. [DOI] [PubMed] [Google Scholar]
- 32.Racoosin EL, Swanson JA. Macrophage colony-stimulating factor (rM-CSF) stimulates pinocytosis in bone marrow-derived macrophages. J. Exp. Med. 1989;170:1635–1648. doi: 10.1084/jem.170.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun S-C, Ganchi PA, Ballard DW, Greene WC. NF-κB controls expression of inhibitor IκBα: evidence for an inducible autoregulatory pathway. Science. 1993;259:1912–1915. doi: 10.1126/science.8096091. [DOI] [PubMed] [Google Scholar]
- 34.Kjellev S, Lundsgaard D, Poulsen SS, Markholst H. Reconstitution of Scid mice with CD4+CD25- T cells leads to rapid colitis: an improved model for pharmacologic testing. Int. Immunopharmacol. 2006;6:1341–1354. doi: 10.1016/j.intimp.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 35.Ambrosini R, Barchiesi A, Di Mizio V, Di Terlizzi M, Leo L, Filippone A, Canalis L, Fossaceca R, Carriero A. Inflammatory chronic disease of the colon: how to image. Eur. J. Radiol. 2007;61:442–448. doi: 10.1016/j.ejrad.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 36.Sartor RB. Mechanisms of disease: pathogenesis of Chron's disease and ulcerative colitis. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006;3:390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- 37.Neuman MG. Immune dysfunction in inflammatory bowel disease. Transl.Res. 2007;149:173–186. doi: 10.1016/j.trsl.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 38.Ivanov I, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 39.Jameson SC. T cell homeostasis: keeping useful T cells alive and live T cells useful. Semin. Immunol. 2005;17:231–237. doi: 10.1016/j.smim.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 40.Li MO, Flavell RA. TGF-beta: a master of all T cell trades. Cell. 2008;134:392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bitzer M, von Gersdorff G, Liang D, Dominguez-Rosales A, Beg AA, Rojkind M, Böttinger EP. A mechanism of suppression of TGF-beta/SMAD signaling by NF-kappa B/RelA. Genes Dev. 2000;14:187–197. [PMC free article] [PubMed] [Google Scholar]
- 42.Li MO, Flavell RA. Contextual regulation of inflammation: a duet by transforming growth factor-beta and interleukin-10. Immunity. 2008;28:468–476. doi: 10.1016/j.immuni.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Hilliard B, Samoilova EB, Liu TT, Rostami AM, Chen Y. Experimental autoimmune encephalomyelitis in nuclear factor-kB-deficient mice: roles of nuclear factor-kB in the activation and differentiation of autoreactive T cells. J. Immunol. 1999;163:2937–2943. [PubMed] [Google Scholar]
- 44.Campbell IK, Gerondakis S, O'Donnell K, Wicks IP. Distinct roles for the NF-kB1 (p50) and c-Rel transcription factors in inflammatory arthritis. J.Clin. Invest. 2000;105:1799–1806. doi: 10.1172/JCI8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J. Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]