Abstract
Performance on the Sternberg working memory task, and MEG cortical response on a variation of the Sternberg task were examined in middle-aged carriers and non-carriers of the APOE ε4 allele. Physical activity was also assessed to examine whether exercise level modifies the relationship between APOE genotype and neurocognitive function. Regression revealed that high physical activity was associated with faster RT in the six- and eight-letter conditions of the Sternberg in ε4 carriers, but not in the non-carriers after controlling for age, gender, and education (N = 54). Furthermore, the MEG analysis revealed that sedentary ε4 carriers exhibited lower right temporal lobe activation on matching probe trials relative to high-active ε4 carriers, while physical activity did not distinguish non-carriers (N = 23). The M170 peak was identified as a potential marker for pre-clinical decline as ε4 carriers exhibited longer M170 latency, and highly physically active participants exhibited greater M170 amplitude to matching probe trials.
Keywords: Exercise, MEG, APOE, Working Memory, M170
1. Introduction
Presence of the ε4 allele of the apolipoprotein E (APOE) gene constitutes a significant risk factor for Alzheimer’s disease (AD) (Corder et al., 1993; Saunders et al., 1993). However, ε4 alone is neither necessary nor sufficient for development of dementia. Although carriers of the ε4 allele account for almost 50% of late-onset AD cases compared with only 15% frequency in the general population (Corder et al., 1993; Saunders et al., 1993; Strittmatter et al., 1993a,b), presence of ε4 does not always result in dementia, suggesting interactive effects of APOE genotype with other genes or lifestyle factors.
One lifestyle factor known to influence healthy brain aging is physical activity. Exercise can protect and even enhance brain function through upregulation of neurotrophic factors (Cotman and Berchtold, 2002), increased neurogenesis (Pereira et al., 2007; van Praag et al., 1999, 2005), increased blood flow (Pereira et al., 2007), reduced oxidative stress (Kiraly and Kiraly, 2005), and even reduction of beta-amyloid, one of the hallmarks of AD (Adlard et al., 2005). Clinical exercise trials in normal aging populations have shown improved cognition (Colcombe and Kramer, 2003), changes in cortical function (Colcombe et al., 2004), and increased brain volume (Colcombe et al., 2006) following exercise. However, less is known about the impact of exercise on AD, or populations genetically at risk for AD such as carriers of the ε4 allele.
Schuit et al. (2001) fostered the notion that ε4 carriers should be a target population for exercise trials. In a prospective study they reported that physical activity was associated with dramatically reduced cognitive decline in the ε4 carriers, while non-carriers of the allele did not exhibit much decline regardless of activity. At face value, the idea that at-risk populations may exhibit the greatest benefit from exercise is both appealing and logical. Although exercise benefits everyone, those genetically at risk for decline might have the greatest capacity to benefit from preventive strategies. The Schuit et al. finding was supported by two additional studies reporting that physical activity was associated with greater impact on cognition in ε4 carriers than in non-carriers (Etnier et al., 2007; Rovio et al., 2005), while at least one study has found that participation in physical activity was associated with reduced risk of AD in non-carriers of the ε4 allele, but not in carriers (Podewils et al., 2005).
The majority of these studies have used clinical diagnosis of dementia (Podewils et al., 2005; Rovio et al., 2005), or changes in Mini Mental State Exam (MMSE) scores (Schuit et al., 2001) as outcome variables, which are not sensitive to pre-clinical cognitive aging. While this represents an important starting point for the study of exercise and dementia, the ideal goal is to prevent or slow onset of decline in normal populations at who are at future risk for AD. As such, identification of measures sensitive to pre-clinical decline and appropriate preventive strategies are crucial. Although Etnier et al. (2007) studied non-demented subjects and reported a positive association between fitness and cognitive performance, their findings extended only to homozygous ε4 carriers, and the trend they observed did not achieve significance in the heterozygous carriers. Cumulatively, these findings illustrate the need for more sensitive research measures in this population.
The current study capitalizes on previously identified measures sensitive to the earliest decline in ε4 carriers, specifically speed of processing during executive function working memory challenge (O’Hara et al., 2007), and measures of cortical activation (Bookheimer et al., 2000; Green and Levey, 1999; Smith et al., 1999; Wetter and Murphy, 2001). Speed of processing was examined using the Sternberg working memory task with letter set sizes of 4, 6, and 8. Main effects were predicted for both exercise level and APOE genotype such that carriers of the ε4 allele were expected to exhibit longer reaction times (RT) relative to non-carriers, and highly physically active participants were expected to exhibit shorter RT. Consistent with the predictions of Etnier et al., Rovio et al., and Schuit et al., an interaction of physical activity level and genotype was predicted with the magnitude of the exercise effect expected to be more pronounced in ε4 carriers.
We also extend the known literature by examining magnetoencephalographic (MEG) measures of cortical activation in a sub-sample of participants during a variation of the Sternberg task, motivated by multiple studies showing ε4-related differences in resting cerebral metabolism (Reiman et al., 1996, 2001, 2004), cerebral activation during cognitive challenge (Bondi et al., 2005; Bookheimer et al., 2000; Smith et al., 1999; Trivedi et al., 2006), and latency of cortical response (Green and Levey, 1999; Wetter and Murphy, 2001).
Although MEG has not previously been used to study APOE-related differences in cortical activation, Maestu et al. (2001) have shown AD patients to exhibit decreased MEG cortical activation in the temporal and parietal regions relative to normal participants during performance of the Sternberg task. Our goal was to examine whether carriers of the ε4 allele exhibit similar deficits in cortical activation during working memory challenge, and whether this relationship is altered by physical activity level. Physical activity and aging studies have previously demonstrated that on executive function and attention tasks physically active individuals exhibit greater cortical activation as assessed by event-related potentials (ERPs) (Hillman et al., 2006) and functional magnetic resonance imaging (fMRI) blood oxygen level dependent (BOLD) response (Colcombe et al., 2004). Greater activation has been attributed to an increased ability of frontal attention circuits to recruit task-relevant brain regions in the aerobically trained participants (Colcombe et al., 2004). As such, ε4 carriers were predicted to exhibit decreased activation in the temporal and parietal regions relative to non-carriers, and physically active participants were expected to exhibit increased amplitude of cortical activation relative to sedentary participants. Once again an interaction between exercise and physical activity level was predicted to reveal the effects of exercise to be greater in ε4 carriers.
Furthermore, latency of event-related potentials have been shown to distinguish cognitively normal carriers of the ε4 allele from non-carriers, with ε4 carriers exhibiting longer latency on both N200 and P300 components (Green and Levey, 1999; Wetter and Murphy, 2001). However, decreased ERP latency has been shown to be associated with physical activity in the elderly. Hillman et al. (2006) reported that both amplitude and latency of the P300 component were sensitive measures of the benefits of physical activity, with the latter reflecting a more maintained speed of processing in the physically active older adults. Again, main effects for genotype and exercise level were predicted with ε4 carriers expected to exhibit longer latency ERPs, while a high level of exercise was expected to be associated with decreased latency, especially in ε4 carriers. Although previous exercise and aging studies have employed target detection tasks to optimally engage the frontal attention circuits, we chose to employ a working memory task known to engage the temporal lobes in normal subjects, a region susceptible to early decline in AD patients (Maestu et al., 2001). As such, we predicted that cortical activation in the temporal region was most likely to distinguish the groups.
2. Methods
2.1. Participants
Seventy-five participants between the ages of 50 and 70 were recruited through newspaper ads, local running events (marathon and 10 km), campus faculty and staff, and other ongoing research studies. After reading and signing informed consent, all participants underwent an initial screening visit to determine physical activity level, cognitive status, APOE genotype and health history. Physical activity level was determined using the Yale Physical Activity Survey for Older Adults (Dipietro et al., 1993). Participants were screened for dementia using the Cambridge Cognitive Exam (CAMCOG; Section B of the CAMDEX-R; Roth et al., 1986). Any participants with a history of neurological or psychiatric disorder were excluded from further participation. A 10 ml blood sample was drawn from the antecubital vein for APOE genotyping.
2.2. Neuropsychological screening
The CAMCOG was administered to determine cognitive status of all participants and screen for cognitive impairment. The maximum score possible was 105 rather than 107 due to removal of two questions, which were considered not applicable given the context of the testing room. Scores ranged from 84 to 101 (mean = 94.8, S.D. = 3.8), and all participants scored within the normal range for their age group (Williams et al., 2003).
2.3. APOE genotype
The APOE gene has three common alleles: ε2, ε3, and ε4, representing specific combinations of alleles at two nearby missense polymorphisms: Cys112Arg (rs429358) and Cys158Arg (rs7412). Allele frequencies in Caucasians are approximately 10, 75, and 15% for the e2, e3, and e4 alleles, respectively. Genotyping was carried out on DNA extracted from whole blood using standard restriction digest procedures. Participants were classified as carriers of the ε4 allele (ε4+) if they were either heterozygous (ε3/ε4) or homozygous (ε4/ε4) for ε4. No statistical distinction was made between heterozygous and homozygous carriers of the ε4 allele as there were only three homozygous carriers in the sample. Non-ε4 carriers (ε4−) were either heterozygous (ε2/ε3) or homozygous (ε3/ε3) for ε3. ε2/ε4 subjects were excluded from behavioral and MEG testing as the ε2 allele is considered protective against dementia, while the ε4 allele conveys increased risk. All researchers collecting and scoring data were blind to APOE genotype.
2.4. Exercise
Physical activity was assessed via interview with the Yale Physical Activity Survey (YPAS) for Older Adults (Dipietro et al., 1993). Administration of the YPAS requires about 25 min, and records both minutes and intensity of weekly physical activity, ranging from household (cooking, cleaning) to recreational (e.g. bowling, dancing etc.) and exercise activities (e.g. aerobic and strength training activities). The scale yields total minutes of activity and total caloric expenditure (kcal) per week. The later was used as our measure of overall physical activity.
2.5. Recruitment for Sternberg behavioral and MEG participants
In order to enrich our sample for ε4 carriers, recruiting advertisements for the study targeted participants with a family history of AD. However, family history was not considered for participation, and was not used for selecting or separating participants into groups. As such, any genetic bias known to influence dementia other than APOE would be expected to be spread equally across all participants (ε4 carriers and non-carriers). Because all participants were volunteers, and as such, a large percentage were motivated by their family history to respond to our recruitment strategies, our sample of recruits included approximately 30% carriers of at least one E4 allele, an increase over the expected population frequency of 20–25%. All participants who were willing and eligible completed the behavioral Sternberg testing, while recruitment into MEG testing was more selective in an attempt to balance the sample for genotype and physical activity as described below. Additional factors excluding participants from MEG testing included claustrophobia due to the confines of the MEG scanner, and any metal in the head or upper extremities (dental work, metal implants, or glasses necessary for vision), which is fairly common in this age group.
Of the 75 participants screened 9 were excluded for health reasons, or because they simply chose not to participate further. Six were excluded for genetic reasons (ε2/ε4, n = 3; unable to determine genotype, n = 3). Of the remaining 60 participants, 54 completed the behavioral Sternberg testing and 23 completed the MEG Sternberg testing. Seventeen participants completed both testing sessions (behavioral and MEG), while six of the MEG participants chose not to participate in the behavioral testing due to personal time constraints.
2.5.1. Behavioral participants
The mean age of the 54 participants completing the behavioral testing was 59.9 (S.D. = 4.6). (Table 1). Thirty participants were male and 24 were female. Thirty-eight participants were ε4− and 16 were ε4+, 3 of which carried two ε4 alleles.
Table 1.
Means and standard deviations for the Sternberg behavioral participants (N = 54) and the MEG participants (N = 23)
| Group | n | M/F | Age | CAMCOG | YPAS exercise (kcal) |
|---|---|---|---|---|---|
| Sternberg participants | |||||
| Overall | 54 | 30/24 | 59.9 (4.6) | 95.5 (3.9) | 8343.4 (4523.4) |
| ε4− | 38 | 18/20 | 60.5 (4.5) | 94.3 (3.8) | 8166.8 (4604.9) |
| ε4+ | 16 | 12/4 | 58.6 (4.8) | 95.1 (4.0) | 8762.8 (4441.0) |
| MEG participants | |||||
| Overall | 23 | 8/15 | 59.5 (5.0) | 94.8 (4.2) | 2554.6 (1895.7) |
| High-active | 14 | 7/7 | 60.3 (4.0) | 95.3 (4.2) | *3388.0 (1838.8) |
| Low-active | 9 | 1/8 | 58.3 (6.4) | 94.0 (4.4) | *1256.7 (1129.2) |
| ε4− | 14 | 4/10 | *61.2 (3.8) | 94.1 (4.0) | 2398.9 (2105.2) |
| ε4+ | 9 | 4/5 | *56.9 (5.8) | 95.8 (4.6) | 2796.7 (1604.1) |
| ε4− high-active | 9 | 3/6 | 60.7 (3.8) | 94.4 (4.6) | 3361.7 (2008.9) |
| ε4− low-active | 5 | 1/4 | 62.2 (4.0) | 93.6 (2.9) | 666.0 (701.8) |
| ε4+ high-active | 5 | 4/1 | 59.6 (4.7) | 96.8 (3.0) | 3438.0 (1706.7) |
| εe4+ low-active | 4 | 0/4 | 53.5 (5.8) | 94.5 (6.3) | 1995.0 (1198.1) |
Represents a significant difference between groups (p < 05). MEG participants in the high-active group scored significantly higher on the exercise portion of the YPAS than low-active participants. ε4+ participants in the MEG testing were younger than ε4− participants.
2.5.2. MEG participants
Due to a smaller sample of participants used for brain imaging, MEG participants were screened further to yield an extreme groups comparison for highly active versus sedentary participants. Participants were considered high-active if they engaged in aerobic exercise at least three times per week, while low-active participants did not participate in regular strenuous aerobic exercise. Aerobic exercise level was determined using the exercise portion of the YPAS (i.e. those items listed under the “Exercise” heading of the questionnaire, and additional activities reported which engage the aerobic system such as racquetball, basketball, etc.). Our goal was to recruit both highly active aerobic exercisers and non-aerobic exercisers, while excluding participants who reported only one or two bouts of light exercise per week. The high-active participants scored significantly higher on the exercise portion of the YPAS (Table 1) than the low-active participants selected (F(1,19) = 8.52, p = .009).
Twenty-three participants completed the MEG testing with a mean age of 59.5 years (S.D. = 5.05) (Table 1). Fourteen participants were designated as high-active, and nine as low-active. Nine participants were ε4+, and one was homozygous (high-active group). Fourteen were ε4−. This yielded an Exercise level (2) × Genotype (2) design, with an n of 9, 5, 5, and 4 (high-active/ε4−, low-active/ε4−, high-active/ε4+, low-active/ε4+, respectively in each cell).
ε4 carriers were significantly younger than non-carriers (F(1,19) = 6.52, p = .019). The Exercise × Genotype interaction term for age approached significance (F(1,19) = 3.99, p=.060) with the low-active e4 carriers being the youngest of the four groups. As such, age was entered as a covariate in all analyses, and subsequently excluded from analyses where age did not significantly contribute.
2.6. Cognitive tasks
Sternberg behavioral and MEG testing occurred on separate visits following the initial screening. The working memory tasks for both are described below.
2.7. Behavioral Sternberg testing
The Sternberg task was administered using Presentation software and a program downloaded from the Neuro-Behavioral Systems website (www.neurobs.com, Sarnthein, J.), then modified as follows. Participants were seated in front of a computer and asked to respond by pressing a button on the keyboard with either their right or left index finger. A visual orientation cue was presented alone in the middle of the display for 1000 ms (“■””) and then remained on the screen during the presentation of a letter string (“T R S ■ F K H”). The letter string was visible for 2000 ms, and consisted of 4, 6, or 8 letters, each represented equally over 100 total trials. Letters were generated randomly from a string of 17 consonants (not including J, M, or X). Following presentation of the letter string was a 3000 ms pause. A probe letter was then presented that matched one of the letters in the previous string 50% of the time. The participant pressed a button with their right hand if the probe letter was part of the preceding string (“yes”), and a button with their left hand if it was not (“no”). The probe letter was visible until the participant responded, or for 10,000 ms. Participants were instructed to answer as quickly and accurately as possible, and they were given six practice trials prior to testing. Trials where the probe letter matched one of the previous stimulus letters were examined for accuracy and reaction time.
2.8. MEG Sternberg testing
A modified version of the Sternberg working memory task was presented visually using Presentation software and a program downloaded from the Neuro-Behavioral Systems website (www.neurobs.com, Sarnthein, J., Sternberg’s working memory task), which was further modified as follows. For the MEG testing, each sequence included five stimulus letters, and two probe letters. The stimulus letters were presented sequentially during MEG testing for further examination of cortical activity during sequential encoding trials with increased working memory load (data not presented here). Although the tasks were similar, the MEG presentation of the task differed from the more traditional Sternberg utilized in the behavioral testing, thus minimizing practice effects for the participants who completed both.
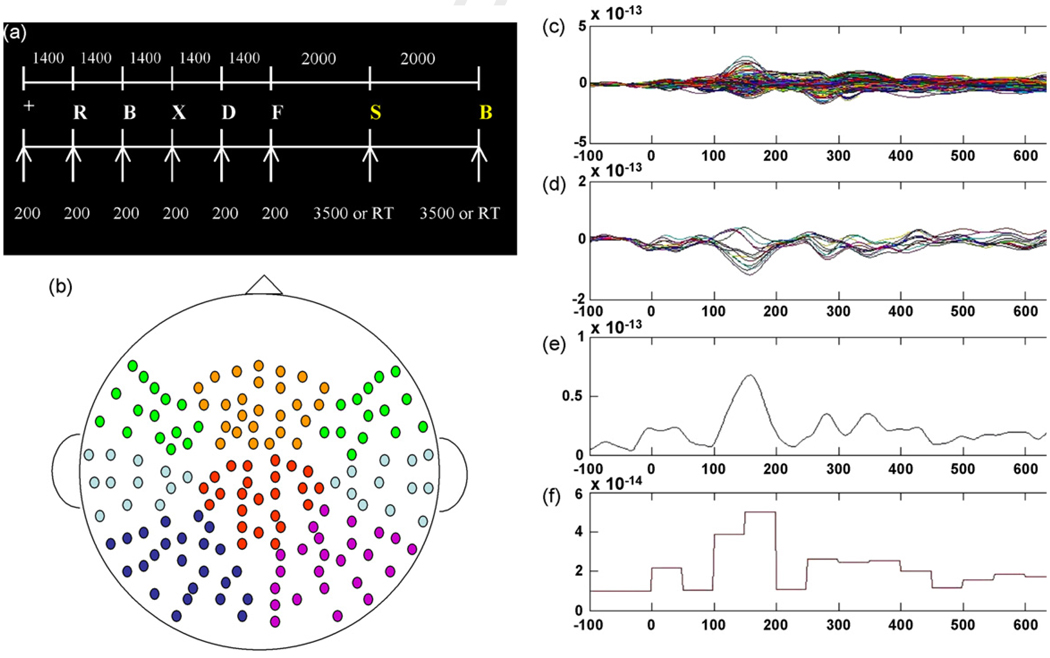
The MEG task consisted of a pre-stimulus cue “+”, followed by the five letters presented for 200 ms each, with a 1400-ms interval between each letter (1600 ms SOA) (see Fig. 1a). Letters subtended 8.2° of the visual angle when projected on the screen in front of them, were white on a black background, and were generated randomly from a set of 21 consonants. Following a 2000 ms pause after the fifth letter, a yellow probe letter was presented visually for a duration of 3500 ms, or until the subject responded. The participants were instructed to press a button with their right thumb if the probe letter matched one of the previous five letters, or to press a button with their left thumb if the probe letter did not. Following another 2000-ms pause, a second probe letter was presented to maximize the number of probe trials in a short time. For each series of trials there were eight stimuli consisting of a pre-stimulus cue, five letters, and two probe trials. The trial sequence was then presented 55 times to produce a total of 110 probe trials, to allow for a sufficient number of trials to yield high SNR neuromagnetic data.
Fig. 1.
(a) MEG Sternberg working memory task. Yellow letters represent probe trials. White numbers represent stimulus durations and ISIs (ms). (b) Channel montage for all eight regions: left frontal, midline frontal, right frontal, left temporal, right temporal, left parietal, right parietal, central-midline. (c) Averaged response to probe trials for all 160 channels for one subject. (d) Channels selected for one of the eight regions. (e) RMS computed for one of the eight regions based on selected channels. (f) Average amplitude of RMS in 50-ms time windows for ANCOVA analyses.
Variable sizes of letter strings were not used for MEG testing because longer letter strings are known to result in longer RT. As such, the size of the preceding letter string (e.g. 4, 6, or 8)would influence latency of the averaged peaks in the subsequent even-trelated fields. Averaging across probe trials for different sized letter strings would therefore distort or smear the temporal sequence of each resultant MEG response. Therefore, for the purpose of acquiring an appropriate number of probe trials for averaging, all trials were five-letter sets. Furthermore, the moderate level of difficulty of the task was designed to yield similar task performance across groups, subsequently ruling out the potential that differences in cortical activation were due simply to differences in task-difficulty between groups.
Participants were instructed in the task prior to the MEG recording. Inside the MEG scanner they had the instructions read to them again while seeing the instructions on the screen in front of them. They were then given three series of practice trials prior to the test trials. The test trials and concurrent MEG recording began when subjects verbally reported understanding the task and were observed performing the task properly. The participants were instructed to answer as quickly and accurately as possible. Testing was conducted in the supine position with an MEG-optimized visual display 35.5 cm above their heads. Optical button pads were placed in each hand for behavioral responses with the right and left thumbs.
2.9. Matching trials
Only trials where the probe letter matched one of the stimulus letters (matching trials) were examined due to greater variability on “non-matching” trials in both RT and MEG response. When the probe letter does not match one of the previous stimulus letters, subjects continue to search working memory for variable periods of time. As such, our analysis focused only matching trials, where a matching of the probe letter to a letter maintained in working could occur.
2.10. MEG data collection and analysis
MEG testing was conducted using a 160-channel whole-head axial gradiometer system (KIT System, Kanazawa, Japan) in a magnetically shielded room. Continuous data were recorded with an online bandpass filter from 1 to 200 Hz with a 60-Hz notch filter at a sampling rate of 500 Hz.
The raw data files were subjected to a noise-reduction algorithm in Matlab 6.0 based on the CALM formula in the MEG160 software (Adachi et al., 2001). Following noise reduction the data were epoched and averaged on “matching” probe trials from 100 ms prior to stimulus onset to 1200 ms post-stimulus onset. Individual trials were visually inspected for blinks and movement artifact. Trials containing blinks or amplitudes of > 3 pT were rejected from analysis. Data further were examined from 0 to 600 ms post-stimulus onset due to excessive eye-blinks following 600 ms in most subjects. The averaged data were then baseline corrected from −100 ms to stimulus onset, low-pass filtered at 20 Hz, and exported into Matlab for further analysis.
The MEG channels were grouped into eight regions of interest including left frontal, midline frontal, right frontal, left temporal, right temporal, center midline, left parietal, and right parietal (Fig. 1b). Root mean square (RMS) across channels for each region was calculated (Fig. 1c–e). RMS average amplitudes were then calculated in 50-ms windows from 0 to 600 ms for each region to generate a time series of 12 average amplitudes for each RMS for statistical analysis (Fig. 1f). Combined with the Exercise × Genotype design, this yielded a 2 × 2 × 12 (time window) design with Exercise and Genotype treated as between subject factors, and repeated measures on the time factor.
2.11. Statistical analyses
2.11.1. Neuropsychological testing
To assess whether physical activity and APOE genotype influenced cognitive status, hierarchical regression was performed. For all 60 participants CAMCOG scores were regressed on physical activity (kcal), genotype (ε4+, ε4−), and the physical activity × Genotype interaction term, after entering age, gender, and education level into the regression equation.
2.11.2. Sternberg Behavioral testing
Performance scores on the behavioral testing were analyzed similarly for the 54 participants, controlling first for age, education, and gender. Percent correct and RT were then regressed independently on physical activity, genotype, and the interaction term. Separate analyses were conducted for the four-letter condition, the six-letter condition, and eight-letter condition on “matching” trials only. When the interaction term achieved significance, simple regressions were conducted separately for the ε4+ and ε4− groups to determine which group exhibited significant relationship between physical activity and performance.
2.11.3. MEG behavioral analysis
Reaction time (RT) and percent correct were examined separately in 2 (Genotype) × 2 (Exercise) ANCOVAs using age and gender as covariates where appropriate.
2.11.4. MEG analysis
For the MEG data, 2 (Genotype) × 2 (Exercise) × 12 (Time) ANCOVAs with repeated measures on time were conducted separately on RMS for each of the eight regions on trials where the probe letter matched one of the previous stimulus letters. Age and gender were run as covariates on all RMS analyses, and reported when significant. Repeated measures analyses where sphericity was violated were Huynh–Feldt corrected, with the adjusted degrees of freedom reported. Bonferroni correction was applied to the MEG analysis to account for eight separate ANCOVAs, one for each of the brain regions. The alpha level (.05) was therefore divided by 8 to yield a critical p value of .00625 to achieve significance. Significant omnibus tests were followed up with Tukey HSD post-hoc comparisons. Furthermore, specific RMS peaks that were shown to distinguish the groups based on the above criteria were examined for latency and subjected to source localization to better characterize the peaks of interest.
3. Results
3.1. Neuropsychological test
Hierarchical regression accounting for age, gender, and years of education revealed that CAMCOG scores were not significantly predicted by physical activity level, APOE genotype, or the interaction. Age alone predicted scores on the CAMCOG (ΔR2 = .063, p = .053).
3.2. Sternberg behavioral testing
3.2.1. Percent correct
Physical activity and genotype did not significantly predict percent correct for any of the three conditions, although the physical activity term approached significance in the eight-letter condition (ΔR2 = .042, p = .097). The interaction term was not significant. Education level predicted correct responses in the six-and eight-letter conditions (ΔR2 = .154, p = .004; ΔR2 = .142, p = .004, respectively).
3.2.2. Reaction time
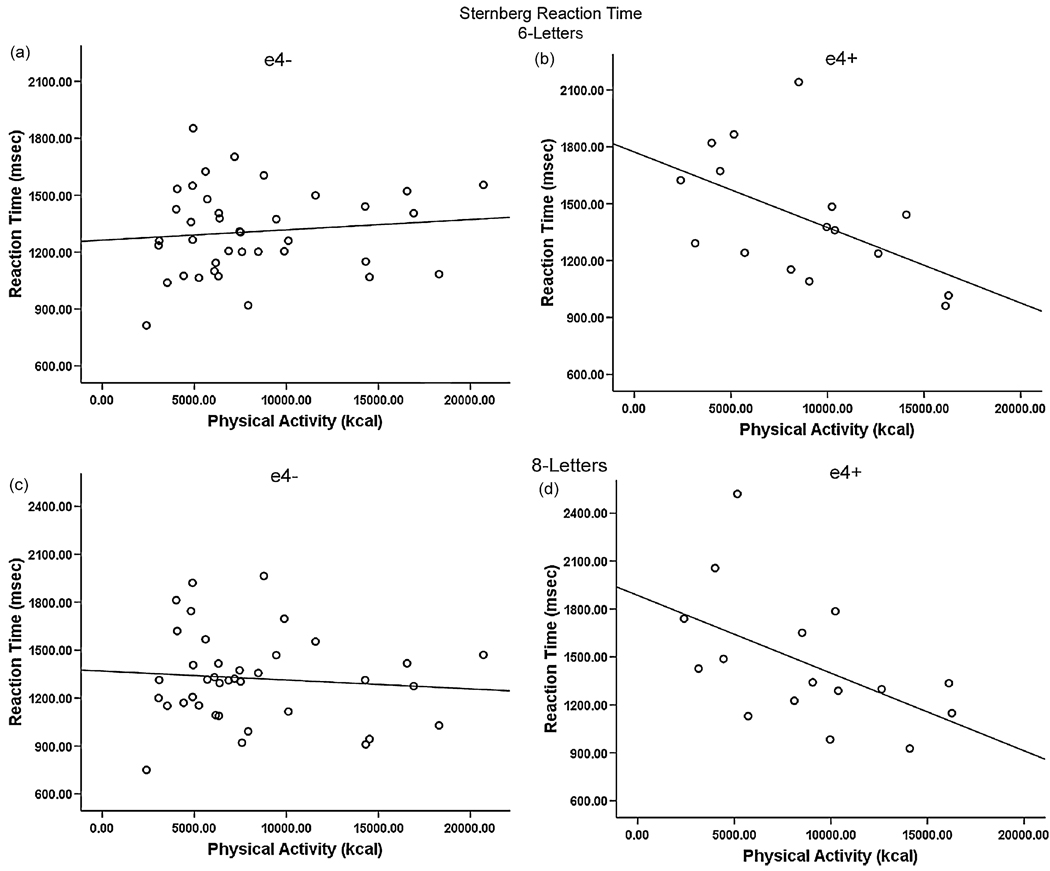
The physical activity × Genotype interaction term significantly predicted RT in both the six- and eight-letter conditions (ΔR2 = .119, p = .012; ΔR2 = .073, p = .048, respectively). Neither physical activity nor Genotype independently contributed when accounting for other variables and the interaction term. Post-hoc simple regressions revealed that physical activity was significantly associated with RT for the ε4+ group, but not the ε4− group in both the six-letter (R2 = .288, p = .032) and eight-letter (R2 = .273, p = .038) conditions. In ε4 carriers greater physical activity levels were associated with faster RT for both the six-letter (Fig. 2 a and b) and eight-letter conditions (Fig. 2 c and d). No other predictors achieved significance for RT.
Fig. 2.
Genotype × Exercise interaction for the six-letter (a and b) and eight-letter (c and d) behavioral Sternberg testing. ε4 carriers (b and d) show decreased RT with increasing physical activity (kcal).
3.3. MEG analysis
3.3.1. MEG task performance
There were no differences between groups on percent correct or RT to the probe trials during MEG testing. Therefore, all groups performed the same on accuracy and speed for the task. The task administered was not optimized to elicit performance differences between groups, as there was only one level of difficulty (five letters). As such, any cortical activation findings are due to differential levels of activation given the same level of task difficulty, and not due to different degrees of task-difficulty between groups.
3.4. Neuromagnetic responses: root mean square (RMS) analysis
Typical MEG data from one participant are illustrated in Fig. 1c and d. Fig. 1c shows the response in all channels, averaged by trial type and filtered. Fig. 1d shows the cortical evoked field for one region of interest (e.g. the right temporal region). Fig. 1e shows the aggregate response within a particular region, calculated as the root mean square across the selected channels, and Fig. 1f represents the average amplitude of RMS in 50-ms windows.
3.5. Right temporal region
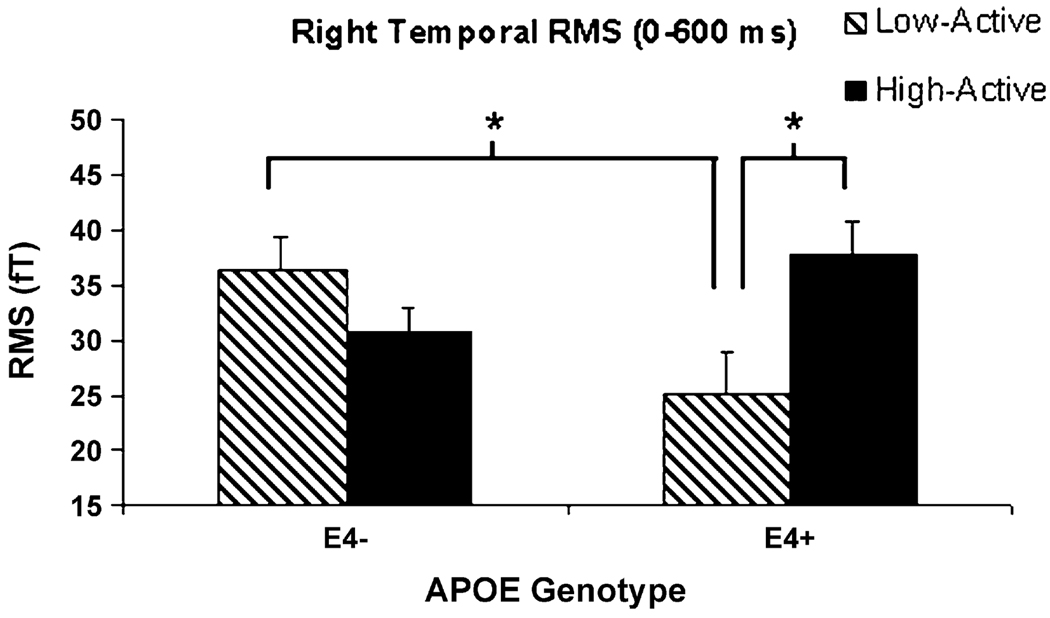
The only region exhibiting significant RMS differences between groups in the design was the right temporal region, where ANCOVA revealed a significant Exercise × Genotype interaction (F(1,19) = 11.73, p = .003). Neither covariate (sex, age) contributed significantly to the analysis, and both were therefore excluded. Low-active ε4 carriers exhibited the lowest overall RMS amplitude (0–600 ms) of the four groups in this region, and post-hoc tests revealed that the low-active ε4+ group exhibited significantly lower RMS than the high-active ε4+ group, and the low-active ε4− group (Fig. 3). High-active and low-active non-carriers did not differ on RMS.
Fig. 3.
Right temporal lobe RMS (0–600 ms). *Sedentary ε4 carriers exhibited decreased RMS amplitude relative to high-active ε4 carriers and sedentary non-carriers.
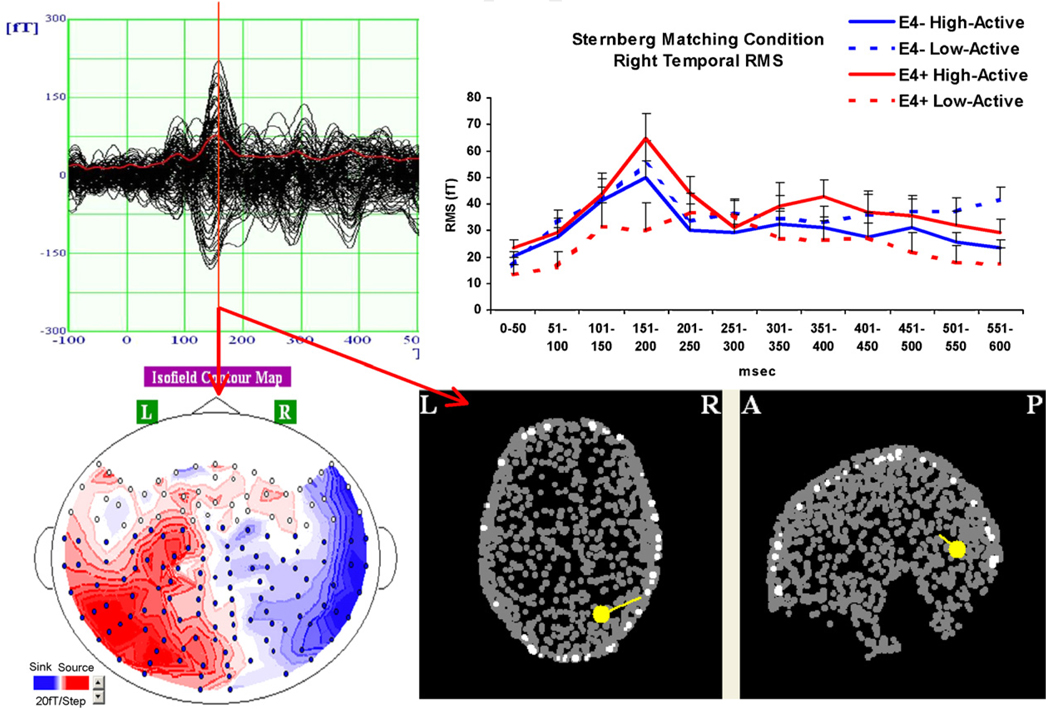
Although the three-way interaction (Exercise × Genotype × Time) did not achieve significance, right temporal activation across the entire 600 ms epoch was examined visually for all four groups to identify potential activation peaks of interest. As illustrated in Fig. 4, the largest difference between groups occurred in the 150–200 ms time window, with the low-active ε4+ group exhibiting a dramatically reduced peak relative to the other three groups. Although the discrepancy between the sedentary ε4 carriers and the other three groups on this peak appears visually dramatic, the lack of a statistically significant contribution of the time variable is likely due a decreased level of activation in the low-active ε4+ group across the entire window. Visual examination of the temporal lobe peak warranted a more focused analysis of the regional activation in this time window. Therefore, a more specific amplitude and latency analysis was conducted of this peak on temporal lobe peak activation.
Fig. 4.
M170 peak contour map and source localization for one high-active non-ε4 carrier indicating an occipito-temporal source. (Top right) Average amplitude RMS to matching probe trials in 50-ms time windows for all four groups.
A temporal lobe peak in this time window on a visual task is consistent with an M170 peak reported in previous studies (Tarkiainen et al., 1999, 2002; Cornelissen, and Salmelin, 2002). A source localization performed on a high-active ε4− subject who exhibited a strong temporal peak in this time window is represented in Fig. 4, and shows an occipito-temporal source consistent with M170 peaks reported in the literature, with the most likely source being the fusiform gyrus.
3.6. M170 analyses
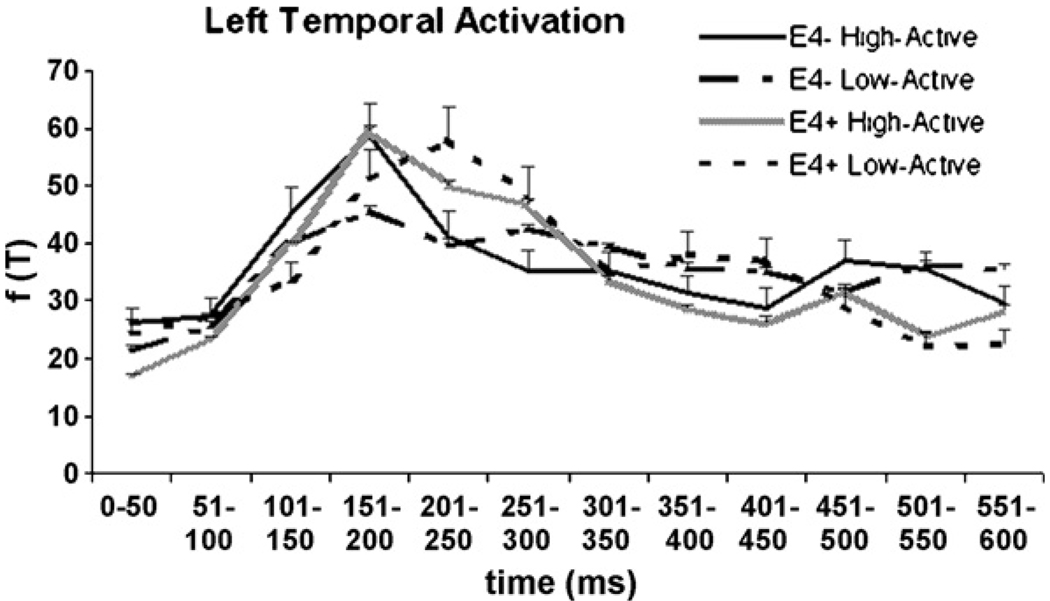
Given the stimulus characteristics of the cognitive task (letters), the M170 peak might be expected to be more pronounced in the left temporal region for most participants, although some subjects do exhibit right hemisphere dominance of M170 to letter stimuli (Tarkiainen et al., 2002). Therefore, despite non-significant differences in left hemisphere activation in the initial design, temporal lobe peaks were examined in both the right and left hemisphere, and nested as such within the ANCOVA design. Left temporal activation for each of the four groups is depicted in Fig. 5.
Fig. 5.
The left temporal RMS for all four groups. The omnibus test revealed no significant differences between groups.
Temporal lobe peaks were further explored by identifying the highest amplitude RMS peaks in the right and left temporal regions similar in latency to the M170 peaks described in the literature. However, as illustrated in Fig. 4 and Fig. 5, some participants did not exhibit clear M170 waveforms that were regionally and temporally consistent with the “classic” peaks described in previous studies. As we could not afford excluding participants who did not exhibit ‘ideal’ M170 peaks (due to the limited sample size), we therefore examined right and left temporal lobe peak activation between 130 and 250 ms. The criteria for peak picking was determined based on the results of Tarkiainen et al., who have shown that visual object and letter stimuli generate an occipital midline peak at 100 ms, followed by a more anterior occipito-temporal peak around 150–200 ms. As illustrated in Fig. 5, some participants exhibited temporal lobe peaks later than 200 ms, and to better characterize temporal lobe activation, the late cut-off for peak picking was set at 250 ms. Therefore, the analysis employed here may be more specifically characterized as examination of the mid-latency inferior temporal lobe peak.
Peak amplitudes and latencies were subjected separately to Genotype (2) × Exercise level (2) × Hemisphere (2) ANCOVAs. Once again age and gender were entered as covariates, but were removed when they did not contribute significantly.
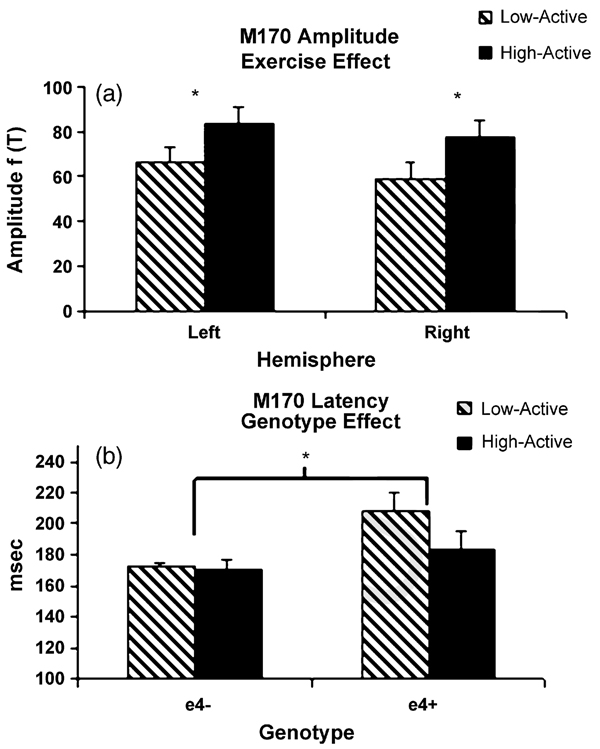
3.6.1. Amplitude
High-active subjects exhibited greater temporal peak amplitude relative to the low-active participants as indicated by the exercise main effect (F(1,19) = 5.48, p = .030)(Fig. 6a). Neither genotype nor hemisphere emerged as significant main effects or interactions.
Fig. 6.
M170 amplitude (a) and latency (b). (a) High-active participants exhibited greater amplitude bilaterally regardless of genotype. (b) ε4 carriers exhibited longer latency than non-carriers. The exercise interaction approached significance with the sedentary ε4 carriers exhibiting the longest latency.
3.6.2. Latency
ε4 carriers exhibited longer M170 latencies bilaterally as indicated by the genotype main effect (F(1,19) = 11.97, p = .003) (Fig. 6b). Both the main effect for exercise (F(1,19) = 3.50, p = .077) and the Exercise × Genotype interaction (F(1,19) = 2.89, p = .105) approached significance. Once again, the hemisphere factor did not contribute significantly.
4. Discussion
Speed of processing and cortical activation during the Sternberg working memory task were examined in middle-aged carriers and non-carriers of the APOE ε4 allele. Physical activity was also assessed to examine whether exercise level modifies the relationship between APOE genotype and neurocognitive function. Our results are consistent with the reports of Schuit et al. (2001), Rovio et al. (2005), and Etnier et al. (2007), as regression revealed an interactive effect of physical activity and APOE genotype on speed of processing after controlling for age, gender, and education. Specifically, physical activity was associated with reduced reaction times in carriers of the ε4 allele, but not in the non-carriers, reinforcing the notion that populations genetically at risk for AD exhibit the most significant protective effects of exercise. Furthermore, a sub-sample of the population performed a variation of the Sternberg task during recording of continuous MEG, once again revealing an Exercise × Genotype interaction with respect to cortical activation. Sedentary ε4 carriers exhibited decreased right temporal lobe activation relative to high-active ε4 carriers, while physical activity did not distinguish non-carriers. The mid-latency temporal lobe peak M170 was identified as a potential marker for pre-clinical decline as it differed with both APOE genotype (latency) and exercise level (amplitude).
The current findings reinforce the notion that the use of executive function tasks in which speed of processing is examined fosters the study of the impact of lifestyle factors and other preventive methods decades prior to onset of potential dementia. The physically active ε4 carriers in this sample appear to have preserved the efficiency of psychomotor processing similar to non-carriers of the allele, while the sedentary carriers required longer RT on the six- and eight-letter matching probe trials. Although physical activity has been known mitigate the age-related slowing of psychomotor speed for many years (Spirduso, 1980), the impact of exercise on AD-related aging has been less clear. The current data suggest that the speed of processing deficits reported previously in cognitively normal carriers of the ε4 allele (O’Hara et al., 2007) may be ameliorated somewhat through regular physical activity.
Potentially even more sensitive to pre-clinical decline than performance measures are latency and amplitude measures of cortical activation (Hillman et al., 2006; Wetter and Murphy, 2001). Our data indicate that ε4 genotype is associated with longer M170 latencies, consistent with Wetter and Murphy (2001) and Green and Levey (1999) who previously reported delayed ERP components in ε4 carriers. Increased exercise was expected to mitigate APOE-related differences based on the ‘classic’ speed of processing benefits in the exercise literature (Spirduso, 1980), and some recent evidence of decreased ERP latency with increased physical activity on executive attention tasks (Hillman et al., 2006). In fact, the physically active participants in the current study did exhibit a trend towards shorter M170 latencies as both the main effect for exercise and the interaction with genotype approached significance.
The data also suggest that higher levels of physical activity are associated with greater amplitude of evoked temporal lobe response during recognition of matching probe letters. High-active participants exhibited greater bilateral M170 peak amplitude than sedentary participants in the temporal region. Increased levels of fitness or physical activity have commonly been associated with greater cortical activation during cognitive challenge (Colcombe et al., 2004; Hillman et al., 2006; Polich and Lardon, 1997), and have been attributed to an increased ability in high-fit participants to recruit task-relevant regions of the cortex (Colcombe et al., 2004). The current data are consistent with this notion as high-active participants exhibited greater M170 amplitude regardless of genotype.
The RMS examination of cortical activation showed a relationship between right temporal amplitude and exercise that was pronounced in carriers of the ε4 allele but not in non-carriers. Sedentary ε4 carriers exhibited the lowest level of right temporal lobe activation sustained across the 600 ms following onset of matching letters, while their high-active counterparts exhibited a cortical activation profile more consistent with the non-carriers (Fig. 3). Although no significant main effects for genotype emerged in the RMS analyses, the interaction with exercise in the right temporal region suggests a decreased level of temporal lobe activation in the sedentary ε4 carriers that is not present in the high-active carriers.
Previous reports of APOE-related differences in cortical activation during cognitive challenge have been varied across studies (for a review see Wierenga and Bondi, 2007). Many fMRI studies have reported greater cortical activation in ε4 carriers (Bondi et al., 2005; Bookheimer et al., 2000), while others have reported reduced activation (Lind et al., 2006; Smith et al., 1999). Greater cortical response has been interpreted as compensatory activation in ε4 carriers necessary to overcome pre-clinical pathological encroachment (Wierenga and Bondi, 2007). Decreased activation in ε4 subjects is generally viewed as a decreased ability to recruit appropriate neural resources. Discrepant findings in the literature are likely due to the variety of cognitive tasks employed and differences in the ages of the populations studied. Although the decreased activation in the right temporal region exhibited by the low-active ε4 carriers in this study might be expected to be accompanied by a compensatory increase in activation in the frontal or other cortical regions, such as that observed by Maestu et al. (2001) in AD patients, no such effect emerged in our data.
Our finding of decreased right temporal RMS amplitude in the sedentary ε4 carriers is consistent with the findings of Borghesani et al. (2007) and Smith et al. (1999), which both reported decreased cortical response in the ventral visual stream of ε4 carriers during tasks requiring visual object or letter recognition. Although Borghesani et al. only reported ε4-related deficits during encoding and not during retrieval as we report here, altered activation of the medial temporal, and inferotemporal region during visual object identification is an emerging trend.
The M170 peak analysis presented here is consistent with functional ε4-related differences in the ventral visual stream, as the source of the M170 peak shown in one ideal subject here and described previously in the literature suggests that the component originates in the occipito-temporal region. Activation of the ventral visual stream in this time window is believed represent object-level analysis of visual stimuli, and acts as a gateway to higher cognitive processes (Tarkiainen et al., 2002). Increased latency of the M170 peak in ε4 carriers may represent a pre-clinical decrease in efficiency of cortical processing in this region, which may be mitigated somewhat by a physically active lifestyle.
Altered latency of cortical processing in this region illustrates the need for additional studies employing EEG and MEG, which afford the ability to examine latency of evoked cortical fields on the order of 1 ms, in stark contrast to haemodynamic measures which require several seconds. Although fMRI studies of abnormal brain function in carriers of the ε4 allele are increasingly abundant, MEG and EEG studies are lacking. The behavioral data (six- and eight-letter Sternberg RT) and MEG data together suggest that speed of processing is similarly compromised, if not more compromised than magnitude of brain activation in ε4 carriers and should be examined further.
The M170 component is also identified as a candidate for future research on pre-clinical cognitive decline and potential intervention strategies as we observed main effects for both APOE genotype (latency) and exercise level (amplitude). The hippocampal and temporal regions are subject to early decline in AD (Braak and Braak, 1991) and nearby cortical tissue reflects early functional changes, for example exhibiting compromised evoked responses that play a central role in visual object recognition. The fusiform gyrus in the temporal lobe has been identified as a primary generator of the M170 (Deffke et al., 2007), and has also been shown to exhibit deficits in cortical activation in ε4 carriers relative to non-carriers (Borghesani et al., 2007). Follow-up studies can examine cognitive paradigms optimally designed to elicit the M170 component, such as face recognition tasks, and study the cortical dynamics of early visual processing in pre-clinical populations at risk for AD. The role of exercise or other potential intervention strategies to prevent decline in carriers of the ε4 allele also warrants further study.
Acknowledgements
This work is in part supported by NIH R01 DC 05660 to D.P., and by NIA AG022791 to S.R. The authors would like to thank Jeff Walker for his excellent technical support on MEG data acquisition and analysis.
Footnotes
Uncited reference
References
- Adachi Y, Shimogawara M, Higuchi M, Haruta Y, Ochiai M. Reduction of non-periodic environmental magnetic noise in MEG measurement by continuously adjusted least squares method. IEEE Transactions on Applied Superconductivity. 2001;11:669–672. [Google Scholar]
- Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. Journal of Neuroscience. 2005;25(17):4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi MW, Houston WS, Eyler LT, Brown GG. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology. 2005;64(3):501–508. doi: 10.1212/01.WNL.0000150885.00929.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, et al. Patterns of brain activation in people at risk for Alzheimer’s disease. New England Journal of Medicine. 2000;343(7):450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghesani PR, Johnson LC, Shelton AL, Peskind ER, Aylward EH, Schellenberg GD, et al. Altered medial temporal lobe responses during visuospatial encoding in healthy apoe*4 carriers. Neurobiology of Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathologica (Berl) 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, et al. Aerobic exercise training increases brain volume in aging humans. Journals of Gerontology Series A Biological Sciences and Medical Sciences. 2006;61(11):1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychological Science. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, et al. Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(9):3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein e type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends in Neurosciences. 2002;25(6):295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Deffke I, Sander T, Heidenreich J, Sommer W, Curio G, Trahms L, et al. Meg/eeg sources of the 170-ms response to faces are co-localized in the fusiform gyrus. Neuroimage. 2007;35(4):1495–1501. doi: 10.1016/j.neuroimage.2007.01.034. [DOI] [PubMed] [Google Scholar]
- Dipietro L, Caspersen CJ, Ostfeld AM, Nadel ER. A survey for assessing physical activity among older adults. Medicine and Science in Sports and Exercise. 1993;25(5):628–642. [PubMed] [Google Scholar]
- Etnier JL, Caselli RJ, Reiman EM, Alexander GE, Sibley BA, Tessier D, et al. Cognitive performance in older women relative to apoe-epsilon4 genotype and aerobic fitness. Medicine and Science in Sports and Exercise. 2007;39(1):199–207. doi: 10.1249/01.mss.0000239399.85955.5e. [DOI] [PubMed] [Google Scholar]
- Green J, Levey AI. Event-related potential changes in groups at increased risk for Alzheimer disease. Archives of Neurology. 1999;56(11):1398–1403. doi: 10.1001/archneur.56.11.1398. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Kramer AF, Belopolsky AV, Smith DP. A cross-sectional examination of age and physical activity on performance and event-related brain potentials in a task switching paradigm. International Journal of Psychophysiology. 2006;59(1):30–39. doi: 10.1016/j.ijpsycho.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Kiraly MA, Kiraly SJ. The effect of exercise on hippocampal integrity: review of recent research. International Journal of Psychiatry in Medicine. 2005;35(1):75–89. doi: 10.2190/HX7L-4B40-PQNY-2A4P. [DOI] [PubMed] [Google Scholar]
- Lind J, Persson J, Ingvar M, Larsson A, Cruts M, Van Broeckhoven C, et al. Reduced functional brain activity response in cognitively intact apolipoprotein e epsilon4 carriers. Brain. 2006;129(Pt 5):1240–1248. doi: 10.1093/brain/awl054. [DOI] [PubMed] [Google Scholar]
- Maestu F, Fernandez A, Simos PG, Gil-Gregorio P, Amo C, Rodriguez R, et al. Spatio-temporal patterns of brain magnetic activity during a memory task in Alzheimer’s disease. Neuroreport. 2001;12(18):3917–3922. doi: 10.1097/00001756-200112210-00013. [DOI] [PubMed] [Google Scholar]
- O’Hara R, Sommer B, Way N, Kraemer HC, Taylor J, Murphy G. Slower speed-of-processing of cognitive tasks is associated with presence of the apolipoprotein epsilon4 allele. Journal of Psychiatric Research. 2007 doi: 10.1016/j.jpsychires.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proceedings of the National Academy of Sciences of the United States of the America. 2007;104(13):5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podewils LJ, Guallar E, Kuller LH, Fried LP, Lopez OL, Carlson M, et al. Physical activity, apoe genotype, and dementia risk: findings from the cardiovascular health cognition study. American Journal of Epidemiology. 2005;161(7):639–651. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- Polich J, Lardon MT. P300 and long-term physical exercise. Electroencephalography Clinical Neurophysiology. 1997;103(4):493–498. doi: 10.1016/s0013-4694(97)96033-8. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Chen K, Alexander GE, Bandy D, Frost J. Declining brain activity in cognitively normal apolipoprotein e epsilon 4 heterozygotes: a foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of the America. 2001;98(6):3334–3339. doi: 10.1073/pnas.061509598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, et al. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein e. New England Journal of Medicine. 1996;334(12):752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, et al. Functional brain abnormalities in young adults at genetic risk for lateonset Alzheimer’s dementia. Proceedings of the National Academy of Sciences of the United States of the America. 2004;101(1):284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, et al. Correlations between apolipoprotein e epsilon4 gene dose and brain-imaging measurements of regional hypometabolism. Proceedings of the National Academy of Sciences of the United States of the America. 2005;102(23):8299–8302. doi: 10.1073/pnas.0500579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovio S, Kareholt I, Helkala EL, Viitanen M, Winblad B, Tuomilehto J, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurology. 2005;4(11):705–711. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, et al. Association of apolipoprotein e allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43(8):1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Schuit AJ, Feskens EJ, Launer LJ, Kromhout D. Physical activity and cognitive decline, the role of the apolipoprotein e4 allele. Medicine and Science in Sports and Exercise. 2001;33(5):772–777. doi: 10.1097/00005768-200105000-00015. [DOI] [PubMed] [Google Scholar]
- Smith CD, Andersen AH, Kryscio RJ, Schmitt FA, Kindy MS, Blonder LX, et al. Altered brain activation in cognitively intact individuals at high risk for Alzheimer’s disease. Neurology. 1999;53(7):1391–1396. doi: 10.1212/wnl.53.7.1391. [DOI] [PubMed] [Google Scholar]
- Spirduso WW. Physical fitness, aging, and psychomotor speed: a review. Journal of Gerontology. 1980;35(6):850–865. doi: 10.1093/geronj/35.6.850. [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of the America. 1993b;90(5):1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Weisgraber KH, Huang DY, Dong LM, Salvesen GS, Pericak-Vance M, et al. Binding of human apolipoprotein e to synthetic amyloid beta peptide: isoform-specific effects and implications for late-onset Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of the America. 1993a;90(17):8098–8102. doi: 10.1073/pnas.90.17.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkiainen A, Cornelissen PL, Salmelin R. Dynamics of visual feature analysis and object-level processing in face versus letter-string perception. Brain. 2002;125(Pt 5):1125–1136. doi: 10.1093/brain/awf112. [DOI] [PubMed] [Google Scholar]
- Tarkiainen A, Helenius P, Hansen PC, Cornelissen PL, Salmelin R. Dynamics of letter string perception in the human occipitotemporal cortex. Brain. 1999;122(Pt 11):2119–2132. doi: 10.1093/brain/122.11.2119. [DOI] [PubMed] [Google Scholar]
- Trivedi MA, Schmitz TW, Ries ML, Torgerson BM, Sager MA, Hermann BP, et al. Reduced hippocampal activation during episodic encoding in middle-aged individuals at genetic risk of Alzheimer’s disease: a cross-sectional study. BMC Medicine. 2006;4:1. doi: 10.1186/1741-7015-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neuroscience. 1999;2(3):266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. Journal of Neuroscience. 2005;25(38):8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetter S, Murphy C. Apolipoprotein e epsilon4 positive individuals demonstrate delayed olfactory event-related potentials. Neurobiology of Aging. 2001;22(3):439–447. doi: 10.1016/s0197-4580(01)00215-9. [DOI] [PubMed] [Google Scholar]
- Wierenga CE, Bondi MW. Use of functional magnetic resonance imaging in the early identification of Alzheimer’s disease. Neuropsychology Review. 2007;17(2):127–143. doi: 10.1007/s11065-007-9025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JG, Huppert FA, Matthews FE, Nickson J. Performance and normative values of a concise neuropsychological test (CAMCOG) in an elderly population sample. International Journal of Geriatric Psychiatry. 2003;18(7):631–644. doi: 10.1002/gps.886. [DOI] [PubMed] [Google Scholar]