Abstract
Objectives/Hypothesis:
High rates of overall survival (OS) and laryngeal preservation were achieved in two sequential phase II clinical trials in patients with stage III/IV laryngeal squamous cell carcinoma (SCC). Patients were treated with chemoradiation after a >50% primary tumor response to one cycle of neoadjuvant chemotherapy (IC). We analyzed outcomes for T4 patients with cartilage invasion from both studies.
Study Design:
Retrospective.
Methods:
Records from 36 patients with T4 SCC of the larynx with cartilage invasion alone (n = 16) or cartilage invasion and extralaryngeal spread (n = 20) were retrospectively reviewed. All were treated with one cycle of cisplatin (100 mg/m2) [or carboplatin (AUC 6)] and 5-fluorouracil (1,000 mg/m2/d for 5 days) (P+5FU). Those achieving >50% response at the primary tumor received chemoradiation (70 Gy; 35 fractions with concurrent cisplatin-100 mg/m2 [carboplatin (AUC 6)] every 21 days for 3 cycles), followed by adjuvant P+5FU for complete histologic responders (CHR). Patients with <50% response after IC underwent total laryngectomy and postoperative radiation.
Results:
Twenty-nine of 36 patients (81%) had >50% response following IC. Of these, 27 received definitive chemoradiation, 23 (85%) obtained CHR, with 58% laryngeal preservation rate. The 3-year OS was 78%, and the disease-specific survival was 80% (median follow-up 69 months). Following chemoradiation, 8/11 (73%) patients with an intact larynx had >75% understandable speech, 6/36 (17%) were g-tube dependent and 6/36 (17%) were tracheostomy dependent.
Conclusions:
Our results suggest that chemo-selection is a feasible organ preservation alternative to total laryngectomy for patients with T4 laryngeal SCC with cartilage invasion.
INTRODUCTION
The Radiation Therapy Oncology Group (RTOG) 91-11 study reported high rates of larynx preservation and disease control and established concurrent chemoradiation as a definitive treatment for patients with advanced laryngeal squamous cell carcinomas.1 Over 90% treated on the RTOG study had T2 or T3 tumors, and patients with large-volume stage T4 disease were excluded, including those with cartilage invasion or >1 cm extension into the base of tongue. Such patients have been historically treated with total laryngectomy followed by radiation therapy.2,3
High rates of laryngeal preservation were also reported in the Department of Veteran's Affairs (VA) Laryngeal Cancer Group Study,4 but with lower overall rates of salvage laryngectomy for T3 patients (28%) compared to patients with T4 primary tumors (56%). Since 1995, our phase II studies in advanced laryngeal cancer have focused on using clinical tumor response to a single cycle of neoadjuvant chemotherapy to select patients for subsequent chemoradiation (University of Michigan Comprehensive Cancer Center [UMCC] 9520).5 We have demonstrated high rates of overall survival and laryngeal preservation in patients who were treated with chemoradiation after attaining a >50% response to one cycle of induction chemotherapy (IC). Based on this paradigm that IC selects for patients most likely to respond to chemoradiation, we designed a follow-up phase II study, (UMCC 0056),6 for stage III/IV laryngeal cancer patients to evaluate whether histologic complete responders to induction chemotherapy could be treated with chemotherapy alone. Patients on this trial, who attained >50% response (but <100% response), were treated identically to those on UMCC 9520 with chemoradiation.
This report analyzes the outcomes of the T4 laryngeal cancer patients treated under these two sequential protocols to determine the overall response rate of a single cycle of induction chemotherapy, used to select T4 patients with cartilage invasion for organ preservation with chemoradiation, and to compare overall survival and laryngeal preservation rates to the rates achieved for patients with T3 tumors treated on the same two protocols.
MATERIALS AND METHODS
Eligibility Criteria and Patient Population
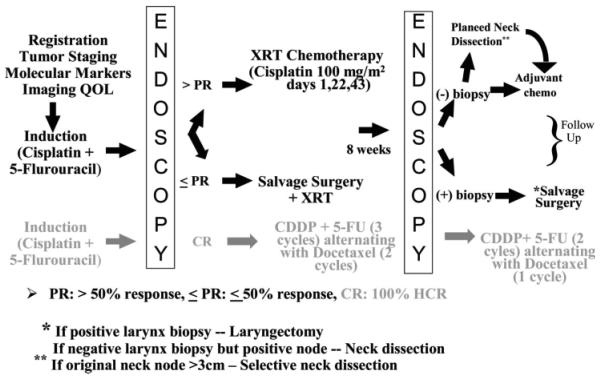
All patients had histologically confirmed previously untreated T4 squamous cell carcinoma of the larynx. Staging included direct laryngoscopy and contrast enhanced computerized tomography (CT). All patients were restaged to correspond with the 2002 American Joint Committee on Cancer staging guidelines7 for T4 tumors. Inclusion criteria for T4 tumors included radiographic evidence of tumor extension through the thyroid cartilage and tumor that invaded tissues beyond the larynx. Patients with a Karnofsky performance status <60% or inadequate medical or lab status to undergo chemotherapy were ineligible. Both UMCC protocols (9520 and 0056) were approved by the institutional review board at the University of Michigan, and all patients gave documented informed consent. The study schema is shown in Figure 1.
Fig. 1.
UMCC protocols 9520 and 0056 study schemas. In black is the schema common to both protocols. The grey shaded schema applies to UMCC 0056 only and was not part of this review.
Treatment Plan
Induction chemotherapy (IC)
Prior to enrollment, patients underwent a history and physical examination, complete blood count with differential and platelets and serum chemistries. For patients with a creatinine clearance ≥60 cc/minute and hearing loss ≤30 dB between 500-2,000 Hz, cisplatin (100 mg/m2) was administered on day 1. Those with a creatinine clearance of 30-59 cc/minute or hearing loss >30 dB received carboplatin (AUC 6) on day 1. Over 5 days, 5-fluorouracil (1,000 mg/m2/day) was administered by continuous infusion.
Tumor assessment
Bidimensional measurements of the primary tumor were determined by direct laryngoscopy under anesthesia and recorded pretreatment and 3 weeks after IC. Response evaluation criteria in solid tumors criteria,8 not in common use at the time this trial was initiated, were not used. In both protocols, patients who had a >50% reduction in the bidimensional product of the primary tumor area were eligible for chemoradiotherapy. Patients who were nonresponders (≤50% response) underwent definitive surgery followed by radiation therapy. In UMCC 0056, patients who were histologic complete responders (100% response) were treated with chemotherapy alone.6 These patients, who numbered only four, did not have T4 tumors and were not included in this analysis.
Concurrent chemoradiotherapy
Concurrent chemoradiation began within 3 weeks after IC. Patients received cisplatin 100 mg/m2 or carboplatin (AUC = 6) on days 1, 22, and 43 concurrent with radiation. Radiation was administered once daily, 5 days per week, 2 Gy per fraction. The dose to the gross disease and to the high/low-risk volumes was 70 Gy. Sub-clinical disease received 70, 60-63, and 50-59 Gy, respectively, all in 35 fractions, using 3D radiotherapy or intensity modulated radiation therapy.9
Tumor assessment following chemoradiotherapy
Eight weeks after the completion of chemoradiation, a direct laryngoscopy with biopsy was performed. Patients without residual disease at the primary site were eligible for two cycles of adjuvant chemotherapy with cisplatin (100 mg/m2) or carboplatin (AUC 6) and 5-fluorouracil (1,000 mg/m2/day) by continuous infusion over 5 days. Each cycle was administered every 21 days. Those with biopsy-proven disease at the primary site underwent total laryngectomy and ipsilateral neck dissection. Patients with no disease at the primary site but clinical evidence of disease in the neck, or with neck nodes greater than 3 cm at time of initial staging, underwent a selective or modified radical neck dissection, as deemed appropriate.
Tumor volume assessments
Volumetric analysis was performed on tumors in which CT scans were available for review (n = 20). The interpretation and contouring was performed by a neuroradiologist (s.m.), using a Microsoft Windows-based personal computer (Microsoft Inc., Redmond, WA) with dual 2 K × 2 K, high-resolution monitors and running BIT-Image software for viewing CT images and making volumetric measurements using a mouse manually to contour the outer margin of any abnormal laryngeal mass on each image. Tumor volume was analyzed in its original continuous scale fashion. The median of the tumor volume was used as a benchmark to represent high tumor volume and low tumor volume groups in the Kaplan-Meier plot depiction of the association between tumor volume and survival.
Quality of life analyses
Quality-of-life (QOL) measurements were collected for patients who remained disease-free for at least 12 months following chemoradiation. We measured gastric tube (g-tube) dependency, persistence of tracheostomy tube, and understandability of speech. The speech QOL instrument administered was the Performance Status Scale for Head and Neck Cancer Patients.10
Statistical analysis
The goal of the analysis was to determine overall success rates of this approach to organ preservation in patients with T4 cancers and compare treatment outcomes between T3 and T4 classes. Treatment outcomes include response to neoadjuvant chemotherapy, response to chemoradiation therapy, time to events, and QOL. Chi-square statistics were used to compare the frequency distribution of induction chemotherapy response, chemoradiation responses, and QOL between T3 and T4 classes. Time-to-event outcomes included overall survival, disease-specific survival, time to recurrences or second primary, and time to indication of surgery at primary site. Overall survival considered all deaths as events. Disease-specific survival defined events as head and neck cancer-related deaths. Time to indication of surgery at primary site (laryngectomy-free survival) was defined as the time to local failure that required laryngectomy. Subjects who were never disease-free were considered as having the event with zero duration. Kaplan-Meier survival estimates and log-rank statistics were used to assess the time-to-event outcomes. All statistical analyses were done using SAS version 9.0 (SAS Institute, Cary, NC). A two-tailed P value of .05 or less was considered statistically significant.
RESULTS
Treatment and Outcome
Twenty-four patients were treated under UMCC 9520, and nine patients were treated under UMCC 0056. Three patients were treated identically to those treated under UMCC 9520 following study closure and were included after petitioning the institutional review board for approval for retrospective analysis. Patient characteristics are described in Table I.
TABLE I.
Patient Characteristics.
| Number | Percentage | |
|---|---|---|
| Total T4 study group | 36 | 100 |
| Extralaryngeal invasion | 20 | 56 |
| Cartilage invasion alone | 16 | 44 |
| Median age, y (range) | 56 (37–76) | |
| Male | 27 | 75 |
| Female | 9 | 25 |
| Disease site | ||
| Glottic | 12 | 33 |
| Supraglottic | 23 | 64 |
| Hypopharynx | 1 | 3 |
| Nodal status | ||
| N0 | 17 | 47 |
| N1 | 5 | 14 |
| N2 | 13 | 36 |
| N3 | 1 | 3 |
| Karnofsky performance status | ||
| 100% | 27 | 75 |
| 90% | 7 | 19 |
| 80% | 2 | 6 |
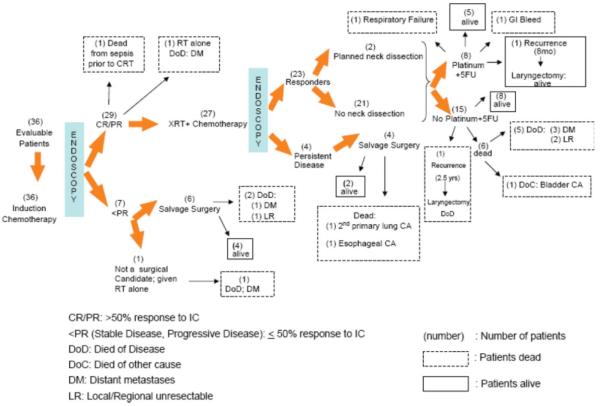
Response data and survival status are shown in Figure 2. All 36 received IC. Twenty-nine of 36 (81%) patients had a ≥50% response at the primary site, and 27 received chemoradiation. One patient died from sepsis unrelated to neutropenia prior to the start of chemoradiation. One patient was treated with radiation alone as his performance deteriorated following induction chemotherapy, and he was no longer a candidate for platinum-based chemotherapy. Seven patients (19%) had <50% response to induction chemotherapy. Of these, six underwent laryngectomy and one was deemed surgically unresectable and was treated with radiation therapy alone.
Fig. 2.
Schema with response data according to treatment.
Of the 27 patients who received chemoradiation, 23 (85%) were complete histologic responders (CHRs). Two of these patients later relapsed and underwent laryngectomy. Two of the 23 CHRs (9%) underwent planned neck dissections; neither had residual tumor in the neck. Six of the 23 CHRs (26%) received two cycles of adjuvant cisplatin (or carboplatin) and 5-fluorouracil, and two received one cycle. Thirteen patients refused adjuvant chemotherapy, and two were not suitable candidates for further treatment due to ototoxicity and persistent neuropathy from previous cisplatin. Four patients (15%) had positive biopsies following chemoradiation; all were salvaged with laryngectomy.
Twenty patients (56%) are alive, 13 with an intact larynx following chemoradiation and seven following salvage laryngectomy. Following several months of successful chemoradiation, two developed local disease. Both were treated with laryngectomy; one remains alive without disease and the other is dead of disease. Within two years of completing chemoradiation, three developed distant metastases and two developed locoregional recurrences, all of whom are dead of disease. Three patients died from other causes following chemoradiation, including gastrointestinal bleeding, bladder cancer, and respiratory failure.
Following early salvage laryngectomy (for failed response to IC), two are dead, one from distant metastases, and one from local regional disease. Of the four patients who underwent late salvage laryngectomy (for failed response to chemoradiation), two are alive and two are dead from second primary cancers (lung and esophagus).
Chemotherapy Compliance and Toxicities
The only limitation to enrollment into either protocol was a Karnofsky performance status <60%. Compliance with the single cycle of induction chemotherapy was 100%. Of 27 who completed chemoradiation, 20 (74%) completed all three cycles of chemotherapy. The seven remaining patients (26%) received two cycles of chemotherapy. Eight patients received adjuvant chemotherapy, six received two cycles, and two received one cycle of platinum and 5-fluorouracil. None of the tracheostomy or g-tube dependent patients received adjuvant therapy. Toxicity data with induction chemotherapy and chemoradiation is reported in Table II.
TABLE II.
Common Adverse Events.
| Number of Patients (%) |
||||
|---|---|---|---|---|
| Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
| Following induction chemotherapy (n = 36) | ||||
| Neutropenia | 1 (3) | 2 (5) | 1 (1) | 0 |
| Febrile neutropenia | 0 | 0 | 0 | 0 |
| Leukopenia | 2 (5) | 4 (11) | 1 (3) | 0 |
| Anemia | 6 (17) | 0 | 0 | 0 |
| Thrombocytopenia | 0 | 1 (3) | 0 | 0 |
| Diarrhea | 3 (8) | 0 | 0 | 0 |
| Mucositis | 3 (8) | 7 (19) | 1 (3) | 0 |
| Nausea | 2 (5) | 0 | 0 | 0 |
| Vomiting | 1 (3) | 0 | 0 | 0 |
| Tinnitus | 0 | 0 | 0 | 0 |
| Sensory neuropathy | 0 | 0 | 0 | 0 |
| During chemoradiation (n = 27) | ||||
| Neutropenia | 1 (4) | 1 (4) | 0 | 0 |
| Febrile neutropenia | 0 | 0 | 0 | 0 |
| Leukopenia | 4 (15) | 4 (15) | 0 | 0 |
| Anemia | 4 (15) | 3 (11) | 0 | 0 |
| Thrombocytopenia | 3 (9) | 3 (9) | 0 | 0 |
| Diarrhea | 0 | 0 | 0 | 0 |
| Mucositis | 14 (52) | 13 (48) | 0 | 0 |
| Nausea | 1 (4) | 2 (7) | 0 | 0 |
| Vomiting | 1 (4) | 3 (11) | 0 | 0 |
| Sensory neuropathy | 1 (4) | 0 | 0 | 0 |
| Tinnitus | 0 | 0 | 0 | 0 |
| Creatinine | 3 (4) | 0 | 0 | 0 |
| Following adjuvant chemotherapy (n = 8) | ||||
| Neutropenia | 0 | 0 | 0 | 0 |
| Febrile neutropenia | 0 | 0 | 1 (13) | 0 |
| Leukopenia | 1 (13) | 0 | 1 (13) | 0 |
| Anemia | 5 (62) | 2 (25) | 0 | 0 |
| Thrombocytopenia | 0 | 0 | 0 | 0 |
| Sensory neuropathy | 0 | 0 | 0 | 0 |
Quality of Life
QOL results are listed in Table III. Patients with T3 lesions from UMCC 9520 and UMCC 0056 were used as a comparison group. Understandability of speech was documented in 11 of the T4 patients, eight of whom had speech that was at least 75% understandable. G-tube dependence and persistent tracheostomy were evaluated for all 36 patients. Six patients (17%) required permanent G-tubes, compared to 4% in the T3 study population (P = .03). Six (17%) had persistent tracheostomy, which was similar to our T3 study population.
TABLE III.
Quality of Life Assessments for Patients Who Received Chemoradiation.
| Understandability of Speech |
UMCC T4 Patients (n = 11), No. (%) |
UMCC T3 Patients (n = 21), No. (%) |
P |
|---|---|---|---|
| 100% | 3 (28) | 7 (33) | |
| 75% | 5 (45) | 8 (38) | |
| 50% | 1 (9) | 5 (24) | |
| 25% | 2 (18) | 1 (5) | |
| 0% | 0 | 0 | |
| G-tube dependence (≥12 mo) | |||
| G-tube dependent/No. evaluated | 6/36 (17) | 3/73 (4) | .03 |
| Persistent tracheostomy (≥12 mo) | |||
| Tracheostomy/No. evaluated | 6/36 (17) | 9/73 (12) | .66 |
UMCC = University of Michigan Comprehensive Cancer Center.
Survival Outcomes
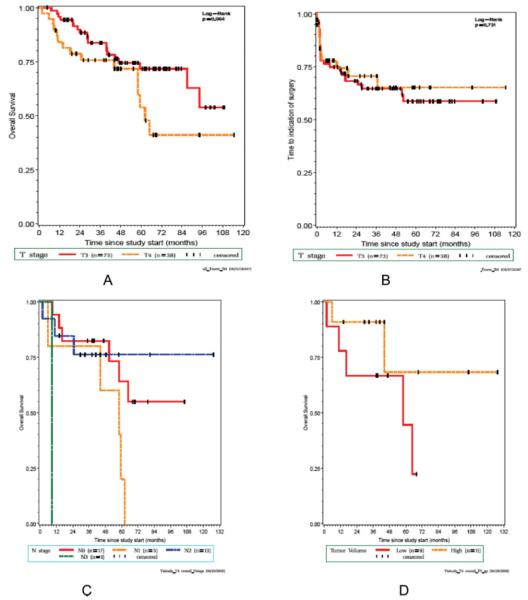
With a median follow up of 69 months (95% CI 44, 74), 3-year overall survival was 78% (95% confidence interval [CI] [60%-88%]) (Fig. 3A), and 3-year, laryngectomy-free survival was 58% (95% CI [40%-73%]) (Fig. 3B). The 3-year, disease-specific survival for those receiving adjuvant chemotherapy was 100% compared to 73% (95% CI 52.61%-86.53%) for those who did not. Figures 3C and 3D depict survival by nodal stage and tumor volume, respectively.
Fig. 3.
(A) Overall survival plots of patients with T4 disease and T3 disease (P = .064). (B) Laryngectomy-free survival plots of patients with T4 disease and T3 disease (P = .731). (C) Overall survival plots for T4 patients by nodal stage (P = .23). (D) Overall survival plots for T4 patients by tumor volume (P = .7); yellow indicates the smaller-volume tumors and red indicates large-volume tumors.
A comparison of survival rates of the UMCC 9520 and 0056 T4 patients versus the UMCC 9520 and UMCC 0056 T3 patients is noted in Table IV.
TABLE IV.
Comparison of Survival Rates of the T4 Study Patients With the T3 Patients Treated With Chemoradiation From UMCC 9520 and UMCC 0056.
| At 3 yrs | UMCC 9520/0056 T4 Patients n = 36 |
UMCC 9520/0056 T3 Patients n = 73 |
|---|---|---|
| Median follow-up, mo | 69 (44–74) | 74 (60–83) |
| Laryngectomy-free survival | 58 (40–73) | 62 (49–72) |
| Disease-free survival | 58 (41–72) | 72 (60–81) |
| Disease-specific survival | 80 (62–90) | 88 (78–94) |
| Overall survival | 78 (60–88) | 83 (72–90) |
The numbers in parentheses refer to the 95% confidence intervals. UMCC = University of Michigan Comprehensive Cancer Center.
Larynx Preservation
Of 36 evaluable patients, six received planned salvage surgery due to nonresponse to IC, four had late salvage surgery following chemoradiation due to persistent disease, and only two had surgery following a prolonged disease-free interval after chemoradiation. One patient with N3 disease was not a surgical candidate. Thus, 12 of 36 patients underwent laryngectomy. The Kaplan-Meier estimated probability of laryngectomy for the T4 patients at 10 years was 55% (95% CI [36%-70%]), and for the T3 patients is 57% (95% CI [44%-68%]). Of the 13 patients who are alive with an intact larynx, seven had cartilage invasion alone and six had both extralaryngeal spread and cartilage invasion.
DISCUSSION
Cartilage invasion and or extralaryngeal spread are often considered contraindications to organ preservation approaches for patients with T4 laryngeal squamous cell carcinomas. Despite these concerns, some highly radio-responsive tumors may be cured despite large volumes or cartilage involvement. Our approach to laryngeal preservation has been to try and select the optimal patient for chemoradiation approaches using clinical response to a single cycle of neoadjuvant chemotherapy as a selection factor.5 Because most patients fear laryngectomy,11 we have typically offered this approach to all T3 or T4 patients even with tumors showing CT evidence of cartilage invasion. On rare occasions, patients with severely compromised pretreatment laryngeal function are offered primary laryngectomy because preservation of such compromised larynxes is unwarranted and improvement in function following chemoradiation is seldom complete. With a 3-year overall survival rate of 78% in patients with T4 cancers, our results strongly support the use of chemoselection as an organ preservation strategy in such patients. Hence, this may be considered as an alternative to up front surgical resection in patients with CT evidence of cartilage invasion. Compared to the best treatment arm (i.e., chemoradiation) of RTOG 91-11,1 our overall and laryngectomy-free survival rates are similar. Furthermore, our T4 subjects with CT evidence of cartilage invasion had larynx preservation rates and survival outcomes comparable to our T3 patient population (Table III). In addition, when tumor volume was evaluated radiographically in a subset of patients, no survival differences were noted between patients with the largest and smallest tumors. This observation argues that the inherent biology of a particular tumor and its sensitivity to chemoradiation is probably more important to ultimate success of a given treatment approach than the presence of either bulky disease or cartilage invasion on imaging studies.
One other retrospective study has evaluated definitive chemoradiotherapy as treatment for high-volume T4 laryngeal cancers. The University of Chicago experience (Knab et al.)12 looked at 32 patients with untreated T4 larynx cancer, 23 of whom had large-volume tumors. All patients were treated with multiple cycles of weekly carboplatin and paclitaxel induction chemotherapy followed by treatment with concomitant paclitaxel, 5-fluorouracil, hydroxyurea, and hyperfractionated radiotherapy. With a median follow-up time of 43 months, the 4-year overall survival for the large-volume subjects was 56%, with 81% larynx preservation. At ≥ 1 year, 13% of patients were G-tube dependent, and 20% had persistent tracheostomy. These preliminary results are encouraging and consistent with our current analysis. However, a cautionary note is warranted as the Chicago experience has only been published in abstract form.
Of particular interest when comparing our results to those of Knab et al. is the difference in the use of induction chemotherapy. Our approach has been to use a single cycle of induction chemotherapy for chemoselection, whereas Knab et al. gave 6 weeks of chemotherapy prior to chemoradiation. Given that the greatest number of disease failures in our trials were in patients with distant metastases, it is interesting to speculate whether additional cycles of platinum and 5-fluorouracil (PF) as induction chemotherapy could have improved our survival outcomes, because only one third of our patients were able to receive additional adjuvant chemotherapy. Two earlier trials demonstrated an overall survival benefit with IC,13,14 and data from the MACH-NC meta-analysis showed a marginal improvement in survival (P = .05) with IC regimens containing cisplatin and 5-FU.15 Currently, improvements in survival have been suggested with the addition of taxanes to PF in IC regimens.16,17 Recently, a study of advanced larynx cancer patients was terminated at our institution (unpublished data) after more than 50% of individuals failed to respond to chemoradiation, all of whom had greater than 50% responses to one cycle of TPF induction chemotherapy. By incorporating a more effective induction regimen, one such explanation for these local failures could be the loss of the discriminating ability of a single neoadjuvant cycle to select patients for immediate laryngectomy.
Adjuvant chemotherapy had no effect on outcome in our original UMCC 9520 study. In general, published meta-analyses demonstrate no survival benefit for its use.15,18 However, the T4 patients in this report may have obtained a benefit from additional chemotherapy following chemoradiation as none of the eight who received adjuvant chemotherapy developed distant metastases and only one had a locoregional failure. Alternatively, these patients may have had better performance status following chemoradiation, and this could be associated with better survival.19,20 We recognize that the number of patients is small, and that most patients declined adjuvant treatment due to prolonged toxicity following chemoradiation. However, it does raise the question whether patients with large-volume disease and/or cartilage invasion with good performance statuses may benefit from additional adjuvant chemotherapy.
QOL was also assessed in our analysis. Some believe that unfavorable T4 tumors with cartilage invasion are unlikely to be cured with organ preservation. Those that are cured often suffer with a dysfunctional larynx with associated aspiration and severe dysphagia, requiring permanent gastrostomy and/or tracheostomy.3 Longitudinal data regarding pretreatment factors associated with tracheostomy and feeding tube dependence were previously published from our institution. In a multivariate analysis of over 700 patients, we found that tracheostomy was a very strong predictor for g-tube dependence.21 Permanent tracheostomy rates of 3% to 7% were related to primary tumor size in the larynx, which included T4 cancers with cartilage invasion. The small percentage of T4 patients in our study that remained tracheostomy dependent was similar to the Chicago experience and that reported by the VA Laryngeal Cancer Study Group. As expected, however, the high volume T4 population had a greater number of patients requiring permanent tracheostomy compared to lower volume tumor patients. For this advanced disease population, our g-tube dependence rate is relatively low (17%). These patients, in general, had a more difficult time tolerating treatment and the majority of them received carboplatin as part of their induction and chemoradiation regimens. Similarly, none received adjuvant chemotherapy.
CONCLUSION
In summary, this is the first published report of patients with T4 tumors with cartilage invasion treated with chemoradiation. Using response to a single cycle of chemotherapy to select our patients for surgery or chemoradiation, our results demonstrate that T4 patients can undergo successful organ preservation with excellent overall survival and larynx preservation rates. Outcomes do not appear to be limited by tumor volume or nodal status, and QOL does not appear to be compromised by treatment with chemoradiation. Although we recognize the limitations of this retrospective analysis, our data suggest that patients with cartilage invasion should be given the opportunity for organ preservation. Additional prospective studies are warranted to verify these findings.
Acknowledgments
This research was supported by National Institutes of Health/National Institute of Dental and Craniofacial Resources grants (R01 DE13346) and (P30 DC 05188), University of Michigan's Head and Neck Cancer SPORE grant (P50 CA97248), and Cancer Center Support Grant P30 CA46592.
Footnotes
Presented at the 43rd Annual ASCO Meeting, Chicago, Illinois, U.S.A., June 2, 2007 and the 7th International Conference on Head and Neck Cancer, San Francisco, California, U.S.A., June 20, 2008.
BIBLIOGRAPHY
- 1.Forastiere AA, Goepfert H, Manor M, et al. Concurrent chemoradiotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 2.Yuen A, Medina JE, Goepfert H, et al. Management of stage T3 and T4 glottic carcinomas. Am J Surg. 1984;148:467–472. doi: 10.1016/0002-9610(84)90371-4. [DOI] [PubMed] [Google Scholar]
- 3.Hinerman RW, Mendenhall WM, Amdur RJ, et al. Carcinoma of the supraglottic larynx: treatment alone or with planned neck dissection. Head Neck. 2002;24:456–467. doi: 10.1002/hed.10069. [DOI] [PubMed] [Google Scholar]
- 4.The Department of Veterans Affairs Laryngeal Cancer Study Group Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med. 1991;324:1685–1690. doi: 10.1056/NEJM199106133242402. [DOI] [PubMed] [Google Scholar]
- 5.Urba S, Wolf G, Eisbruch A, et al. Single-cycle induction chemotherapy selects patients with advanced laryngeal cancer for combined chemoradiation: a new treatment paradigm. J Clin Oncol. 2006;24:593–598. doi: 10.1200/JCO.2005.01.2047. [DOI] [PubMed] [Google Scholar]
- 6.Worden FP, Wolf GT, Eisbruch A, et al. Chemo-selection of patients for organ preservation in advanced laryngeal cancer: failure of chemotherapy as the sole treatment for complete histologic responders to neoadjuvant chemotherapy. J Clin Oncol. 2006;24(suppl):18S. abstract 5560. [Google Scholar]
- 7.American Joint Committee on Cancer . In: AJCC Cancer Staging Manual and Handbook. 6th ed. Greene FL, Page DL, Fleming ID, et al., editors. Springer-Verlag; New York, NY: 2002. pp. 301–346. [Google Scholar]
- 8.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 9.Eisbruch A, Marsh LH, Martel MK, et al. Comprehensive irradiation of head and neck cancer using conformal multisegmental fields: assessment of target coverage and noninvolved tissue sparing. Int J Radiat Oncol Biol Phys. 1998;41:559–568. doi: 10.1016/s0360-3016(98)00082-0. [DOI] [PubMed] [Google Scholar]
- 10.List MA, Ritter-Sterr C, Lansky SB. A performance status scale for head and neck cancer patients. Cancer. 1990;66:564–569. doi: 10.1002/1097-0142(19900801)66:3<564::aid-cncr2820660326>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 11.Dropkin MJ. Body image and quality of life after head and neck cancer. Semin Oncol. 1988;15:61–69. [Google Scholar]
- 12.Knab RB, Salama JK, Stenson KM, et al. Definitive chemo-radiation for T4 laryngeal squamous cell carcinoma. Int J Rad Onc Biol Phys. 2006;66(suppl):S14. abstract. [Google Scholar]
- 13.Paccagnella A, Orlando A, Marchiori C, et al. Phase III trial of initial chemotherapy in stage III or IV head and neck cancers: a study by the Gruppo di Studio sui Tumori della Testa e del Collo. J Natl Cancer Inst. 1994;86:265–272. doi: 10.1093/jnci/86.4.265. [DOI] [PubMed] [Google Scholar]
- 14.Domenge C, Hill C, Lefebvre JL, et al. Randomized trial of neoadjuvant chemotherapy in oropharyngeal carcinoma. French Groupe d'Etude des Tumeurs de la Tete et du Cou (GETTEC) Br J Cancer. 2000;83:1594–1598. doi: 10.1054/bjoc.2000.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pignon JP, Bourhis J, Domenge C, Designe L. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet. 2000;355:949–955. [PubMed] [Google Scholar]
- 16.Hitt R, Lopez-Pousa A, Martinez-Trufero J, et al. Phase III study comparing cisplatin plus fluorouracil to paclitaxel, cisplatin, and fluorouracil induction chemotherapy followed by chemoradiotherapy in locally advanced head and neck cancer. J Clin Oncol. 2005;23:8636–8645. doi: 10.1200/JCO.2004.00.1990. [DOI] [PubMed] [Google Scholar]
- 17.Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–1715. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 18.Bourhis J, Le Maitre A, Baujat B, Audry H, Pignon JP. Individual patients' data meta-analyses in head and neck cancer. Curr Opin Oncol. 2007;19:188–194. doi: 10.1097/CCO.0b013e3280f01010. [DOI] [PubMed] [Google Scholar]
- 19.Jordhoy MS, Fayers P, Loge JH, et al. Quality of life in advanced cancer patients: the impact of sociodemographic and medical characteristics. Br J Cancer. 2001;85:1478–1485. doi: 10.1054/bjoc.2001.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Movsas B, Scott C, Watkins-Bruner D. Pretreatment factors significantly influence quality of life in cancer patients: a radiation therapy oncology group (RTOG) analysis. Int J Radiat Oncol Biol Phys. 2006;65:830–835. doi: 10.1016/j.ijrobp.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Cheng S, Terrell J, Bradford C, et al. Variables associated with feeding tube placement in head and neck cancer. Arch Otolaryngol Head Neck Surg. 2006;132:1–8. doi: 10.1001/archotol.132.6.655. [DOI] [PubMed] [Google Scholar]