Abstract
Objectives
To test an easily administered, noninvasive technology to identify vulnerability to mental stress ischemia.
Background
Myocardial ischemia provoked by emotional stress (MSI) in patients with stable coronary artery disease (CAD) predicts major adverse cardiac events. A clinically useful tool to risk stratify patients on this factor is not available.
Methods
Patients with documented CAD (n=68) underwent single photon emission computed tomography (SPECT) myocardial perfusion imaging concurrent with pulse wave amplitude assessment by peripheral arterial tonometry (PAT) during a mental stress protocol of sequential rest and anger stress periods. Heart rate and blood pressure were assessed, and blood was drawn for catecholamine assay, during rest and stress. MSI was defined by the presence of a new perfusion defect during anger stress (n=26) and the ratio of stress to rest PAT response was calculated.
Results
Patients with MSI had a significantly lower PAT ratio than those without MSI (0.76 ± 0.04 vs. 0.91 ± 0.05, p=0.03). An ROC curve for optimum sensitivity/specificity of PAT ratio as an index of MSI produced a sensitivity of 0.62 and a specificity of 0.63. Among patients taking angiotensin converter enzyme (ACE) inhibitors, the sensitivity and specificity of the test increased to 0.86 and 0.73 (respectively); 90% of patients without MSI were correctly identified.
Conclusions
PAT in concert with ACE inhibition may provide a useful approach to assess risk for MSI. Future studies should help determine how best to utilize this approach for risk assessment in the clinical setting.
Keywords: Mental stress, myocardial ischemia, peripheral arterial tonometry
INTRODUCTION
Emotionally stressful episodes can trigger acute heart failure, myocardial infarction (MI) and sudden cardiac death (c.f., 1–4). Furthermore, both laboratory and real-world studies have shown that emotional stress can precipitate frank myocardial ischemia (MSI) (6) in patients with coronary artery disease (CAD). MSI is most often asymptomatic and accounts for up to 75% of total ischemic burden (6–8). In the laboratory, MSI has been observed in 30–60% of patients with CAD (c.f., 8), depending on whether the ischemia is indexed by a decline in left ventricular ejection fraction, by new ventricular wall motion abnormalities, or by a decrement in myocardial perfusion. Vulnerability to MSI has prognostic significance above standard risk models that include positive exercise imaging procedures, with studies demonstrating between a 2.4–3.0 fold increased risk of acute coronary events over a 2–4 year (9–11), and a 3.0 rate ratio of death at 5-years (13).
Despite the prognostic significance of MSI for patients with CAD, risk stratification algorithms that include this factor have not been implemented in the clinical setting. One obstacle to such implementation has been that detection requires a detailed protocol that includes nuclear myocardial perfusion imaging (8). Considerations of logistics, expense, and risks for patients have precluded the widespread use of this methodology for screening. Hence, an easily administered, noninvasive test would facilitate the determination of the additional risk above that determined by exercise testing that is associated with vulnerability to MSI in patients with known CAD. Such a test would be useful in the clinical setting to identify CAD patients who might benefit from a cardiac care strategy specifically designed to address the elements of their disease related to emotional stress.
In addition to its ability as a provocateur of ischemia, emotional stress increases vascular smooth muscle tone. This effect is observed in epicardial coronary vessels (14), in the coronary microvascular bed (15), and in the peripheral circulation (16). Since change in peripheral microvascular resistance reflects change in coronary microvascular resistance under given conditions (17), the observed effects of emotional stress on the peripheral circulation demonstrate one avenue by which MSI-associated risk stratification might be more easily accomplished in the clinical setting. Peripheral arterial tonometry (PAT) is a technique for non-invasively assessing arterial pulse wave amplitude (PWA) in the peripheral microcirculation on a continuous basis (18–19). This technology thereby provides a means for measuring the effects of either environmental or pharmacologic probes on microvascular smooth muscle tone. In the present study, we tested the utility of this method for predicting vulnerability to MSI in a group of patients with chronic stable CAD, and whether ACE inhibition therapy - known to affect vascular performance - affects PAT prediction of MSI.
Mental and emotional stress also provokes an increase in neurohormonal output, most readily observed as an increase in circulating sympathetic markers (2; 6; 20). To determine whether sympathetic factors were linked to changes in peripheral arterial tone during emotional stress, we also measured reactive changes in levels of circulating catecholamines.
METHODS
Participants
Study participants (n=68) were recruited from the Cardiology Clinics of Yale-New Haven Hospital and VA Connecticut Healthcare System from January 2004 through June 2006. The study was approved by the Institutional Review Boards at both medical facilities. The main inclusion criterion was an existing diagnosis of stable CAD, based on a positive exercise myocardial perfusion study (N=10), a history of myocardial infarction (N=10), or history of percutaneous (N=31) or surgical (N=24) revascularization. Exclusion criteria were: ACS or coronary revascularization in the preceding 6 months; history of major cardiac arrhythmia, or use of a pacemaker or ICD; uncompensated heart failure; presence of any other incapacitating or life-threatening illness; major psychiatric disorder or abuse of psychoactive substances; or chronic use of benzodiazepines or other sedatives. Patients meeting these criteria were approached during routine clinic visits. The study was described to them, and informed consent obtained. The population enrolled was homogeneous with regard to severity of CAD, with all patients falling into NY Heart Association Class I–II.
Medical chart review and patient interview were used to obtain demographic information and determine cardiovascular risk profile. Participants were classified as diabetic if they carried a clinical diagnosis of Type I or II diabetes mellitus. Those with a recent history of systolic pressure >140 mm Hg or diastolic pressure >90 mm Hg, or currently taking medication for high blood pressure were classified as hypertensive, while those with total cholesterol ≥200 mg/dl, LDL ≥130 mg/dl, or taking cholesterol lowering medications were classified as having hypercholesterolemia. Tobacco use was also determined.
Procedures
Participants were asked to eat a light breakfast and take their normal medications before reporting to the VACT Neurocardiac Research Laboratory at 9 AM on the study day. Intravenous (IV) access and ECG leads were secured, and a baseline myocardial perfusion imaging (MPI) study was performed by single-photon emission computed tomography (SPECT) with technetium-99-sestamibi (Tc-99m). The participant was then placed in a relaxed recumbent position. A blood pressure cuff was placed on the arm with IV access, and a PAT noninvasive plethysmographic probe (Itamar Medical, Caesarea, Israel) was placed on the middle finger of the opposing arm.
The PAT is a computer driven, automated system for assessment of beat-to-beat PWA. The plethysmographic probe applies a uniform pressure field around the fingertip; the field is automatically adjusted for the subject’s baseline diastolic pressure to prevent venous pooling of blood, and to unload arterial wall tension. Hence, any pulsatile volume change in the fingertip is due only to arterial perfusion (18). The probe is attached to a pressure transducer and through it to the main system, which amplifies the transducer signal and band-pass filters it in the frequency range of 0.3 to 30 Hz. The system then sends the signal to a dedicated computer, which records the amplitude of each pulse wave as a continuous tracing, providing a measure of the micro-arterial smooth muscle tone in the fingertip. As tone increases, PWA decreases. The PAT and associated hardware is small and portable.
The experiment employed standard laboratory emotional stress procedures (c.f., 4–5; 14–16), and included a resting baseline condition followed by an anger condition. During the resting baseline, the patient was instructed to close their eyes and imagine being in a restful setting. The resting baseline condition lasted for 15 minutes. Approximately 10-minutes into this condition a 4mL blood sample was collected into refrigerated tubes containing reduced glutathione (for catecholamine analysis) and placed on ice.
After completion of the resting condition, the anger condition was initiated. Participants were instructed to recall a recent incident that had made them irritated, aggravated, or frankly angry. They were then instructed to describe this incident in detail to the interviewer, who asked follow-up questions throughout the condition that were designed to make the experience of anger more vivid. Approximately 90 seconds into this 10-minute condition, Tc-99m was injected for assessment of myocardial perfusion during the condition. Approximately 2 minutes into the condition, a 4mL blood sample was collected into refrigerated tubes containing reduced glutathione (for catecholamine analysis) and placed on ice; an additional sample was collected at the end of the task. Upon completion of the condition, all equipment and leads were removed. A SPECT MPI scan was performed approximately 30-minutes later, after which the subject was released to home. Promptly after completion of the study protocol, centrifugation of blood samples was performed at 3000 rpm for 5 minutes, at which time plasma was withdrawn and aliquotted. All aliquots were stored at −80°C until batch analysis.
Measures
Myocardial perfusion images were analyzed in the standard fashion using a previously published Wackers-Liu software package developed at Yale University (21). Two experienced nuclear cardiologists (RS, AS) blindly interpreted all SPECT studies by visual analysis, and each region was coded as normal, reversible or partially reversible (ischemic), or fixed. Disagreements were adjudicated by a third reader (RL).
PWA was recorded continuously throughout the experiment. Heart rate, blood pressure, and ECG were recorded at 5-minute intervals during the resting baseline and at 1-minute intervals during the anger period. Average hemodynamic parameters were calculated for heart rate (HR) and systolic (SBP)/diastolic (DBP) blood pressure during the resting baseline. Rate pressure product (RPP) was determined for each minute of the anger period. Change in HR and SBP/DBP from baseline to anger was calculated by subtracting the baseline average from the reading for the interval with the greatest RPP during anger.
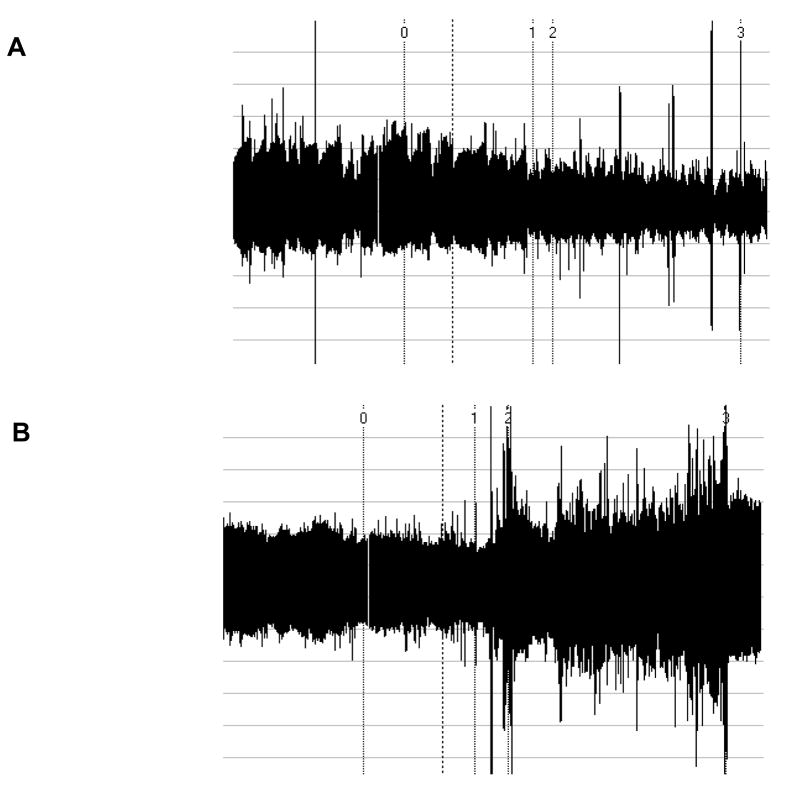
PAT ratio (PWAanger/PWArest), which provides an index of change in microarterial tone during anger stress, was automatically calculated by the computer system. A 1-minute representative segment from the resting baseline period and a 30-second to 1-minute segment with the lowest PWA from the anger period (indicating maximum reaction to the anger condition) was selected for this calculation. Figure 1 displays examples of PWA traces that demonstrate increased and unchanged PAT ratios during anger stress.
Figure 1.
Examples of PWA traces as a function of time. Numeral 0 in each trace indicates the relaxation period and numeral 1 indicates the beginning of the mental stress task. A. Trace resulting in an abnormal PAT ratio, with relative vasoconstriction during mental stress. B. Trace resulting in a normal PAT ratio, with relative vasodilatation during mental stress.
Blood was assayed for levels of epinephrine and norepinephrine, measured using reverse-phase high-performance liquid chromatography (ESA Inc, Chelmsford, MA) and electrochemical detection (Coulochem II) after alumina extraction. The intra-assay coefficient of variation for this method is 1–2%, and the inter-assay coefficient of variation varied from 10% for norepinephrine to 25% for low levels of epinephrine (<25 pg/ml). Samples were run in duplicate for each subject.
Statistical Analysis
MSI was defined by the presence of a new myocardial perfusion defect on SPECT MPI during anger stress, compared to the baseline scan. Participants with (MSI+) vs. without (MSI−) ischemia during anger were compared on age, baseline LVEF, and demographic characteristics. All BP data were normally distributed and comparisons were made by Student’s t test. Nonparametric tests were used for analyses involving HR, RPP, and catecholamine levels due to their significant positive skew. Average PAT ratios are reported as mean ± SEM. All other results are reported as mean ± SD except where data are not normally distributed, in which case they are reported as median (interquartile range).
MSI+ and MSI− groups were compared on PAT ratio by Student’s t test. A receiver operating characteristics (ROC) curve was generated for the relationship between PAT ratio and MSI to find a threshold value for PAT ratio with maxima of sensitivity and specificity. These results were compared with existing findings that suggested a threshold of <0.8 for an abnormal PAT ratio (22). A group of patients at or below this threshold was identified using the results of the ROC curve and analysis. Cross-tabulation of PAT test results with SPECT-MPI results was performed using Fisher’s Exact Test to evaluate significance and concordance of these indices. All analyses were performed using SAS statistical software (23).
RESULTS
Of the total study sample, 34% (26 of 68 patients) demonstrated a new mild (N=22) to moderate (N=4) perfusion defect during anger (MSI+), which is consistent with other studies that have used anger stress (c.f., 8). Demographic information is provided in Table 1. MSI+ patients did not differ significantly from MSI− patients with respect to age, LVEF, medical comorbidity, or cardiovascular medications. HR, SBP, DBP, and RPP, are shown in Table 2. Hemodynamic parameters increased significantly during anger for MSI+ and MSI− groups (P < 0.001), and there was no significant difference between the two groups on these parameters at baseline or during anger.
Table 1.
Demographics of the study group.A
| Variable | Overall (n=68) | MSI+ (n=22) | MSI− (n=46) |
|---|---|---|---|
| Age (years) | 65.9 ± 8.9 | 64.9 ± 6.9 | 66.2 ± 9.7 |
| Female | 8 (12%) | 1 (5%) | 7 (15%) |
| Nonwhite | 11 (16%) | 3 (14%) | 8 (17%) |
| LVEF | 54 (18) | 53 (18) | 54 (17) |
| Comorbidities | |||
| Hypertension | 59 (87%) | 20 (91%) | 39 (85%) |
| Diabetes | 18 (27%) | 8 (36%) | 10 (22%) |
| Hypercholesterolemia | 65 (96%) | 22 (100%) | 43 (94%) |
| Obesity | 23 (34%) | 7 (32%) | 16 (35%) |
| History of Smoking | 45 (66%) | 16 (73%) | 29 (63%) |
| Actively Smoking | 14 (21%) | 2 (9%) | 12 (26%) |
| Medications | |||
| ACE Inhibitor | 35 (52%) | 12 (55%) | 23 (50%) |
| Beta Blocker | 51 (75%) | 16 (73%) | 35 (76%) |
| Calcium Channel Agents | 16 (24%) | 6 (27%) | 10 (22%) |
| Statin | 61 (90%) | 20 (91%) | 41 (89%) |
| Aspirin | 47 (69%) | 15 (68%) | 32 (70%) |
Values are displayed as n (%), mean ± SD (age), or median (interquartile range) (LVEF). There were no significant differences between the ischemic and non-ischemic groups with regard to demographics or comorbidities (P = NS).
Table 2.
Hemodynamics at baseline and during anger recall stress.A
| MSI | PAT | ACE | |||||
|---|---|---|---|---|---|---|---|
| VARIABLE | Overall (n=68) | Positive (n=22) | Negative (n=46) | Positive (n=29) | Negative (n=39) | Positive (n=35) | Negative (n=33) |
| Baseline | |||||||
| HR (bpm) | 57 (11) | 58 (11) | 56 (12) | 55 (10) | 57 (12) | 57 (11) | 56 (13) |
| SBP (mm Hg) | 131 ± 24 | 131 ± 29 | 130 ± 21 | 124 ± 30 | 135 ± 16 | 129 ± 23 | 132 ± 25 |
| DBP (mm Hg) | 74 ± 12 | 73 ± 9 | 74 ± 14 | 71 ± 16 | 75 ± 9 | 74 ± 10 | 73 ± 15 |
| MAP (mm Hg) | 94 ± 11 | 94 ± 12 | 94 ± 10 | 93 ±12 | 95 ± 9 | 93 ± 11 | 95 ± 10 |
| RPP (bpm*mm Hg) | 7166 (2424) | 6862 (3624) | 7183 (1917) | 6912 (2068) | 7680 (2305) | 7068 (2583) | 7400 (2305) |
| Mental Stress | |||||||
| HR (bpm) | 68 (14) | 64 (18) | 68 (11) | 64 (13) | 69 (15) | 67 (16) | 68 (8) |
| SBP (mm Hg) | 162 ± 21 | 161 ± 23 | 162 ± 20 | 161 ± 22 | 163 ± 20 | 161 ± 23 | 163 ± 18 |
| DBP (mm Hg) | 90 ± 11 | 87 ± 12 | 91 ± 10 | 87 ± 12 | 92 ± 10 | 89 ± 12 | 91 ± 9 |
| MAP (mm Hg) | 114 ± 12 | 112 ± 14 | 115 ± 11 | 112 ± 14 | 115 ± 11 | 113 ± 15 | 115 ± 9 |
| RPP (bpm*mm Hg) | 10910 (2697) | 10901 (5424) | 10910 (2454) | 10400 (2527) | 11224 (2659) | 11020 (2870) | 10640 (2522) |
Values are displayed as mean ± SD or median (interquartile range). All groups experienced highly significant increases in all hemodynamic parameters during mental stress compared to baseline conditions (P < 0.001). There were no significant differences between the MSI +/−, PAT +/−, or ACE +/− groups at similar conditions or from baseline to mental stress.
PAT and MSI
The average PAT ratio for the study population was 0.86 ± 0.04, indicating on average, a peripheral vasoconstriction response during anger. MSI+ participants had an average PAT ratio of 0.76 ± 0.04, while MSI− participants had an average PAT ratio of 0.91 ± 0.05 (p=0.03). MSI+ patients therefore demonstrated significantly greater peripheral vasoconstriction during anger stress.
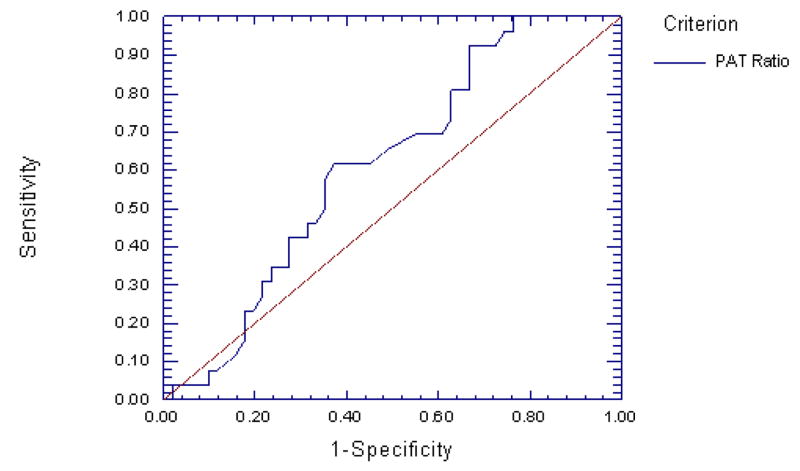
To determine the optimum sensitivity/specificity threshold for PAT as an index of MSI, an ROC curve was generated (Figure 2). The estimated area under the curve (AUC) was 0.613 with a standard error of 0.065 (one-sided p = 0.04). Using the standard approach of selecting the point at which the false positive values begin to exceed the number of false negative values, this curve produced a maximum of sensitivity vs. 1-specificity at a value of 0.78 to define a positive test, with a sensitivity of 0.62 and a specificity of 0.63 at this value. This threshold produced a positive predictive value of 0.46 and a negative predictive value of 0.76. There were no significant differences in AUC when comparing active smoking, obesity, and diabetes.
Figure 2.
ROC curve for PAT ratio predicting mental stress ischemia. The AUC is 0.613 (SE, 0.065, one-sided P = 0.04), indicating that PAT ratio has diagnostic utility in predicting MSI.
The study population was divided into groups at or below (PAT+) and above (PAT−) the PAT ratio threshold of 0.78. PAT+ and PAT− groups demonstrated a significant increase in hemodynamic parameters from baseline to anger stress (p < 0.001), and there was no significant difference between the two groups on these parameters at baseline or during anger (see Table 2). Cross-tabulation of these groups as a function of MSI revealed that 47% of those who were PAT+ were classified as MSI+ and 23% of those who were PAT− were classified as MSI+. We evaluated the concordance in a 2 × 2 contingency table using the Fisher’s test to determine the independence of frequencies. The overall concordance using this approach was 64% (i.e., the test properly diagnosed 64% of the subjects, p=0.05).
PAT, MSI and ACE Inhibition
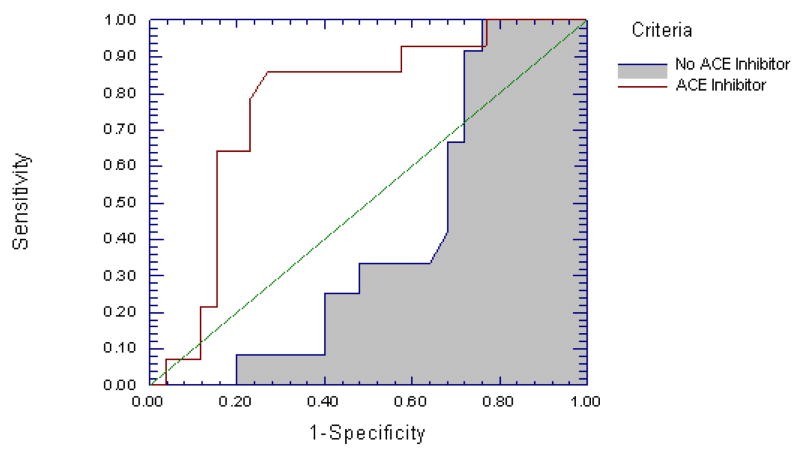
ACE inhibitors are prescribed for patients with cardiovascular disease because they lower arteriolar resistance venous capacitance - they affect microvascular performance. We therefore next generated separate ROC curves for participants taking, and not taking ACE inhibitors. For those on ACE inhibitors the AUC was 0.768 (standard error 0.081, one-sided p < 0.001), versus 0.408 (standard error 0.095, one-sided p = 0.17) for those not on ACE inhibitors (p = 0.004 for difference in AUC, Figure 3). In the group taking ACE inhibitors, the sensitivity and specificity of the test increased to 0.86 and 0.73 (respectively), at the established threshold of 0.78, and the PAT accurately identified 63% of patients as MSI+ (PAT+, MSI+), and 90% as MSI− (PAT−, MSI−) with overall concordance as defined above of 78% (p< 0.001). These values were also maxima for the ROC curve. Despite this difference in the performance of PAT ratio as a test of MSI, there was no difference in average PAT ratio between those taking and not taking ACE inhibitors (0.88 ± 0.07 vs. 0.83 ± 0.04, ns), nor was there a difference in percent of patients who were MSI+ vs. MSI− on this class of medications (see Table 1).
Figure 3.
ROC curves for PAT ratio predicting mental stress ischemia for those who were taking ACE inhibitors versus those who were not. The AUCs are 0.768 (SE = 0.081, one-sided P < 0.001) when ACE inhibitors are present and 0.408 (SE = 0.095, one-sided P = 0.17) when they are absent (P = 0.004 for difference in AUC). PAT ratio performs significantly better as a test for MSI in those who are taking ACE inhibitors.
PAT, Catecholamines, and ACE Inhibition
Norepinephrine and epinephrine at baseline and during anger stress was examined as a function of PAT findings and ACE inhibition. PAT+ and PAT− groups each demonstrated a significant increase in norepinephrine from baseline to anger (see Table 3) however, when examined in light of ACE inhibition, those on ACE inhibitors in the PAT+ group showed this effect while those on ACE inhibitors in the PAT− group did not. In the PAT+ group, patients taking ACE inhibitors also had significantly higher norepinephrine at baseline (327±175 vs. 204±106, p<0.05) and during anger (358±183 vs. 229±90, p<0.03) than patients in this group not taking ACE inhibitors; this difference was not seen in the PAT− group. The PAT+ and PAT− groups also demonstrated a significant increase in epinephrine from baseline to anger stress (see Table 4).
Table 3.
Norepinephrine at baseline and anger recall as a function of ACE inhibition (ACE +/−) and PAT response
| Group | Condition | p Value | |
|---|---|---|---|
| Subgroup | Baseline | Mental Stress | |
| PAT + (n=29) | 267 (156) | 295 (157) | 0.001 |
| PAT − (n=39) | 387 (325) | 400 (247) | 0.04 |
| p value | 0.13 | 0.08 | |
| ACE + (n=35) | 388 (311) | 385 (226) | 0.06 |
| ACE − (n=33) | 281 (212) | 324 (209) | 0.002 |
| p value | .08 | .17 | |
| PAT + (n=29) | |||
| ACE + (n=15) | 327 (175) | 358 (183) | 0.02 |
| ACE − (n=14) | 204 (106) | 229 (90) | 0.05 |
| p value | 0.05 | 0.03 | |
| PAT − (n=39) | |||
| ACE + (n=20) | 435 (381) | 406 (257) | 0.54 |
| ACE − (n=19) | 338 (253) | 394 (244) | 0.03 |
| p value | 0.45 | 0.92 | |
Table 4.
Epinephrine at baseline and anger recall as a function of ACE inhibition (ACE +/−) and PAT response
| Group | Condition | p Value | |
|---|---|---|---|
| Subgroup | Baseline | Mental Stress | |
| PAT + | 19.5 (26.0) | 26.7 (27.4) | 0.02 |
| PAT − | 23.0 (28.4) | 36.6 (49.5) | 0.0009 |
| p value | 0.57 | 0.63 | |
| ACE + | 19.6 (17.5) | 28.6 (25.9) | 0.001 |
| ACE − | 23.5 (35.0) | 36.4 (53.6) | 0.01 |
| p value | 0.83 | 0.80 | |
| PAT + | |||
| ACE + | 15.9 (13.3) | 24.3 (21.9) | 0.14 |
| ACE − | 23.4 (35.2) | 29.2 (33.0) | 0.06 |
| p value | 0.90 | 0.68 | |
| PAT − | |||
| ACE + | 22.4 (20.0) | 31.8 (28.7) | 0.004 |
| ACE − | 23.7 (35.8) | 41.6 (65.3) | 0.09 |
| p value | 0.67 | 0.37 | |
DISCUSSION
In this study of patients with chronic stable CAD, we found that a threshold PAT ratio of 0.78 when paired with ACE inhibitor therapy correctly identified 63% of those subjects who evidenced myocardial ischemia during anger stress - MSI, and 90% of those who did not. The sensitivity and specificity of the test in subjects with ongoing ACE therapy was 0.86 and 0.73 respectively. PAT therefore provided a relatively easy method to identify risk for MSI within this group of patients, and in particular, to identify those not at risk. The availability of easily administered procedures for risk stratification associated with MSI, by overcoming obstacles to the determination of MSI associated risk clinically, could have an important impact on the care of patients with CAD, since MSI is associated with early morbidity (9–11) and 5-year mortality (12). The PAT is FDA approved for the diagnosis of obstructive sleep apnea, and the cost and portability of the device provide for home testing in this regard. If the findings of this study are extended and replicated, PAT technology could lead to routine screening of CAD patients for MSI as a component of primary and secondary prevention. Patients identified as not at risk for MSI could be reassured, while those identified as at risk could be referred for further testing and/or specific interventions such as stress management, that have been found to reduce the occurrence of MSI while improving overall event-free survival, and reducing the costs of cardiologic care (24–25).
This study also contributes to an understanding of the pathophysiology underlying MSI. Prior studies have shown that anger provokes vasoconstriction in epicardial coronary arteries with significant stenotic disease (14; 26), and we previously reported impaired coronary flow reserve in the microvascular bed of patients with stable CAD during laboratory stress (15). The current findings also highlight the importance of vascular performance as a factor underlying MSI, while demonstrating that the peripheral effects of emotional stress can be a useful indicator of comparable effects in the coronary vasculature. In addition, the importance of sympathetic factors was highlighted, since patients who were PAT+ (those who demonstrated a pronounced peripheral vasoconstriction) also demonstrated a neurohormonal profile that was marked by a more robust increase in norepinephrine, compared to those who were PAT− (10.5% vs. 3.3%). This finding is consistent with earlier validation work accomplished with the PAT device (18–19; 22). Acute emotional stress is known to increase neurohormonal output overall, and through sympatho-neuronal processes, of norepinephrine in particular (2; 6; 20). Norepinephrine, as part of the overall response to emotional stress may therefore play an essential role in the provocation of MSI among patients with CAD.
The ability of PAT ratio to predict vulnerability to MSI was heightened among patients taking ACE inhibitors as part of their cardiologic care. The failure of standard pharmacologic agents to protect patients from emotion provoked ischemia in the lab has been described previously for β-blockers, and the failure with regard to that class of agents was thought to be a function of unopposed α-adrenergic effects on the vasculature (5). The response measured by PAT is particularly sensitive to changes in circulating norepinephrine (18–19; 22). Within the PAT+ group, we found significantly higher levels of norepinephrine during both baseline and anger among patients taking ACE inhibitors, compared to patients not on ACE inhibition; this was not the case for the PAT− group. The use of ACE inhibitors results in positive remodeling of the arterial vasculature (c.f., 27–28), a reduction in the bioavailability of angiotensin-II, and an increase in the circulating levels of norepinephrine, which serves to maintain vasomotor tone (c.f., 29–30). Under these conditions the vessel wall may become more sensitive to the influences of norepinephrine. As a result, those patients who increased their norepinephrine during anger may have had a systemic vascular response they were more likely to evidence both a PAT+ response (peripheral vasoconstriction) and an MSI+ response (coronary vasoconstriction). Hence, the increased importance of norepinephrine for maintenance of vascular tone may help reveal both a central (cardiac) and peripheral vulnerability to the effects of emotional stress among patients.
While the results of the current study hold promise for the establishment in the clinical setting of risk stratification by emotional stress testing with an ACE-PAT test, further work is needed. The sample reported here was relatively small, and studies that replicate these findings are needed. These replications must further more clearly determine CAD severity in the study group, and test the effect of ACE inhibitor washout on PAT response and its ability to predict vulnerability to MSI.
In summary, the importance of MSI as a prognostic indicator for major adverse cardiac events and the ability to intervene successfully with patients who demonstrate this form of ischemia highlights the need for a clinically useful tool to assess patient vulnerability. Our preliminary data with the ACE-PAT test shows the potential of this tool for such purposes.
Acknowledgments
This work was supported by R01 awards (HL59619-01 and HL071116-01) from the National Heart, Lung, and Blood Institute, and by a Merit Review award from the Department of Veterans Affairs to Dr Soufer.
Abbreviations
- CAD
Coronary artery disease
- MSI
Mental stress induced ischemia
- SPECT
Single photon emission computed tomography
- PAT
Peripheral arterial tonometry
- PWA
Pulse wave amplitude
- ACE
Angiotensin converting enzyme
- ACS
Acute coronary syndrome
- IV
Intravenous
- MPI
Myocardial perfusion imaging
- SBP/DBP
Systolic/diastolic blood pressure
- HR
Heart rate
- RPP
Rate pressure product
- LVEF
Left ventricular ejection fraction
- ROC
Receiver operating characteristics curve
Footnotes
The authors have no conflicts of interest.
Literature Citations
- 1.Sharkey SW, Lesser JR, Zenovich AG, Maron MS, Lindberg J, Longe TF, Maron BJ. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation. 2005;111:472–9. doi: 10.1161/01.CIR.0000153801.51470.EB. [DOI] [PubMed] [Google Scholar]
- 2.Wittstein IS, Thiemann DR, Lima JAC, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ, Chanmpion HC. Neurohormonal features of myocardial stunning due to sudden emotional stress. New Engl J Med. 2005;352:539–48. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 3.Strike PC, Steptoe A. Behavioral and emotional triggers of acute coronary syndromes: a systematic review and critique. Psychosom Med. 2005;67:179–86. doi: 10.1097/01.psy.0000155663.93160.d2. [DOI] [PubMed] [Google Scholar]
- 4.Lampert R, Joska T, Burg MM, Batsford WP, McPherson CA, Jain D. Emotional and physical precipitants of ventricular arrhythmia. Circulation. 2002;106:1800–05. doi: 10.1161/01.cir.0000031733.51374.c1. [DOI] [PubMed] [Google Scholar]
- 5.Burg MM, Jain D, Soufer R, Kerns RD, Zaret BL. Role of behavioral and psychological factors in mental stress-induced silent left ventricular dysfunction in coronary artery disease. J Am Coll Cardiol. 1993;22:440–8. doi: 10.1016/0735-1097(93)90048-6. [DOI] [PubMed] [Google Scholar]
- 6.Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2192–217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- 7.Schang SJ, Pepine CJ. Transient asymptomatic S-T segment depression during daily activity. Am J Cardiol. 1977;39:396–402. doi: 10.1016/s0002-9149(77)80095-7. [DOI] [PubMed] [Google Scholar]
- 8.Burg MM, Vashist A, Soufer R. Mental stress ischemia: Present status and future goals. J Nucl Cardiol. 2005;12:523–9. doi: 10.1016/j.nuclcard.2005.06.085. [DOI] [PubMed] [Google Scholar]
- 9.Jain D, Burg MM, Soufer R, Zaret BL. Prognostic implications of mental stress induced silent left ventricular dysfunction in patients with stable angina pectoris. Am J Cardiol. 1995;76:31–5. doi: 10.1016/s0002-9149(99)80796-6. [DOI] [PubMed] [Google Scholar]
- 10.Jiang W, Babyak M, Krantz DS, Waugh RA, Coleman RE, hanson MM, Frid DJ, McNulty S, Morris JJ, O’Connor CM, Blumenthal JA. Mental stress-induced myocardial ischemia and cardiac events. JAMA. 1996;275:1651–6. doi: 10.1001/jama.275.21.1651. [DOI] [PubMed] [Google Scholar]
- 11.Krantz DS, Santiago HT, Kop WJ, Bairey-Merz CN, Rozanski A, Gottdeiner JS. Prognostic value of mental stress testing in coronary artery disease. Am J Cardiol. 1999;84:1292–7. doi: 10.1016/s0002-9149(99)00560-3. [DOI] [PubMed] [Google Scholar]
- 13.Sheps DS, McMahon RP, Becker L, Carney RM, Freedland KE, Cohen JD, Sheffield D, Goldberg AD, Ketterer MW, Pepine CJ, Raczynski JM, Light K, Krantz DS, Stone PH, Knatterud GL, Kaufmann PG. Mental stress induced ischemia and all-cause mortality in patients with coronary artery disease. Circulation. 2002;105:1780–4. doi: 10.1161/01.cir.0000014491.90666.06. [DOI] [PubMed] [Google Scholar]
- 14.Yeung AC, Vekshtein VI, Krantz DS, Vita JA, Ryan TJ, Jr, Ganz P, Selwyn AP. The effect of atherosclerosis on the vasomotor response of coronary arteries to mental stress. N Engl J Med. 1991;325:1551–6. doi: 10.1056/NEJM199111283252205. [DOI] [PubMed] [Google Scholar]
- 15.Arrighi JA, Burg M, Cohen IS, Soufer R. Myocardial blood flow response during mental stress in patients with coronary artery disease. Lancet. 2000;356:310–1. doi: 10.1016/S0140-6736(00)02510-1. [DOI] [PubMed] [Google Scholar]
- 16.Ghiadoni L, Donald AE, Cropley M, Mullen MJ, Oakley G, Taylor M, O’Connor G, Betteridge J, Klein N, Steptoe A, Deanfield JE. Mental stress induces transient endothelial dysfunction in humans. Circulation. 2000;102:2473–8. doi: 10.1161/01.cir.102.20.2473. [DOI] [PubMed] [Google Scholar]
- 17.Takase B, Uehata A, Akima T, Nagai T, Nishioka T, Hamabe A, Satomura K, Ohsuzu F, Kurita A. Endothelium-dependent flow-mediated vasodilation in coronary and brachial arteries in suspected coronary artery disease. Am J Cardiol. 1998;82:1535–9. doi: 10.1016/s0002-9149(98)00702-4. [DOI] [PubMed] [Google Scholar]
- 18.Rozanski A, Qureshi E, Bauman M, Reed G, Pillar G, Diamond G. Peripheral arterial responses to treadmill exercise amoung healthy subjects and atherosclerotic patients. Circulation. 2001;103:2084–9. doi: 10.1161/01.cir.103.16.2084. [DOI] [PubMed] [Google Scholar]
- 19.Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP, Karas RH, Udelson JE. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168–74. doi: 10.1016/S0002-8703(03)00094-2. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg AD, Becker LC, Bonsall R, Cohen JD, Ketterer MW, Kaufman PG, Krantz DS, Light KC, McMahon RP, Noreuil T, Pepine CJ, Raczynski J, Stone PH, Strother D, taylor H, Sheps DS. Ischemic, hemodynamic, and neurohormonal responses to mental and exercise stress. Experience from the Psychophysiological Investigations of Myocardial Ischemia Study (PIMI) Circulation. 1996;94:2402–9. doi: 10.1161/01.cir.94.10.2402. [DOI] [PubMed] [Google Scholar]
- 21.Liu YH, Sinusas AJ, Shi CQ, Shen MY, Dione DP, Heller EN, Wackers FJ. Quantification of technetium 99m-labeled sestamibi single-photon emission computed tomography based on mean counts improves accuracy for assessment of relative regional myocardial blood flow: experimental validation in a canine model. J Nucl Cardiol. 1996;3:312–20. doi: 10.1016/s1071-3581(96)90091-4. [DOI] [PubMed] [Google Scholar]
- 22.Chouraqui P, Schnall RP, Dvir I, Rozanski A, Qureshi E, Arditti A, Saef J, Feigin PD, Sheffy J. Peripheral arterial tonometry: A comparison with stress TI-201 SPECT myocardial imaging for detecting myocardial ischemia. J Am Coll Cardiol. 2002;40:2195–200. doi: 10.1016/s0735-1097(02)02591-3. [DOI] [PubMed] [Google Scholar]
- 23.The SAS System for Windows, version 8.02. SAS Institute; Cary, NC: 2006. [Google Scholar]
- 24.Blumenthal JA, Jiang W, Babyak MA, Krantz DS, Frid DJ, Coleman RE, Waugh R, Hanson M, Appelbaum M, o’Connor C, Morris JJ. Stress management and exercise training in cardiac patients with myocardial ischemia. Arch Int Med. 1997;157:2213–23. [PubMed] [Google Scholar]
- 25.Blumenthal JA, Babyak M, Jiang W, Wei J, O’Connor C, Waugh R, Eisenstein E, Mark D, Sherwood A, Woodley PS, Irwin RJ, Reed G. Usefulness of psychosocial treatment of mental stress induced ischemia in men. Am J Cardiol. 2002;89:164–8. doi: 10.1016/s0002-9149(01)02194-4. [DOI] [PubMed] [Google Scholar]
- 26.Boltwood MD, Taylor CB, Burke MB, Grogin H, Giacomini J. Anger report predicts coronary artery vasomotor response to mental stress in atherosclerotic segments. Am J Cardiol. 1993;72:1361–5. doi: 10.1016/0002-9149(93)90180-k. [DOI] [PubMed] [Google Scholar]
- 27.Dzau VJ, Bernstein K, Celermajer D, Cohen J, Dahlof B, Deanfield J, Diez J, Drexler H, Ferrari R, Van Gilst W, Hansson L, Hornig B, Husain A, Johnston C, lazar H, Lonn E, Luscher T, Mancini J, Mimran A, Pepine C, Rabelink T, Remme W, Ruilope L, Ruzicka M, Schunkert H, Swedberg K, Unger T, Vaughan D, Weber M. Pathophysiologic and therapeutic importance of tissue ACE: a consensus report. Cardiovasc Drugs Ther. 2002;16:149–60. doi: 10.1023/a:1015709617405. [DOI] [PubMed] [Google Scholar]
- 28.Peach MJ. Renin-angiotensin system: Biochemistry and mechanisms of action. Physiol Rev. 1977;57:313–70. doi: 10.1152/physrev.1977.57.2.313. [DOI] [PubMed] [Google Scholar]
- 29.Dendorfer A, Thornagel A, Raasch W, Grisk O, Tempel K, Dominiak P. Angiotensin II induces catecholamine release by direct ganglionic excitation. Hypertension. 2002;40:348–54. doi: 10.1161/01.hyp.0000028001.65341.aa. [DOI] [PubMed] [Google Scholar]
- 30.Raasch W, Bartels T, Gieselberg A, Dendorfer A, Dominiak P. Angiotensin I-converting enzyme inhibition increases cardiac catecholamine content and reduces monoamine oxidase activity via an angiotensin type I receptor-mediated mechanism. J Pharmacol Exp Ther. 2002;300:428–34. doi: 10.1124/jpet.300.2.428. [DOI] [PubMed] [Google Scholar]