Abstract
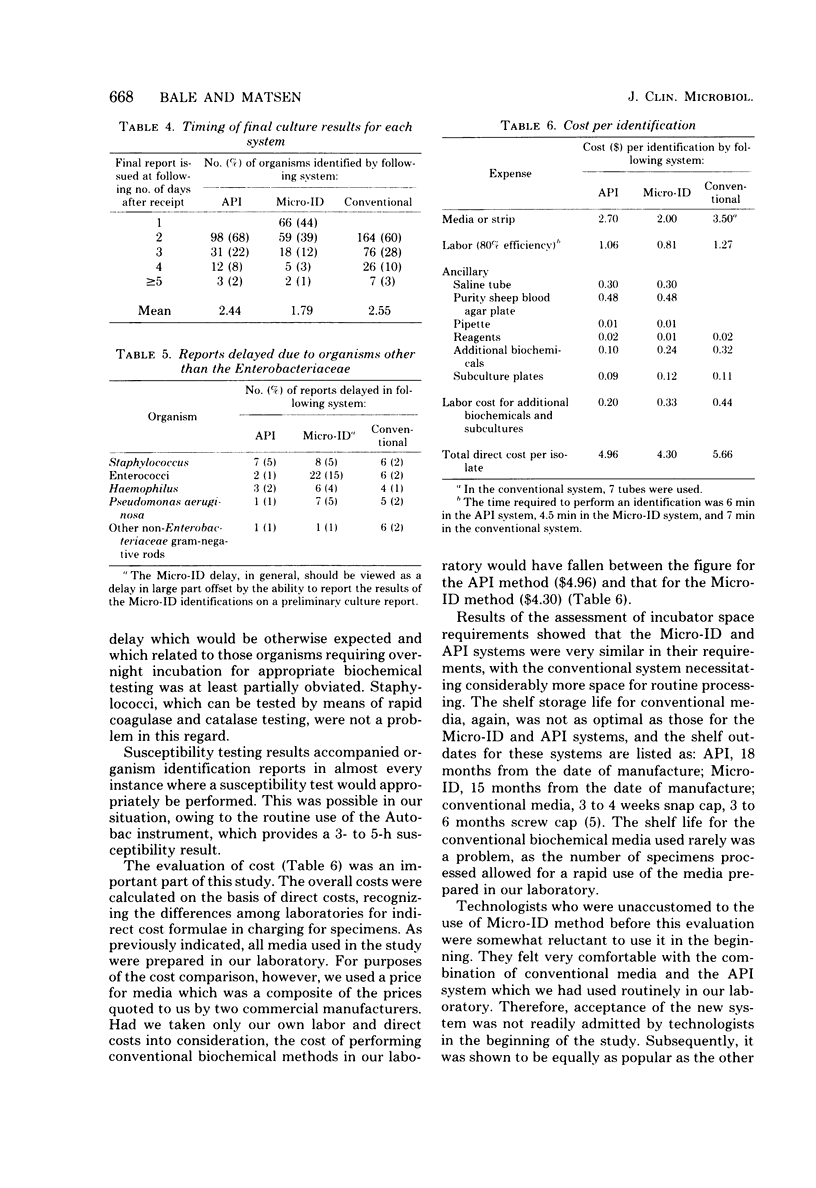
A total of 730 Enterobacteriaceae strains isolated from 567 cultures were evaluated by a rapid kit method (Micro-ID; General Diagnostics, Morris Plains, N.J.; 4 h), an overnight incubation kit method (API 20E; Analytab Products, Plainview, N.Y.), and conventional biochemical test methodology (mostly overnight incubation and some rapid methods) to compare the amount of laboratory effort required, timing, and cost parameters. We assessed the amount of technologist time expended, the time sequence of culture reporting to physicians, the number of isolates requiring repeat testing or additional biochemical testing, the number of cultures held due to the need for identification of other organisms, the cost of total work-up, etc. Cultures evaluated included urines, respiratory cultures, wound cultures, body fluids, genital cultures, and cultures from miscellaneous categories. A total of 64% of the Enterobacteriaceae strains processed by the Micro-ID method could be identified within 24 h of receipt of the specimens in the clinical laboratories, in contrast to the need for an additional day required by the API or conventional biochemical methods. The Micro-ID method also required less technologist time (4.5 min) for set-up and interpretation than did either the API method (6 min) or conventional methods (7 min). Total direct costs (June 1981) per organism identified were: Micro-ID, $4.30; API 20E, $4.96; conventional biochemicals with commercially prepared media, $5.66. An estimate of 80% technologist time efficiency was made in all procedures.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge K. E., Gardner B. B., Clark S. J., Matsen J. M. Comparison of micro-ID, API 20E, and conventional media systems in identification of Enterobacteriaceae. J Clin Microbiol. 1978 Jun;7(6):507–513. doi: 10.1128/jcm.7.6.507-513.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazevic D. J., Mackay D. L., Warwood N. M. Comparison of Micro-ID and API 20E systems for identification of Enterobacteriaceae. J Clin Microbiol. 1979 May;9(5):605–608. doi: 10.1128/jcm.9.5.605-608.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edberg S. C., Atkinson B., Chambers C., Moore M. H., Palumbo L., Zorzon C. F., Singer J. M. Clinical evaluation of the MICRO-ID, API 20E, and conventional media systems for identification of Enterobacteriacea. J Clin Microbiol. 1979 Aug;10(2):161–167. doi: 10.1128/jcm.10.2.161-167.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marymont J. H., 3rd, Marymont J. H., Jr, Gavan T. L. Performance of enterobacteriaceae identification systems. An analysis of College of American Pathologists Survey data. Am J Clin Pathol. 1978 Sep;70(3 Suppl):539–547. [PubMed] [Google Scholar]



