Abstract
siRNA and antisense oligonucleotides, AON, have similar size and negative charge and are often packaged for in vitro delivery with cationic lipids or polymers–but exposed positive charge is problematic in vivo. Here we demonstrate loading and functional delivery of RNAi and AON with non-ionic, nano-transforming polymersomes. These degradable carriers are taken up passively by cultured cells after which the vesicles transform into micelles that allow endolysosomal escape and delivery of either siRNA into cytosol for mRNA knockdown or else AON into the nucleus for exon skipping within pre-mRNA. Polymersome-mediated knockdown appears as efficient as common cationic-lipid transfection and about half as effective as Lentivirus after sustained selection. For AON, initial results also show that intramuscular injection into a mouse model of muscular dystrophy leads to the expected protein expression, which occurs along the entire length of muscle. The lack of cationic groups in antisense polymersomes together with initial tests of efficacy suggests broader utility of these non-viral carriers.
Keywords: Polymersome, siRNA, AON, Antisense, Exon skipping, Muscular dystrophy
1. Introduction
Antisense agents are finding wide application in vitro as tools for discovery of protein function [1] as well as in gene-specific therapies for various diseases [1,2]. Delivery can be a problem even though antisense oligos are far smaller than plasmids. Small interfering RNAs (siRNA) are typically ∼23 bp in length and utilize a cell's own RNA interference pathways to catalyze the degradation of targeted mRNA transcripts [3], whereas antisense oligonucleotides (AON) are single-stranded and can act additionally by altering splicing of pre-mRNA [4]. While many diseases might be approached by direct knockdown, at least one disease that now appears amenable to AON-induced exon skipping is Duchenne Muscular Dystrophy (DMD). Recent cell and animal studies with viruses and various transfection reagents demonstrate that AON-induced splicing of the dystrophin transcript allows expression of this protein that is otherwise blocked by the disease mutations [5-11] in cells cultured from human patients with DMD [12-17] and also in the mdx mouse that bears a nonsense mutation in exon-23 of the dystrophin gene [5,8,9,18]. Efficacy of such therapies has been increased by recent advances in the bio-stability of AONs, with 2′O-methyl modifications defining one important class of particularly stable AONs [19], but stability against degradation does not overcome the significant challenge of efficient delivery of functional RNA molecules into cells either in vitro or in vivo.
In vitro challenges of oligonucleotide delivery typically include cellular uptake and escape from the internalizing endolysosomes. In vivo challenges include avoiding clearance by the liver and spleen and achieving permeation of the target tissue. Encapsulation can in principle provide protection against oligo degradation and clearance as well as supply a means for cell entry and release into the cytosol [5,6,20,21]. Controlled release polymer vesicles or ‘polymersomes’ (Psomes) seem to possess some of the needed features. An aqueous lumen allows for loading, and release can be achieved through either oxidation-sensitive [22] or hydrolysis-sensitive block copolymer amphiphiles [23,24]. With polyethylene glycol (PEG) as a non-ionic hydrophilic chain that minimizes or at least delays clearance-activating interactions with plasma proteins and cells, correctly proportioned PEG-polycaprolactone can assemble into ‘OCL’ polymersomes and PEG-(polylactic acid) can make ‘OLA’ polymersomes that release encapsulants as the polyester chains degrade [25]. Non-ionic polymer vesicles already appear both biocompatible and immunocompatible [23-28] – which are concerns for either viruses or cationic compounds used to deliver nucleic acid. Polymersomes generally circulate in vivo for much longer than lipid vesicles and cationic carriers [25-28]. In addition, copolymer degradation can generate surfactants that promote endolysosomal release as already exploited in the nuclear delivery of a DNA-intercalating drug [25].
Here we describe oligo-loaded, controlled release polymersomes and demonstrate efficient delivery of antisense into cells (Fig. 1). Fluorescent-oligos and fluorescent-copolymer help in visualizing cellular uptake as well as nuclear delivery. Knockdown of the lamin-A/C protein in cultured cancer cells is demonstrated with siRNA-Psomes, whereas with AON-Psomes we demonstrate nuclear localization of an exon-skipping AON as well as expression and suitable membrane-localization of dystrophin in mdx mouse muscle. Expression of dystrophin provides an initial functional readout of AON-directed splicing, while in vitro studies of uptake kinetics and nuclear localization highlight the intrinsic ability of controlled release polymersomes to deliver antisense molecules.
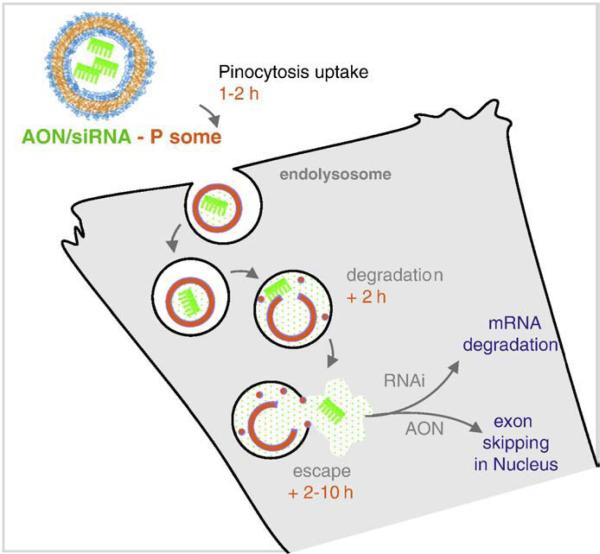
Fig. 1.
Cell uptake and endolysosomal escape of polymersome encapsulated oligonucleotides. Based on recent studies (Ahmed et al. [25]), polymer vesicles of ∼100 nm are taken up non-specifically via pinocytosis over several hours. Controlled release polymer vesicles degrade in the low pH lumen of the lysosome, releasing their payload, and eventually lysing the confining lipid bilayer. The released payload is then free to diffuse through the cell.
2. Materials and methods
2.1. Materials
Dialysis tubing was from Spectrum (Rancho Dominguez, CA), chloroform was from Fisher Scientific (Suwanee, CA), absolute alcohol, DMSO, PKH26 and PKH67 dyes, phosphate buffered saline (PBS) were from Sigma-Aldrich (St. Louis, MO), and tetramethyl rhodomine carboxyl azide (TMRCA), fluorescein-5-carbonyl azide and Alexa Fluor anionic dextran were from Molecular Probes (Eugene, OR).
2.2. Antisense siRNA and AON
Lentiviral shRNA against the human lmna gene targeted the sequence 5′-CGACTGGTGGAGATTGACAAT-3′ (Sigma, TRCN0000061833). FITC labeled duplexed 23mer siRNAs were from Sigma, targeting the sequence 5′-CTGGACTTCCAGAAGAACAT-3′. The AON used in this study was a 5′ 6-FAM labeled 2′O-methyl 20-mer oligoribonucleotide, 5′-UCCAUUCGGCUCCAAACCGG-3′ (Proligo, Boulder, CO). Each base was phosphorothioated and contained a methoxy group at the 2′ carbon so as to reduce nuclease degradation and increase hybridization to the target pre-mRNA [14]. To assess stability of labeled AON, AON-Psomes were incubated at 37 °C for almost 2 weeks and then the mobility of AON was examined by electrophoresis of aliquots (in 2% agarose gel) at day-0, 1, 3, 5, 12; no shifts were seen.
2.3. Polymer vesicle preparation and loading
The Supplement tabulates the various block copolymers PEG-polycaprolactone (OCL), PEG-polylactic acid (OLA), and inert PEG-polybutadiene (OB) similar to those described previously [23] (Table S1). Biodegradable OCL blend polymersomes composed of 25:75 w/w (OB plus OCL) are prepared by mixing solutions of copolymers (up to 5.0 mg/ml) dissolved in DMSO with PBS solution (15:85 v/v). Copolymer solutions were prepared fresh for each use to prevent degradation of OCL or OLA assemblies. At room temperature, copolymer solutions were added slowly to oligonucleotide solutions in DI water to reach the required concentration and vortexed briefly. The mixed solution was dialyzed (3.5 kDa) for 24 h in cold PBS to extract the DMSO, during which time vesicles form. A second dialysis step (300 kDa) is required to remove the free AON/siRNA. Free AON/siRNA was dialyzed in parallel to ensure complete removal. Extrusion through nano-porous filters was used to generate 100-nm vesicles [25,28]. For control studies, negatively charged dextran of similar molecular weight to oligos (10 or 40 kDa) was loaded. Stability of AON loaded vesicles was evaluated as a function of pH (5.5 and 7.4) and temperature (4 and 37 °C). OCL polymersomes had a final concentration of 1 mg/ml copolymer and 2 μg/ml AON. OLA polymersomes had a final concentration of 400 μg/ml copolymer and 6 μg/ml siRNA. Details on fluorescent polymer are in the Supplementary data.
2.4. Quantitation of loading efficiency into polymersomes
Vesicle-encapsulant (siRNA, AON) suspensions were monitored after vesicle formation (DMSO removal) using spectrofluorimetry, both during and after the second dialysis step to remove free encapsulant. The excitation/emission maxima (wavelengths in nm) of FITC-siRNA and -AON are 492/520 and those of TMR-tagged vesicles are 545/578. To establish the dialysis time (at 4 °C), non-degradable vesicles were loaded with the negatively charged FITC-dextran (10 or 40 kDa), and the percentage of the initial 520 emission was measured as dialysis progressed (Fig. S1). Without encapsulation, complete extraction by dialysis was complete within hours; with encapsulation, dialysis was typically extended to 48 h. FITC-siRNA/AON was used to make a calibration curve, so that the encapsulant after the second dialysis step could be determined.
2.5. Cell cultures
All culture reagents were from GIBCO (Grand Island, NY) unless otherwise noted. A549 cells and C2C12 murine skeletal myocytes cells were maintained in maintained in 75-cm2 polystyrene tissue culture flasks. C2C12 growth medium consists of high glucose DMEM supplemented with 20% fetal bovine serum (FBS), 0.5% chick embryo extract, and 0.5% penicillin/streptomycin (P/S) (10,000 units/ml and 10,000 mg/ml, respectively); A549 growth medium consists of Ham's F12 with 10% FBS and 1% P/S. Cells were passaged every 2−3 days. For myotube experiments, micropatterned slides [29] or collagen-coated six well tissue culture plates were seeded with 105 cells. One day later, differentiation media was used (DMEM supplemented with 10% horse serum and 0.5% penicillin/streptomycin), and cells were differentiated for 10 days to obtain mature myotubes. DM medium was replaced every alternate day. AON-loaded polymersomes were added to cultured cells, and after 3 h of incubation the medium was replaced with fresh medium. Imaging details are in the Supplementary data.
2.6. Intramuscular injection in mdx-mice and immunostaining
Tibialis anterior (TA) muscles of mdx mice (6−8weeks of age) were injected at mid-muscle with a 30 μl solution of either free AON (control, 5.0 μg AON total) or AON-polymersome (5.0 μg AON and 1.5 mg/ml polymer concentration). Post-injection, the mice (duplicates) were divided in two groups. One group was sacrificed 12 h later to study AON nuclear delivery and the latter group was sacrificed after 3 weeks for dystrophin expression. TA muscles were snap frozen in OCT medium and stored at −70 °C. Approximately 50 cryo-sections (of 7 μm thickness) were obtained to cover the entire length of each TA muscle. The mouse-on-mouse (MOM) kit from Vector Labs was used for immunostaining with the mouse monoclonal Dys-2 antibody (Novacastra. Newcastle, UK), following the manufacturer's protocol. In brief, tissue sections were fixed in methanol for 1 min, and allowed to air dry. Fixed sections were washed with PBS and incubated for 1 h in a working solution of the MOM Mouse Ig blocking reagent. Sections were washed in PBS and incubated for 1 h with Dys-2 antibody in MOM diluent (1:100). Sections were washed again in PBS, followed by application of MOM biotinylated anti-mouse IgG and fluorochrome avidin-DCS (1:1000). After washing with PBS and counterstaining nuclei with Hoechst 33342, the slides were mounted using gel-mount (Biomedia; Sigma). Similar methods were used in immunostaining muscle sections for the integral membrane protein CD47 [30] with rat-derived mIAP301 (BD Pharmingen).
Images in paired experiments were taken at the same time under constant imaging conditions and constant camera settings (see Supplementary data for microscope details) with analyses done in either ImagePro or Image J (NIH) preserving relative intensities. For analyses of dystrophin expression in muscle sections, images were thresholded by minimizing the variance between the foreground and background, with the threshold selected based on the AON-Psome image and then applied to the free-AON image.
3. Results
3.1. Encapsulation, degradation, and controlled release of oligos by nano-polymersomes
As with lipid vesicles, a variety of methods can be used to encapsulate reagents within polymer vesicles, but methods that are efficient with materials will be dictated by the properties of the block copolymer as well the encapsulant, especially solubility and molecular weight. Both OCL-based polymersomes and OLA polymersomes were formed by a cosolvent dialysis method that exploits DMSO's miscibility with water and its hydrophobic interactions with membrane-forming amphiphiles [31,32]. In brief, copolymer is dissolved in DMSO, oligonucleotides are added in PBS, and vesicle formation proceeds during dialysis (with a 3.5 kDa cut-off membrane) at 4 °C as the DMSO is extracted and the solvent quality for the hydrophobic block is gradually reduced. Unencapsulated ‘free’ oligo is removed in a second step of dialysis (with a 300 kDa cut-off membrane). Some of the OCL based polymersomes are sufficiently large to visualize the encapsulation of fluorescent AON (Fig. 2A), but these are subsequently sized down to cell-deliverable, 100 nm vesicles by extrusion and sterile-filtration through nanoporous filters as routinely done with liposomes. OLA copolymer generates nano-polymersomes during cosolvent dialysis and is seen to colocalize with fluorescently labeled 23-mer siRNA (Fig. 2B).
Fig. 2.
Encapsulation, degradation, release, and lysis of antisense with controlled release polymersomes (Psome). (A) Fluorescence microscope images of AON (green) loaded in biodegradable OCL-based Psome (red, due to fluorescent copolymer) after formation of large unilamellar vesicles. Inset shows edge-bright intensity profile for Psome membrane and the filled lumen profile for AON, indicating a lack of membrane interaction. (B) Fluorescence images of siRNA (green) loaded into fluorescently-labeled OLA nano-polymersomes (red). (C) Transition from vesicles to micelles occurs over tens of hours as 100 nm vesicles are lost and 40 nm micelles emerge. The indicated fit of the decay has a t = 3 h offset to account for stable 100−110 nm vesicles, and then a micellization time constant of t = 2 h. (D) Release kinetics from self-porating vesicles are consistent with degradation kinetics, and increase with increasing temperature; at 4 °C, leakage of oligo is undetectable for days. Release is accelerated under acidic conditions, typical of endolysosomes inside cells. (E) Degradable polymersomes are membrane lytic at concentrations of ∼100 mg/ml, which are attainable in endolysosomes containing one or more polymersomes. Red cell hemolysis is the standard assay used. TX 100 is the non-ionic detergent triton X-100 at just 10 mg/ml and shown here to rapidly lyse membranes compared to lysis times for 100 mg/ml copolymers of 10−20 h. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Quantitation of loading efficiency into the Psomes by spectrofluorimetry reveals up to 30% encapsulation of total added fluorescent siRNA by OLA Psomes and at least 20% loading of fluorescent-AON into OCL Psomes. This encapsulation efficiency is discussed in more depth later but appears similar to that of oligonucleotides in nano-complexes with the commercially available cationic lipid delivery vehicle Lipofectamine 2000 (LF2k) (Table S2) as well as that of other drugs in both polymersomes and liposomes [33,34].
Both OLA and OCL have been shown to yield hydrolytically unstable assemblies, with tunable degradation times that depend on both the chemistry and sizes of the diblock chains [23,35]. Dynamic Light Scattering (DLS) shows that the initial vesicles are 100 nm and indeed sufficiently small for extended circulation as well as efficient cell entry (Fig. 2C), but after 5−6 h at 37 °C, the average hydrodynamic radius of both formulations decreases to ∼60 nm, suggesting a mixture of vesicles and micelles [23,25]. From 18−48 h, this average size decreases to ∼40−50 nm, consistent with a transformation to small, surfactant-like micelles that have presumably released their oligos.
Detailed release kinetics for both OCL and OLA polymersomes has been presented previously [23,36], and so here we confirmed the expected release during dialysis at physiological temperature in neutral or acidic saline, as well as at 4 °C. At low temperatures, these carriers are stable for extended periods, consistent with recent studies [25]. At physiological temperatures the hydrophobic polyester blocks degrade, allowing for release of the encapsulants over hours (Fig. 2D). The kinetics are pH dependent, showing a more rapid release (3 h) at the relevant endolysosomal pH ∼5.0. Compared to recent studies with degradable polymersomes, the release kinetics of AON reported here are more rapid by several-fold and the pH-dependence appears less dramatic [23,25]. The differences likely reflect the use here of lower molecular weight copolymers that yield less robust membranes [37], but the systems here nonetheless prove suited to both in vivo and in vitro delivery of oligonucleotides.
Degradation of the polymersomes not only leads to release of encapsulants, but as previously demonstrated [25], the degradation of the hydrophobic block is critical for release of the encapsulant from the endolysosome: the degraded copolymer – at high concentration – acts effectively as a surfactant to lyse a confining lipid bilayer (Fig. 2E) [38]. Non-degradable vesicles formed completely from PEG-polybutadiene (Table S1) do not lyse cell membranes, but ∼100 mg/ml of both OLA and OCL does lyse cells. Such membrane-lytic concentrations are readily achievable when considering a 200 nm endolysosome surrounding a 100 nm Psome [25,38]. Transient uptake of these degradable polymersomes is thus expected to promote endolysosomal escape and should lead to antisense activity.
3.2. siRNA knockdown of lamin A/C using degradable OLA nano-polymersomes
To study knockdown by siRNA-Psomes in vitro, we used human A549 lung cancer epithelial cells that we had examined previously for both pinocytic uptake and delivery with drug-loaded polymersomes [25,39]. The siRNA used here targeted the mRNA coding for the lamin A/C proteins, which can be important nuclear-structure proteins in differentiated cells but are not essential (i.e. knockdown is not intrinsically cytotoxic). We use here a sequence (see Methods) that has shown efficacy in previous studies of siRNA [40]. After a 6 h (saturating) dose of siRNA loaded OLA-Psomes, delivery of both fluorescent-polymersomes and fluorescent-siRNA was imaged directly via two-color epifluorescence microscopy (Fig. 3A). Copolymer and siRNA appeared to colocalize to an endolysosomal compartment as previously shown with Psome delivered doxorubicin [25].
Fig. 3.
Delivery of siRNA and knockdown of lamin-A/C. (A) A549 cancer cells (outlined) take up fluorescently-labeled polymersomes (red, due to PKH26 dye) loaded with siRNA (green) within several hours. (B,C) Polymersome delivery of siRNA induces knockdown of the lamin A/C proteins just as effectively as Lipofectamine 2000 (LF2k). Naked siRNA does not induce measurable changes in lamin A/C expression, while lentiviral delivery followed by antibiotic selection results in about 2-fold greater knockdown.
Functional knockdown of the lamin A/C proteins was assessed in confluent cultures by both indirect immunofluorescence (Fig. 3B), as well as with a fluorescent microplate reader (Fig. 3C). For comparison, we use the highly effective transfection reagent LF2k as well as viral delivery of an shRNA using lentiviral particles followed by antibiotic selection with puromycin. For quantitation by the plate reader, total fluorescence from Hoechst-stained nuclei was used to normalize the immunofluorescence signal, and a baseline was established by subtracting signal from immunostaining with only secondary antibody. None of the treatments showed any signs of cytotoxicity at the doses used, which for degradable Psomes is consistent with past reports that show only the high concentrations achieved within an endolysosome (∼100 mg/ml) are lytic [23].
As expected, naked siRNA shows no knockdown of the lamin A/C proteins relative to untreated control cultures – both by imaging and by the plate reader. On the other hand, lentiviral delivery of shRNA followed by selection establishes a maximum for RNAi induced silencing of lamin A/C, with a knockdown of 70−90%. OLA-Psomes show a knockdown of 40% that is equal in efficiency to LF2 k. While the latter cationic system has been extensively optimized for in vitro delivery of DNA and RNA, a similar previous formulation, Lipofectamine™, has had few if any applications in vivo [41] whereas tail-vein injections of dual-drug loaded Psomes have been shown to effectively shrink tumors [25].
3.3. In vitro uptake of polymersomes by myotubes
Compared to plasmids intended for gene therapy, antisense oligos that might be used in gene repair are relatively small molecules and their ability to target gene transcripts irrespective of size is a particular advantage for a target such as the pre-mRNA coding for the muscle protein dystrophin, which is the largest gene known [7,9,13,42].
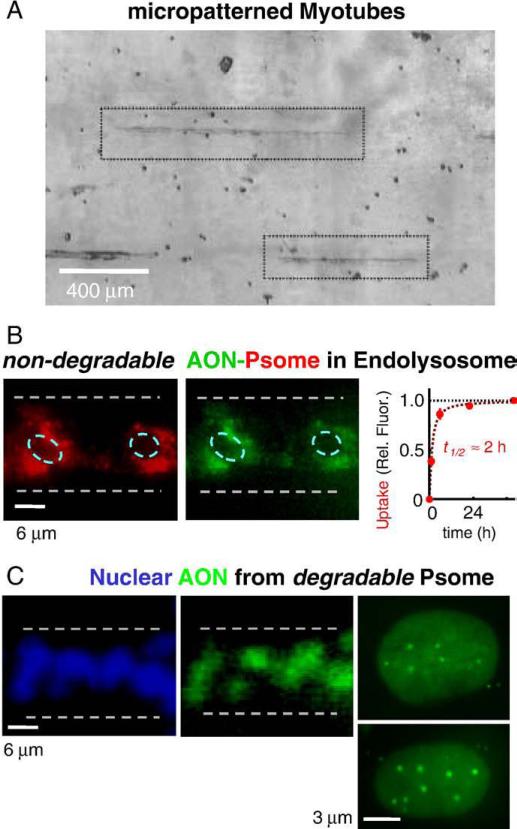
To begin our muscle delivery studies, mouse-derived C2C12 muscle cells were grown and differentiated on micro-patterned collagen strips [29], thereby generating a sparse monolayer of well-separated myotubes that could be clearly visualized (Fig. 4A). Quantitation of the uptake kinetics of non-degradable PEO-PBD polymersomes into such patterned myotubes is simplified relative to the typical high density cultures with multi-layers of myotubes. Saturation in vesicle uptake occurred within hours, with a time constant of 1.5 h, and perinuclear localization was sustained for at least 4 days (Fig. 4B) with no signs of apoptosis or necrosis. Similar to early times points for the OLA-Psomes in the cancer cells, these inert vesicles appeared as perinuclear punctates with no nuclear localization, consistent with Psome or nanoparticle localization to endolysosomes (e.g.[25,43]).
Fig. 4.
Uptake of degradable and nondegradable polymersomes in micropatterned C2C12 myotubes. (A) Micro patterning of myotubes minimizes cell layering and provides well-defined geometries for studying cellular uptake and localization. (B) Non-degradable PEG-PBD vesicles (red, due to fluorescent copolymer) were taken up by quiescent myotubes over a few hours, and showed a perinuclear localization for several days. (C) Biodegradable OCL-Psomes showed efficient nuclear delivery of fluorescent-AON within 24 h. High magnification images of myonuclei show clear and diffuse nuclear uptake as well as a few punctuate localizations. The diffuse AON is highly mobile, while punctates show little recovery after FRAP (see Fig. S2).
3.4. Degradable polymersomes foster delivery and release of AON with nuclear localization
As emphasized previously, degradable copolymers lead not only to release of encapsulants (Figs. 1 and 2), but degraded copolymer at high concentrations acts as an effective surfactant to porate lipid membranes so that uptake is followed by endolysosomal escape. With the OCL-based Psomes, entry into myotubes occurred within 1.5 h and escape occurred after another ∼2−4 h or more, with localization of fluorescent-AON to the nucleus (Fig. 4C; Fig. S2A). As expected, free AON did not show any significant nuclear delivery into cultured myotubes, and it was also verified – with both the non-dividing myotubes and the proliferating A549 cells – that AON added together with but not encapsulated within degradable Psomes does not lead to cell uptake (Fig. S3). Positive nuclei were seen with standard in vitro delivery by PEI (polyethylene imine)-complexed AON, which presumably exploits a cationic sponge effect to escape endolysosomes.
High magnification images of AON-positive fluorescent nuclei (Fig. 4C, rightmost panels) show a broadly diffuse nucleoplasm and 3 to 20 bright accumulations that appear similar to previous reports with fluorescent RNAs [44]. To clarify the nature of the interactions of these diffuse and localized pools, the mobility of AON was assessed by Fluorescence Recovery After Photobleaching (FRAP), using a pulsed dye laser for rapid bleaching. With the diffuse AON (red circle in Fig. S2), FRAP shows essentially complete recovery of fluorescence within about 5 s and a t1/2 ∼2 s. In contrast, recovery after FRAP of fluorescent-AON within the nuclear bodies (blue circle) is minimal and indicates strong binding. Since base pairing interactions are generally temperature dependent, we also compared diffusion at 22 °C to 42 °C, but no difference was measured. As discussed below, the FRAP studies clearly establish dynamic localization of AON within the nucleoplasm, which is at least consistent with the need for on–off binding to effect function on the pre-mRNA target.
3.5. Efficient delivery of AON-polymersomes in mdx mice
AON has recently been demonstrated to facilitate exon skipping of targeted pre-mRNAs, which provides an exciting alternative to therapy by full gene replacement – especially for large genes such as dystrophin. In this therapeutic modality delivered AON binds to specifically targeted splicing sites, preventing the incorporation of the targeted exon(s) into the mature mRNA (Fig. 5A). Careful selection of the skipped exons can yield a truncated, but in frame, gene product that provides sufficient rescue of function [9,10].
Fig. 5.
Intramuscular AON delivery and Dystrophin expression in mdx mice. (A) Mechanism of action of exon-skipping AON: after endolyosomal escape, AON enters the nucleus and binds target premRNA at spliceosomal recognition sites. This causes the splicosomal machinery to fail at these sites, resulting in an mRNA that lacks the targeted exons which are degraded along with introns. (B) Nuclear delivery of Psome-AON or free AON (green) into nuclei (blue) of TA muscle sections 12 h postinjection. (C) Indirect immunofluorescence images of dystrophin expression (Dys-2 antibody) in the midsections of TA muscles from mdx mice 3 weeks post intramuscular injections. Polymersome delivered AON induces visibly more expression of dystrophin than free AON. (D) Low magnification collages of original and binarized mid- or end-sections that have been thresholded to illustrate the %-cross sectional area of dystrophin staining in polymersome-AON and free AON treated muscles (at least 5 random sections were analyzed per mouse with <5% variation). Psome-AON treated muscle shows roughly twice the dystrophin positive area (see Materials and methods for details).
To evaluate whether the controlled release Psomes introduced above would allow AON delivery in vivo, we examined the intramuscular delivery of encapsulated versus free AON in mdx mice. The AON sequence used here was selected based on prior work with isolated muscle fibers, and is reported in Materials and methods [45]. The dystrophin-deficient mdx mouse is a widely used animal model for muscular dystrophy as it has a tissue pathophysiology similar to human muscular dystrophy, particularly in sharing leaky plasma membranes [46]. In addition, the mdx mouse expresses a dystrophin pre-mRNA with a premature stop codon in exon-23, which makes this one exon a target for skipping; subsequent protein expression then provides a functional test for AON delivery. Intramuscular injections also test principles of delivery such as dispersion separate from issues of in vivo circulation and tissue targeting [47]. In fact, free AON does not circulate more than a few minutes following systemic injection although polymersomes can circulate for many hours [28,48].
Following intramuscular injection into tibialis anterior (TA) muscle, nuclear localization of polymersome-delivered AON was readily apparent in muscle within 12 h (Fig. 5B) whereas free AON showed relatively little evidence of nuclear localization. Delivery efficiency was quantified by calculating the ratio, defined by an intensity threshold, of AON positive nuclei to the total number of Hoechst labeled nuclei (∼10,000 nuclei counted in randomly selected fields from at least a dozen sections from each animal injected). AON-Psomes gave a mean delivery efficiency of over 50% and showed a relatively even distribution along the entire muscle length, while using a relatively low dose of AON compared to past studies [9]. In contrast, free AON showed less than 10% efficiency and appeared primarily localized to the nuclei of mid-section muscle in close proximity to the injection site.
3.6. Dystrophin expression in mdx mice with AON-polymersomes
Three weeks after a single intramuscular injection of the AON formulations, dystrophin expression was visualized by indirect immunostaining. If the AON delivered here successfully skips the defective exon 23 in the mdx mouse, then a protein that is only 71 amino acids shorter than full-length dystrophin is expected to be expressed [9,45]. In this initial characterization, immunodetection of dystrophin protein was done on various muscle sections along the length of the muscle using antibodies that recognize sites distal to the induced splice sites.
Dystrophin expression after delivery of AON-Psomes was visible across muscle sections at the periphery of myotubes, qualitatively similar to that of normal muscle (Fig. 5C), similar to that of the ubiquitous integral membrane protein CD47 (Fig. S4), and similar also to that seen in other recent studies of normal or diseased mouse muscle [49,50]. These high magnification images of the wild type control and AON-Psome treated mdx muscle are representative in showing unstained gaps between adjacent myotubes, consistent with immunostaining of dystrophin at the membrane within the cells rather than non-specific staining of the extracellular matrix. In comparison, untreated sections stained with secondary antibody alone, as well as primary and secondary antibody, showed no systematic immunostaining of dystrophin at the myotube membrane (Fig. S4B). Diffuse staining was also seen in muscle sections after injection with either empty vesicles or free AON; the latter treatment, although not optimized here, tended to show some membrane staining as in past reports [10].
The muscle mid-section was the site of injection, and so to assess biodistribution along the length of the muscle, sections from along the entire muscle length were immunostained, imaged and quantified. Specifically, 16-bit images of muscle sections imaged under identical setting were binarized to estimate the percent area that appears dystrophin positive (Fig. 5D, S5). Membrane-localized dystrophin expression was observed not only across the muscle mid-section but also toward the ends of the muscles with AON-Psomes (Fig. 5D, top panels), and the total area of dystrophin positive muscle with AON-Psomes was consistently near ∼35% area – about twice that of the free AON control (15−20%). Further efforts to count distinctly circumscribed muscle fibers (∼4000 muscle fibers for AON-Psomes; two-person, double-blind assessment) suggested 3-fold more positive fibers than with free-AON in an equal number of randomly chosen sections spanning similar cross-sectional areas.
The broad distribution of dystrophin expression throughout the muscle suggests these stealthy degradable polymersomes enhance the perfusion of AON along the entire muscle length compared to previous studies using AON alone [5,7]. The nontoxic biocompatibility of the copolymer is also evident in the lack of obvious degeneration observed in polymer treated mdx muscles compared to polymer free controls. Free AON samples show in an intensity analysis that some dystrophin expression occurs above uninjected controls, but the brightest areas of immunostaining generally occurred in the regions of the ‘needle track’ where the muscle cell membranes were locally disrupted. In comparison, delivery of AON with degradable polymersomes provides significant dystrophin expression, indicating the effective delivery without active disruption of the cell membrane.
4. Discussion
Non-viral delivery of antisense molecules for therapeutic purposes has been greatly hampered by the lack of a suitable in vivo delivery system. One recent study [51] highlights the problems involved with delivery of naked dsRNAs: in a mouse model of age-related macular degeneration (AMD), which is caused by aberrant growth of blood vessels across the retina, a host of non-specific dsRNA molecules were found to reduce vascularization to the same extent as a targeted siRNA. The authors identified this as an immune response induced by activation of a toll-like receptor present in the tissue, a potential hazard for in vivo delivery of siRNAs in other tissues as well. Carriers are necessary to overcome this; and the most common systems to date involve the complexation of DNA/RNA with charged lipids (lipoplexes), charged polymers (polyplexes, such as PEI), and surfactants (such as Pluronic F-127). Highly charged carrier systems are problematic due to rapid clearance from the systemic circulation as well as inflammatory reactions [8,11]. While PEG-ylated PEI carriers show high transfection capacity for AON delivery in vitro and are apparently non-toxic both in vitro [45] and also after intramuscular injection in vivo [7], even the small surface charge of these PEG–PEI complexes will likely limit circulation and perfusion into large muscles.
PEG-based Pluronic F-127 is one other non-ionic carrier system for oligos that has shown some success following both systemic and intramuscular injections. Delivery of AON with this carrier reportedly yields dystrophin-positive fibers throughout the skeletal muscles of the mdx mouse including the hind-limb muscle [9,10]. Whether antisense-Pluronic complexes are vesicles or some other type of micellar assembly appears unclear in the cited studies, but it is also true that degradable Psomes here generate micelles that – at high concentration achievable after cell uptake – have important bioactive function in endolysosomal escape of AON (Fig. 4C). More recently, the cationic diblock copolymer PBD-P4VPQI was used to encapsulate plasmid DNA for in vitro delivery, but despite successful encapsulation, the highly charged nature of the copolymer results in a non-viable in vivo delivery system [52]. Our use here of non-ionic degradable biocompatible nano-polymersomes represents a proof of principle for the suitability of such vehicles in the delivery of antisense molecules in vivo.
100% PEG-ylated, non-ionic block copolymers have been shown by many groups to reproducibly generate – through various formation processes – a number of well-defined nano-morphologies, including vesicles. Here, co-entrapment of 20−30% of added oligonucleotides (Fig. 2A,B) at the end of Psome formation could proceed, we believe, through vesicular, emulsion-like intermediates. If a spherical vesicle is DMSO-expanded to 200 nm radius with DMSO-thinned walls (∼5 nm, instead of the typical ∼10 nm per [25]), the relative volume entrapped per polymer is (lumen volume)/(wall volume)=2003/(2053−2003)≈13, and so 5 mg/ml copolymer (∼0.5% v/v) as used here (see Materials and methods) would entrap 6.5% of a homogeneous solution. Extraction of DMSO would tend to decrease vesicle radius and thicken the wall (both two-fold), but entrapment of 20−30% added antisense requires an additional mechanism of 3 to 5 fold enrichment. Because DMSO denatures nucleic acid structure (suppressing DNA melting temperature, Tm, by 20 °C for 15% v/v solutions[53]) and also leads to denser solutions of nucleic acid (by 3% for 15% v/v), amphiphile-stabilized inhomogeneities of concentrated oligonucleotides seem plausible in the quaternary mixtures here of {water/DMSO/copolymer/oligonucleotide}–complexation has indeed been reported recently in a {water/DMSO/polysaccharide/oligonucleotide} system [54]. DMSO is also known to unfold proteins and drive their precipitation [55]. Inhomogeneities are thus postulated here to template membranes and nucleate vesicles better than solution, with an estimated difference in nucleation free energies of only ΔΔG=–kBT logn(3 to 5)≈−1.1 to −1.6 kBT. While the proposed mechanisms motivate a future, detailed study, aggregates of at least one concentrated encapsulant (doxorubicin) within Psomes have been visualized by electron microscopy following a pH-dependent precipitation method [25].
Control over the release kinetics of both hydrophobic and hydrophilic payloads can also be achieved by altering the physicochemical properties of the parental diblocks. Here, the capacity for controlled vesicle degradation was exploited to maintain carrier integrity during the hours necessary for cellular uptake, followed by degradation inside the cell and subsequent release from the endolysosomal pathway into the cytosol. The surfactant generated by the degradation of the carrier provides a means of escape of the payload from the confining endolysosomal compartment and facilitates the desired spatial relocalization of AON to the nucleus as well as functionally active siRNA in the cytosol. In the nucleus, the dynamics of the nucleoplasmic pool of the dystrophin-directed AON studied by spot-FRAP show that AON is almost entirely mobile with a t1/2∼2 s corresponding to a diffusion constant of Ddys-AON≅0.15 μm2/s per a previously established method [56]. Diffusion times of small molecules in the nucleus vary depending on the probes used as well as the measurement method; Molenaar et al [44] used 2′OMe(U)22 probes to the poly(A)+ tail of nuclear mRNAs and found DAAA-AON≈0.2 Ddys-AON, whereas negative control 2′OMe(A)18 against a non-expressed (HCMV) mRNA diffuses considerably faster with Dfree-AON≈11 Ddys-AON. Our results therefore seem consistent with some level of AON binding pre-mRNA.
Toxicity of in vivo drug carrier systems is always a potential concern. Previous studies with very similar polymersome systems as here indicate a maximum tolerated dose that exceeds 35 mg/ml after systemic injection and no measurable cytotoxicity to either cardiovascular or muscle cells in vitro with C2C12 skeletal muscle cells, BAEC endothelial cells, A7r5 aortic smooth muscle cells [25,57]. It is also important to note that in the in vivo studies presented here, the final concentration of copolymer injected into mdx mice was comparatively low (at 1 mg/ml), and as such more polymersomes could certainly be injected to attain either a higher dose or protracted release [25], avoiding multiple injections and toxicity to tissues [23,25]. Here we show that long-circulating Psomes might be advantageous for other methods of delivery such as intramuscular injection. Unlike PEG–PEI complexes or free AON, polymersomes appear capable of effecting stealthy perfusion throughout the injected muscle with subsequent induction of dystrophin expression efficiently along the entire muscle. Therapeutic exon-splicing seems promising with recent human studies from others already showing detectable – but relatively limited – effects after free AON was injected into the TA muscle [58]. More generally, achieving spatially distributed expression with high efficiency of functional activity and no toxicity would seem to be at least three key attributes for any antisense carrier system.
5. Conclusion
Polymersomes have been shown here to deliver functional siRNA into cancer cells in vitro as well as AON into muscle in vivo, appearing similar in pathways to polymersomes that deliver chemotherapeutic agents [25]. Further structure-function studies of the particular muscle disease model used here are certainly needed, but the broader goal here was to illustrate the potential for encapsulation and intracellular delivery of the various types of antisense oligonucleotides with non-ionic, controlled release nano-polymersomes.
Supplementary Material
Supplemental Tables
Table S1 Properties of degradable and non-degradable diblock copolymer amphiphiles.
Table S2 Mean hydrodynamic radius of aggregates, RNAi loading efficiency, and range of Zeta Potentials.
Supplemental Methods
2.7. Labeling of polymersomes For tracking copolymer fate in cells the fluorophore tetramethyrhodamine (TMR) was chemically conjugated to OB copolymers. The hydroxyl end of the PEG was covalently attached to TMR through a rearrangement of an acyl azide [29]. Briefly, a TMR acyl azide (Molecular Probes) was heated in toluene at 80 °C to cause rearrangement to an isocyanate. Simultaneously, 0.5 mg of OB was added in a molar ratio of 10:1 dye: copolymer for 12 h. 20 mg of NH3OH was then added to the stirred solution for 2 h to deprotect the nonfluorescent urethane derivative, which turned the solution color from pink to a deep red. For cell culture studies, polymersomes were alternatively labeled by adding the membrane-labeling fluorescent dyes PKH26 or PKH67 directly to a vesicle suspension.
2.8. Fluorescence microscopy, FRAP, and spectrofluorimetry Morphological and quantitative fluorescent measurements of vesicles, cells and tissue sections were made after imaging with an Olympus IX71 inverted fluorescence microscope with a 20x air and a 60x oil objective and a Cascade CCD camera [30]. Photo-bleaching studies were conducted using a pulsed dye laser (Photonic Instruments, St. Charles, IL), on a NIKON TE300 inverted fluorescence microscope, imaged with a 60x oil immersion objective. Image J was used for image analysis. The reported fluorescence recovery curves have been normalized to correct for photo bleaching during data collection.
Supplemental Figures and Legends
Figure S1. Measurements of retained anionic dextrans during dialysis after formation of polymersomes (see Methods). Closed points are for dialysis into DI water and open points are for PBS. Fits to y = (100% - A) + A(1-exp(-t/τ)) yield τ ∼ 6−12 hr, which indicates that 48 h of dialysis should extract >98% of the polymer.
Figure S2. FRAP measurements of dystrophin-specific fluorescent-AON within C2C12 muscle nuclei.
Figure S3. Delivery of FITC-AON into myotubes (see Fig.4) is successful when complexed with PEI (Exgen 500™; Fermentas, Inc., Hanover, MD) but not when delivered as free AON or with non-encapsulating, degradable Psomes. Similar images were obtained at 6 h and 20 h as well as with A549 cells.
Figure S4. Immunostaining controls for dystrophin in muscle sections. (A) Co-staining for the ubiquitous integral membrane protein, CD47. (B) Non-specific background with secondary antibody.
Figure S5. Further examples of treated mdx immunostaining, imaging, binarization, and quantitation. (A) Image analysis, and (B) Dystrophin positive membranes relative to free AON.
Acknowledgements
This work was funded by grants from NIH-NIBIB/NIAMS, MDA, NSF, and Penn-Drexel NTI.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.jconrel.2008.10.020.
References
- 1.Dillon CP, Sandy P, Nencioni A, Kissler S, Rubinson DA, Van Parijs L. Rnai as an experimental and therapeutic tool to study and regulate physiological and disease processes. Annual Review of Physiology. 2005;67(1):147–173. doi: 10.1146/annurev.physiol.67.040403.130716. [DOI] [PubMed] [Google Scholar]
- 2.Lu PY, Xie F, Woodle MC, Leaf Huang M-CHAEW. Advances in Genetics. Vol. 54. Academic Press; 2005. In vivo application of RNA interference: from functional genomics to therapeutics; pp. 115–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 4.Lavrovsky Y, Chen S, Roy AK. Therapeutic potential and mechanism of action of oligonucleotides and ribozymes. Biochemical and Molecular Medicine. 1997;62(1):11–22. doi: 10.1006/bmme.1997.2631. [DOI] [PubMed] [Google Scholar]
- 5.Mann CJ, Honeyman K, Cheng AJ, Ly T, Lloyd F, Wilton SD. Antisense-induced exon skipping and synthesis of dystrophin in the mdx mice. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:42–47. doi: 10.1073/pnas.011408598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wells KE, Fletcher S, Mann CJ, Wilton SD, Wells DJ. Enhanced in vivo delivery of antisense oligonucleotides to restore dystrophin expression in adult mdx mouse muscle. FEBS Letters. 2003;552:145. doi: 10.1016/s0014-5793(03)00904-9. [DOI] [PubMed] [Google Scholar]
- 7.Williams JH, Sirsi SR, Latta DR, Lutz GJ. Induction of dystrophin expression by exon skipping in mdx mice following intramuscular injection of antisense oligonucleotides complexed with PEG–PEI copolymers. Molecular Therapy. 2006;14(1):88–96. doi: 10.1016/j.ymthe.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 8.Bremmer-Bout M, Aartsma-Rus A, Meijer EJD, Kaman WE, Janson AAM, Vossen RHAM, Ommen G-JBV, Dunnen JTD, Deutekom JCTV. Targeted exon skipping in transgenic hDMD mice: a model for direct preclinical screening of human-specific antisense oligonucleotides. Molecular Therapy. 2004;10(2):232–240. doi: 10.1016/j.ymthe.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 9.Lu QL, Mann CJ, Lou F, Bou-Gharios G, Morris GE, Xue S-A, Fletcher S, Partridge TA, Wilton SD. Functional amounts of dystrophin produced by skipping the mutated exon in the mdx dystrophic mouse. Nature Medicine. 2003;9:1009–1014. doi: 10.1038/nm897. [DOI] [PubMed] [Google Scholar]
- 10.Lu QL, Rabinowitz A, Chen YC, Yokota T, Yin H, Alter J, Jadoon A, Bou-Gharios G, Partridge T. Systemic delivery of antisense oligoribonucleotide restores dystrophin expression in body-wide skeletal muscles. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(1):198–203. doi: 10.1073/pnas.0406700102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoen PACT, Wees CGCVD, Aartsma-Rus A, Turk R, Goyenvalle A, Danos O, Garcia L, Ommen G-JBV, Dunnen JTD, Deutekom JCTV. Gene expression profiling to monitor therapeutic and adverse effects of antisense therapies for Duchenne muscular dystrophy. Pharmacogenomics. 2006;7(3):281–297. doi: 10.2217/14622416.7.3.281. [DOI] [PubMed] [Google Scholar]
- 12.Aartsma-Rus A, Bremmer-Bout M, Janson AA, Dunnen JTD, G.J. VO, J.C. VD. Targeted exon skipping as a potential gene correction therapy for Duchenne muscular dystrophy. Neuromuscular Disorders. 2002;12(Suppl 1):S71–S77. doi: 10.1016/s0960-8966(02)00086-x. [DOI] [PubMed] [Google Scholar]
- 13.Aartsma-Rus A, Janson AAM, Kaman WE, Bremmer-Bout M, Van Ommen G-JB, Den Dunnen JT, Van Deutekom JCT. Antisense-induced multiexon skipping for duchenne muscular dystrophy makes more sense. The American Journal of Human Genetics. 2004;74:83–92. doi: 10.1086/381039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aartsma-Rus A, Janson AAM, Kaman WE, Bremmer-Bout M, Dunnen JTD, Baas F, Ommen G-JBV, Deutekom JCTV. Therapeutic antisense-induced exon skipping in cultured muscle cells from six different DMD patients. Human Molecular Genetics. 2003;12(8):907–914. doi: 10.1093/hmg/ddg100. [DOI] [PubMed] [Google Scholar]
- 15.Van Deutekom JC, Bremmer-Bout M, Janson AA, Ginjaar IB, Baas F, Dunnen JTD, Ommen GJV. Antisense-induced exon skipping restores dystrophin expression in DMD patient derived muscle cells. Human Molecular Genetics. 2001;10(15):1547–1554. doi: 10.1093/hmg/10.15.1547. [DOI] [PubMed] [Google Scholar]
- 16.Tidball JG, Spencer MJ. Skipping to new gene therapies for muscular dystrophy. Nature Medicine. 2003;9:997–998. doi: 10.1038/nm0803-997. [DOI] [PubMed] [Google Scholar]
- 17.Wilton SD, Fletcher S. Modification of pre-mRNA processing: application to dystrophin expression. Current Opinion in Molecular Therapeutics. 2006;8(2):130–135. [PubMed] [Google Scholar]
- 18.Alter J, Lou F, Rabinowitz A, Yin H, Rosenfeld J, Wilton SD, Partridge TA, Lu QL. Systemic delivery of morpholino oligonucleotide restores dystrophin expression bodywide and improves dystrophin pathology. Nature Medicine. 2006;12(2):175–176. doi: 10.1038/nm1345. [DOI] [PubMed] [Google Scholar]
- 19.Kurreck J. Antisense technologies: Improvement through novel chemical modifications. European Journal of Biochemistry. 2003;270:1628–1644. doi: 10.1046/j.1432-1033.2003.03555.x. [DOI] [PubMed] [Google Scholar]
- 20.Dass CR. Vehicles for oligonucleotide delivery to tumours. Journal of Pharmacy and Pharmacology. 2002;54:3–27. doi: 10.1211/0022357021771887. [DOI] [PubMed] [Google Scholar]
- 21.Lorenz P, Baker BF, Bennett CF, Spector DL. Phosphorothioate antisense oligonucleotides induce the formation of nuclear bodies. Molecular Biology of the Cell. 1998;9:1007–1023. doi: 10.1091/mbc.9.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Napoli A, Valentini M, Tirelli N, Müller M, Hubbell JA. Oxidation-responsive polymeric vesicles. Nature Materials. 2004;3:183–189. doi: 10.1038/nmat1081. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed F, Discher DE. Self-porating polymersomes of PEG-PLA and PEG-PCL: hydrolysis-triggered controlled release vesicles. Journal of Controlled Release. 2004;96(1):37–53. doi: 10.1016/j.jconrel.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 24.Discher DE, Eisenberg A. Polymer vesicles. Science. 2002;297(5583):967–973. doi: 10.1126/science.1074972. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed F, Pakunlu RI, Srinivas G, Brannan A, Bates F, Klein ML, Minko T, Discher DE. Shrinkage of a rapidly growing tumor by drug-loaded polymersomes: pH-triggered release through copolymer degradation. Molecular Pharmaceutics. 2006;3(3):340–350. doi: 10.1021/mp050103u. [DOI] [PubMed] [Google Scholar]
- 26.Roth CM, Sundaram S. Engineering synthetic vectors for improved DNA delivery: insights from intracellular pathways. Annual Review Of Biomedical Engineering. 2004;6:397–426. doi: 10.1146/annurev.bioeng.6.040803.140203. [DOI] [PubMed] [Google Scholar]
- 27.Meyer O, K.D, K H, B S, J.W. P, M.C. W, D. P. Cationic liposomes coated with polyethylene glycol as carriers for oligonucleotides. The Journal of Biological Chemistry. 1998;273(25):15621. doi: 10.1074/jbc.273.25.15621. [DOI] [PubMed] [Google Scholar]
- 28.Photos P, Discher BM, Bacakova L, Bates FS, Discher DE. Polymersomes in vivo: increased stealth with both PEG density and molecular weight. Journal of Controlled Release. 2003;90:323–334. doi: 10.1016/s0168-3659(03)00201-3. [DOI] [PubMed] [Google Scholar]
- 29.Griffin MA, Sen S, Sweeney HL, Discher DE. Adhesion-contractile balance in myocyte differentiation. Journal of Cell Science. 2004;117:5855–5863. doi: 10.1242/jcs.01496. [DOI] [PubMed] [Google Scholar]
- 30.Subramanian S, Boder ET, Discher DE. Phylogenetic divergence of CD47 interactions with human signal regulatory protein alpha reveals locus of species specificity. Implications for the binding site. Journal of Biological Chemistry. 2007;282(3):1805–1818. doi: 10.1074/jbc.M603923200. [DOI] [PubMed] [Google Scholar]
- 31.Bonora S, Markarian SA, Trincheroa A, Grigorian KR. DSC study on the effect of dimethysulfoxide (DMSO) and diethylsulfoxide (DESO) on phospholipid liposomes. Thermochimica Acta. 2005;433(1−2):19–26. [Google Scholar]
- 32.Moldovan D, Pinisetty D, Devireddy RV. Molecular dynamics simulation of pore growth in lipid bilayer membranes in the presence of edge-active agents. Applied Physics Letters. 2007;91(20):4104–4107. [Google Scholar]
- 33.Meng F, Engbers GHM, Feijen J. Biodegradable polymersomes as a basis for artificial cells: encapsulation, release and targeting. Journal of Controlled Release. 2005;101(1−3):187–198. doi: 10.1016/j.jconrel.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 34.Yamauchi M, Kusano H, Saito E, Iwata T, Nakakura M, Kato Y, Aoki N. Development of wrapped liposomes: novel liposomes comprised of polyanion drug and cationic lipid complexes wrapped with neutral lipids. Biochimica et Biophysica Acta. 2006;1758:90–97. doi: 10.1016/j.bbamem.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 35.Geng Y, Discher DE. Hydrolytic degradation of poly(ethylene oxide)-block-polycaprolactone worm micelles. Journal of the American Chemical Society. 2005;127(37):12780–12781. doi: 10.1021/ja053902e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Discher DE, Ahmed F. Polymersomes. Annual Review of Biomedical Engineering. 2006;8(1):323–341. doi: 10.1146/annurev.bioeng.8.061505.095838. [DOI] [PubMed] [Google Scholar]
- 37.Bermudez H, Brannan AK, Hammer DA, Bates FS, Discher DE. Molecular weight dependence of polymersome membrane structure, Elasticity, and Stability. Macromolecules. 2002;35(21):8203–8208. [Google Scholar]
- 38.Andrei C, Dazzi C, Lotti L, Torrisi MR, Chimini G, Rubartelli A. The secretory route of the leaderless protein interleukin 1 involves exocytosis of endolysosome-related vesicles. Molecular Biology of the Cell. 1999;10(5):1463–1475. doi: 10.1091/mbc.10.5.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai S, Vijayan K, Cheng D, Lima EM, Discher DE. Micelles of different morphologies–advantages of worm-like filomicelles of PEO–PCL in paclitaxel delivery. Pharmaceutical Research. 2007;24(11):2099–2109. doi: 10.1007/s11095-007-9335-z. [DOI] [PubMed] [Google Scholar]
- 40.Harborth J, Elbashir SM, Bechert K, Tuschl T, Weber K. Identification of essential genes in cultured mammalian cells using small interfering RNAs. Journal of Cell Science. 2001;114(24):4557–4565. doi: 10.1242/jcs.114.24.4557. [DOI] [PubMed] [Google Scholar]
- 41.Felgner PL, Tsai YJ, Sukhu L, Wheeler CJ, Manthorpe M, Marshall J, Cheng SH. Improved cationic lipid formulations for in vivo gene therapy. Annals of the New York Academy of Science. 1995;772:126–139. doi: 10.1111/j.1749-6632.1995.tb44738.x. [DOI] [PubMed] [Google Scholar]
- 42.Hoffman EP, Brown RH, Kunkel LM. Dystrophin: the protein product of the duchenne muscular dystrophy locus. Cell. 1987;51(6):919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 43.Suh J, Wirtz D, Hanes J. Efficient active transport of gene nanocarriers to the cell nucleus. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(7):3878–3882. doi: 10.1073/pnas.0636277100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Molenaar C, Abdulle A, Gena A, Tanke HJ, Dirks RW. Poly(A)+ RNAs roam the cell nucleus and pass through speckle domains in transcriptionally active and inactive cells. The Journal of Cell Biology. 2004;165(2):191–202. doi: 10.1083/jcb.200310139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sirsi SR, Williams JH, Lutz GJ. Poly(ethylene imine)-poly(ethylene glycol) copolymers facilitate efficient delivery of antissense oligonucleotides to nuclei of mature muscle cells of mdx mice. Human Gene Therapy. 2005;16:1307–1317. doi: 10.1089/hum.2005.16.1307. [DOI] [PubMed] [Google Scholar]
- 46.Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fletcher S, Honeyman K, Fall AM, Harding PL, Johnsen RD, Wilton SD. Dystrophin expression in the mdx mouse after localised and systemic administration of a morpholino antisense oligonucleotide. The Journal of Gene Medicine. 2006;8:207–216. doi: 10.1002/jgm.838. [DOI] [PubMed] [Google Scholar]
- 48.Fischer D, Osburg B, Petersen H, Kissel T, Bickel U. Effect of poly(ethylene imine) molecular weight and pegylation on organ distribution and pharmacokinetics of polyplexes with oligodeoxynucleotides in mice. Drug Metabolism and Disposition. 2004;32:983–992. [PubMed] [Google Scholar]
- 49.Allikian MJ, Hack AA, Mewborn S, Mayer U, Mcnally EM. Genetic compensation for sarcoglycan loss by integrin alpha7beta1 in muscle. Journal of Cell Science. 2004;117(Pt 17):3821–3830. doi: 10.1242/jcs.01234. [DOI] [PubMed] [Google Scholar]
- 50.Chen J, Shi W, Zhang Y, Sokol R, Cai H, Lun M, Moore BF, Farber MJ, Stepanchick JS, Bonnemann CG, Chan YM. Identification of functional domains in sarcoglycans essential for their interaction and plasma membrane targeting. Experimental Cell Research. 2006;312(9):1610–1625. doi: 10.1016/j.yexcr.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 51.Kleinman ME, Yamada K, Takeda A, Chandrasekaran V, Nozaki M, Baffi JZ, Albuquerque RJC, Yamasaki S, Itaya M, Pan Y, Appukuttan B, Gibbs D, Yang Z, Kariko K, Ambati BK, Wilgus TA, Dipietro LA, Sakurai E, Zhang K, Smith JR, Taylor EW, Ambati J. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452(7187):591–597. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Korobko AV, Backendorf C, Vandermaarel JRC. Plasmid DNA encapsulation within cationic diblock copolymer vesicles for gene delivery. Journal Of Physical Chemistry. 2006;110(30):14550–14556. doi: 10.1021/jp057363b. [DOI] [PubMed] [Google Scholar]
- 53.Markarian SA, Asatryan AM, Grigoryan KR, Sargsyan HR. Effect of diethylsulf-oxide on the thermal denaturation of DNA. Biopolymers. 2006;82(1):1–5. doi: 10.1002/bip.20454. [DOI] [PubMed] [Google Scholar]
- 54.Anada T, Sakurai K, Shinkai S. A new polynucleotide-polysaccharide complex and its application to functional oligonucleotide delivery. Trends in Glycoscience and Glycotechnology. 2005;17(94):49–58. [Google Scholar]
- 55.Arakawa T, Kita Y, Timasheff SN. Protein precipitation and denaturation by dimethyl sulfoxide. Biophysical Chemistry. 2007;131(1−3):62–70. doi: 10.1016/j.bpc.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 56.Yguerabide J, Schmidt JA, Yguerabide EE. Lateral mobility in membranes as detected by fluorescence recovery after photobleaching. Biophysical Journal. 1982;39:69–75. doi: 10.1016/S0006-3495(82)84459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee JC-M, Bermudez H, Discher BM, Sheehan MA, Won Y-Y, Bates FS, Discher DE. Preparation, stability, and in vitro performance of vesicles made with diblockcopolymers. Biotechnology and Bioengineering. 2001;73:135–145. doi: 10.1002/bit.1045. [DOI] [PubMed] [Google Scholar]
- 58.van Deutekom JC. Local dystrophin restoration with antisense oligonucleotide PRO051. New England Journal Of Medicine. 2007;357(26):2677–2686. doi: 10.1056/NEJMoa073108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Tables
Table S1 Properties of degradable and non-degradable diblock copolymer amphiphiles.
Table S2 Mean hydrodynamic radius of aggregates, RNAi loading efficiency, and range of Zeta Potentials.
Supplemental Methods
2.7. Labeling of polymersomes For tracking copolymer fate in cells the fluorophore tetramethyrhodamine (TMR) was chemically conjugated to OB copolymers. The hydroxyl end of the PEG was covalently attached to TMR through a rearrangement of an acyl azide [29]. Briefly, a TMR acyl azide (Molecular Probes) was heated in toluene at 80 °C to cause rearrangement to an isocyanate. Simultaneously, 0.5 mg of OB was added in a molar ratio of 10:1 dye: copolymer for 12 h. 20 mg of NH3OH was then added to the stirred solution for 2 h to deprotect the nonfluorescent urethane derivative, which turned the solution color from pink to a deep red. For cell culture studies, polymersomes were alternatively labeled by adding the membrane-labeling fluorescent dyes PKH26 or PKH67 directly to a vesicle suspension.
2.8. Fluorescence microscopy, FRAP, and spectrofluorimetry Morphological and quantitative fluorescent measurements of vesicles, cells and tissue sections were made after imaging with an Olympus IX71 inverted fluorescence microscope with a 20x air and a 60x oil objective and a Cascade CCD camera [30]. Photo-bleaching studies were conducted using a pulsed dye laser (Photonic Instruments, St. Charles, IL), on a NIKON TE300 inverted fluorescence microscope, imaged with a 60x oil immersion objective. Image J was used for image analysis. The reported fluorescence recovery curves have been normalized to correct for photo bleaching during data collection.
Supplemental Figures and Legends
Figure S1. Measurements of retained anionic dextrans during dialysis after formation of polymersomes (see Methods). Closed points are for dialysis into DI water and open points are for PBS. Fits to y = (100% - A) + A(1-exp(-t/τ)) yield τ ∼ 6−12 hr, which indicates that 48 h of dialysis should extract >98% of the polymer.
Figure S2. FRAP measurements of dystrophin-specific fluorescent-AON within C2C12 muscle nuclei.
Figure S3. Delivery of FITC-AON into myotubes (see Fig.4) is successful when complexed with PEI (Exgen 500™; Fermentas, Inc., Hanover, MD) but not when delivered as free AON or with non-encapsulating, degradable Psomes. Similar images were obtained at 6 h and 20 h as well as with A549 cells.
Figure S4. Immunostaining controls for dystrophin in muscle sections. (A) Co-staining for the ubiquitous integral membrane protein, CD47. (B) Non-specific background with secondary antibody.
Figure S5. Further examples of treated mdx immunostaining, imaging, binarization, and quantitation. (A) Image analysis, and (B) Dystrophin positive membranes relative to free AON.