Abstract
Background:
Ultrasound has been used therapeutically for accelerating fracture healing since many years. However, the controversy on the exact mechanism of osteoinduction still continues. In this study, we try to bring out the exact biomolecular mechanism by which ultrasound induces fracture healing.
Materials and Methods:
The study was conducted in two phases: animal experiments and clinical study. In the first phase, we induced fractures on the left tibia of Wistar strain rats under anaesthesia. They were divided into two groups. One of the groups was given low-intensity, pulsed ultrasound (30 MW/cm2) 20 min a day for 10 days. Tissue samples and radiographs were taken weekly for 3 weeks from both the groups. In the second phase of our study, ten patients with fractures of the distal end of the radius (ten fractures) were included. Five of these were treated as cases, and five were treated as controls. Ultrasound was given 30 MW/cm2 for 20 min every day for 2 weeks. The patients were assessed radiologically and sonologically before and after ultrasound therapy. Tissue samples were studied with thymidine incorporation test with and without adding various neurotransmitter combinations.
Results:
Radiological findings revealed that there was an increased callus formation in the ultrasound group. At the cellular level, there was an increased thymidine incorporation in the ultrasound group. When various neurotransmitters were added to the cells, there was an increased thymidine incorporation in the ultrasound group.
In the second phase of the study, radiological and sonological assessments showed that there was an increased callus formation in the ultrasound group. In cytological study, thymidine incorporation was found to be increased in the ultrasound group.
Conclusions:
The results of animal and clinical studies demonstrated an early and increased callus formation in the ultrasound group. Cytological studies revealed increased thymidine incorporation, suggesting increased osteoblastic activity.
Keywords: Fracture healing, neurotransmitter, thymidine incorporation, ultrasound
INTRODUCTION
Ultrasound has been used therapeutically for accelerating fracture healing for the past many years. Ever since its clinical use for the first time, there was a growing interest in this modality of osteoinduction. However, the controversy on the exact mechanism of osteoinduction still continues. The US Food and Drug Administration (FDA) has approved the use of ultrasound in fresh fractures (1994)1,2 and for established nonunions (2000).3,4 The role of chemical messengers like neurotransmitters in osteoinduction has evoked new research interest in this field.5–7 In this study, we try to bring out the exact biomolecular mechanism by which ultrasound induces fracture healing. If we could elucidate the exact mechanism of osteoinduction at the cellular or molecular level, we may be able to modify fracture healing as such.
MATERIALS AND METHODS
The study protocol was cleared by the institutional review board. The study was conducted in two phases. In the first phase, we induced fractures of the left tibia in Wistar strains of rats under ether anaesthesia. The fractures were splinted with special plastic splints. The rats were divided into two groups. The first group of 10 rats, defined as test group, was given low-intensity, pulsed ultrasound (30 MW/cm2) 20 min a day for 10 days. In the second group, of 10 rats, defined as control group, ultrasound treatment was not offered. Tissue samples and serial radiographs were obtained weekly for 3 weeks from both groups. The tissue samples were taken by aspiration from the fracture site [Figures 1 and 2].
Figure 1.
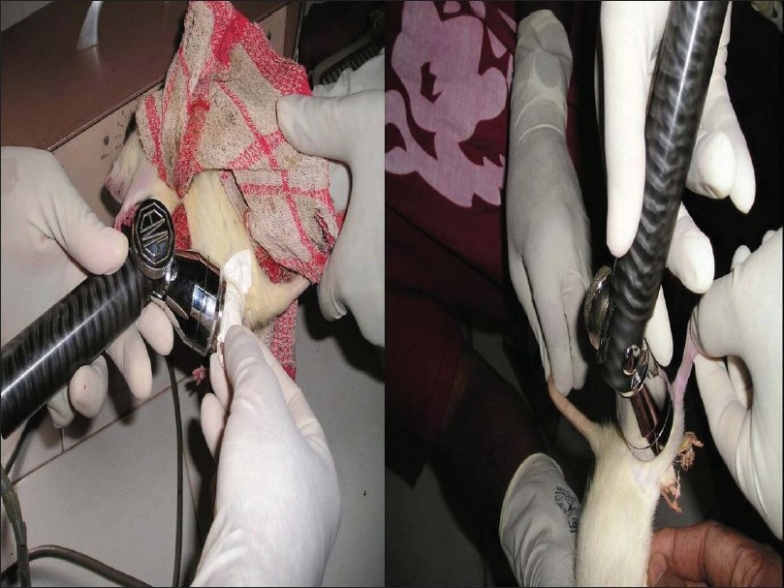
Therapeutic ultrasound in test group
Figure 2.
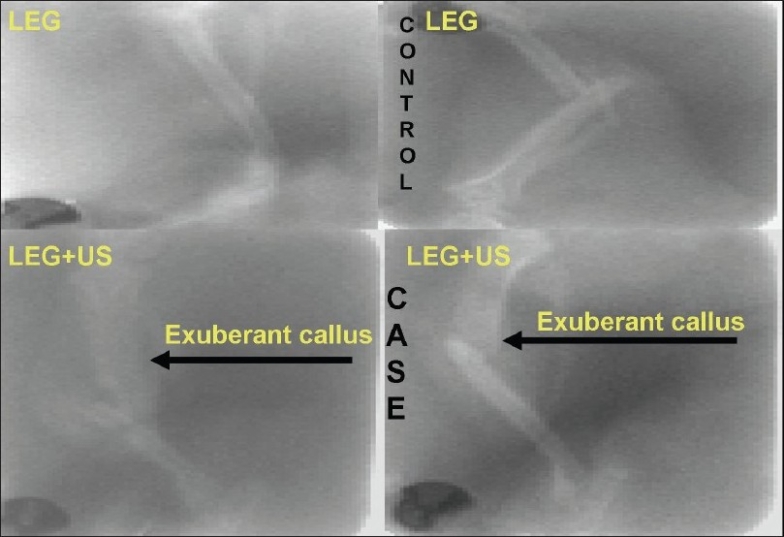
Radiological evaluation at 2 weeks. Note the exuberant callus in the test group
Cells were taken in the phosphate buffer solution (PBS), and their viability was tested with Trifan blue stain. Then the cells were cultured in Rosewaal Pasteur Memorial Institute (RPMI) medium for 24 h. Radioactive thymidine8 incorporation test was done on these cells. The tissue aspirated from the fracture site was incubated with radioactive thymidine, and radioactivity was measured in a scintillation counter. The results of the uptake study in the test and control groups were compared statistically. Cells were again cultured with various neurotransmitter combinations, such as serotonin (5HT), dopamine (D)+, butaclomol (BU), GABA+ bicuculline (BI), and radioactive thymidine incorporation test was done; the results compared between both groups.
In the second phase of the study, 10 patients with fracture of the distal radius (extraarticular simple fractures) were included. Five of these fractures were designated as test group, and the rest five were treated as controls. The test group was subjected to low-intensity, pulsed ultrasound applied at the fracture site 20 min daily for 2 weeks. Those belonging to the control group were not given ultrasound therapy. The patients were assessed radiologically, sonologically, and through cell studies before and after ultrasound therapy exactly as in the first phase. The cytological study was conducted with the aspirate from the fracture site exactly as in the first phase.
RESULTS
In the first phase of study, there was an increased callus formation in the ultrasound group when assessed radiologically by measuring the dimensions of the callus at the fracture site in the anteroposterior and lateral views using a scale.9–11 At the cellular level, there was an increased thymidine incorporation in the ultrasound group, as expressed by the scintillation counts shown in Tables 1–3.
Table 1.
Radioactive thymidine incorporation test expressed in scintillation counts
| Test | Control | |
|---|---|---|
| First week | 8737 | 7190 |
| Second week | 8863 | 6583 |
| Third week | 9285 | 7814 |
Table 3.
Radioactive thymidine incorporation test in cells after administration of 5HT, D+BU and GABA+BI
| Patient | RTI in patients | RTI in cells after 5HT administration | RTI in cells after D + BU administration | RTI in cells after GABA + BI administration | ||||
|---|---|---|---|---|---|---|---|---|
| Test | Control | Test | Control | Test | Control | Test | Control | |
| 1 | 8750 | 5679 | 11 006 | 7486 | 11 608 | 7184 | 9433 | 5936 |
| 2 | 9319 | 4357 | 79 970 | 6052 | 56 605 | 7200 | 70871 | 5285 |
| 3 | 7844 | 4532 | 32 533 | 6573 | 33 832 | 6943 | 33062 | 5487 |
| 4 | 6567 | 4578 | 43 453 | 5489 | 41 326 | 5467 | 45632 | 4378 |
| 5 | 7786 | 5371 | 39 763 | 6913 | 39 552 | 4774 | 37194 | 5279 |
RTI: Radioactive thymidine incorporation
Table 2.
Radioactive thymidine incorporation test with neurotransmitters
| Neurotansmitters | 1 week | 2 week | 3 week | |||
|---|---|---|---|---|---|---|
| Test | Control | Test | Control | Test | Control | |
| Serotonin | 9499 | 119 | 6674 | 6607 | 8490 | 7752 |
| Dopamine + Butaclomol | 9510 | 8622 | 9613 | 8061 | 7582 | 6161 |
| GABA + Bicuculline | 10403 | 2451 | 8916 | 6447 | 9030 | 6228 |
In the second phase of the study, there was an increased callus formation when assessed sonologically in the ultrasound group [Figure 3]. And, at the cellular level, there was increased thymidine incorporation in the ultrasound group. By using the radioactive thymidine incorporation test [Graph 1], increased osteoblastic activity was observed when neurotransmitter combinations were added to the cells from fracture site in ultrasound group.
Figure 3.
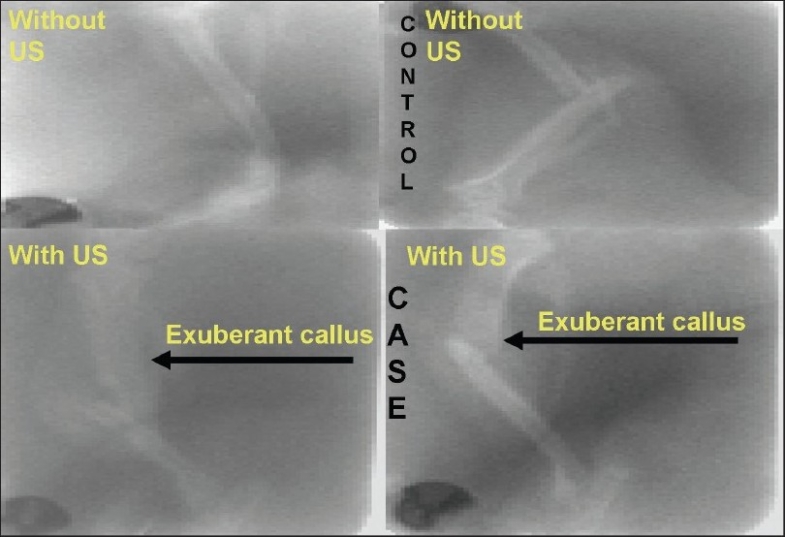
Difference in callus observed without ultrasound and with ultrasound
Graph 1.
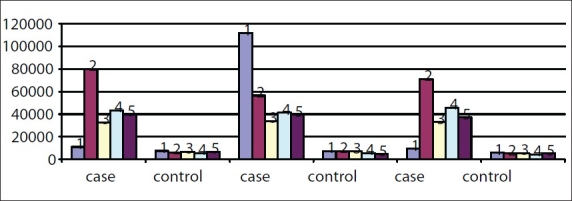
Radioactive study after neurotransmitter administration composite comparison. 5HT D + BU GABA + BI
These experimental and clinical observations strongly suggest that the radioactive thymidine incorporation was significantly higher in the test group after ultrasound therapy and after neurotransmitter administration.
DISCUSSION
Low-intensity, pulsed ultrasound has been proved in previous studies in the literature to significantly enhance fracture healing. In our study, the level of radioactivity12,13 was an index of osteoblastic activity at the fracture site. In the test group, the radioactive12 thymidine incorporation was significantly higher when compared with that in the control group. This is a definite evidence to suggest that ultrasound increases cell replication at the fracture site.
We tried to elucidate the mechanism by which ultrasound enhances healing by experimentally adding neurotransmitters at the fracture site in both the test group and controls. The radioactive thymidine incorporation was markedly increased in the test group with all the three different sets of neurotransmitters added.11 Because the neurotransmitters act through their respective receptors, this might indicate that the receptor levels of these neurotransmitters were significantly increased at the fracture site by ultrasound administration.14
Thus, we postulate that ultrasound enhances fracture healing by increasing the receptor activity of neurotransmitters and hence the neurotransmitter activity at the fracture site. This also indicates that neurotransmitters play a role in inducing fracture healing. How neurotransmitters act at the molecular or subcellular level in enhancing the healing process remains to be studied in detail. Also the present study has to be supplemented by further studies in larger populations to authenticate the proposed mechanism by which ultrasound induces fracture healing. In the light of the concrete base thus achieved, this could pave the way for an entirely new frontier in fracture management and impetus for further research.
CONCLUSION
We conclude that low-intensity, pulsed ultrasound acts by upregulating the receptor activity of neurotransmitters at the fracture site, which probably enhances fracture healing.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Heckman JD, Ryaby JP, McCabe J, Frey JJ, Kilcoyne RF. Acceleration of tibial fracture-healing by non-invasive, low-intensity pulsed ultrasound. J Bone Joint Surg Am. 1994;76:26–34. doi: 10.2106/00004623-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Kristiansen TK, Ryaby JP, McCabe J, Frey JJ, Roe LR. Accelerated healing of distal radial fractures with the use of specific, low-intensity ultrasound: A multicenter, prospective, randomized, double-blind, placebo-controlled study. J Bone Joint Surg Am. 1997;79:961–73. doi: 10.2106/00004623-199707000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Maylia E, Nokes LD. The use of ultrasonics in orthopaedics: A review. Technol Health Care. 1999;7:1–28. [PubMed] [Google Scholar]
- 4.Ziskin MC. Applications of ultrasound in medicine-comparison with other modalities. In: Rapacholi MH, Grandolfo M, Rindi A, editors. Ultrasound: medical applications, biological effects, and hazard potential. New York: Plenum Press; 1987. pp. 49–59. [Google Scholar]
- 5.Dyson M. Therapeutic applications of ultrasound. In: Nyborg WL, Ziskin MC, editors. Biological effects of ultrasound. New York: Churchill Livingstone; 1985. pp. 121–33. [Google Scholar]
- 6.Wells PNT. Surgical applications of ultrasound. In: Nyborg WL, Ziskin MC, editors. Biological effects of ultrasound. New York: Churchill Livingstone; 1985. pp. 157–67. [Google Scholar]
- 7.Kaufman JJ, Einhorn TA. Ultrasound assessment of bone. J Bone Miner Res. 1993;8:517–25. doi: 10.1002/jbmr.5650080502. [DOI] [PubMed] [Google Scholar]
- 8.Maliekal TT, Sudha B, Paulose CS. Kinetic parameters of thymidine kinase and DNA synthesis during rat liver regeneration: Role of thyroid hormones. Life Sci. 1997;60:1867–74. doi: 10.1016/s0024-3205(97)00147-1. [DOI] [PubMed] [Google Scholar]
- 9.Moed BR, Kim EC, van Holsbeeck M, Schaffler MB, Subramanian S, Bouffard JA, et al. Ultrasound for the early diagnosis of tibial fracture healing after static interlocked nailing without reaming: Histologic correlation using a canine model. J Orthop Trauma. 1998;12:200–5. doi: 10.1097/00005131-199803000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Moed BR, Subramanian S, van Holsbeeck M, Watson JT, Cramer KE, Karges DE, et al. Ultrasound for the early diagnosis of tibial fracture healing after static interlocked nailing without reaming: clinical results. J Orthop Trauma. 1998;12:206–13. doi: 10.1097/00005131-199803000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Brown BS. How safe is diagnostic ultrasonography? Can Med Assoc J. 1984;131:307–11. [PMC free article] [PubMed] [Google Scholar]
- 12.Biju MP, Pyroja S, Rajesh Kumar NV, Paulose CS. Brain stem GABA receptor functional regulation during rat liver cell proliferation. Neurochem Res. 2002;27:905–10. doi: 10.1023/a:1020391514995. [DOI] [PubMed] [Google Scholar]
- 13.Paulose CS, Renuka TR, Eswar Sankar PN. Neurotransmitter receptor gene expression: Insulin secretion and cell proliferation, Neurobiology in the Post Genomic era. New Delhi: Narosa Publishing House; 2004. [Google Scholar]
- 14.Ignatius J, John PS, Paulose CS, Thomas SK, Khan R, Gireesh G. Upregulation of neurotransmitter receptors: A possible mechanism for accelerated fracture healing. J Kerala Orthop Assoc (KOA) 2006;20:31–9. [Google Scholar]


