Abstract
Background
Relationships between environment and cortical-limbic-striatal pathways are not well-researched in child bipolar I disorder (BP-I).
Methods
This was a controlled, blindly rated magnetic resonance imaging study of children with DSM-IV BP-I, manic or mixed type, compared with matched typically developing children (TC).
Results
There were 47 subjects (21 BP-I, 26 TC) aged 14.0 ± 3.1 (BP-I onset age 8.8 ± 4.2). Total intracranial volume was greater in male subjects (n = 28) versus female subjects (n = 19) [F(1,44) = 24.3, p <.001], controlling for age. Volumes were not significantly different in BP-I and TC groups, after accounting for multiple comparisons, in the medial orbital frontal cortex, rostral anterior cingulate cortex, hippocampus, amygdala (AMG), or nucleus accumbens (NAcc). Across subjects (n = 47), a greater number of independent life events (ILE) was associated with smaller AMG [F(1,36) = 7.8, p = .009] and NAcc [F(1,36) = 9.4, p = .004] volumes, controlling for total intracranial volume (TICV), group, age, sex, and family psychopathology. Use of stimulant medication at the time of the scan was associated with larger AMG volume [F(1,41) = 9.0, p = .005], controlling for TICV, group, age, and sex. In male subjects, the age × group interaction was a significant predictor in general linear models of AMG (p = .028) and NAcc (p = .030) volumes. Effects of low maternal warmth were not significant.
Conclusions
Findings suggest that ILE affect AMG and NAcc volume, but further research is needed to examine specificity to child BP-I. Furthermore, differential age × group (child BP-I vs. TC) effects only in male subjects are consistent with differential brain development by sex.
Keywords: Adolescent, bipolar I disorder, child, independent life events, MRI
According to the 2000 U.S. census (1) there are 29,040,538 children ages 7–13 years old. Data from the largest ever National Institutes of Health-funded epidemiology study of bipolar I disorder (BP-I) (2), in which over 43,000 individuals were interviewed, found a 5% prevalence of BP-I in the youngest age group (12–29-year-olds). With these figures, there are an estimated 1,452,027 individuals ages 7–13 with BP-I in the United States. This number is expected to increase, because of data supporting that age of onset of BP-I is decreasing in more recently born cohorts (3). Even with the caveats of interpreting generational earlier age of onset (the so-called “anticipation” phenomenon) (4) and the lack of community prevalence data under the age of 12, these data forcefully argue for investigating the etiopathogenesis, including environmental effects on neuro-imaging in child BP-I.
However, to our knowledge, familial and environmental influences on cortical-limbic-striatal volumes, in a sample with child BP-I, has not previously been reported. To gain perspective on environmental and genetic factors, both family psychopathology and environmental factors should be examined within the same sample. These are especially cogent questions during childhood, because this is when peak brain growth occurs (5). In addition, recent work suggests a greater influence of environmental versus genetic factors during the early school-age years (6).
Prior work has shown that two measures of environmental adversity, low maternal warmth and greater number of independent life events (ILE), significantly differentiated child bipolar I disorder (BP-I) subjects from typically developing children (TC) (7,8). Recently published data found familial aggregation for child BP-I probands was 7–8 times higher than that for adult BP-I probands (3). Furthermore, even when accounting for familial psychopathology, low maternal warmth (which is akin to expressed emotion) remained a significant predictor of worse course of child BP-I over 8-year follow-up (9,10). On the basis of these findings, it was hypothesized that environmental adversity would affect cortical-limbic-striatal pathways that have been related to mood and substance use disorders (SUD) (11).
None of these magnetic resonance imaging (MRI) findings have been previously published.
Methods and Materials
Subject Ascertainment
Subjects were a subset of participants in the National Institute of Mental Health (NIMH)-funded “Phenomenology and Course of Pediatric Bipolar Disorders” study (10). Subjects with child BP-I were obtained between 1995 and 1998 from designated outpatient child psychiatric and pediatric facilities by consecutive new case ascertainment (12). Subjects in the TC group were obtained through a random survey that matched these subjects to the child BP-I subjects by age, sex, socioeconomic status (SES), ethnicity, and zip code (12).
Inclusion and Exclusion Criteria
Inclusion criteria common to both groups were 7–16 years old, male subjects and female subjects, and good physical health.
At baseline, subjects in the BP-I group had first episode DSM-IV BP-I, mixed or manic phase, for ≥ 2 weeks with at least one cardinal symptom (elation and/or grandiosity). The cardinal symptom criterion avoided diagnosing BP-I only by symptoms that overlapped with attention-deficit/hyperactivity disorder (12). To ensure clinical impairment, a Children’s Global Assessment Scale (CGAS) (13) score ≤ 60 was needed (0 is worst, 100 is best, ≤ 60 is clinical impairment) (12). All DSM-IV severity and duration criteria were fulfilled. This child BP-I phenotype has been validated by unique symptoms (12), longitudinal stability (9,10,14), and familial aggregation (3).
At baseline, subjects in the TC group had no current or lifetime DSM-IV diagnoses and a CGAS score ≥ 70 (12).
Exclusion criteria for both groups were IQ < 70, adopted status, pervasive developmental disorders, schizophrenia, epilepsy or other major medical or neurological disorder, and baseline substance dependency or pregnancy. Subjects who developed substance use disorders (SUD) or became pregnant during prospective follow-up were continued in the study. Subjects in the BP-I group could not exhibit manic symptoms only while receiving antidepressant, stimulant, or other mania-inducing medications. There were no family psychopathology exclusions in either group.
Rationales for the inclusion and exclusion criteria are described in detail elsewhere (12).
Assessment Instruments
Comprehensive assessment by blind research nurses included the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) (15), given separately to mothers about their children and to children about themselves. The WASH-U-KSADS is a semi-structured interview with excellent reliability for mania symptoms, mood diagnoses, daily cycling, and time frames (κ values .82 – 1.00) (15). Different raters were used for the parent and child within each family to avoid bias from knowing what one informant reported when assessing the other (16).
The Psychosocial Schedule for School Age Children—Revised (PSS-R) (17) was administered separately to each informant (16). The PSS-R contains comprehensive measures of child interactions with parents, siblings, peers, and teachers and also assesses marital relationships. Measurements of maternal warmth are included in the PSS-R (17). Both continuous and categorical measures of maternal warmth were used in analyses. For the categorical analyses, a score of 1 (mutual concern and affection, close relationship) on maternal warmth indicated high warmth, and scores of 2–5 indicated low warmth (9,10).
The Life Events Checklist (LEC), which is embedded in the PSS-R, was used for assessment of life events. Like the WASH-U-KSADS and the PSS-R, the LEC was administered by experienced research nurses to parents about their children and children about themselves. The LEC consists of 57 items. Each of these items is grouped into one of the following categories: independent of the child (e.g., a parent died), dependent on the child (e.g., failed a school course), or uncertain (e.g., a sibling goes to boarding school, which might or might not be due to the subject’s illness). It was previously shown that child BP-I subjects had significantly more independent, dependent, and uncertain life events than TC subjects (8). Although MRI scans were conducted a mean 2.7 years after baseline, the number of ILEs at the baseline assessment were used in the analyses.
MRI Methods
The MRI data were collected by aligning two three-dimensional T1-weighted magnetization prepared rapid gradient echo (MPRAGE) sequences (.94 × .94 × 1.5 mm3 voxels) acquired on a 1.5-T Siemens Sonata scanner (Siemens Medical Solutions, Malvern, Pennsylvania). The MRI exclusions included SUD, loss of consciousness > 5 min, and certain braces, tattoos, and medical/neurological conditions (18). All scans were rated blind to whether subjects were in the BP-I or TC group.
MRI Data Processing
Cortical reconstruction and volumetric segmentation were performed with the imaging analysis suite FreeSurfer (https://surfer.nmr.mgh.harvard.edu). Details regarding the specific automated methods are presented in a series of articles (e.g., 19–22) but are described here in brief. First, variations in image intensities were normalized for each subject’s three-dimensional image volume (23). Extra-cerebral voxels were then removed in a “skull-strip” of the brain with a hybrid watershed/surface deformation process (24). Remaining voxels were classified, with intensity and continuity information, into gray and white matter regions (20). Subcortical white matter and deep gray matter structures were segmented with an automated approach (25,26). When compared with manual raters, this method demonstrated approximately 80% volume overlap for the right and left hippocampus and approximately 70% volume overlap with the left and right amygdala; volume overlap was not reported for the nucleus accumbens (25). The white matter segmentation was split into hemispheres, and the gray-white junction was tessellated. This surface was deformed and inflated outward to locate the approximate pial surface and subsequently refined to obtain a representation of the gray-cerebrospinal fluid boundary. Any geometric inaccuracies or topological defects were corrected with a combination of automatic and manual methods (22,27). Cortical volumes were derived from estimations of volume between the gray-white and pial surface. Parcellation of the cortex into gyral and sulcal structures was obtained through the overlaying of an atlas constructed by Bayesian algorithms, comparisons between this procedure and manual methods yielded intraclass correlations of .83 and .91 for the left and right medial orbital frontal cortices, and .81 and .84 for left and right rostral anterior cingulate cortices (28). Freesurfer morphometric procedures have demonstrated strong test-retest reliability across scanner manufacturers and field strengths (29).
This study was approved by the Human Studies Committee at Washington University in St. Louis. After complete description of the study, written informed consent was obtained from parents and written assent was obtained from children.
Statistical Analyses
Comparisons of demographic data in the BP-I and TC groups were made with t tests for continuous variables and χ2 tests for categorical variables. Left and right region of interest (ROI) measurements were combined. Additional analyses of left and right regions were conducted if analysis of the combined volume was significant. Comparisons between BP-I and TC groups were made for gray matter medial orbital frontal cortex (mOFC), gray matter rostral anterior cingulate cortex (rACC), hippocampus (HC), amygdala (AMG), and nucleus accumbens (NAcc) volumes with general linear models. Comparisons of these brain structure volumes were also made for subjects by level of maternal warmth and by number of ILE. All models controlled for total intracranial volume (TICV), age at MRI scan, and sex. Comparisons of BP-I and TC groups also controlled for CGAS score, and the maternal warmth and ILE analyses also controlled for familial BP-I and/or recurrent major depressive disorder.
Because age at MRI scan varied widely in both the BP-I and TC groups, the interaction of age and group was analyzed in general linear models of ROI volumes that also covaried for age and group.
Significance levels were determined with the Bonferroni method of correcting for multiple comparisons. This resulted in a Bonferroni corrected significance level of p < .01 for comparisons of brain structure volumes by group, maternal warmth, ILE, and familial psychopathology.
Results
There were 21 BP-I and 26 TC subjects. Demography and severity characteristics of the two groups are presented in Table 1. At baseline, all BP-I subjects were in their first episode of DSM-IV BP-I, manic or mixed state.
Table 1.
Demography and Severity Characteristics in BP-I Versus TC Subjects
| BP-I (n = 21) |
TC (n = 26) |
|||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | p | |
| Age at MRI Scan (yrs) | 14.1 | 3.1 | 13.8 | 3.1 | .3 | NS |
| Age at Baseline (yrs) | 11.3 | 3.2 | 11.3 | 2.8 | .1 | NS |
| Age of Mania Onset (yrs) | 8.8 | 4.2 | — | — | — | — |
| Duration of Baseline Mania Episode (yrs) |
2.5 | 1.8 | — | — | — | — |
| CGAS at MRI Scan | 56.8 | 17.5 | 88.9 | 8.9 | 7.7 | <.001a |
| % | n | % | n | χ2 | p | |
| Sex | ||||||
| Male | 52.4 | 11 | 65.4 | 17 | .8 | NS |
| Female | 47.6 | 10 | 34.6 | 9 | ||
| Handedness | ||||||
| Right | 81.0 | 17 | 76.9 | 20 | F.E. | NS |
| Left | 19.0 | 4 | 23.1 | 6 | ||
| Race | ||||||
| Caucasian | 81.0 | 17 | 92.4 | 24 | 1.3 | NS |
| African-American | 9.5 | 2 | 3.8 | 1 | ||
| Other | 9.5 | 2 | 3.8 | 1 | ||
BP-I, bipolar I disorder; TC, typically developing control; MRI, magnetic resonance imaging; CGAS, Children’s Global Assessment Scale; F.E., Fisher’s Exact test.
Severity was included as a covariate in analyses comparing BP-I and TC groups.
Subjects were scanned a mean 2.7 years after baseline. At the time of scanning, 71.4% of BP-I subjects were in a mood episode. Mood states at the time of the scan were manic or hypomanic (38.1%), depressed (33.3%), and mixed manic (0.0%). No subject had post-traumatic stress disorder at any rating time. Volumes were not significantly different between subjects with and subjects without a mood diagnosis at the time of the scan.
Subjects were assessed in the research unit, but all clinical care was given by their own community practitioners. At the time of the scan, 11 (52.4%) BP-I subjects were taking at least one psychotropic medication (33.3% stimulant, 28.6% neuroleptic, 23.8% anticonvulsant, 9.5% antidepressant, 0.0% lithium). No TC subjects were taking psychotropic medication. Use of stimulant medication at the time of the scan was associated with larger AMG volume [F(1,41) = 9.0, p = .005], controlling for TICV, group, age, and sex.
The TICV was significantly greater in male subjects compared with female subjects [F(1,44) = 24.3, p < .001], controlling for age.
The BP-I subjects had smaller mOFC volume compared with TC subjects [F(1,41) = 5.7, p = .022]. This difference, however, did not remain after Bonferroni correction. There were no significant differences in rACC, HC, AMG, or NAcc volumes in BP-I compared with TC subjects.
For all subjects (n = 47), a greater number of ILE was significantly associated with smaller AMG [F(1,36) = 7.8, p = .009] and NAcc [F(1,36) = 9.4, p = .004] volumes, controlling for TICV, group, age, gender, and familial BP-I and/or recurrent major depressive disorder. Separate analysis of left and right AMG showed no significant difference in volumes after Bonferroni correction [left: F(1,36) = 5.7, p = .023; right: F(1,36) = 7.0, p = .012]. A greater number of ILE was significantly associated with smaller left NAcc volume [F(1,36) = 9.2, p = .005] but was not significantly associated with right NAcc volume [F(1,36) = 2.4, p = .128]. Differences in AMG and NAcc volumes by number of ILE were more pronounced in male subjects (AMG: p = .073; NAcc: p = .020) compared with female subjects (AMG: p= .153; NAcc: p = .400), although no differences reached statistical significance, likely due to small sample size.
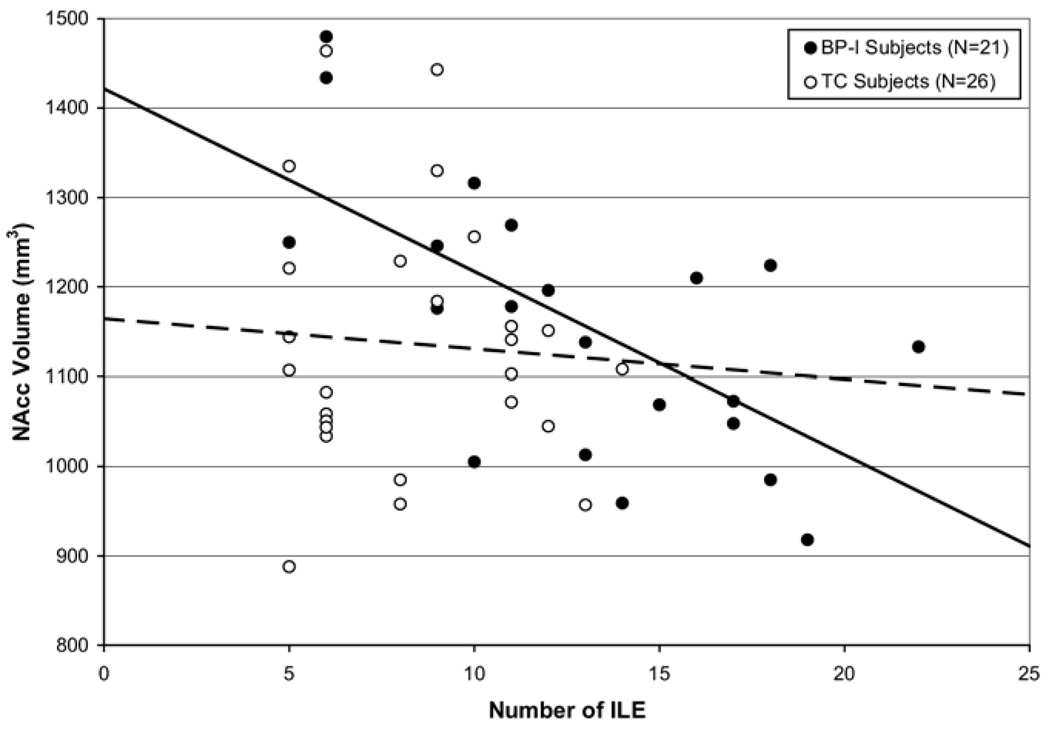
In the BP-I group (Figure 1), subjects with a greater number of ILE had significantly smaller NAcc volume [F (1,14) = 11.3, p = .005], controlling for TICV, age, sex, and familial BP-I and/or recurrent major depressive disorder. Separate analysis of left and right NAcc found that a greater number of ILE was significantly associated with smaller left NAcc volume [F(1,14) = 11.1, p = .005] but was not associated with smaller right NAcc volume [F(1,14) = 1.9, p = .199]. By contrast, there were no differences in mOFC, rACC, HC, AMG, or NAcc volumes by number of ILE in TC subjects. Of note, BP-I subjects had a significantly greater number of ILE than TC subjects (t = 3.9, p < .001). The maximum number of ILE in the TC group was 14. When BP-I subjects with >14 ILE were removed from the analysis, there was no longer a significant association between number of ILE and NAcc volume after Bonferroni correction [F(1,6) = 6.8, p = .041], although this might have been a result of small sample size (n = 13).
Figure 1.
Nucleus accumbens (NAcc) volumes by number of independent life events (ILE) in bipolar I disorder (BP-I) and typically developing control (TC) subjects. The solid line illustrates the relationship between number of ILE and NAcc volume in BP-I subjects, whereas the dashed line illustrates the relationship between number of ILE and NAcc volume in TC subjects. The BP-I subjects with a greater number of ILE had significantly smaller NAcc volume [F(1,14) = 11.3, p =.005], controlling for total intracranial volume, age, sex, and familial BP-I and/or recurrent major depressive disorder. The NAcc volume did not differ in TC subjects by number of ILE [F(1,17) = .6, p = .45].
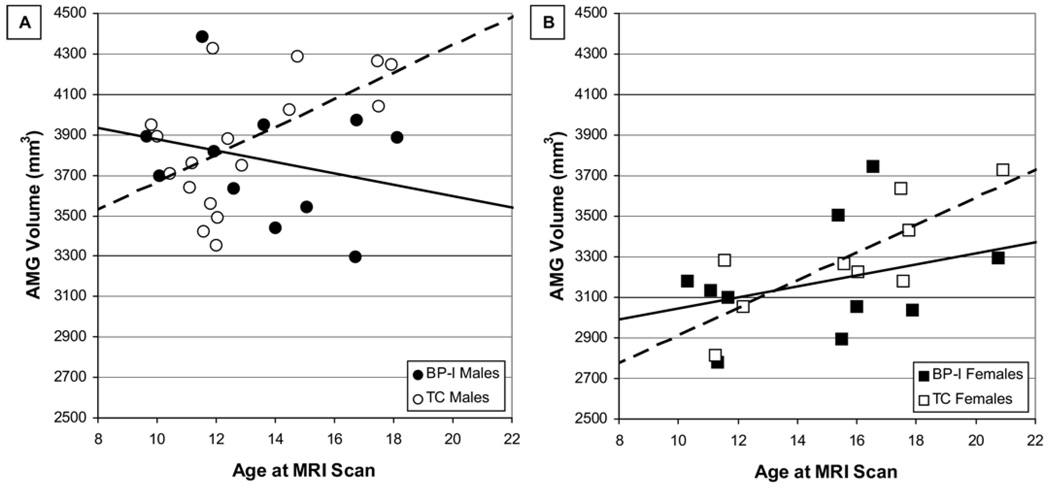
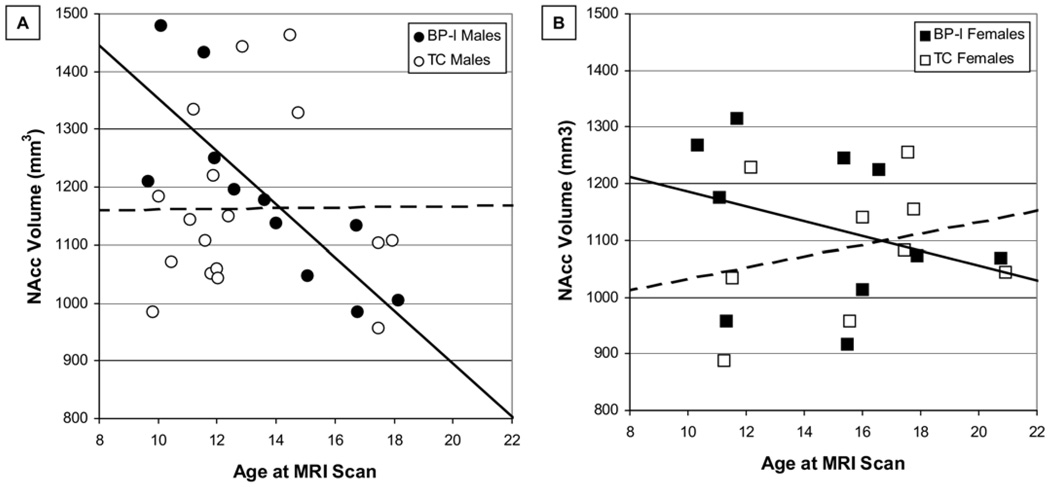
As shown in Figure 2 and Figure 3, there were significant age × group interactions in models of AMG [F(1,27) = 5.5, p = .028] and NAcc [F(1,27) = 5.3, p = .030] volumes in male subjects but not in female subjects. Age × group interactions in models of mOFC, rACC, and HC were not significant.
Figure 2.
Amygdala (AMG) volume by age and sex in bipolar I disorder (BP-I) and typically developing control (TC) subjects. (A) The solid line illustrates the relationship between age and AMG volume in male BP-I subjects, whereas the dashed line illustrates the relationship between age and AMG in male TC subjects. Significant predictors in a general linear model of AMG volume in male subjects were group (p = .046) and the age × group interaction (p = .028). (B) The solid line illustrates the relationship between age and AMG volume in female BP-I subjects, whereas the dashed line illustrates the relationship between age and AMG in female TC subjects. Age was a significant predictor in a general linear model of AMG volume in female subjects (p = .016). MRI, magnetic resonance imaging.
Figure 3.
Nucleus accumbens (NAcc) volume by age and sex in BP-I and TC subjects. (A) The solid line illustrates the relationship between age and NAcc volume in male BP-I subjects, whereas the dashed line illustrates the relationship between age and NAcc volume in male TC subjects. Significant predictors in a general linear model of NAcc volume in male subjects were age (p = .034), group (p = .025), and the age × group interaction (p = .030). (B) The solid line illustrates the relationship between age and NAcc volume in female BP-I subjects, whereas the dashed line illustrates the relationship between age and NAcc volume in female TC subjects. There were no significant predictors of NAcc volume in a general linear model that included age, group, and the age × group interaction. Abbreviations as in Figure 1 and Figure 2.
There were no significant differences in mOFC, rACC, HC, AMG, or NAcc volumes by maternal warmth.
All analyses detailed in the preceding text were also conducted on a subset of subjects that were matched by sex. The matched group consisted of n = 20 BP-I and n = 20 TC subjects, each group with 11 male subjects and 9 female subjects. Results of all analyses on this matched subset were consistent with the findings for the whole sample, with the following exceptions. In the matched group, stimulant use was significantly associated with greater NAcc volume [F(1,34) = 8.3, p = .007], and the age × group interactions in the general linear models of AMG [F(1,35) = 3.0, p = .092] and NAcc [F(1,35) = 3.0, p = .092] volumes were not significant.
Discussion
These data, supporting environmental influences on limbic areas, are consistent with the significant effect of another environmental factor, low maternal warmth, on relapse rates in child BP-I, even when controlling for family psychopathology (9,10). Given the known high familial aggregation in relatives of child BP-I probands (3), these ILE data emphasize the need for examining gene–environment interactions in child BP-I populations. Furthermore, investigation of relationships among ILE, NAcc volume, and onset of SUD is warranted, given the high rate of SUD in child BP-I samples (10) and the significance of early adolescent environmental factors on later SUD (30).
Various volumetric differences between BP-I and TC have been reported for prepubertal and adolescent onset BP (31–38). Speculations on reasons for these differences across samples include sample size and MRI methods. In addition, different diagnostic methods and socioeconomic background might influence phenotypic and morphometric presentations. Given the relationship of AMG activation to fear, future studies of frightening ILE on AMG volume would be informative (39).
Sexually dimorphic findings presented in Figure 2 and Figure 3 are consistent with data on sex differences in normative developmental trajectories across this age group (5,40–43).
The increased NAcc volume in the matched sample in subjects receiving stimulants is consistent with an increase in NAcc volume for methamphetamine users (44).
There are, to our knowledge, no prior studies of the relationship between environmental adversity and MRI in child BP-I for comparison.
The meaning of volumetric differences between child BP-I and TC is not discernible from this study. But, these findings might inform avenues of future research.
Limitations include relatively small sample size and lack of a control group with a non-mood diagnosis, such as schizophrenia or conduct disorder, to examine specificity. In addition, at the time of scanning, this study was underpowered to fully examine medication exposures and variable mood states. Finally, manual tracing was not performed.
Acknowledgments
This work was supported by National Institute of Mental Health (NIMH) Grants R01 MH-53063 and R01 MH-57451 and from the Nathan Cummings Research Foundation to Dr. Geller.
Dr. Geller has received research support from NIMH and the Nathan Cummings Research Foundation. Dr. DelBello has received research support from NIMH, National Institute on Drug Abuse, National Alliance for Research on Schizophrenia and Depression, Thrasher Foundation, Eli Lilly, Janssen, Pfizer, AstraZeneca, Shire, Somerset Pharmaceuticals, BMS, GSK, Repligen, and Abbott. She has served as a consultant for Pfizer, Eli Lilly, AstraZeneca, Glaxo-SmithKline, and the France Foundation (CME company). Dr. DelBello has served on the Speakers’ bureaus for AstraZeneca, Pfizer, Eli Lilly, Bristol Myers Squibb, and the France Foundation (CME company). Dr. Csernansky has received research grants from the NIMH and the National Institute on Aging, has received royalties from Medtronic for a patent held jointly with Washington University School of Medicine, and has been a paid consultant to Eli Lilly, Sanofi-Aventis, and Houston-Pharma. Dr. Harms, Dr. Wang, Ms. Tillman, and Ms. Bolhofner have no biomedical financial interests or potential conflicts of interest.
References
- 1.U.S. Census Bureau. [Accessed May 20, 2008];American Factfinder. Available at: http://factfinder.census.gov/servlet/DTTable?_bm=y&-geo_id=01000US&-ds_name=DEC_2000_SF1_U&-_lang=en&-state=dt&-mt_name=DEC_2000_SF1_U_P014&-format=&-CONTEXT=dt.
- 2.Grant BF, Stinson FS, Hasin DS, Dawson DA, Chou SP, Ruan WJ, Huang B. Prevalence, correlates, and comorbidity of bipolar I disorder and axis I and II disorders: Results from the national epidemiologic survey on alcohol and related conditions. J Clin Psychiatry. 2005;66:1205–1215. doi: 10.4088/jcp.v66n1001. [DOI] [PubMed] [Google Scholar]
- 3.Geller B, Tillman R, Bolhofner K, Zimerman B, Strauss NA, Kaufmann P. Controlled, blindly rated, direct-interview family study of a prepubertal and early-adolescent bipolar I disorder phenotype: Morbid risk, age at onset, and comorbidity. Arch Gen Psychiatry. 2006;63:1130–1138. doi: 10.1001/archpsyc.63.10.1130. [DOI] [PubMed] [Google Scholar]
- 4.Goossens D, Del-Favero J, Van Broeckhoven C. Trinucleotide repeat expansions: Do they contribute to bipolar disorder? Brain Res Bull. 2001;56:243–257. doi: 10.1016/s0361-9230(01)00657-8. [DOI] [PubMed] [Google Scholar]
- 5.Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lenroot RK, Schmitt JE, Ordaz SJ, Wallace GL, Neale MC, Lerch JP, et al. Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence [published online ahead of print November 27] Hum Brain Mapp. 2007 doi: 10.1002/hbm.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geller B, Bolhofner K, Craney JL, Williams M, DelBello MP, Gundersen K. Psychosocial functioning in a prepubertal and early adolescent bipolar disorder phenotype. J Am Acad Child Adolesc Psychiatry. 2000;39:1543–1548. doi: 10.1097/00004583-200012000-00018. [DOI] [PubMed] [Google Scholar]
- 8.Tillman R, Geller B, Nickelsburg MJ, Bolhofner K, Craney JL, DelBello MP, Wigh W. Life events in a prepubertal and early adolescent bipolar disorder phenotype compared to attention-deficit hyperactive and normal controls. J Child Adolesc Psychopharmacol. 2003;13:243–251. doi: 10.1089/104454603322572570. [DOI] [PubMed] [Google Scholar]
- 9.Geller B, Tillman R, Craney JL, Bolhofner K. Four-year prospective outcome and natural history of mania in children with a prepubertal and early adolescent bipolar disorder phenotype. Arch Gen Psychiatry. 2004;61:459–467. doi: 10.1001/archpsyc.61.5.459. [DOI] [PubMed] [Google Scholar]
- 10.Geller B, Tillman R, Bolhofner K, Zimerman B. Child bipolar I disorder: Prospective continuity with adult bipolar I disorder; characteristics of second and third episodes; predictors of 8-year outcome. Arch Gen Psychiatry. 2008;65:1125–1133. doi: 10.1001/archpsyc.65.10.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caetano SC, Olvera RL, Glahn D, Fonseca M, Pliszka S, Soares JC. Fronto-limbic brain abnormalities in juvenile onset bipolar disorder. Biol Psychiatry. 2005;58:525–531. doi: 10.1016/j.biopsych.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 12.Geller B, Zimerman B, Williams M, Bolhofner K, Craney JL, Frazier J, Beringer L. DSM-IV mania symptoms in a prepubertal and early adolescent bipolar disorder phenotype compared to attention-deficit hyperactive and normal controls. J Child Adolesc Psychopharmacol. 2002;12:11–25. doi: 10.1089/10445460252943533. [DOI] [PubMed] [Google Scholar]
- 13.Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, Aluwahlia S. A Children’s Global Assessment Scale (C-GAS) Arch Gen Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 14.Geller B, Craney JL, Bolhofner K, Nickelsburg MJ, Williams M, Zimerman B. Two-year prospective follow-up of children with a prepubertal and early adolescent bipolar disorder phenotype. Am J Psychiatry. 2002;159:927–933. doi: 10.1176/appi.ajp.159.6.927. [DOI] [PubMed] [Google Scholar]
- 15.Geller B, Zimerman B, Williams M, Bolhofner K, Craney JL, DelBello MP, Soutullo C. Reliability of the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) mania and rapid cycling sections. J Am Acad Child Adolesc Psychiatry. 2001;40:450–455. doi: 10.1097/00004583-200104000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Tillman R, Geller B, Craney JL, Bolhofner K, Williams M, Zimerman B. Relationship of parent and child informants to prevalence of mania symptoms in children with a prepubertal and early adolescent bipolar disorder phenotype. Am J Psychiatry. 2004;161:1278–1284. doi: 10.1176/appi.ajp.161.7.1278. [DOI] [PubMed] [Google Scholar]
- 17.Puig-Antich J, Lukens E, Brent D. Psychosocial Schedule for School Age Children–Revised in 1986 and 1987. Pittsburgh: Western Psychiatric Institute and Clinic; 1986. [Google Scholar]
- 18.Shellock FG. [Accessed May 20, 2008];Magnetic resonance (MR) procedure screening form for patients. 2002 Available at: http://www.ufbi.ufl.edu/facilities/amris/spectro/3t/MRIscreeningform.pdf.
- 19.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 20.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 21.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischl B, Liu A, Dale AM. Automated manifold surgery: Constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- 23.Dale AM, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: A linear approach. J Cogn Neurosci. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- 24.Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 25.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 26.Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23 suppl 1:S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 27.Segonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging. 2007;26:518–529. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- 28.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 29.Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: The effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 30.Kendler KS, Schmitt E, Aggen SH, Prescott CA. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Arch Gen Psychiatry. 2008;65:674–682. doi: 10.1001/archpsyc.65.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DelBello MP, Zimmerman ME, Mills NP, Getz GE, Strakowski SM. Magnetic resonance imaging analysis of amygdala and other subcortical brain regions in adolescents with bipolar disorder. Bipolar Disord. 2004;6:43–52. doi: 10.1046/j.1399-5618.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 32.Wilke M, Kowatch RA, DelBello MP, Mills NP, Holland SK. Voxel-based morphometry in adolescents with bipolar disorder: First results. Psychiatry Res. 2004;131:57–69. doi: 10.1016/j.pscychresns.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Chang K, Karchemskiy A, Barnea-Goraly N, Garrett A, Simeonova DI, Reiss A. Reduced amygdalar gray matter volume in familial pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:565–573. doi: 10.1097/01.chi.0000159948.75136.0d. [DOI] [PubMed] [Google Scholar]
- 34.Frazier JA, Chiu S, Breeze JL, Makris N, Lange N, Kennedy DN, et al. Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am J Psychiatry. 2005;162:1256–1265. doi: 10.1176/appi.ajp.162.7.1256. [DOI] [PubMed] [Google Scholar]
- 35.Frazier JA, Breeze JL, Makris N, Giuliano AS, Herbert MR, Seidman L, et al. Cortical gray matter differences identified by structural magnetic resonance imaging in pediatric bipolar disorder. Bipolar Disord. 2005;7:555–569. doi: 10.1111/j.1399-5618.2005.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahn MS, Breeze JL, Makris N, Kennedy DN, Hodge SM, Herbert MR, et al. Anatomic brain magnetic resonance imaging of the basal ganglia in pediatric bipolar disorder. J Affect Disord. 2007;104:147–154. doi: 10.1016/j.jad.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 37.Chiu S, Widjaja F, Bates ME, Voelbel GT, Pandina G, Marble J, et al. Anterior cingulate volume in pediatric bipolar disorder and autism. J Affect Disord. 2008;105:93–99. doi: 10.1016/j.jad.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 38.Frazier JA, Hodge SM, Breeze JL, Giuliano AJ, Terry JE, Moore CM, et al. Diagnostic and sex effects on limbic volumes in early-onset bipolar disorder and schizophrenia. Schizophr Bull. 2008;34:37–46. doi: 10.1093/schbul/sbm120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bertolino A, Arciero G, Rubino V, Latorre V, De Candia M, Mazzola V, et al. Variation of human amygdala response during threatening stimuli as a function of 5'HTTLPR genotype and personality style. Biol Psychiatry. 2005;57:1517–1525. doi: 10.1016/j.biopsych.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 40.Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: Ages 4–18 years. J Comp Neurol. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 41.Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, et al. Quantitative magnetic resonance imaging of human brain development: Ages 4–18. Cereb Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- 42.Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, Rapoport JL. Sexual dimorphism of the developing human brain. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1185–1201. doi: 10.1016/s0278-5846(97)00158-9. [DOI] [PubMed] [Google Scholar]
- 43.Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: A structural MRI study. Dev Med Child Neurol. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- 44.Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TD, et al. Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry. 2005;162:1461–1472. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]