Abstract
We describe a novel ultrasensitive cell-based immunocytotoxicity assay for detecting less then 1 pg/ml of Clostridium difficile toxins in porcine clinical samples. The assay is simple to perform with a turnaround time of approximately 3 hours and capable of detecting less then 1 pg/ml of toxin A. Using this assay, we were able to detect the presence of C. difficile toxins in the fecal and serum specimens of experimentally infected piglets.
Keywords: Clostridium difficile, toxins, diagnosis, cytotoxicity
Clostridium difficile is a gram-positive, spore forming, anaerobic bacterium. It is the leading cause of antibiotic-associated diarrhea, the severity of which ranges from mild diarrhea to life threatening pseudomembranous colitis [1]. Pathogenic C. difficile strains excrete exotoxins A (TcdA) and B (TcdB) that have been intimately linked to its pathogenicity. Both TcdA and TcdB are enterotoxic, capable of inducing intestinal epithelial damage and increasing mucosal permeability, and hence are thought to be responsible for the pathogenesis of C. difficile-associated colitis [2].
The diagnosis of C. difficile infection remains a challenge [3]. The current diagnostic modalities mainly consist of the detection of the C. difficile organisms and of their toxins in fecal samples. Isolation of C. difficile from stool culture is seldom carried out clinically because it is labor-intensive and time-consuming [4]. One method commonly used is the detection of the enzyme glutamate dehydrogenase (GDH) of C. difficile, but this approach cannot distinguish the toxigenic strains from non-toxigenic ones. Other methods, such as real-time PCR for detecting bacterial genes, are under evaluation for the diagnosis of C. difficile-associated disease [5], but require sophisticated equipment and training. These assays, which detect the organism, are associated with an inherent problem in that 10% to 30% of hospitalized patients are colonized with toxigenic or non-toxigenic C. difficile without disease [6]. It is therefore more desirable to detect toxins which are thought to be the cause of C. difficile-associated diarrhea (CDAD) [7]. The widely used enzyme immunoassays (EIAs) are based on monoclonal antibodies (MAbs) that recognize TcdA and / or TcdB. EIAs are rapid and easy, but suffer from low to moderate sensitivity [8]. The cytotoxin B assay is the “gold standard” for the laboratory diagnosis of C. difficile infection due to its high sensitivity and specificity [9]. It mainly detects the presence of TcdB, which is far more potent than TcdA in causing cytopathic changes in cultured cells. The drawbacks of cytotoxin B assay are technical complexity, slow turnaround time (24 − 72 hr) and the requirement for a cell culture facility [9]. Given the dramatic increase of cases and severity of CDAD in recent years, a rapid and easy to perform assay with high sensitivity and specificity for the diagnosis of C. difficile infection is an urgent need.
Here we report a novel cell-based immunocytotoxicity assay for detecting C. difficile toxins. We generated an anti-C. difficile toxin A (TcdA) monoclonal antibody, named A1H3, which substantially enhanced the activity of TcdA on Fc gamma receptor I (FcγRI)-expressing cells [10]. We applied A1H3, in combination with an electronic sensing system, to develop a real-time and ultrasensitive assay for the detection of biological activity of C. difficile toxins. The assay was easy-to-perform and particularly sensitive for TcdA at a level of 0.1 to 1 pg/ml, with a short turnaround time of 3 hr.
The mRG1−1, an engineered CHO cell line expressing murine FcγRI-α-chain [11], was provided by Dr. Daniel Conrad (Virginia Commonwealth University). The highly purified recombinant holotoxins TcdA and TcdB used in this study have equivalent biological activities to native toxins [12]. A1H3 is a mouse anti-TcdA MAb of IgG2a isotype generated in our laboratory.
Gnotobiotic piglets were maintained within sterile isolators as previously described [13]. Piglets were inoculated orally with 1×106 to 108 of C. difficile (NAP1/027 strain) spores (n=12) at the age of 2 to 5 days. The fecal samples were collected at day 0 before inoculation and daily post-inoculation thereafter. The specimens were stored in aliquots at −20°C until further use. For sample processing, stool aliquots were thawed on ice and diluted in PBS (1:10, wt/vol). The supernatant was then harvested by centrifugation and passed through a 0.45 μm filter.
The real-time cell electronic sensoring (RT-CES, or xCELLigence) system [14] (Roche Applied Science, Indianapolis, IN) was employed to monitor the dynamic response of mRG1−1 to C. difficile toxin stimulation via measurement of cell index. CI is a parameter to describe electronic impedance, which corresponds to the number of cells attaching to the bottom of microelectrode-embedded microplate (E-plate) wells. In addition, the CI value is positively affected by the extent of cells spreading on the bottom [14]. C. difficile toxins disrupt cell attachment and cause cell rounding (i.e. reduce cell spreading), thus lowering the CI values.
A 16-well E-plate was seeded with mRG1−1 cells (2×104/well) before being placed on the RT-CES device station. Cells were either grown overnight before the addition of toxins or biological samples in the absence or presence of a saturating dose of A1H3, or mixed with these reagents directly before being added into the E-plates. To block toxin activity, rabbit antiserum against TcdA (generated in our laboratory) or goat antiserum against both TcdA and TcdB (TechLab Inc.) was applied. The dynamic change in impedance as a result of cell attachment was recorded using a parameter of cell index (CI).
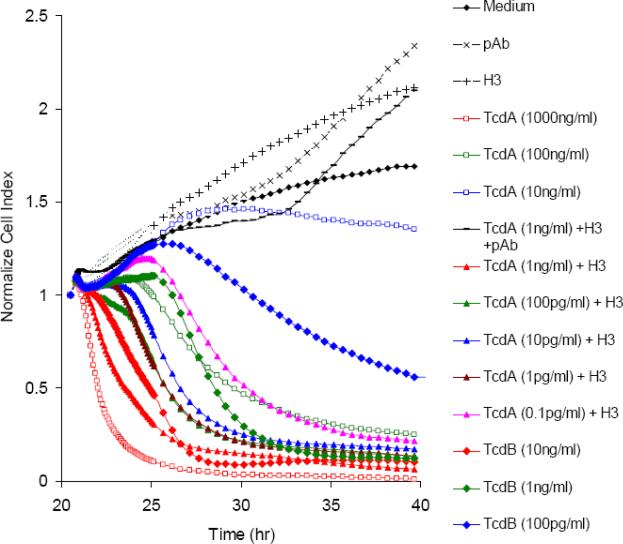
The RT-CES system was employed for a real-time detection of C. difficile toxin activity. As shown in Fig 1A, a dynamic response recorded by RT-CES revealed that A1H3 enhanced the sensitivity of mRG1−1 cells to the cytotoxic effect of TcdA by a factor of at least 1000. A rapid decrease in CI (shown in red lines in Fig 1A) within hours following the addition of toxins was observed in cells treated with 1000 ng/ml of TcdA, 10 ng/ml of TcdB, or TcdA at 1 ng/ml in the presence of A1H3. After 20 h of incubation, TcdA at a dose as low as 0.1 pg/ml was sufficient to render a complete loss of CI when A1H3 was present. This was in sharp contrast to the observation that TcdA alone at 10 ng/ml only resulted in a partial loss of CI as compared to the medium control (Fig 1A). The cytotoxic effect on mRG1−1 cells by TcdA / A1H3 was completely blocked by rabbit-anti-TcdA sera, confirming that the loss of CI was TcdA-specific (Fig 1A). TcdB at doses of 10 or 1 ng/ml also sharply decreased electronic resistance, whereas a lower dose (100 pg/ml) resulted in a slow reduction of CI overtime (Fig 1A). A1H3 neither cross-reacts with TcdB nor enhances its biological activity (data not shown). Nevertheless, the cytotoxic effect of TcdB on mRG1−1 cells was significantly higher than that of TcdA in the absence of A1H3 (Fig 1).
Fig 1. Real-time monitoring of cytotoxic effect of C. difficile toxins on mRG1−1 using RT-CES system.
(A) The mRG1−1 cells were seeded on 16-well E-plates at the 0 hr time point. After an overnight culture, the cells were exposed to the indicated amount of toxins in the absence or presence of A1H3 (H3). The control groups included the cells with PBS, rabbit antiserum, or A1H3 alone. (B) The freshly thawed mRG1−1 cells were seeded on 16-well E-plates simultaneously with a mixture of the same amount of TcdA and TcdB in the absence or presence of A1H3 (H3). In the serum blocking experiment, the rabbit-anti-TcdA serum (pAb) or goat anti-TcdA and –TcdB serum (polyAb) was mixed with the toxins and A1H3 and then added to the cells. The dynamic changes in CI were recorded by RT-CES at a 15-min interval. The data shown here is from the representative experiments.
One of the disadvantages of a tissue-culture-based assay for detection of C. difficile toxins is the slow turnaround time [9]. To overcome this, the freshly thawed mRG1−1 cells from cryopreservation were added together with the toxins to E-plates. As shown in Fig 1B, an increase in CI value over time was observed in control cells (PBS vehicle treatment). In contrast, the CI remained low when cells were treated with a mixture of TcdA and TcdB at doses of 0.1 to 1 ng/ml or higher, indicating the intoxicated cells had a reduced ability attach to the bottom of E-plates. The presence of A1H3 substantially enhanced the sensitivity of the assay, allowing the detection of toxin activity at 1 pg/ml within 4 hr (Fig. 1B). The goat antiserum against both TcdA and TcdB blocked the cytotoxic activities of the toxins and allowed cells to attach to the bottom of wells, as indicated by an increase in CI (Fig 1B).
To determine whether the immunocytotoxicity assay can be used to detect toxin activities in biological samples, mRG1−1 cells on E-plates were treated with supernatant of fecal samples from C. difficile challenged piglets. Figure 2A shows the representative data from one piglet. CI remained low when cells were treated with a 100-fold diluted fecal sample from a piglet 3 days post infection, whereas that from the same piglet before bacterial inoculation at such a dilution did not block the increase of the CI (Fig 2A). The presence of A1H3 allowed the detection of toxin activity in the 1000-fold diluted fecal sample within 2 to 3 hr (Fig 2A). Anti-serum against TcdA and TcdB completely neutralized the toxin activities in fecal samples, confirming that the low CI values caused by fecal samples were due to C. difficile toxins (Fig 2A). These data demonstrated that the immunocytotoxicity assay was capable of rapidly detecting C. difficile toxins in highly diluted fecal samples from a C. difficile infected piglet.
Fig 2. Detection of toxins biological specimens.
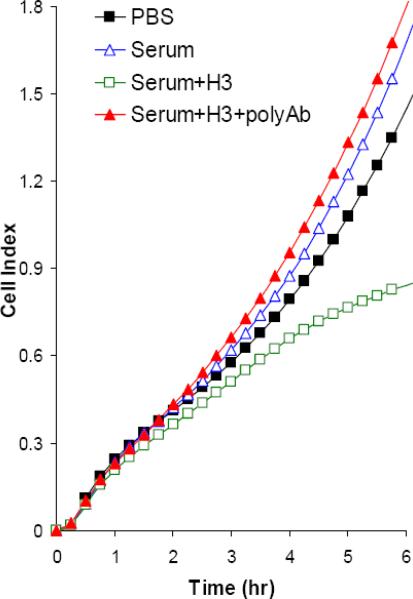
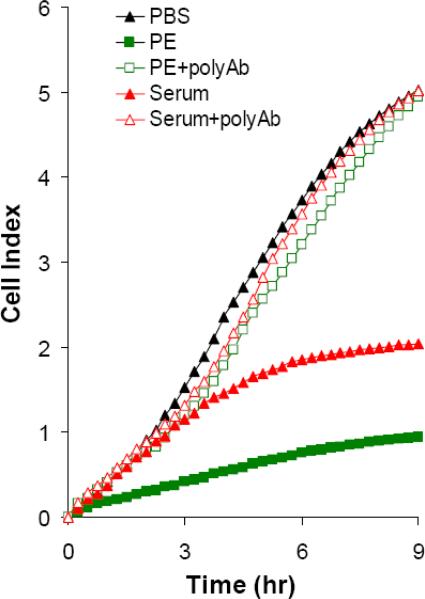
(A) Cells were mixed with diluted (100 or 1000 times) fecal samples from C. difficile pre-(F-N) or post- (F-P) inoculated gnotobiotic piglets (n=12) in the presence or absence A1H3. (B and C) Serum and pleural effusion from severely infected piglets (n=5) were diluted with PBS (final dilution 30 times) and then mixed with the freshly thawed mRG1−1 cells with or without A1H3 (H3). Serum sample from piglet #7 (B). Serum and pleural effusion (PE) samples from piglet #11 (C). In the serum blocking experiment, the goat antiserum against both TcdA and TcdB (polyAb) was mixed with the samples and mRG1−1 cells before being added into E-plate. The dynamic changes of CI were recorded by RT-CES. The data shown here is from the representative specimens.
Life-threatening cases of CDAD are often accompanied by systemic complications [15]. It has been suggested that a possible cause might be the toxins entering into circulation and disseminating systemically [16]. We have observed that the severe cases of C. difficile infection in experimental piglets are associated with systemic complications (unpublished data). We therefore measured the toxin activities in serum from the severely infected piglets using the immunocytotoxicity assay. The serum alone failed to inhibit the increase of CI as compared to that in PBS group, suggesting that the amount of toxins in the serum, if any, was not high enough to block the cell attachment (Fig 2B). However, in the presence of A1H3, the ascent of CI was partially inhibited by the serum sample (Fig 2B). The inhibitory effect was reverted by anti-sera against C. difficile toxins (Fig 2B), indicating that it was indeed a result of the toxins. Similarly, the serum and pleural effusion from another severely infected piglet reduced the ascent of CI, which was reverted by the anti-serum against C. difficile toxins (Fig 2C). Furthermore, these samples caused the rounding of mRG1−1 cells after an overnight culture only when A1H3 was present (data not shown). The anti-serum against the toxins blocked such cytopathic effects of these samples (data not shown). These data demonstrated that a low level of toxins disseminated in the circulation of the severely affected piglets, which might explain the systemic complications seen in these piglets. Systemic complications are also observed in severe cases of C. difficile infected human patients, but whether these complications are associated with the toxins in circulation remains to be determined. Our ultrasensitive immunocytotoxicity assay may offer such a determination. .
In summary, we report here a novel cell-based immunocytotoxicity assay for detecting biological activities of C. difficile toxins and we tested the assay using porcine clinical samples. Compared to the “gold standard” Cytotoxin B assay, this newly developed method is substantially more sensitive for detecting TcdA. We utilized A1H3, an anti-TcdA MAb, which substantially augments the cytotoxic activity of TcdA on FcγRI expressing cells. In addition, by utilizing freshly thawed cells and the monoclonal antibody, we reduced the turnaround time of the assay to 2−4 hr. Since the cryopreserved cells were applied directly from the freezer, a cell culture facility and expertise in cell culture techniques were no longer required. A CO2 incubator was not needed when a pH-buffered medium was used (data not shown). Furthermore, the assay is easy to perform. After mixing samples with cells and reagents into E-plates, the results were obtained in a real-time and automatic fashion. The immunocytotoxicity assay reported here is a rapid and easy-to-perform method with superior sensitivity and specificity for detecting the biological activity of C. difficile toxins, and therefore it has great potential for the diagnosis of C. difficile infection.
Acknowledgments
This work was supported by NIH N01AI30050, and in part by NIH K01DK076549 to HF and National High Technology Development Program of China (2007AA021702) to JW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflicts of interest.
Reference
- 1.Bartlett JG. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med. 2002;346:334–9. doi: 10.1056/NEJMcp011603. [DOI] [PubMed] [Google Scholar]
- 2.Kelly CP, LaMont JT. Clostridium difficile infection. Annu Rev Med. 1998;49:375–90. doi: 10.1146/annurev.med.49.1.375. [DOI] [PubMed] [Google Scholar]
- 3.Wilkins TD, Lyerly DM. Clostridium difficile testing: after 20 years, still challenging. J Clin Microbiol. 2003;41:531–4. doi: 10.1128/JCM.41.2.531-534.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartlett JG. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann Intern Med. 2006;145:758–64. doi: 10.7326/0003-4819-145-10-200611210-00008. [DOI] [PubMed] [Google Scholar]
- 5.Peterson LR, Manson RU, Paule SM, et al. Detection of toxigenic Clostridium difficile in stool samples by real-time polymerase chain reaction for the diagnosis of C. difficile-associated diarrhea. Clin Infect Dis. 2007;45:1152–60. doi: 10.1086/522185. [DOI] [PubMed] [Google Scholar]
- 6.McFarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989;320:204–10. doi: 10.1056/NEJM198901263200402. [DOI] [PubMed] [Google Scholar]
- 7.Kelly CP, LaMont JT. Clostridium difficile--more difficult than ever. N Engl J Med. 2008;359:1932–40. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 8.Planche T, Aghaizu A, Holliman R, et al. Diagnosis of Clostridium difficile infection by toxin detection kits: a systematic review. Lancet Infect Dis. 2008 doi: 10.1016/S1473-3099(08)70233-0. [DOI] [PubMed] [Google Scholar]
- 9.Chang TW, Lauermann M, Bartlett JG. Cytotoxicity assay in antibiotic-associated colitis. J Infect Dis. 1979;140:765–70. doi: 10.1093/infdis/140.5.765. [DOI] [PubMed] [Google Scholar]
- 10.He X, Sun X, Wang J, et al. Antibody-enhanced, Fc{gamma}R-mediated endocytosis of Clostridium difficile toxin A. Infect Immun. 2009 doi: 10.1128/IAI.01577-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho S, Conrad DH. A new multivalent B cell activation model--anti-IgD bound to Fc gamma RI: properties and comparison with CD40L-mediated activation. Int Immunol. 1997;9:239–48. doi: 10.1093/intimm/9.2.239. [DOI] [PubMed] [Google Scholar]
- 12.Yang G, Zhou B, Wang J, et al. Expression of recombinant Clostridium difficile toxin A and B in Bacillus megaterium. BMC Microbiology. 2008;8:192. doi: 10.1186/1471-2180-8-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krakowka S, Morgan DR, Kraft WG, Leunk RD. Establishment of gastric Campylobacter pylori infection in the neonatal gnotobiotic piglet. Infect Immun. 1987;55:2789–96. doi: 10.1128/iai.55.11.2789-2796.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abassi YA, Jackson JA, Zhu J, O'Connell J, Wang X, Xu X. Label-free, real-time monitoring of IgE-mediated mast cell activation on microelectronic cell sensor arrays. J Immunol Methods. 2004;292:195–205. doi: 10.1016/j.jim.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 15.Siemann M, Koch-Dorfler M, Rabenhorst G. Clostridium difficile-associated diseases. The clinical courses of 18 fatal cases. Intensive Care Med. 2000;26:416–21. doi: 10.1007/s001340051175. [DOI] [PubMed] [Google Scholar]
- 16.Hamm EE, Voth DE, Ballard JD. Identification of Clostridium difficile toxin B cardiotoxicity using a zebrafish embryo model of intoxication. Proc Natl Acad Sci U S A. 2006;103:14176–81. doi: 10.1073/pnas.0604725103. [DOI] [PMC free article] [PubMed] [Google Scholar]