Abstract
Single-walled carbon nanotubes (SWCNT) show unique properties find applications in micro devices; electronics to biological systems specially drug delivery and gene therapy. However the manufacture and extensive use of nanotubes raises concern about its safe use and human health. Very few studies have been carried out on toxicity of carbon nanotubes in experimental animals and humans, thus resulted in limiting their use. The extensive toxicological studies using in vitro and in vivo models are necessary and are required to establish safe manufacturing guidelines and also the use of SWCNT. These studies also help the chemists to prepare derivative of SWCNT with less or no toxicity. The present study was undertaken to determine the toxicity exhibited by SWCNT in rat lung epithelial cells as a model system. Lung epithelial cells (LE cells) were cultured with or without SWCNT and reactive oxygen species (ROS) produced were measured by change in fluorescence using dichloro fluorescein (DCF). The results show increased ROS on exposure to SWCNT in a dose and time dependent manner. The decrease in glutathione content suggested the depletion and loss of protective mechanism against ROS in SWCNT treated cells. Use of rotenone, the inhibitor of mitochondrial function have no effect on ROS levels suggested that mitochondria is not involved in SWCNT induced ROS production. Studies carried out on the effect of SWCNT on superoxide dismutase (SOD-1 and SOD-2) levels in LE cells, indicates that these enzyme levels decreased by 24 hours. The increased ROS induced by SWCNT on LE cells decreased by treating the cells with 1 mM of glutathione, N-Acetyl Cysteine, and Vitamin C. These results further prove that SWCNT induces oxidative stress in LE cells and shows loss of antioxidants.
Keywords: LE Cells, Single-Walled Carbon Nanotubes, Reactive Oxygen Species (ROS), Superoxide Dismutase
1. INTRODUCTION
Single-walled carbon nanotubes (SWCNT’s) have unique chemical and physical properties and are composed solely of carbon atoms, made of sheets of graphite rolled up to give tube like structures. The growing use of these materials will very soon find applications in energy storage, molecular electronics, micro devices, atomic force microscopy, mechanical instruments, and others. Applications of SWCNT’s in the field of biotechnology such as biosensors to drug delivery including gene therapy have recently started to emerge and hence they are expected for a large scale industrial production.1–3 However, the use of SWCNT in various industries, especially biological applications raises serious concern about the safety use and human health. SWCNT could become airborne during manufacturing process and handling and would result in inhalation and dermal exposure of workers to particles causing unknown toxic response. Though limited toxicological studies were carried out on activated carbon, graphite, and carbon fibers, quite often the industrial workers suffers from asthma and other respiratory diseases, suggests that SWCNT’s may have similar environmental hazard. The use of SWCNT’s is hindered by few toxic studies that have been carried out on animal models and cell culture studies. Therefore extensive toxicological studies are required on cell and animal models to establish safety guidelines in manufacturing process and during the application of SWCNT
Studies from our laboratory and also others have shown the toxic effect of SWCNT on keratinocytes and other cells including animal models.4–13 Studies carried out on the effect of SWCNT’s on human keratinocyte cells, indicated that the free radicals are formed with depletion of antioxidants and induction of oxidative stress.6,9 Oxidative stress in turn induced the accumulation of peroxide products, decreased cell viability6 and activation of transcription factor NFκB in these cells and related signaling in HaCaT cells.9 SWCNT was found to elicit acute inflammation and onset of progressive fibrosis and granulomas in lung of C57BL/6 mice.7 In a rat model studies SWCNT has been reported to induce the formation of multiple granulomas, causing lung toxicity and injury. However, animal studies failed to correlate well with toxicity profile of the lavage fluid, cell proliferation, dose response relationship, and non-uniform distribution of lesions and the mechanism of toxicity.5,8 The present study was undertaken to study the toxic effect of SWCNT on rat lung epithelial cells (LE cells) as the lung may be affected next to skin in industrial workers due to inhalation. Further extensive studies are required on various cell culture models as, it helps to understand the basic mechanism of toxicity exhibited by SWCNT. Such studies may also be useful to establish standard procedure in manufacturing principles and to develop counter measures to reduce the toxicity.
2. EXPERIMENTAL DETAILS
SWCNT (Catalogue Number 652512-G), Rotenone, Glutathione, N-Acetyl Cysteine, Ascorbic acid (Vitamin C), 3-(4,5-dimethylthiozol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), Glutathione assay kit were purchased from Sigma Chemical Co. (St. Louis, MO, USA). 2,7-dichlorofluoroscein diacetate (DCF-DA) was purchased from Molecular probes (Invitrogen Corp., Carlsbad, CA, USA), Rat lung epithelial cells (RL 65, ATCC; CRL-10354) were purchased from American Type Culture Collection (Manassas, VA). Dulbecco’s minimum essential medium (DMEM), Phosphate buffer saline (PBS), Fetal calf serum were obtained from Gibco (Invitrogen Corp., Carlsbad, CA, USA), Penicillin and Streptomycin were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Superoxide dismutase-1 (SOD-1; sc 11407) and superoxide dismutase-2 (SOD-2; sc 18503) antibodies were obtained from Santacruz Biotechnology Inc. (Santacruz, CA, USA).
In our studies, SWCNT particles were suspended in Dimethylformamide (DMF) and sonicated for 5 minutes and henceforth in all control experiments the cells were treated with equivalent volume of DMF.
2.1. Cell Culture and Treatments
Rat lung epithelial cell (LE cells) cultures were maintained in DMEM supplemented with 10% fetal calf serum, Penicillin (100 μg/ml) and Streptomycin (100 μg/ml) under the atmosphere of 5% CO2, 95% air in humidified incubator at 37 °C. The cells were incubated with or without SWCNT or substances in 96 well plates or 6 well plates for time intervals as indicated in the figure legends.
2.2. Measurement of Intracellular ROS
Oxygen radicals collectively called as reactive oxygen species plays a key role in cytotoxicity. Increased ROS levels in cells by chemical compound reflect toxicity and cell death. The level of ROS present in living cells were quantified essentially as described earlier.14 Equal number of rat lung epithelial cells (10,000 number/well in 96 well plate in Hanks Balanced Salt Solution) were treated with 10 μM DCF-DA (2,7-dichlorofluoroscein diacetate) for 3 hours. Cells were washed with phosphate buffered saline and treated with different concentrations of SWCNT’s. Following incubation at indicated time intervals the intensity of fluorescence is measured at excitation and emission of wavelength at 485/527 nm, respectively and expressed as fluorescence units.
2.3. MTT Assay
MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay, was first described by Mosmann in 1983,15 was performed as described earlier with minor modifications.9 Rat LE cells (2000/well) were cultured in 96 wells plate and were incubated with different concentrations of SWCNT’s for 72 hours at 37 °C. The cells were washed with PBS and MTT at a final concentration (125 μg) was added and incubated further for 3 hrs at 37 °C. Then absorbance was measured at 570 nm.
2.4. Effect of Rotenone on SWCNT Induced ROS
The major site of ROS production in a cell is mitochondrial electron transport chain, or the group of enzymes mixed function oxidases that uses cytochrome P450. To determine the role of mitochondria in SWCNT induced ROS production, the LE cells were incubated with rotenone (Sigma) the inhibitor of electron transport chain. Cells were incubated with or without 10 μM rotenone for 3 hours and 24 hours. Then the cells are washed and incubated with SWCNT (10 μg) and DCF-DA for 3 h. The fluorescence was determined as described above.
2.5. Glutathione Assay
Reduced glutathione (GSH) is the major free sulfhydryl groups containing molecule, present in cells and is involved in detoxification of xenobiotics, removal of ROS and maintenance of oxidation state of protein sulfhydryl groups. It is the key antioxidant present in most of the cells. Increased ROS may deplete the concentration of GSH in cells and tissues. The concentration of GSH in cells was measured by glutathione assay kit as per the instructions provided by the manufacturer. In brief equal number of cells were grown in 6 well plates and treated in triplicates with or without SWCNT (10 μg/ml) and incubated for 6 hours at 37 °C. The cells were scraped and homogenized in PBS and deproteinized with 5% 5-sulfosalicylic acid and centrifuged to remove protein precipitate. The supernatant was treated with 5. 5′-dithiobis (2-nitrobenzoic acid; DTNB). GSH reduce DTNB to TNB and oxidized to GSSG. Oxidized GSSG present in cells react with added NADPH to give GSH, which later also reacts with DTNB to give TNB. The total TNB formed is measured by absorption at 412 nm in a spectrophotometer.
2.6. Immuno Blot Analysis of SOD-1 and SOD-2
It is a well known fact that ROS plays an important role in pathophysiology of various diseases from neurological disorders to cancer.16 These constantly produced ROS are scavenged by superoxide dismutase (SOD), glutathione peroxidase, and catalase. SOD specifically processes superoxide anion (O2−) and produces hydrogen peroxide. Three types of SOD’s have been isolated of which the two major ones SOD-1 and SOD-2 are involved in detoxification of ROS.17 Hence immunoblot analysis of SOD-1 and SOD-2 was done in cell lysate from cells treated with SWCNT for 24 h. Control and SWCNT treated cells were washed with chilled PBS in presence of protease inhibitor and cell extracts were made in PBS by homogenization. Protein concentration was measured by Bradford’s method as described earlier.18 Protein (75 μg) was mixed with sample buffer and boiled for 5 minutes and resolved on sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to nylon membranes. The membranes were then probed with SOD-1 or SOD-2 antibodies, followed by washing thrice with PBS and incubated with second antibody coupled to horse radish peroxidase. To visualize the appearance of the protein bands the membrane was probed with chemiluminiscence reagent by standard procedure as described earlier.9
2.7. Effect of Antioxidants on SWCNT Induced ROS Production
The cells have preventive mechanism to reduce or neutralize the ROS constantly formed. While in the presence of toxic compounds this preventive mechanism is reduced which can be restored by the addition of compounds with known antioxidant activity. The effect of antioxidants also leads to understand the counter measure strategy. LE cells were treated with 1 mM concentrations of glutathione (GSH), N-Acetyl cysteine (NAC), and Vitamin C (Ascorbic acid) for 24 h. These conditioned cells were treated with DCF for 3 h and washed and incubated with or with out SWCNT (10 μg/ml) for additional 3 h. ROS formed in cells were measured by change in DCF fluorescence as described above.
3. RESULTS AND DISCUSSION
3.1. SWCNT Induces Oxidative Stress in LE Cells
Studies carried out on the effect of SWCNT on rat LE cells suggest that SWCNT induces ROS in a dose dependent manner (Fig. 1(a)). SWCNT increased ROS by 6 fold as compared to control at concentration as low as 2.5 μg. SWCNT was also found to increase ROS in a time dependent manner and is all most 7 fold higher than the control values in 6 hours at 1 μg/ml (Fig. 1(b)). Recent studies carried out from our laboratory also show that SWCNT induces ROS in a dose dependent manner in human keratinocytes 9. Our recent studies in BJ Foreskin cells treated with SWCNT have shown significant increase in ROS at concentrations of 6 μg/ml in a dose and time dependent manner.14 However, the studies on LE cells suggest that low concentrations of SWCNT (0.1–1 μg) induced non-significant amounts of ROS (data not shown) and this difference may be due to difference in the uptake of SWCNT or difference in the interaction of SWCNT with different cell types. Under normal conditions, the ROS generation occurs naturally in all most all cells. Cells or organisms are constantly exposed to environmental free radicals, UV, gamma radiation, light, smog, tobacco smoke, certain organic compounds, drugs, heavy metal ions, free iron, alcohol, induce ROS, and there by increase in normal values, causing toxicity and certain kinds of diseases.19
Fig. 1.
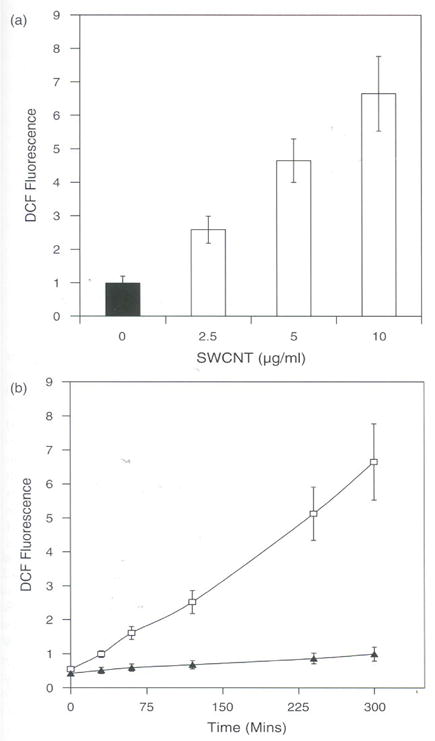
(a) SWCNT induces ROS in rat LE Cells. Equal number of cell (10,000) per well was seeded in a 96 wells plate in DMEM containing 10% FCS and penicillin (100 μg/ml), streptomycin (100 μg/ml) and incubated at 37°C in humidified chamber under 95% carbon dioxide and 5% oxygen for 18 h. Then cells were washed with Hank’s Balanced Salt Solution (HBSS) and incubated in the presence of 10 μM DCF for 3 h in HBSS. Cells were then washed with HBSS and incubated with different concentrations of SWCNT (2.5.. 5.0, and 10.0 μg per ml) in DMEM and further incubated for 3 hours. Fluorescence was measured at the end of 3 h and expressed as fluorescence units mean ± SD of 8 wells and the figure is a representative from three experiments performed independently, (b) Time course induction of ROS by SWCNT in rat LE cells. Equal number of cells (10,000) per well was seeded in a 96 wells plate in DMEM containing 10% FCS and penicillin (100 μg/ml), streptomycin (100/μ/ml) and incubated at 37°C in humidified chamber under 95% carbon dioxide and 5% oxygen for 18 h. Then cells were washed with Hank’s Balanced Salt Solution (HBSS) and incubated in the presence of 10 μM DCF for 3 h in HBSS. Cells were then washed with HBSS and incubated with SWCNT (5,0 μg per ml) in DMEM, Fluorescence was measured at different time intervals (30, 60, 120, 240, and 300 mins) and the values are expressed as fluorescence units mean ± SD of 8 wells and the figure is a representative from three experiments performed independently.
3.2. SWCNT Decreases Cell Viability
To study the extent of damage caused by SWCNT on cell viability, MTT assay was carried in LE cells treated with various concentrations of SWCNT and the results suggest that the cell viability decreases with increase in the concentration SWCNT by 72 hours compared to control cells. Only 40% of cells found to be viable at the concentration of 10 μg/ml of SWCNT and was found to be statistically significant (Fig. 2). Studies from our laboratory on other cells (Hela, HI299, A 549, and HaCaT cells) also show cell viability decreased on exposure to SWCNT’s by more than 90%.9 Overproduction of ROS is known to induce signals that lead to cell death. The present result correlates well with the observation of increased ROS and loss of cell viability. In a recent report using various nano-materials differing in size and shape reflect that SWCNT treatment can induce significant ROS and influence cell viability.13 Further, loss of cell viability was shown to be due to apoptosis and necrosis by MWCNT however the precise mechanism yet to be worked out.20 Our previous report showing activation of NF KappaB in HaCaT cells do provide partial insight to the mechanism of apoptosis. Involvement of NF kappaB in apoptosis has been extensively studied with various treatments and in variety of cell lines. Taken together all this it is suggested that oxidative stress induced by SWCNT in LE cells may trigger signals that inhibit cell proliferation leading to cell death via apoptosis or necrosis.
Fig. 2.
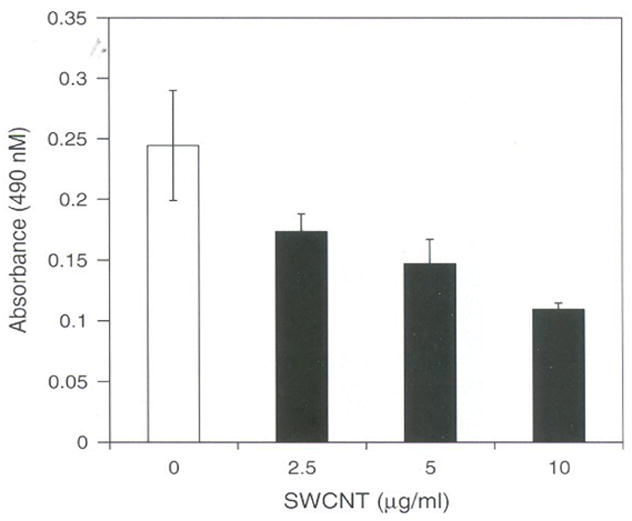
MTT assay effect of SWCNT’s on cell viability. Equal number of cells (5000) per well was seeded in a 96 wells plate in DMEM containing 10% FCS and penicillin (100 μg/ml), streptomycin (100 μg/ml) and incubated at 37°C in humidified chamber under 95% carbon dioxide and 5% oxygen for 18 h. Later cells were incubated with different concentrations of SWCNT (2.5. 5.0, and 10.0 μg per ml) in DMEM containing 10% FCS and incubated further for 72 hours. The cells viability was assayed by MTT dye uptake and the results are expressed as absorbance measured at 570 nm mean ± SD of 8 wells and the figure is a representative from three experiments performed independently.
3.3, Rotenone has no Effect on SWCNT Induced ROS Production
The potential source of ROS may be from numerous chemical reactions such as auto-oxidation, photochemical oxidation, and enzymatic reactions.21–24 The source of ROS produced in LE cells exposed to SWCNT may be contributed by the enzyme systems of electron transport located in mitochondria. Mitochondria generate hydrogen peroxide, superoxide anion up to 2% of total oxygen consumption24 and it occurs mainly at complex I.25 Other important extra mitochondrial enzymatic reactions capable of generating ROS in cells are due to the activity of mixed-function oxidases (NADPH oxidase, Monoamine oxidase, and Xanthine oxidase) that contains cytochrome P450.26
To gain insight into the source of ROS produced due to SWCNT toxicity, the LE cells were treated with rotenone an inhibitor of mitochondrial electron transport complex I for 3 hours and 24 hours. Results of such a study (Fig. 3) show that rotenone have no effect on SWCNT induced ROS production both at 3 hours and 24 hours, suggested that the mitochondria is not involved in ROS generation. However, rotenone treated in most cell types including lung slices show considerably reduced ROS production suggesting the involvement of mitochondria in these studies.27 Since mitochondria was not the source of ROS induced by SWCNT in LE cells, it therefore suggest that antioxidant systems involved in scavenging the oxidative radicals in the cytosol could be the essential target for further studies.
Fig. 3.
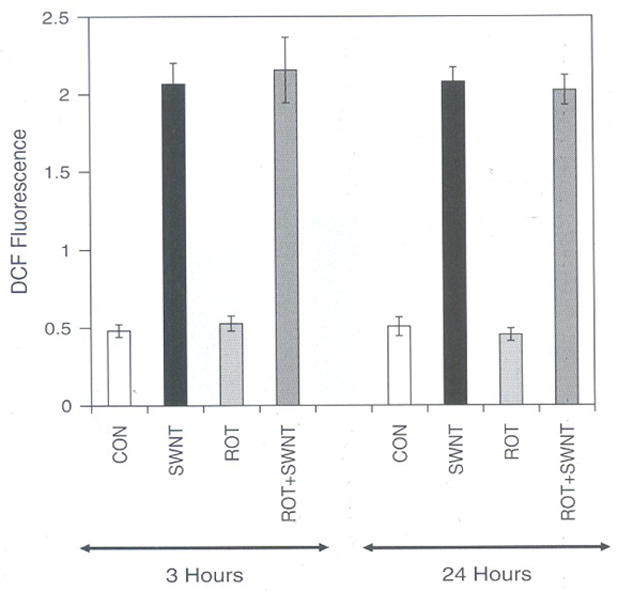
Effect of Rotenone (Mitochondrial Electron transport chain inhibitor) on SWCNT induced ROS in rat LE Cells. Equal number of cells (10,000) per well was seeded in a 96 wells plate and incubated in CO2 and oxygen for 3 h or 24 h with or with out 10 μM Rotenone in DMEM containing 10% FCS and penicillin (100 μg/ml), streptomycin (100 μg/ml) cells were then washed with Hank’s Balanced Salt Solution (HBSS). Cells were incubated in the presence of 10 μM DCF for 3 h in HBSS. Cells were then washed with HBSS and incubated with or with out SWCNT (10.0 μg per ml) in DMEM. Fluorescence was measured at the end of 3 h and expressed as fluorescence units mean ± SD of 8 wells and the figure is a representative from three experiments performed independently.
3.4. SWCNT Depletes Cellular Glutathione Levels
Because ROS production is a natural process the cells have evolved some protective mechanisms.28 The protective mechanisms are multiple antioxidant defenses that include both non-enzymatic and enzymatic mechanisms. Non-enzymatic mechanisms include vitamin C, vitamin E, Glutathione that neutralizes ROS. The most common antioxidant mechanism present in all most all cells is GSH system which includes mainly a factor reduced GSH, glutathione peroxidase and glutathione reductase, and nicotinamide adenosine dinucleotide phosphate. This system effectively removes hydrogen peroxide and other radicals.19 All these protective mechanisms are limited and hence increased ROS often depletes the protective mechanism present in cells.29 In the present study, the effect of SWCNT treatment to LE cells considerably decreased total GSH at 10 μg concentration. The decrease in glutathione was approximately more than 65% as compared to control by 6 h (Fig. 4). Similar findings on variety of cell systems using various compounds have suggested that toxic effect of these compounds was due to induction of ROS and due to depletion of GSH. The toxic effect exhibited by glutamate on C6 rat glioma cells was found to be due to increased ROS and reduced intracellular glutathione concentration by more than 85% of control levels and lead to cytolysis and apoptosis.30 The total loss of GSH by SWCNT justifies the cell death as shown by MTT assay. Apoptosis of Fibroblast cells are found to be induced by oxidative stress by multiwalled carbon nanotubes could be due to depletion of GSH.20
Fig. 4.
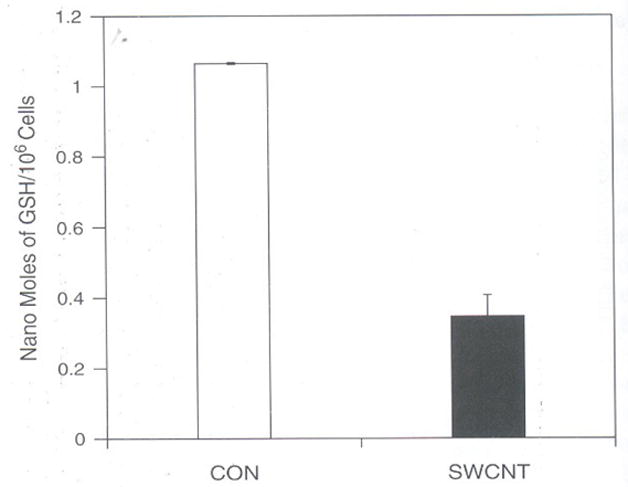
SWCNT depletes GSM levels in rat LE Cells. Equal number of cells (106) per well was seeded in a 6 wells plate in DMEM containing 10% FCS and penicillin (100 μg/ml), streptomycin (100 μg/ml) and incubated at 37°C for 3 h in humidified chamber under 95% carbon dioxide and 5% oxygen with or without SWCNT (10.0 μg per ml) in DMEM containing 10% FCS. The cells were scraped and homogenized in PBS and deproteinized with 5% 5′-sulfosalicylic acid and centrifuged to remove protein precipitate. The supernatant was treated with 5,5′-dithiobis (2-nitrobenzoic acid; DTNB). The total TNB formed is measured in a spectrophotometer at 412 nm which is proportional to the concentration of GSH. The results are expressed as nano moles of GSH present/106 mean ± SD of 3 wells and the figure is a representative from three experiments performed independently.
3.5. SWCNT Decreases SOD-1 and SOD-2 Levels
The important cellular enzymes SOD along with glutathione peroxidase and catalase play an important role in scavenging ROS regularly formed during normal physiological conditions. Effect of SWCNT on LE cells suggest that SWCNT induces ROS in a dose and time dependent manner (Figs. 1(a) and (b)). The increase in the levels of ROS and decreased cell viability by SWCNT suggests that there may be a loss of scavenging SOD enzymes. Immunoblot analysis clearly show significant decrease in the levels of SOD-1 and SOD-2 in LE cells treated with SWCNT for 24 h. The protein levels of SOD-1 and SOD-2 decreased by 40% and 30%, respectively as compared to control (Fig. 5). However, the levels of SOD-1 and SOD-2 levels were unchanged at earlier time points of exposure to SWCNT, up to 12 hours (Data not shown). Since the levels of GSH also observed to decrease by 24 h suggest that a concerted fall in the levels of antioxidant levels by SWCNT in LE cells. The loss of SOD-1 and SOD-2 under excessive oxidative stress is documented in various studies that generally induce ROS.31 These conditions of decreased antioxidants and ROS scavenging systems by oxidative stress developed due to SWCNT treatment to LE cells could allow in inducing the pathways that leads to cell death.
Fig. 5.
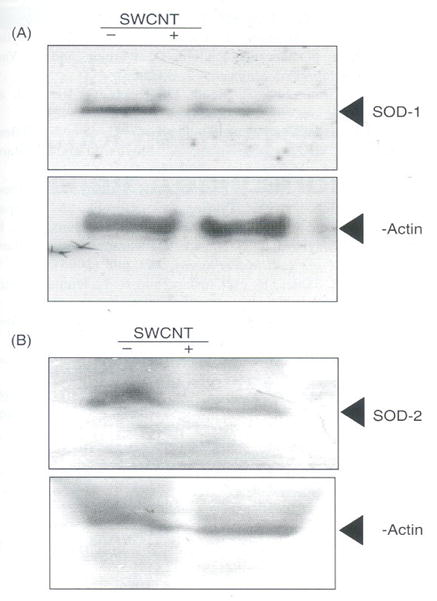
Effect of SWCNT’s on SOD-1 and SOD-2. Equal number of rat LE cells (106 cells) per well was seeded in a 6 wells plate in DMEM containing 10% FCS arid penicillin (100 μg/ml), streptomycin (100 μg/ml) incubated at 37°C in humidified chamber under 95% carbon dioxide and 5% oxygen for 18 h. Later cells were incubated with or without SWCNT (10.0 μg per ml) in DMEM containing 10% FCS and incubated further for 24 hours. Cell was homogenized in PBS and 75 μg of protien was analyzed on SDS-PAGE and transferred to nylon membrane and probed with SOD-1 (A) and SOD-2 (B) antibodies (1:3000) and the figure is a representative from three experiments performed independently.
3.6. Antioxidants Decreases SWCNT Induced ROS
Under normal conditions the cells constantly produce ROS and the excess of ROS generated are removed by antioxidants and other scavenging enzyme systems. As SWCNT induced the ROS sustained even after six hours and SOD-1 and SOD-2 decreased considerably by 24 hours prompted us to study the effect of antioxidants supplemented externally in cell culture systems to restore the increased ROS by SWCNT. The results of such a study presented in Figure 6, suggests that all the antioxidants (GSH, NAC, Vitamin C) used at 1 mM concentration decreased the ROS induced by SWCNT significantly as compared to control LE cells. Thus the data presented also confirms that SWCNT induces ROS and can be protected by the addition of exogenous antioxidants. Similar antioxidant effect of NAC and GSH, which suppressed the production of ROS in paraquat induced ROS in Swiss 3T3 cells has been reported.32 Iron rich SWCNT’s also caused significant loss of intracellular GSH and accumulation of lipid hydroperoxides in both zymosan and PMA stimulated RAW 264.7 maerophagcs.32 Therefore it seems that the induction of ROS by carbon nano-materials could be through a common signaling pathway that needs to be extensively studied to reveal the increase in ROS induced by SWCNT. Recent study carried out on RAW 264.7 macrophages show that ultrasonicated well dispersed iron rich SWCNT (26% iron) and purified SWCNT (0.23% of iron) were unable to induce intracellular production of superoxide radicals or nitric oxide. However, in the presence of zymosan, iron rich non-purified SWCNT induced hydroxyl radicals significantly compared to purified SWCNT’s, and hence concluded that the presence of iron in SWCNT may be an important factor that determines the oxidative stress responses of macrophages.32 However, extensive studies carried out using wide range of carbon materials on fibroblast cells suggested that SWCNT without iron also can induce toxicity and it is ranked as the most effective molecules in inhibiting the cell survival.33
Fig. 6.
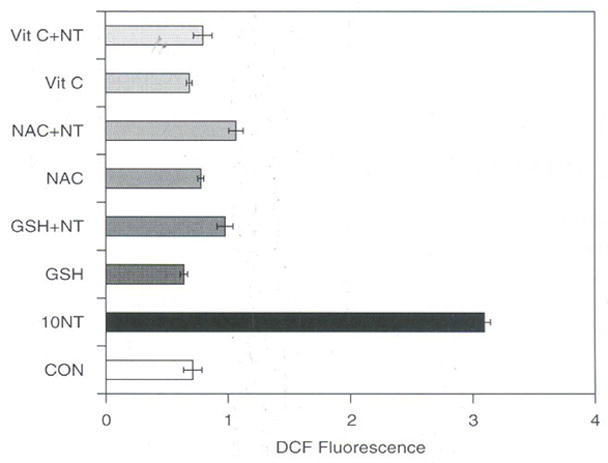
Effect of antioxidants on SWCNT induced ROS in rat LE Cells. Equal number of cells (10,000) per well was seeded in a 96 wells plate in DMEM containing 10% FCS and penicillin (100 μg/ml), streptomycin (100 μg/ml) and incubated at 37°C in humidified chamber under 95% carbon dioxide and 5% oxygen for 18 h. Then cells were washed with Hank’s Balanced Salt Solution (HBSS) and incubated in the presence of 10 μM DCF for 3 h in HBSS. Cells were then washed with HBSS and incubated with different concentrations of SWCNT (2.5, 5.0, and 10.0 μg per ml) and with or without 1 mM final concentration of antioxidants (GSH, NAC, and Vitamin C) in DMEM and further incubated for 3 h in DMEM. Fluorescence was measured at the end of 3 h and expressed as fluorescence units mean ± SD of 8 wells and the figure is a representative from three experiments performed independently.
4. CONCLUSION
Health and toxic effects associated with SWCNT’s are largely unknown, and hence multi-disciplinary and coordinated approach is required to establish toxicity and health hazard effects caused. Due to their small size, nanoparticles may pass into cells directly through the cell membrane or penetrate between or through cells and translocate to other parts of the body. In the present study, the effect of SWCNT’s on rat lung epithelial cells was examined. As low as 2.5 μg of SWCNT induce oxidative stress in these cells and oxidative stress is sustained may be because of stable polymeric nature of SWCNT. The increase in ROS by SWCNT was found to be in a dose and time dependent manner. Due to continuous increase in ROS the protective antioxidant mechanism in cells such as GSH was depicted by more than 65% compared to control levels. As a consequence the number of viable cells decreased in by more than 60% on exposure to SWCNT by 72 hours. Both SOD-1 and SOD-2 the scavenging system that helps to remove ROS also decreased significantly in the LE cells exposed to SWCNT. Antioxidants like GSH, NAC, and Vitamin C was found to counteract the ROS and these overall results indicate the generation of oxidative stress induced by SWCNT. Thus the results of the present study clearly suggest that SWCNT are toxic to LE cells and the toxicity is due to increased oxidative stress leading to cell death. Further studies are in progress to understand the mechanism of induction of oxidative stress and cell death caused by SWCNT in LE cells.
Acknowledgments
This work was supported by NASA funding NCC 9-165: NCC-1-02038: NAG 9-1414: NIH/RCMI RR03045-18 (GR).
References and Notes
- 1.Bianco A, Kostarelos K, Partidos CD, Prato M. Chem Commun. 2004;1:14. doi: 10.1039/b410943k. [DOI] [PubMed] [Google Scholar]
- 2.Ramanathan T, Fisher FT, Ruoff RS, Brinson LC. Chem Mater. 2005;17:1290. [Google Scholar]
- 3.Bianco A, Kostarelos K, Prato M. Curr Opin Chem Biol. 2005;9:674. doi: 10.1016/j.cbpa.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Oberdörster G, Maynard A, Donaldson K, Castranova V, Fitzpatrick J, Ausman K, Carter J, Karn B, Kreyling W, Lai D, Olin S, Monteiro-Riviere N, Warheit D, Yang H. 2005;2:8. doi: 10.1186/1743-8977-2-8. www.particleandfibretoxicology.com. [DOI] [PMC free article] [PubMed]
- 5.Warheit D, Laurence B, Reed K, Roaeh D, Reynold G, Webb T. Toxicol Sci. 2004;77:117. doi: 10.1093/toxsci/kfg228. [DOI] [PubMed] [Google Scholar]
- 6.Shvedova AA, Castranova V, Kisin ER, Schwegler-Berry D, Murray AR, Gandelsman VZ, Maynard A, Baron P. J Toxicol Environ Health A. 2003;66:1909. doi: 10.1080/713853956. [DOI] [PubMed] [Google Scholar]
- 7.Shvedova AA, Kisin ER, Mercer R, Murray AR, Johnson VJ, Potapovich AI, Tyurina YY, Gorelik O, Arepalli S, Schwegler-Berry D, Hubbs AF, Antonini J, Evans DE, Ku BK, Ramsey D, Maynard A, Kagan VE, Castranova V, Baron P. Am J Physiol Lung Cell Mal Physiol. 2005;289:698. doi: 10.1152/ajplung.00084.2005. [DOI] [PubMed] [Google Scholar]
- 8.Lam CW, James JT, McCluskey R, Hunter RL. Toxicol Sci. 2004;77:126. doi: 10.1093/toxsci/kfg243. [DOI] [PubMed] [Google Scholar]
- 9.Manna SK, Sarkar S, Barr J, Wise K, Barrera EV, Jejelowo, Rice-Ficht AC, Ramesh GT. Nano Lett. 2005;5:1676. doi: 10.1021/nl0507966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xing X, He X, Peng J, Wang K, Tan W. J Naaosci Nanotechnol. 2005;10:1688. doi: 10.1166/jnn.2005.199. [DOI] [PubMed] [Google Scholar]
- 11.Wei-Xian Z, Tina M. Environ Sci Technol. 2003;37:102A. doi: 10.1021/es0323998. [DOI] [PubMed] [Google Scholar]
- 12.Mihail CR, Barbara K. Environ Sci Technol. 2005;39:106A. [Google Scholar]
- 13.Jia G, Wang H, Yan L, Wang X, Pei R, Yan T, Zhao Y, Guo X. Environ Sci Technol. 2005;39:1378. doi: 10.1021/es048729l. [DOI] [PubMed] [Google Scholar]
- 14.Sarkar S, Sharma C, Yog R, Periakaruppan A, Jejelowo O, Thomas R, Barrera EV, Rice-Ficht AC, Wilson BL, Ramesh GT. J Nanosci Nanotechnol. 2007;7:584. [PMC free article] [PubMed] [Google Scholar]
- 15.Mosmann T. J Immanol Meth. 1983;65:55. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 16.Chan PH. J Cereb Blood Flow Metab. 2001;21:2. doi: 10.1097/00004647-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Murklund SL. Prog Brain Res. 1993;96:97. doi: 10.1016/s0079-6123(08)63260-4. [DOI] [PubMed] [Google Scholar]
- 18.Bradford MM. Anal Biochem. 1976;72:248. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 19.Wu D, Cederbaum J. Biol Chem. 2003;278:1115. doi: 10.1074/jbc.M207856200. [DOI] [PubMed] [Google Scholar]
- 20.Ding L, Stilwell J, Zhang T, Elboudwarej O, Jiang H, Selegue JP, Cooke PA, Gray JW, Chen EF. Nono Lett. 2005;12:2448. doi: 10.1021/nl051748o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chance B, Sies H, Boveris A. Physiol Rev. 1979;59:527. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 22.Loschen G, Flohe L, Chance B. FEBS Lett. 1971;18:261. doi: 10.1016/0014-5793(71)80459-3. [DOI] [PubMed] [Google Scholar]
- 23.Boveris A, Cadenas E. FEBS Lett. 1975;54:311. doi: 10.1016/0014-5793(75)80928-8. [DOI] [PubMed] [Google Scholar]
- 24.Boveris A, Oshino N, Chance B. Biochem J. 1972;128:617. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kehrer JP. Toxicol. 2000;149:43. doi: 10.1016/s0300-483x(00)00231-6. [DOI] [PubMed] [Google Scholar]
- 26.Kamala H, Hirata H. Cell Signal. 1999;11:1. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- 27.Paddenberg R, Ishaq B, Goldenberg A, Faulhammer P, Rose R, Weissmann N, Braun-Dullaeus RC, Kummer W. Am J Physiol Lung Cell Mol Physiol. 2003;284:710. doi: 10.1152/ajplung.00149.2002. [DOI] [PubMed] [Google Scholar]
- 28.Yu BP. Physiol Rev. 1994;74:139. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]
- 29.Wiseman H, Halliwell B. Biochem J. 1996;313:17. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higuchi Y, Matsukawa S. Free Radic Biol Med. 1998;24:418. doi: 10.1016/s0891-5849(97)00273-6. [DOI] [PubMed] [Google Scholar]
- 31.Wallace DC, Melov S. Nat Genet. 1998;19:105. doi: 10.1038/448. [DOI] [PubMed] [Google Scholar]
- 32.Kagan VE, Tyurian YY, Tyurin VA, Konduru NV, Potapovich AI, Osipov AN, Kishm ER, Sewegler-Berry D, Mercer R, Castranova V, Shvedova AA. Toxicol Lett. 2006;165:88. doi: 10.1016/j.toxlet.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Tian FR. PhD Thesis. Max Planks Ins fur Metallforschung; Stuttgart: 2006. [Google Scholar]


