Abstract
Purpose
Morin is a flavone that exhibits antiproliferative, antitumor, and anti-inflammatory effects through a mechanism that is not well understood. Because of the role of transcription factor nuclear factor-κB (NF-κB) in the control of cell survival, proliferation, tumorigenesis, and inflammation, we postulated that morin mediates its effects by modulating NF-κB activation.
Experimental Design
We investigated the effect of morin on NF-κB pathway activated by inflammatory agents, carcinogens, and tumor promoters. The effect of this flavone on expression of NF-κB – regulated gene products involved in cell survival, proliferation, and invasion was also examined.
Results
We showed by DNA-binding assay that NF-κB activation induced by tumor necrosis factor (TNF), phorbol 12-myristate 13-acetate, lipopolysaccharide, ceramide, interleukin-1, and H2O2 was suppressed by morin; the suppression was not cell type specific. The suppression of NF-κB by morin was mediated through inhibition of IκBα (inhibitory subunit of NF-κB) kinase, leading to suppression of phosphorylation and degradation of IκBα and consequent p65 nuclear translocation. Morin also inhibited the NF-κB – dependent reporter gene expression activated by TNF, TNF receptor (TNFR) 1, TNFR1-associated death domain, TNFR-associated factor 2, NF-κB – inducing kinase, IκB kinase, and the p65 subunit of NF-κB. NF-κB – regulated gene products involved in cell survival [inhibitor of apoptosis (IAP) 1, IAP2, X chromosome-linked IAP, Bcl-xL, and survivin], proliferation (cyclin D1and cyclooxygenase-2), and invasion (matrix metalloproteinase-9) were down-regulated by morin. These effects correlated with enhancement of apoptosis induced by TNF and chemotherapeutic agents.
Conclusion
Overall, our results indicate that morin suppresses the activation of NF-κB and NF-κB – regulated gene expression, leading to enhancement of apoptosis. This may provide the molecular basis for the ability of morin to act as an anticancer and anti-inflammatory agent.
Although consumption of various fruits and vegetables has been linked with decreased incidences of cancer and various other chronic illnesses, the identity of the active components in these fruits and vegetables and their molecular targets are less clear. Morin (3,5,7,2′,4′-pentahydroxyflavone; Fig. 1A) is a flavone originally isolated from members of the Moraceae family, such as mulberry figs and others Chinese herbs. It has also been isolated as yellow pigment from almond hulls and old fustic (Chlorophora tinctoria). Over the years, numerous activities have been assigned to this flavone. Morin has been shown to inhibit the growth of COLO205 cells in nude mice (1), exhibit intestinal anti-inflammatory activity in the acute phase of the trinitrobenzenesulfonic acid model of rat colitis (2, 3), inhibit azoxymethane-induced aberrant crypt foci in rats (4), exhibit chemopreventive effects on chemically induced rat tongue carcinogenesis (5), and exert anti-inflammatory effects and reduce the incidence of lipopolysaccharide (LPS)–induced septic shock (6). Morin has also been shown to suppress phorbol ester–induced transformation of hepatocytes (7).
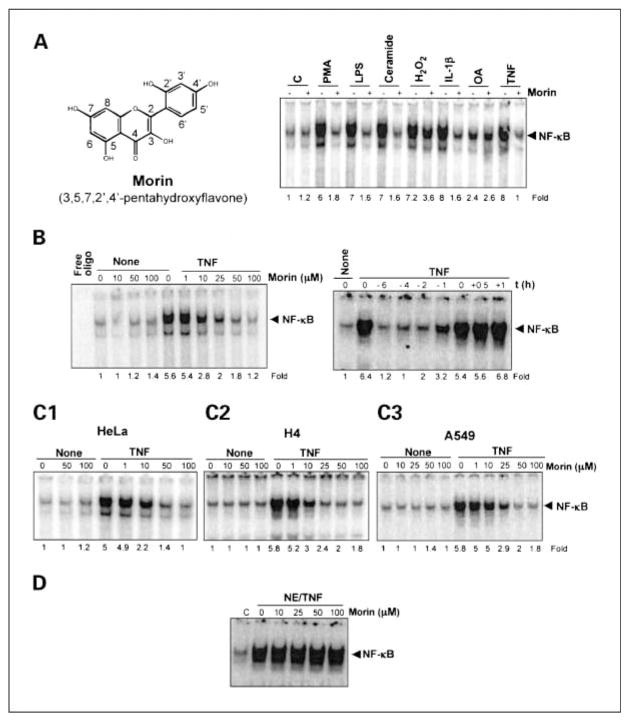
Fig.1.
A, left, structure of morin (3,5,7,2′,4′-pentahydroxyflavone); right, effect of morin on PMA-, LPS-, ceramide-, H2O2-, IL-1β, OA-, and TNF-induced NF-κB activation. KBM-5 cells (2 × 106/mL) were cultured in RPMI1640 complete (10% fetal bovine serum and antibiotics) medium, harvested at 40% confluence, and treated with morin (50 μmol/L) for 4 h. Cells were then stimulated with PMA (25 ng/mL), serum-activated LPS (100 ng/mL), ceramide (10 μmol/L), H2O2 (250 μmol/L), IL-1β (100 ng/mL), OA (500 nmol/L), and TNF (100 pmol/L) for1h, and then tested for NF-κB activation as described in Materials and Methods. Results are representative of three independent experiments. Effect of morin on TNF-induced NF-κB activation. B, left, KBM-5 cells (2 × 106/mL) were preincubated for 4 h with different concentrations (0–100 μmol/L) of morin followed by 1h of incubation with100 pmol/L TNF. After these treatments, nuclear extracts were prepared and then assayed for NF-κB as described in Materials and Methods. Right, KBM-5 cells were either preincubated, coincubated, or postincubated with 50 μmol/L morin at 37°C for the indicated times and then stimulated with 100 pmol/L TNF at 37°C for1h. After these treatments, nuclear extracts were prepared and then assayed for NF-κB. C, effect of morin on activation of NF-κB induced by TNF in different cell lines. Human epithelial (HeLa; C1), human neuronal (H4; C2), and lung carcinoma (A549; C3) cells were incubated at 37°C with different concentrations of morin for 4 h and then stimulated with100 pmol/L TNF for1h. After these treatments, nuclear extracts were prepared and then assayed for NF-κB by electrophoretic mobility shift assays. Results are representative of three independent experiments. D, in vitro effect of morin on DNA binding of NF-κB protein. Nuclear extracts (NE) were prepared from100 pmol/L TNF-treated KBM-5 cells; 12 μg/sample NE protein was treated with the indicated concentrations of morin for 4 h at 37°C and then assayed for NF-κB by electrophoretic mobility shift assays. Results are representative of three independent experiments.
Morin also suppresses the proliferation of a wide variety of tumor cells, including oral squamous cell carcinoma (8), leukemia (9), and colon cancer (10) cell lines, and induces differentiation of keratinocytes (11). How morin mediates these effects is not fully understood. Morin has been shown to induce p21 and activate caspases (1), suppress AKT activation and induce stress kinase activation (8), abolish peroxisome proliferator-activated receptor activity (11), inhibit P-glycoprotein (12), inhibit lipooxygenase-1 (13), suppress inducible nitric oxide synthase and cyclooxygenase-2 (COX-2) expression in macrophages (14), and inhibit the release of inflammatory cytokines, such as interleukin (IL)-6, IL-8, and tumor necrosis factor (TNF) from mast cells (15).
We postulated that morin may mediate its various activities through suppression of the transcription factor nuclear factor-κB (NF-κB) for the following reasons: (a) NF-κB is activated by various carcinogens and tumor promoters; (b) NF-κB activation has been implicated in trinitrobenzenesulfonic acid–induced rat colitis, in azoxymethane-induced aberrant crypt foci, and in chemically induced carcinogenesis; (c) NF-κB has been linked with LPS-induced septic shock; (d) NF-κB activation has been linked with cell proliferation and apoptosis; and (e) several gene products modulated by morin are regulated by NF-κB (16, 17).
NF-κB is a heterodimeric protein complex of members of the Rel (p50)/NF-κB (p65) protein family. NF-κB is primarily composed of proteins with molecular masses of 50 kDa (p50) and 65 kDa (p65) and is retained in the cytoplasm by the inhibitory subunit, IκBα (inhibitory subunit of NF-κB; ref. 18). In its unstimulated form, NF-κB is activated by a wide variety of inflammatory stimuli, including TNF, IL-1, phorbol 12-myristate 13-acetate (PMA), H2O2, ceramide, LPS, and γ-radiation. Most of these agents induce the phosphorylation-dependent degradation of IκBα proteins, allowing active NF-κB to translocate to the nucleus, where it regulates gene expression. The phosphorylation of IκBα is mediated through the activation of the IκBα kinase (IKK) complex consisting of IKK-α, IKK-β, IKK-γ (also called NF-κB essential modulator), IKK-associated protein 1, FIP-3 (type 2 adenovirus E3 14.7-kDa interacting protein), HSP90, and ELKS (19).
Because morin can inhibit inflammation, inhibit tumor promotion, suppress tumor growth, and down-regulate the expression of certain genes regulated by NF-κB, we postulated that morin modulates the activation of NF-κB and NF-κB–regulated gene expression induced by carcinogens, inflammatory agents, and immune modulators. We found that morin inhibited NF-κB activation through the suppression of IκBα phosphorylation and degradation and inhibition of IKK. It also suppressed the expression of the NF-κB–regulated gene products and potentiated apoptosis induced by TNF and chemotherapeutic agents.
Materials and Methods
Reagents
Morin was purchased from Sigma Chemicals (St. Louis, MO). A 10 mmol/L solution of morin was prepared in DMSO, stored as small aliquots at −20°C, and then diluted as needed in the cell culture medium. Bacteria-derived human recombinant TNF, purified to homogeneity with a specific activity of 5 × 107 units/mg, was kindly provided by Genentech (South San Francisco, CA). Penicillin, streptomycin, RPMI 1640, DMEM, fetal bovine serum, and Lipofect AMINE 2000 were obtained from Invitrogen (Grand Island, NY). PMA, okadaic acid (OA), H2O2, and anti– β-actin antibody were obtained from Sigma Chemicals. The antibodies anti-p65, anti-p50, anti-IκBα, anti–inhibitor of apoptosis (IAP) 1, anti-IAP2, anti-Bcl-xL, anti–cyclin D1, anti–matrix metalloproteinase-9 (MMP-9), AKT, and anti–poly(ADP-ribose) polymerase were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-COX-2 and anti–X chromosome-linked IAP antibodies were obtained from BD Biosciences (San Diego, CA). Phospho-specific anti-IκBα (Ser32/36) antibodies were purchased from Cell Signaling (Beverly, MA). Anti–IKK-α, anti–IKK-β, and phospho-AKT (Ser473) antibodies were kindly provided by Imgenex (San Diego, CA). A polyclonal antibody that recognizes the Ser536 phosphorylated form of p65 was obtained from Rockland Laboratories (Gilbertsville, PA). Survivin antibody was purchased from R&D Systems (Minneapolis, MN).
Cell lines
Human epithelial cells (HeLa), human neuronal cells (H4), human lung carcinoma cells (A549), and human embryonic kidney (A293) were obtained from American Type Culture Collection (Manassas, VA). Jurkat and A549 cells were cultured in RPMI 1640, and A293 cells were cultured in DMEM supplemented with 10% fetal bovine serum, 100 units/mL penicillin, and 100 μg/mL streptomycin.
Electrophoretic mobility shift assay
To determine NF-κB activation, we did electrophoretic mobility shift assays as described (20). For supershift assays, nuclear extracts prepared from TNF-treated cells were incubated with antibodies against either p50 or p65 of NF-κB for 30 min at 37°C before the complex was analyzed by electrophoretic mobility shift assays. The dried gels were visualized, and radioactive bands were quantified by using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA) using ImageQuant software.
Western blot analysis
To determine the levels of protein expression in the cytoplasm or nucleus, we prepared extracts of each (21) from TNF-treated cells and fractionated them by SDS-PAGE. After electrophoresis, the proteins were electrotransferred to nitrocellulose membranes, blotted with each antibody, and detected by using the enhanced chemiluminescence reagent (Amersham, Piscataway, NJ). The bands obtained were quantified using NIH image software (Bethesda, MD).
IKK assay
The IKK assay was done by a method described previously (22). Briefly, IKK complex from whole-cell extracts was precipitated with antibody against IKK-α followed by treatment with protein A/G-Sepharose beads (Pierce, Rockford, IL). After a 2-h incubation, the beads were washed with lysis buffer and then assayed in kinase assay mixture containing 50 mmol/L HEPES (pH 7.4), 20 mmol/L MgCl2, 2 mmol/L DTT, 20 μCi [γ-32P]ATP, 10 μmol/L unlabeled ATP, and 2 μg of substrate glutathione S-transferase-IκBα (amino acids 1–54). After incubation at 30°C for 30 min, the reaction was terminated by boiling with SDS sample buffer for 5 min. Finally, the protein was resolved on 10% SDS-PAGE, the gel was dried, and the radioactive bands were visualized by PhosphorImager. To determine the total amounts of IKK-α and IKK-β in each sample, 50 μg of the whole-cell protein were resolved on 7.5% SDS-PAGE, electrotransferred to a nitrocellulose membrane, and then blotted with either anti–IKK-α or anti–IKK-β antibody.
NF-κB–dependent reporter gene expression assay
The effect of morin on TNF-, TNF receptor (TNFR)–, TNFR1-associated death domain (TRADD)–, TNFR-associated factor 2 (TRAF2)–, NF-κB–inducing kinase (NIK)–, IKK-, and p65-induced NF-κB–dependent reporter gene transcription was analyzed by secretory alkaline phosphatase (SEAP) assay as described previously (22).
Immunocytochemistry for NF-κB p65 localization
The effect of morin on the nuclear translocation of p65 was examined by immunocytochemical method as described (23).
Cytotoxicity assay
Cytotoxicity was assayed by the modified tetrazolium salt 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay as described previously (23).
Live/dead assay
To measure the effect of morin on apoptosis induced by TNF and chemotherapeutic agents, we also used the live/dead assay (Molecular Probes, Eugene, OR), which determines intracellular esterase activity and plasma membrane integrity as described previously (23).
Results
Our aim in the current study was to investigate the effect of morin on the NF-κB activation pathway, NF-κB–regulated gene products, and NF-κB–regulated cellular responses. The concentration of morin and NF-κB activators used and the time of exposure had minimal effect on the viability of these cells. The structure of morin is shown in Fig. 1A (left). Most of the studies were carried out using human chronic myeloid leukemia (KBM-5) cells because these cells express both types of TNFRs. Due to difference in NF-κB activation between the cell types, we investigated the effect of morin on TNF-induced NF-κB activation in epithelial cells (HeLa), neuronal cells (H4), and lung carcinoma cells (A549).
Morin blocks NF-κB activation induced by PMA, LPS, H2O2, OA, ceramide, IL-1β, and TNF
We first investigated the effect of morin on the activation of NF-κB by PMA, LPS, H2O2, OA, ceramide, IL-1β, and TNF, which are potent activators of NF-κB with different mechanisms of action (24). As shown in Fig. 1A (right), all these inflammatory agents and carcinogens activated NF-κB, and morin pretreatment suppressed this activation. NF-κB activation by OA was less pronounced and was not significantly affected by morin. Under these conditions, morin suppressed NF-κB activation induced by H2O2 by ~50%. These results suggest that morin acts at a step in the NF-κB activation pathway that is common to all these agents. The effect of morin on the TNF-induced NF-κB activation was studied in detail because this pathway has been well characterized.
Morin inhibits TNF-dependent NF-κB activation in a dose-dependent manner
Because TNF is one of the most potent activators of NF-κB and the mechanism of activation of NF-κB is relatively well established, we first examined the effect of morin on TNF-induced NF-κB activation in human myeloid KBM-5 cells. Cells were incubated with different concentrations of morin for 4 h and then exposed to TNF. Morin by itself did not activate NF-κB, but TNF-induced NF-κB activation was inhibited by morin in a dose-dependent manner (Fig. 1B, left). Under these conditions, cells were fully viable when treated with morin.
Morin inhibits TNF-dependent NF-κB activation in a time-dependent manner
To determine the minimum time required to suppress TNF-induced NF-κB activation by morin, we incubated the cells with morin for 6, 4, 2, or 1 h before TNF treatment, or at the same time as TNF treatment, or 30 min or 1 h after the TNF treatment. NF-κB activation was suppressed only when cells were pretreated with morin for 1 h or longer (Fig. 1B, right).
Because various combinations of Rel/NF-κB protein can constitute an active NF-κB heterodimer that binds to a specific sequence in the DNA (25), to show that the retarded band visualized by electrophoretic mobility shift assays in TNF-treated cells was indeed NF-κB, we incubated nuclear extracts from TNF-stimulated cells with antibody to either the p50 (NF-κB1) or the p65 (RelA) subunit of NF-κB. Both shifted the band to a higher molecular mass, thus suggesting that the TNF-activated complex consisted of p50 and p65 subunits. Excess unlabeled NF-κB (100-fold) caused disappearance of the band, but the mutant oligonucleotide of NF-κB did not affect NF-κB-binding activity (data not shown).
Suppression of TNF-dependent NF-κB activation by morin is not cell type specific
Because the mechanism of NF-κB activation in leukemic cells may differ from that in epithelial cells (26), we investigated the effect of morin on TNF-induced NF-κB activation in different cell types. Epithelial cells (HeLa), neuronal cells (H4), and lung carcinoma cells (A549) were preincubated with different concentrations of morin for 4 h and then stimulated with 100 pmol/L TNF for 1 h. After these treatments, nuclear extracts were prepared and then assayed for NF-κB. Morin alone did not activate NF-κB, but it inhibited TNF-mediated NF-κB activation in all cell types (Fig. 1C1-C3).
Morin does not interfere with the binding of NF-κB to the DNA
Several agents have been described that suppress NF-κB binding to the DNA [e.g., CAPE (27), herbimycin (28), plumbagin (29), ethyl caffeate (30), and N-tosyl-L-phenylalanine chloromethyl ketone (31)]. We investigated whether morin also mediates its effects through a similar mechanism. Nuclear extracts from TNF-treated cells were incubated with different concentrations of morin for 30 min and then examined for NF-κB binding to the DNA. Morin had no effect on the DNA binding of NF-κB protein (Fig. 1D), indicating that morin mediates its effects through a mechanism different from that of CAPE and other agents.
Morin inhibits TNF-dependent IκBα degradation
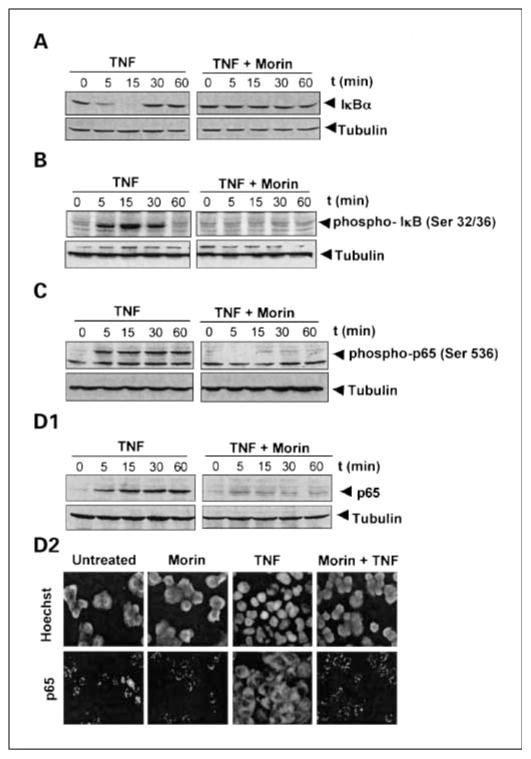
The translocation of NF-κB to the nucleus is preceded by the proteolytic degradation of IκBα (16). To determine whether inhibition of TNF-induced NF-κB activation was due to inhibition of IκBα degradation, we pretreated cells with morin and then exposed them to TNF for different times and measured cytoplasmic IκBα by Western blot analysis. We found that TNF induced IκBα degradation, which preceded NF-κB translocation in control cells by as much as 5 min, but in morin-pretreated cells TNF had no effect on IκBα degradation (Fig. 2A). Thus, morin inhibits TNF-induced NF-κB activation through inhibition of IκBα degradation.
Fig. 2.
Effect of morin on the inhibition of TNF-induced IκBα degradation (A) and phosphorylation (B). Cells were preincubated with 50 μmol/L morin for 4 h at 37°C and then stimulated with 100 pmol/L TNF for different times at 37°C. Cytoplasmic extracts were prepared and analyzed in 10% SDS-PAGE and assayed for IκBα and phospho-IκBα. C, effect of morin on TNF-induced p65 phosphorylation. KBM-5 cells (2 × 106/mL), either untreated or pretreated with 50 μmol/L morin for 4 h, were stimulated with TNF (100 pmol/L) for different times. Nuclear extracts were prepared and assayed for p65 phosphorylation by Western blot analysis using antibody directed against Ser536 residue. D1, effect of morin on TNF-induced p65 translocation. KBM-5 cells (2 × 106/mL), either untreated or pretreated with 50 μmol/L morin for 4 h, were stimulated with TNF (100 pmol/L) for different times. Nuclear extracts were prepared and assayed for p65 translocation by Western blot analysis. D2, KBM-5 cells were treated with or without morin (50 μmol/L) and then stimulated with TNF (100 pmol/L) for 30 min. Cells were subjected to immunocytochemical analysis as described in Materials and Methods. Results are representative of three independent experiments.
Morin inhibits TNF-dependent IκBα phosphorylation
The proteolytic degradation of IκBα is known to require phosphorylation at Ser32 and Ser36 residues (18). To determine the effects of morin on TNF-induced IκBα phosphorylation, we next assayed the TNF-induced phosphorylated form of IκBα by Western blot analysis, using an antibody that recognizes the serine-phosphorylated form of IκBα. TNF induced IκBα phosphorylation, and this phosphorylation was almost completely suppressed by morin (Fig. 2B).
Morin inhibits TNF-induced p65 phosphorylation
The phosphorylation of p65 has been implicated in NF-κB–mediated transcription. We therefore examined whether morin affects TNF-induced p65 phosphorylation. As shown in Fig. 2C, TNF induced p65 phosphorylation and morin inhibited it.
Morin inhibits TNF-induced nuclear translocation of p65
We also analyzed the effect of morin on TNF-induced nuclear translocation by Western blot analysis. As shown in Fig. 2D1, TNF induced the nuclear translocation of p65 in a time-dependent manner, and morin suppressed TNF-induced p65 nuclear translocation almost completely.
To further confirm the effect of morin on the suppression of nuclear translocation of p65, we did an immunocytochemistry assay and found that p65 is localized in the cytoplasm in untreated cells. TNF induced nuclear translocation of p65, and treatment with morin clearly suppressed p65 translocation to the nucleus (Fig. 2D2).
Morin inhibits TNF-induced IKK activation
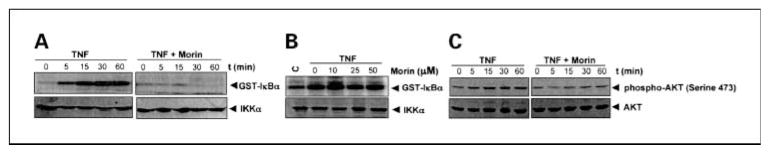
Because IKK is required for TNF-induced phosphorylation of IκBα (18) and, as we have shown here, morin inhibits the phosphorylation of IκBα, we next determined the effect of morin on TNF-induced IKK activation. We found that morin completely suppressed the TNF-induced activation of IKK (Fig. 3A). TNF or morin had no effect on the expression of IKK protein.
Fig. 3.
A, effect of morin on the TNF-induced activation of IKK. KBM-5 cells (4 × 106/mL) were pretreated with 50 μmol/L morin for 4 h and then activated with100 pmol/L TNF for the indicated times. Whole-cell extracts were prepared, and extracts were immunoprecipitated with antibodies against IKK-α and IKK-β. The immune complex kinase assay was then done as described in Materials and Methods. Thereafter, the immunocomplex kinase assay was done as described in Materials and Methods. To examine the effect of morin on the level of expression of IKKα, 50 μg of cell extract protein were analyzed in 10% SDS-PAGE and assayed for IKKα by Western blot analysis. B, to detect the direct effect of morin on the TNF-induced activation of IKK, cells were stimulated with100 pmol/L TNF for1h, and then whole-cell extracts were prepared. The extracts were then incubated with different concentrations of morin and immunoprecipitated with anti-IKKα and IKKβ antibodies. The immunocomplex kinase assay was then done. C, effect of morin on TNF-induced AKT activation. Cells (2 × 106/mL) were incubated with either medium or 50 μmol/L morin for 4 h and then treated with 100 pmol/L TNF for the indicated times. Whole-cell extracts were prepared, and phospho-AKT (Ser473) and AKT were detected by Western blot using phospho-specific anti-AKT and anti-AKT antibodies. Results are representative of three independent experiments.
To evaluate whether morin directly binds to IKK protein or suppresses its activation, we incubated untreated and TNF-treated whole-cell extracts with different concentrations of morin. Results of the immunocomplex kinase assay showed that morin did not directly affect the activity of IKK (Fig. 3B), suggesting that morin modulates TNF-induced IKK activation.
Morin inhibits TNF-induced activation of AKT
Because AKT activation is required for TNF-induced NF-κB activation (32), it is possible that morin suppressed TNF-induced IKK activation through the suppression of AKT. When we activated the morin-pretreated cells with TNF for different times, we found that morin completely suppressed TNF-induced activation of AKT (Fig. 3C).
Morin represses TNF-induced NF-κB–dependent reporter gene expression
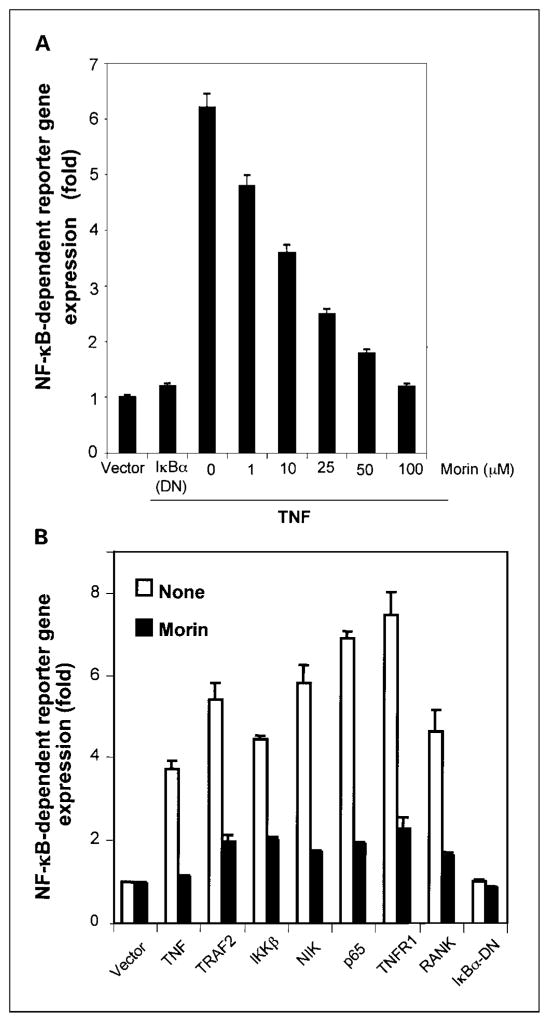
Because DNA binding does not always correlate with NF-κB–dependent gene transcription (33), we investigated the effect of morin on gene transcription. To determine the effect of morin on TNF-induced NF-κB–dependent reporter gene expression, we transiently transfected cells with the NF-κB–regulated SEAP reporter construct, incubated them with morin, and then stimulated the cells with TNF. As shown in Fig. 4A, TNF induced the NF-κB reporter activity, and this activity was inhibited by morin in a dose-dependent manner.
Fig. 4.
A, morin inhibits the NF-κB – dependent reporter gene expression nduced by TNF. A293 cells were transiently cotransfected with NF-κB binding sequence-containing plasmid linked to SEAP, IκBα-DN, and β-galactosidase genes. After 6 h of transfection, cells were washed and treated with different concentrations of morin for 4 h and then stimulated with 100 pmol/L TNF for an additional 12 h. Culture supernatant was collected and assayed for SEAP, and the pellet was extracted and assayed for β-galactosidase. B, morin inhibits TRAF2-, IKK-, NIK-, p65-, TNFR1-, and receptor activator of NF-κB (RANK)– mediated NF-κB – dependent reporter gene expression. A293 cells were transiently transfected with the indicated plasmids along with an NF-κB binding site containing plasmid linked to the SEAP gene and then either untreated or treated with morin (50 μmol/L) for 4 h and then incubated for 12 h. Cell supernatants were assayed for SEAP activity as described in Materials and Methods. Results are expressed as fold activity over the vector-transfected control. Results are representative of three independent experiments.
Morin represses NF-κB–dependent reporter gene expression induced by TNFR1, TRAF2, TRADD, NIK, IKK, and receptor activator of NF-κB
TNF-induced NF-κB activation is mediated through sequential interaction of the TNFR with TRADD, TRAF2, NIK, and IKK, resulting in phosphorylation of IκBα (34). To determine the effect of morin on NF-κB activation induced by plasmids expressing TNFR1, TRADD, TRAF2, NIK, IKK, and p65, cells were transfected with the expression plasmid and then monitored for NF-κB–dependent SEAP expression. Morin suppressed TNFR1-, TRADD-, TRAF2-, NIK-, IKK-, p65-, and receptor activator of NF-κB ligand–induced NF-κB reporter gene expression (Fig. 4B). These results suggested that the morin effect occurs at a step upstream from p65.
Morin represses the expression of TNF-induced NF-κB–dependent antiapoptotic gene products
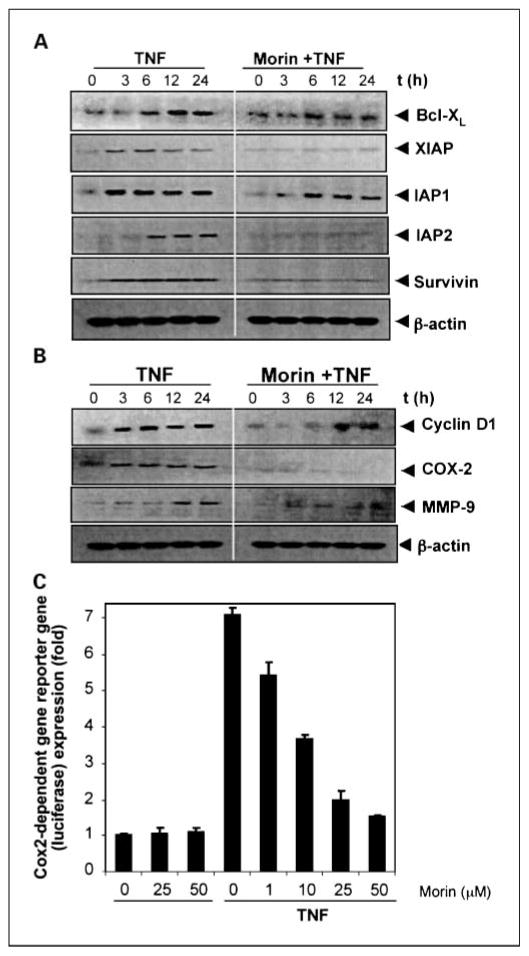
Because NF-κB also regulates the expression of several antiapoptotic proteins, including Bcl-xL, X chromosome-linked IAP, IAP1/IAP2, and survivin (16), and we tested the ability of morin to modulate the expression of these antiapoptotic gene products induced by TNF. We found that TNF induced Bcl-xL, X chromosome-linked IAP, IAP1, IAP2, and survivin expression in a time-dependent manner and that morin blocked TNF-induced expression of all these antiapoptotic proteins (Fig. 5A).
Fig. 5.
A, morin inhibits the expression of TNF-induced antiapoptotic proteins. KBM-5 cells were incubated with 25 μmol/L morin for 4 h and then treated with 1nmol/L TNF for the indicated times. Whole-cell extracts were prepared and analyzed by Western blotting using the indicated antibodies. B, morin inhibits TNF-induced cyclin D1, COX-2, and MMP-9. KBM-5 cells were incubated with 25μmol/L morin for 4 h and then treated with 1nmol/L TNF for the indicated times. Whole-cell extracts were prepared and analyzed by Western blotting using the relevant antibodies. For these experiments, we used three separate gels; based on molecular weight, each gel was sliced into two, and each slice was stripped and reprobed with different antibodies. C, morin inhibits COX-2– induced reporter gene expression. H1299 cells were transfected with the COX-2 luciferase expression vector and β-galactosidase gene. After 6 h of transfection, cells were treated with different concentrations of morin for 4 h and then stimulated with 1nmol/L TNF for 12 h. The luciferase and β-galactosidase enzyme activities were measured. Results are representative of three independent experiments.
Morin suppresses the expression of TNF-induced NF-κB–dependent gene products involved in the proliferation and metastasis of tumor cells
TNF has been shown to induce cyclin D1, COX-2, and MMP-9, all of which have NF-κB binding sites in their promoters (16). Therefore, we determined whether morin could inhibit TNF-induced cyclin D1, COX-2, and MMP-9. Cells were pretreated with morin and then treated with TNF for different times. Whole-cell extracts were prepared and analyzed by Western blot analysis for the expression of cyclin D1, COX-2, and MMP-9 (Fig. 5B). TNF induced cyclin D1, COX-2, and MMP-9 expression in a time-dependent manner and indeed morin blocked TNF-induced expression of cyclin D1, COX-2, and MMP-9, further strengthening our demonstrations that morin blocks TNF-induced NF-κB–regulated gene products.
Morin represses TNF-induced COX-2 promoter activity
COX-2 promoter is known to contain a NF-κB binding site, which is activated in response to TNF (35), so we investigated whether morin can suppress the COX-2 promoter activity. As shown in Fig. 5C, TNF induced the COX-2 promoter activity and morin suppressed it.
Morin potentiates apoptosis induced by TNF and chemotherapeutic agents
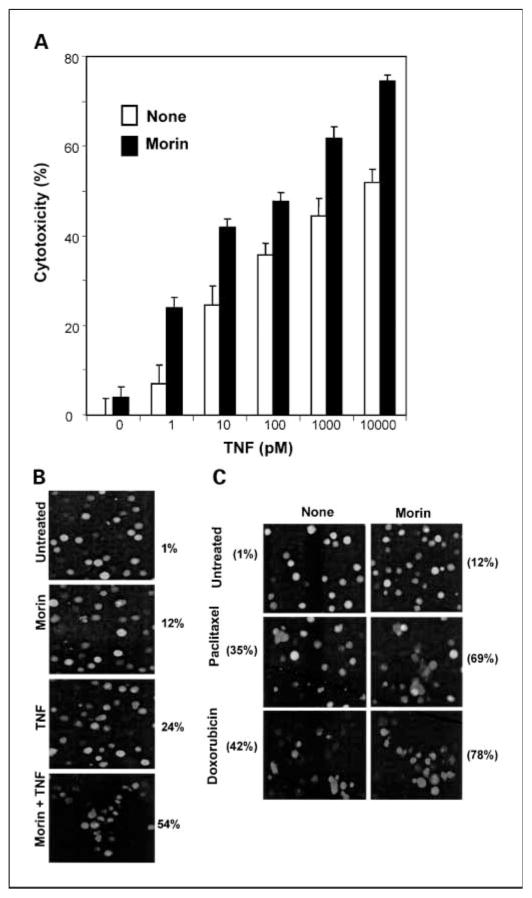
Activation of NF-κB inhibits TNF-induced apoptosis (36). Our results to this point suggested that morin might enhance the apoptosis induced by TNF and other cytotoxic agents through suppression of NF-κB–regulated antiapoptotic gene products. We therefore investigated whether suppression of NF-κB by morin affects TNF-induced apoptosis. Results of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay showed that TNF was cytotoxic to cells and that morin enhanced that cytotoxicity (Fig. 6A).
Fig. 6.
A, effect of morin on the cell death induced by TNF. KBM-5 cells were treated with or without 50 μmol/L morin for 4 h and then treated with different concentrations of TNF and incubated at 37°C for 72 h. Cell viability was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay as described in Materials and Methods. B, KBM-5 cells were treated with or without 50 μmol/L morin for 4 h and then stimulated with 1nmol/L TNF for 72 h. Cell viability was assayed by the live/dead assay as described in Materials and Methods. C, effect of morin on the cell death induced by various chemotherapeutic agents. Cells were pretreated with 50 μmol/L morin for 4 h and then stimulated with 5 nmol/L paclitaxel or 300 nmol/L doxorubicin for 72 h. Cell viability was assayed by the live/dead assay.
We then further investigated whether this enhanced cytotoxicity was due to apoptosis. The live/dead assay, which measures membrane integrity and esterase staining, indicated that morin up-regulated TNF-induced apoptosis from 12% to 54% (Fig. 6B). Similarly, morin enhanced paclitaxel-induced apoptosis from 35% to 69% and enhanced apoptosis induced by doxorubicin from 42% to 78% (Fig. 6C). These results together suggest that morin enhanced the apoptotic effects of TNF and chemotherapeutic agents.
Discussion
Because morin has been shown to exhibit anti-inflammatory and antitumor activities linked to NF-κB activation (1–10), we investigated whether morin modulates the NF-κB activation pathway. We found that morin can suppress NF-κB activation induced by inflammatory stimuli and carcinogens and that the suppression was not cell type specific. The inhibition of NF-κB activation involved the suppression of IKK activation, leading to suppression of phosphorylation and degradation of IκBα and consequent p65 nuclear translocation. Morin also inhibited the NF-κB–dependent reporter gene expression activated by TNF signaling elements. TNF-induced NF-κB – regulated gene products involved in the regulation of apoptosis, proliferation, and invasion were all down-regulated by morin. Morin also potentiated the apoptosis induced by TNF and various chemotherapeutic agents.
This is the first report to suggest that morin can suppress NF-κB activation induced by TNF, IL-1β, LPS, PMA, and ceramide. Only 50% suppression of NF-κB activation induced by H2O2 was noted. Because these agents activate NF-κB by different mechanisms, our results suggest that morin must act at a step common to all these agents. IKK is needed for NF-κB activation by all these agents. We found that morin does not affect the activity of IKK directly but blocks the activation of IKK. What activates IKK is not fully understood, but the roles of AKT (37), transforming growth factor–activated kinase 1 (38), mitogen-activated protein kinase/extracellular signal-regulated kinase kinase kinase 1 (39), mitogen-activated protein kinase/extracellular signal-regulated kinase kinase kinase 3 (40), and glycogen synthase kinase-3β (41) have been implicated. We found that morin abolished TNF-induced AKT activation. Whether suppression of TNF-induced IKK activation by morin is due to suppression of AKT activation is not clear. TNF has been shown to also activate transforming growth factor–activated kinase 1, which is linked to IKK activation (42).
It is not clear whether morin also modulates TNF-induced NF-κB activation through inhibition of transforming growth factor–activated kinase 1.
Although several flavones have been shown to exhibit anti-inflammatory activities (43), very little is known about the mechanism. The flavones apigenin and luteolin have both been shown to suppress the TNF-induced intercellular adhesion molecule-1 through the down-regulation of NF-κB (44, 45). Morin is a 3,5,7,2′,4′-pentahydroxyflavone, whereas apigenin is 5,7,4′-trihydroxyflavone and luteolin is 5,7,3′,4′-tetrahydroxyflavone. However, 3,5,7,4′-tetrahydroxyflavone (kaempferol) and quercetin (3,5,7,3,4′-pentahydroxyflavone) have been found to be less active in blocking NF-κB activation (44–46). Thus, which hydroxyl groups are critical for NF-κB suppression is unclear. The suppression of TNF-induced AKT activation by morin agrees with a report (45) that showed similar results with apigenin and luteolin.
We found that morin down-regulated the expression of several NF-κB–regulated gene products that are involved in cell survival, cell proliferation, and invasion. TNF-induced IAP1 expression is suppressed by luteolin (47). We found that morin potentiated apoptosis induced by TNF, paclitaxel, and doxorubicin through the down-regulation of antiapoptotic gene products. Although there is no prior report concerning morin, Shi et al. (47) showed that luteolin can sensitize colon cancer cells to TNF. Morin has been shown to suppress inflammation-induced tumorigenesis in various rodent models, including the trinitrobenzenesulfonic acid model of colitis (2, 3), azoxymethane-induced aberrant crypt foci formation (4), and chemically induced tongue carcinogenesis (5). Apparently, all these effects are mediated through suppression of NF-κB activation as shown here. Similarly, suppression of LPS-induced septic shock (6) could also be mediated through inhibition of LPS-induced NF-κB activation. The ability of morin to inhibit P-glycoprotein (12), suppress inducible nitric oxide synthase and COX-2 expression (14), and inhibit the release of inflammatory cytokines, such as IL-6, IL-8, and TNF (15), is very likely mediated through suppression of NF-κB activation.
Overall, our results show the use of morin in targeting one of the central pathways involved in inflammation and tumorigenesis. The results with morin obtained here are similar to that reported previously with flavopiridol, a synthetic flavone, which we showed also interferes with NF-κB pathway (21). Despite promising preclinical studies with this cyclin-dependent kinase inhibitor in chronic lymphocytic leukemia and other diseases, previous clinical trials with this agent have been disappointing. A recent study, however, showed that flavopiridol when administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia (48). Whether the dose of morin used in our studies is relevant to that in vivo situation is unclear. No data are available to correlate the dose of morin used in our studies to that found in the plasma. In rodents, dietary administration of morin up to 5% (w/w) for 13 weeks (49) or i.p. injection (75 mg/kg body weight; ref. 50) showed no toxic effect. Thus, the lack of toxicity of morin, combined with its efficacy as described here, warrants further preclinical studies preceeding human trials.
Acknowledgments
We thank Michael S. Worley for carefully reading the manuscript and providing valuable editorial comments.
Grant support: Clayton Foundation for Research (B.B. Aggarwal), NIH grant CA91844 (B.B. Aggarwal) and RCMI 5G12 RR 003045-19 (G.T. Ramesh), National Science Foundation grant HRD0401587 (G.T. Ramesh), and National Aeronautics amd Space Administration grant NCC9-165 (G.T. Ramesh).
References
- 1.Chen YC, Shen SC, Chow JM, Ko CH, Tseng SW. Flavone inhibition of tumor growth via apoptosis in vitro and in vivo. Int J Oncol. 2004;25:661–70. [PubMed] [Google Scholar]
- 2.Ocete MA, Galvez J, Crespo ME, et al. Effects of morin on an experimental model of acute colitis in rats. Pharmacology. 1998;57:261–70. doi: 10.1159/000028250. [DOI] [PubMed] [Google Scholar]
- 3.Galvez J, Coelho G, Crespo ME, et al. Intestinal anti-inflammatory activity of morin on chronic experimental colitis in the rat. Aliment Pharmacol Ther. 2001;15:2027–39. doi: 10.1046/j.1365-2036.2001.01133.x. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka T, Kawabata K, Honjo S, et al. Inhibition of azoxymethane-induced aberrant crypt foci in rats by natural compounds, caffeine, quercetin, and morin. Oncol Rep. 1999;6:1333–40. doi: 10.3892/or.6.6.1333. [DOI] [PubMed] [Google Scholar]
- 5.Kawabata K, Tanaka T, Honjo S, et al. Chemopreventive effect of dietary flavonoid morin on chemically induced rat tongue carcinogenesis. Int J Cancer. 1999;83:381–6. doi: 10.1002/(sici)1097-0215(19991029)83:3<381::aid-ijc14>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 6.Fang SH, Hou YC, Chang WC, Hsiu SL, Chao PD, Chiang BL. Morin sulfates/glucuronides exert anti-inflammatory activity on activated macrophages and decreased the incidence of septic shock. Life Sci. 2003;74:743–56. doi: 10.1016/j.lfs.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Hsiang CY, Wu SL, Ho TY. Morin inhibits 12-O-tetra-decanoylphorbol-13-acetate-induced hepatocellular transformation via activator protein 1 signaling pathway and cell cycle progression. Biochem Pharmacol. 2005;69:1603–11. doi: 10.1016/j.bcp.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Brown J, O’Prey J, Harrison PR. Enhanced sensitivity of human oral tumours to the flavonol, morin, during cancer progression: involvement of the Akt and stress kinase pathways. Carcinogenesis. 2003;24:171–7. doi: 10.1093/carcin/24.2.171. [DOI] [PubMed] [Google Scholar]
- 9.Krol W, Dworniczak S, Pietsz G, et al. Synthesis and tumoricidal activity evaluation of new morin and quercetin sulfonic derivatives. Acta Pol Pharm. 2002;59:77–9. [PubMed] [Google Scholar]
- 10.Ranelletti FO, Ricci R, Larocca LM, et al. Growth-inhibitory effect of quercetin and presence of type-II estrogen-binding sites in human colon-cancer cell lines and primary colorectal tumors. Int J Cancer. 1992;50:486–92. doi: 10.1002/ijc.2910500326. [DOI] [PubMed] [Google Scholar]
- 11.Thuillier P, Brash AR, Kehrer JP, et al. Inhibition of peroxisome proliferator-activated receptor (PPAR)-mediated keratinocyte differentiation by lipoxygenase inhibitors. Biochem J. 2002;366:901–10. doi: 10.1042/BJ20020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ikegawa T, Ohtani H, Koyabu N, et al. Inhibition of P-glycoprotein by flavonoid derivatives in Adriamycin-resistant human myelogenous leukemia (K562/ADM) cells. Cancer Lett. 2002;177:89–93. doi: 10.1016/s0304-3835(01)00761-3. [DOI] [PubMed] [Google Scholar]
- 13.Ratty AK, Sunamoto J, Das NP. Interaction of flavonoids with 1,1-diphenyl-2-picrylhydrazyl free radical, liposomal membranes and soybean lipoxygenase-1. Biochem Pharmacol. 1988;37:989–95. doi: 10.1016/0006-2952(88)90499-6. [DOI] [PubMed] [Google Scholar]
- 14.Raso GM, Meli R, Di Carlo G, Pacilio M, Di Carlo R. Inhibition of inducible nitric oxide synthase and cyclo-oxygenase-2 expression by flavonoids in macrophage J774A.1. Life Sci. 2001;68:921–31. doi: 10.1016/s0024-3205(00)00999-1. [DOI] [PubMed] [Google Scholar]
- 15.Kempuraj D, Madhappan B, Christodoulou S, et al. Flavonols inhibit proinflammatory mediator release, intracellular calcium ion levels, and protein kinase Cθ phosphorylation in human mast cells. Br J Pharmacol. 2005;145:934–44. doi: 10.1038/sj.bjp.0706246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aggarwal BB. Nuclear factor-κB: the enemy within. Cancer Cell. 2004;6:203–8. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Kumar A, Takada Y, Boriek AM, Aggarwal BB. Nuclear factor-κB: its role in health and disease. J Mol Med. 2004;82:434–48. doi: 10.1007/s00109-004-0555-y. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh S, May MJ, Kopp EB. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–60. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 19.Ducut Sigala JL, Bottero V, Young DB, Shevchenko A, Mercurio F, Verma IM. Activation of transcription factor NF-κB requires ELKS, an IκB kinase regulatory subunit. Science. 2004;304:1963–7. doi: 10.1126/science.1098387. [DOI] [PubMed] [Google Scholar]
- 20.Chaturvedi MM, Mukhopadhyay A, Aggarwal BB. Assay for redox-sensitive transcription factor. Methods Enzymol. 2000;319:585–602. doi: 10.1016/s0076-6879(00)19055-x. [DOI] [PubMed] [Google Scholar]
- 21.Takada Y, Aggarwal BB. Flavopiridol inhibits NF-κB activation induced by various carcinogens and inflammatory agents through inhibition of IκBα kinase and p65 phosphorylation: abrogation of cyclin D1, cyclooxygenase-2, and matrix metalloprotease-9. J Biol Chem. 2004;279:4750–9. doi: 10.1074/jbc.M304546200. [DOI] [PubMed] [Google Scholar]
- 22.Takada Y, Aggarwal BB. Betulinic acid suppresses carcinogen-induced NF-κB activation through inhibition of IκBα kinase and p65 phosphorylation: abrogation of cyclooxygenase-2 and matrix metalloprotease-9. J Immunol. 2003;171:3278–86. doi: 10.4049/jimmunol.171.6.3278. [DOI] [PubMed] [Google Scholar]
- 23.Takada Y, Gillenwater A, Ichikawa H, Aggarwal BB. Suberoylanilide hydroxamic acid potentiates apoptosis, inhibits invasion, and abolishes osteoclastogenesis by suppressing nuclear factor-κB activation. J Biol Chem. 2006;281:5612–22. doi: 10.1074/jbc.M507213200. [DOI] [PubMed] [Google Scholar]
- 24.Takada Y, Mukhopadhyay A, Kundu GC, Mahabeleshwar GH, Singh S, Aggarwal BB. Hydrogen peroxide activates NF-κB through tyrosine phosphorylation of IκBα and serine phosphorylation of p65: evidence for the involvement of IκBα kinase and Syk protein-tyrosine kinase. J Biol Chem. 2003;278:24233–41. doi: 10.1074/jbc.M212389200. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh S, Karin M. Missing pieces in the NF-κB puzzle. Cell. 2002;109 (Suppl):S81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 26.Bonizzi G, Piette J, Merville MP, Bours V. Distinct signal transduction pathways mediate nuclear factor-κB induction by IL-1βin epithelial and lymphoid cells. J Immunol. 1997;159:5264–72. [PubMed] [Google Scholar]
- 27.Natarajan K, Singh S, Burke TR, Jr, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-κB. Proc Natl Acad Sci U S A. 1996;93:9090–5. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahon TM, O’Neill LA. Studies into the effect of the tyrosine kinase inhibitor herbimycin A on NF-κB activation in T lymphocytes. Evidence for covalent modification of the p50 subunit. J Biol Chem. 1995;270:28557–64. doi: 10.1074/jbc.270.48.28557. [DOI] [PubMed] [Google Scholar]
- 29.Sandur SK, Ichikawa H, Sethi G, Ahn KS, Aggarwal BB. Plumbagin (5-hydroxy-2-methyl-1,4-naphthoqui-none) suppresses NF-κB activation and NF-κB-regulated gene products through modulation of p65 and IκBα kinase activation, leading to potentiation of apoptosis induced by cytokine and chemotherapeutic agents. J Biol Chem. 2006;281:17023–33. doi: 10.1074/jbc.M601595200. [DOI] [PubMed] [Google Scholar]
- 30.Chiang YM, Lo CP, Chen YP, et al. Ethyl caffeate suppresses NF-κB activation and its downstream inflammatory mediators, iNOS, COX-2, and PGE2 in vitro or in mouse skin. Br J Pharmacol. 2005;146:352–63. doi: 10.1038/sj.bjp.0706343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finco TS, Beg AA, Baldwin AS., Jr Inducible phosphorylation of IκBα is not sufficient for its dissociation from NF-κB and is inhibited by protease inhibitors. Proc Natl Acad Sci U S A. 1994;91:11884–8. doi: 10.1073/pnas.91.25.11884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-κB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–5. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 33.Nasuhara Y, Adcock IM, Catley M, Barnes PJ, Newton R. Differential IκB kinase activation and IκBα degradation by interleukin-1β and tumor necrosis factor-α in human U937 monocytic cells. Evidence for additional regulatory steps in κB-dependent transcription. J Biol Chem. 1999;274:19965–72. doi: 10.1074/jbc.274.28.19965. [DOI] [PubMed] [Google Scholar]
- 34.Hsu H, Shu HB, Pan MG, Goeddel DV. TRADD- TRAF2 and TRADD-FADD interactions define two distinct TNF receptor1signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto K, Arakawa T, Ueda N, Yamamoto S. Transcriptional roles of nuclear factor κB and nuclear factor-interleukin-6 in the tumor necrosis factor α-dependent induction of cyclooxygenase-2 in MC3T3-1 cells. J Biol Chem. 1995;270:31315–20. doi: 10.1074/jbc.270.52.31315. [DOI] [PubMed] [Google Scholar]
- 36.Giri DK, Aggarwal BB. Constitutive activation of NF-κB causes resistance to apoptosis in human cutaneous T cell lymphoma HuT-78 cells. Autocrine role of tumor necrosis factor and reactive oxygen intermediates. J Biol Chem. 1998;273:14008–14. doi: 10.1074/jbc.273.22.14008. [DOI] [PubMed] [Google Scholar]
- 37.Gustin JA, Maehama T, Dixon JE, Donner DB. The PTEN tumor suppressor protein inhibits tumor necrosis factor-induced nuclear factor κB activity. J Biol Chem. 2001;276:27740–4. doi: 10.1074/jbc.M102559200. [DOI] [PubMed] [Google Scholar]
- 38.Takaesu G, Surabhi RM, Park KJ, Ninomiya-Tsuji J, Matsumoto K, Gaynor RB. TAK1 is critical for IκB kinase-mediated activation of the NF-κB pathway. J Mol Biol. 2003;326:105–15. doi: 10.1016/s0022-2836(02)01404-3. [DOI] [PubMed] [Google Scholar]
- 39.Lee FS, Peters RT, Dang LC, Maniatis T. MEKK1 activates both IκB kinase α and IκB kinase β. Proc Natl Acad Sci U S A. 1998;95:9319–24. doi: 10.1073/pnas.95.16.9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang J, Lin Y, Guo Z, et al. The essential role of MEKK3 in TNF-induced NF-κB activation. Nat Immunol. 2001;2:620–4. doi: 10.1038/89769. [DOI] [PubMed] [Google Scholar]
- 41.Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3β in cell survival and NF-κB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 42.Sakurai H, Miyoshi H, Toriumi W, Sugita T. Functional interactions of transforming growth factor β-activated kinase 1 with IκB kinases to stimulate NF-κB activation. J Biol Chem. 1999;274:10641–8. doi: 10.1074/jbc.274.15.10641. [DOI] [PubMed] [Google Scholar]
- 43.Theoharides TC, Alexandrakis M, Kempuraj D, Lytinas M. Anti-inflammatory actions of flavonoids and structural requirements for new design. Int J Immunopathol Pharmacol. 2001;14:119–27. [PubMed] [Google Scholar]
- 44.Chen CC, Chow MP, Huang WC, Lin YC, Chang YJ. Flavonoids inhibit tumor necrosis factor-α-induced up-regulation of intercellular adhesion molecule-1 (ICAM-1) in respiratory epithelial cells through activator protein-1 and nuclear factor-κB: structure-activity relationships. Mol Pharmacol. 2004;66:683–93. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 45.Ruiz PA, Haller D. Functional diversity of flavonoids in the inhibition of the proinflammatory NF-κB, IRF, Akt signaling pathways in murine intestinal epithelial cells. J Nutr. 2006;136:664–71. doi: 10.1093/jn/136.3.664. [DOI] [PubMed] [Google Scholar]
- 46.Natarajan K, Manna SK, Chaturvedi MM, Aggarwal BB. Protein tyrosine kinase inhibitors block tumor necrosis factor-induced activation of nuclear factor-κB, degradation of IκBα, nuclear translocation of p65, and subsequent gene expression. Arch Biochem Bio-phys. 1998;352:59–70. doi: 10.1006/abbi.1998.0576. [DOI] [PubMed] [Google Scholar]
- 47.Shi RX, Ong CN, Shen HM. Luteolin sensitizes tumor necrosis factor-α-induced apoptosis in human tumor cells. Oncogene. 2004;23:7712–21. doi: 10.1038/sj.onc.1208046. [DOI] [PubMed] [Google Scholar]
- 48.Byrd JC, Lin TS, Dalton JT, et al. Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood. 2007;109:399–404. doi: 10.1182/blood-2006-05-020735. Epub 2006 Sep 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cho YM, Onodera H, Ueda M, Imai T, Hirose M. A 13-week subchronic toxicity study of dietary administered morin in F344 rats. Food Chem Toxicol. 2006;44:891–7. doi: 10.1016/j.fct.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 50.Rotelli AE, Guardia T, Juarez AO, de la Rocha NE, Pelzer LE. Comparative study of flavonoids in experimental models of inflammation. Pharmacol Res. 2003;48:601–6. doi: 10.1016/s1043-6618(03)00225-1. [DOI] [PubMed] [Google Scholar]