Abstract
Purpose
Although genital tactile stimulation is regarded as a precursor to sexual arousal and a recognized initiator of central nervous system arousal, specific afferent neural pathways transmit sensory stimuli of arousal, beginning at the epithelial level on the clitoris and following the course of arousal stimuli through the central nervous system. Limited knowledge exists of the pathway from the cutaneous receptors of nerves originating in the epithelial tissue of the clitoris and continuing to spinal cord afferents. Such information may contribute to an understanding of sexual arousal, particularly in female vertebrates. We further defined the neural pathways and mechanisms responsible for arousal originating in the epithelium of the clitoris as well as related neural pathways to the spinal cord in a murine model.
Materials and Methods
We performed a comprehensive review of the published relevant clinical and histological material from human and nonhuman vertebrate studies. In 29 adult female C57B1/6 mice the distribution of pelvic nerves and vessels was mapped. Gross dissection of 4 female mice was facilitated by resin injection of the vascular system in 2. Neuronal tracing was performed in 25 mice that received clitoral injection of wheat germ agglutinin-horseradish peroxidase into the clitoris and were sacrificed after 72 to 96 hours. The spinal cord and periclitoral tissue were removed and fixed. Immunohistochemistry was performed.
Results
Gross anatomy of the mouse clitoris showed that pudendal and hypogastric nerves have a major role in the innervation of the external genitalia. Neuronal tracing revealed that the greatest nerve density was noted in the L5/6 spinal cord. The distribution extended from S1 to L2 with no labeling seen in the L3 spinal cord. Wheat germ agglutinin-horseradish peroxidase labeling was seen caudal in levels S1 through L4 and rostral in L2.
Conclusions
Understanding the neuroanatomy of the clitoris using a murine model may provide a valuable tool for the study of sexual arousal disorders and the further understanding of sexual function related to neural pathologies and trauma.
Keywords: clitoris, female, neurons, afferent, mice, spinal cord
The dorsal horn of the spinal cord and the dorsal column nuclei are the places where information from the body surface and underlying tissues reaches the central nervous system. The first stage in the integration of sensory messages takes place here, where the major somatosensory systems originate, and this is the first place where the brain can exert control over the messages through various descending pathways.1
The particular path that delivers afferent stimuli from the clitoris epithelium has been poorly delineated and it is of interest to those who participate in many types of sexual medicine and research. A thorough understanding of the neuroanatomy and physiology inclusive of the afferent spinal pathway of clitoral arousal would be particularly helpful for the strategic design of female reconstructive surgery. Historically studies to clarify sexual function and dysfunction involving the clitoris have been less comprehensive than studies of its male counterpart, the penis, in investigations of basic reproductive neuroanatomy and physiology. There are few studies detailing the antegrade paths of epithelial innervation through the spinal cord.2–5 Detailed studies of clitoral anatomy in animals are rare6–9 and in humans they are also problematic. Generally the study of human clitoral anatomy is based on cadaveric studies, often using elderly postmenopausal women.6,10 Some clitoral anatomy studies have been performed in healthy pre-menopausal women using magnetic resonance imaging8 and in human fetal tissue.11
General neuroanatomical studies of the female reproductive organs have given little attention to the sensory innervation of the female external genitalia. Previous studies of the pudendal nerve in female humans12 and in other animals13–16 have typically overlooked the distal pudendal nerve branches innervating the clitoris that include the clitoris corpus cavernosus nerve, the perineal nerve and the dorsal nerve of the clitoris. It is likely that this fine innervation of the clitoris and external genitalia transmits the sensory stimulation that influences the vasocongestive events of clitoral sexual arousal.
We studied the innervation of the murine clitoris using microdissection. The study was supplemented by identification of the central projections from the clitoral area using discrete injections of WGA-HRP, an antegrade axonal tracer, beneath the skin of the external genitalia and into the clitoris. Our aim was to achieve a better understanding of sexual arousal in female vertebrates by studying the sensorial innervation of the mouse external genitalia and clitoris.
MATERIALS AND METHODS
Adult female C57B1/6 mice (Jackson Laboratories, Bar Harbor, Maine) were delivered at ages 5 to 6 weeks and housed at the Rockefeller University animal care facility until they were sacrificed for experiments. The environment was maintained at 22C with 12 hours of darkness. All procedures followed the protocol approved by the Rockefeller University Animal Care and Use Committee.
Gross anatomical dissection was done in 4 female mice, of which 2 had the vascular systems injected with colored resin. Heparin was added to the animals before we started the protocol. The circulatory system was cleaned by saline solution previous to injection through the left ventricle and the right ventricle was opened to allow the solutions to drain. Microphil (Flowtech, Carver, Massachusetts) was subsequently injected, also through the left ventricle, and the right ventricle was clamped to keep the silicone in the vasa. The pelvic organs were identified along with the blood vessels supplying these structures and the distribution of the nerves between the cavity and the pelvic viscera. The presence of neural fibers was detected by a conventional silver staining technique.
For the neuronal tracing procedure a 4.0% solution of HP conjugated to WGA (Vector Laboratories, Burlingame, California) was used to label central afferent projections of the mouse clitoris. Preliminary parametric studies varied the injection volume (1 to 5 µl) and the transport time (3 to 5 days) required to reliably deposit and limit WGA-HRP to the clitoris and achieve optimal labeling in the spinal cord. Overall 25 female mice received clitoral injections of WGA-HRP. They were anesthetized with 7.5 mg Nembutal® per 100 gm body weight intraperitoneally. While the female was lying on the back, WGA-HRP was slowly delivered through the needle of a 5.0 µl syringe (Hamilton, Reno, Nevada) into the clitoris. The neural tracer was deposited into the clitoris or in the skin ventral to the preputial glans in all 25 animals.
After the appropriate time following WGA-HRP injection (72 to 96 hours) the animals were deeply anesthetized with 0.3 ml Nembutal® per animal intraperitoneally and per-fused intracardially with saline, followed by cold 1% paraformaldehyde/ 1.25% glutaraldehyde. The spinal cord was exposed by dorsal laminectomy, including the thoracic through the last sacral segment, and then simply divided. Segment identification was done by examining the histological sections and determining segmental boundaries as the midpoints between the dorsal root. After removal they were post-fixed in the same fixative for 5 hours and then transferred to sucrose phosphate buffer (10% weight per volume, pH 7.4) overnight for cryoprotection. Additionally, the periclitoral area was removed intact, post-fixed and cryoprotected in the same manner. To visualize and delimit injection sites the clitoral block was frozen sectioned into serial 40 µm horizontal sections and immediately reacted using a modified tetramethyl benzidine protocol,17 mounted on gelatin coated slides and counterstained with thionin or simply cleared and coverslipped. Spinal cords were blocked into 4 equal sections, embedded in gelatin, frozen sectioned transversely at 40 µm, collected into 4 alternate series and reacted using the same tetramethyl benzidine protocol. All levels were examined for labeling, including T10-L1. Sections were mounted on gelatin coated slides, counterstained with thionin and coverslipped with Permount®.18,19
Two animals with the largest and smallest clitoral injections (3 and 1 µl, respectively) were selected for detailed analysis of the pattern of terminal labeling in the spinal cord. In each case WGA-HRP injections successfully targeted and were limited to the clitoris. Using the largest and smallest injections specific to our target of interest we closely defined the relationship between the injection zone and the spinal cord labeling pattern, which was the same in the 2 animals. In each animal the distribution of afferent fibers in the spinal cord was mapped under dark field illumination. Beginning with the first section in which WGAHRP labeled fibers were present, the entire rostrocaudal distribution of labeling was assessed in every fifth section in 1 of the alternate series (800 µm apart) using the Neurolucida ® computer based morphometry system at a final magnification of 250×. For each section all WGA labeled fibers were drawn regardless of location, size or contiguity to ensure the complete assessment of afferent distribution. Because the entire rostrocaudal range of labeling in each animal was sampled, this method allowed complete assessment of afferent projections in the transverse and horizontal planes. Spinal segmental boundaries were determined using the midpoints between dorsal root entrances.20 Digital light micrographs were obtained using an MDS 290 digital camera system (Kodak®). The brightness and contrast of these images were adjusted in Adobe® Photoshop®.
From 1 mouse 1 of every 10 sections (400 µm apart) was lightly counterstained with hematoxylin and eosin, and from the other mouse 1 complete set of sections was stained with slightly modified Bielschowsky silver stain.21 Sections were exposed to 20% silver nitrate for 2 hours, followed by 2 minutes of incubation with ammoniacal silver. They were assessed microscopically until fibers were seen. Finally, slides from all procedures were dehydrated through graded alcohols and cleared in xylene. Coverslips were applied with Permount.
RESULTS
Gross microdissection of the pelvic cavity revealed that the clitoris consisted of a cylindrical erectile organ that was internally located between the preputial glans and ventral to the urethra. A fibrous tunica albuginea ensheathed the corporeal body, made up of vascular smooth muscle and collagen connective tissue. The clitoral body was formed by sponge-like cavernous tissue and composed of a meshwork of interconnected cavernous spaces surrounded by a fibrous tunica albuginea. The cavernous space was lined by vascular endothelium and separated by trabeculae containing bundles of smooth muscle. The urethra was surrounded by erectile tissue.
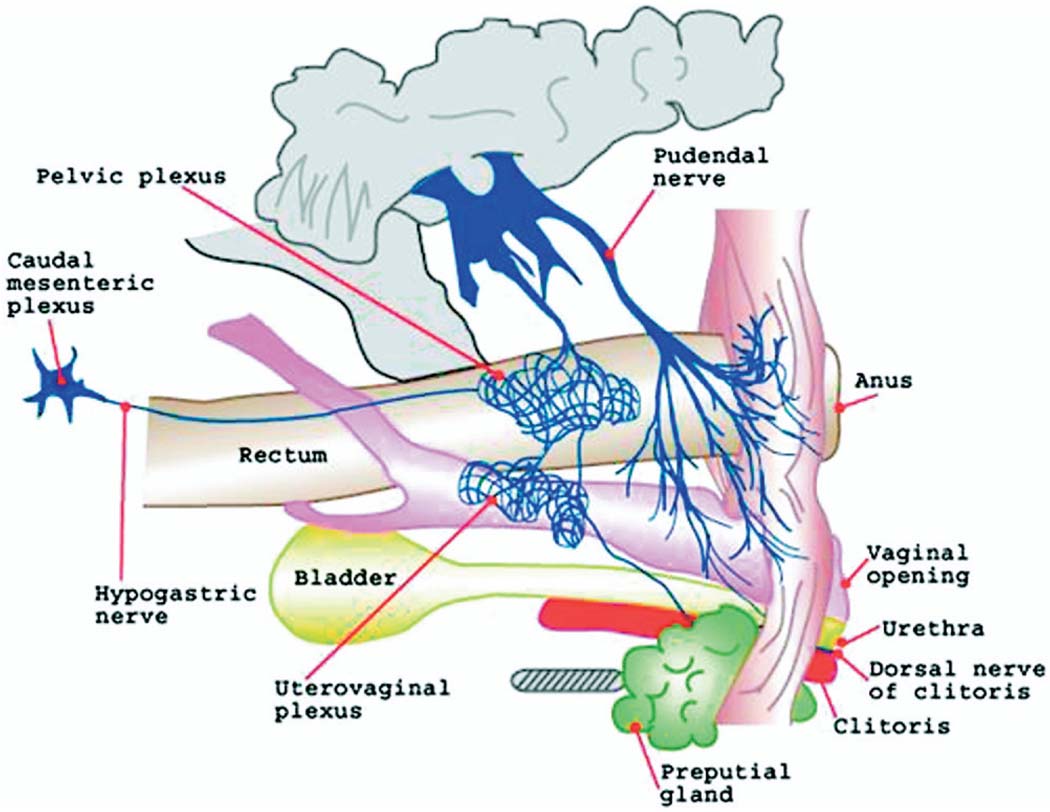
Gross dissection also revealed the innervation of structures in the pelvic cavity, including the vulva, bladder, urethra, vagina and rectum (fig. 1). The penetration of colored resin in the vascular system was adequate and it served as a reference to dissect the nerves in the genital region.
FIG. 1.
Dissection of female mouse pelvic cavity shows innervation of clitoris and preputial glans
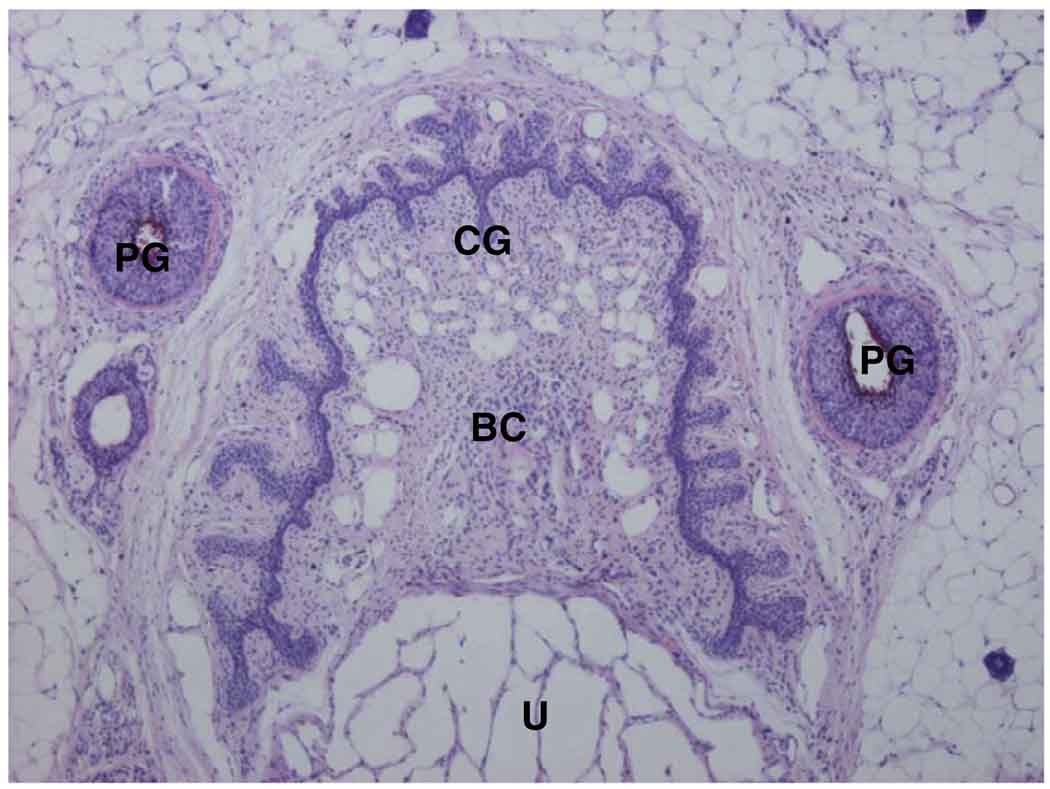
The dorsal nerve of the clitoris, the clitoris corpus cavernosus nerve and the pudendal nerve branches studied were also based on gross anatomical investigations and microdissection. The clitoral nerve that innervates the clitoris bifurcated into a dorsal nerve that coursed along the cranioventral border of the corpus clitoridis toward the glans with a deeper one to the corpus cavernosum clitoridis. These peripheral sensory afferents of the clitoris merged with the pudendal nerve. Two nerve branches entered the ischiorectal fossa, including 1 branch to the clitoris and the perineal region, and 1 to the muscles of that region. The clitoris was also innervated with fibers coming from the uterovaginalis plexus. The plexus was located close to the internal iliac veins. We found nerve fibers (hypogastric nerve) connecting the plexus mesenteric caudalis to the plexus pelvinus. Also, ventral nerve fibers from the plexus pelvinus innervated the uterus and vagina (plexus uterovaginalis). Histological characterization using Masson’s stain showed that the clitoris was composed of the clitoral glans located distal and the corpus or body of cavernous tissue (fig. 2).
FIG. 2.
Transverse section of mouse clitoris and preputial glans. U, urethra. BC, body of clitoris. PG, preputial glans. CG, clitoral glans. H & E, reduced from ×4.
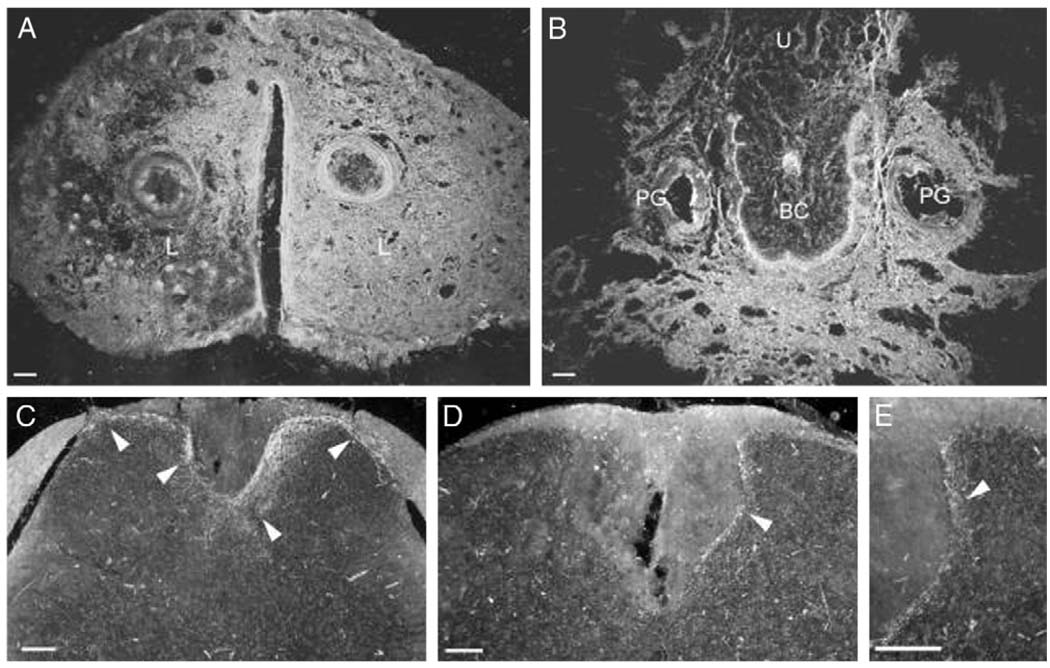
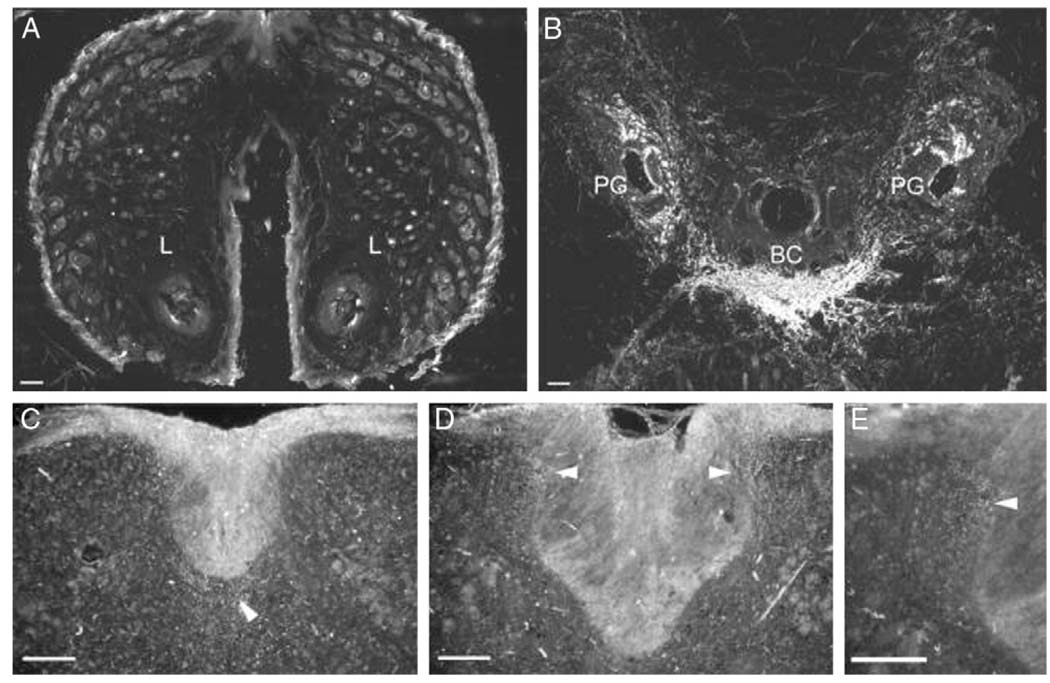
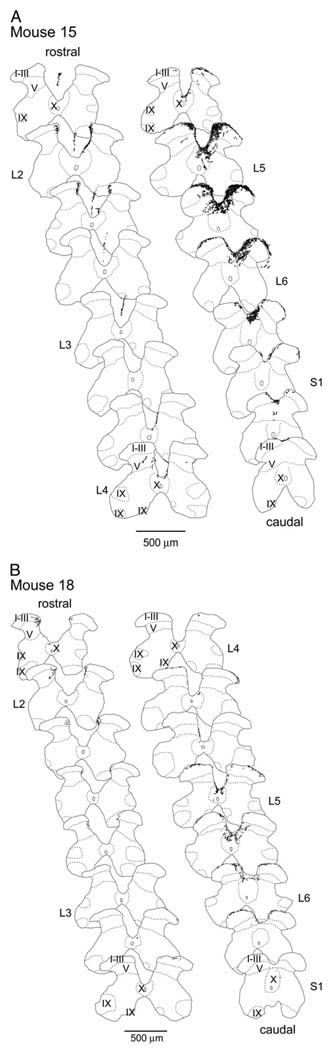
Neuronal tracing resulted in an afferent pattern of labeling in the spinal cord, consistent with pudendal and hypogastric innervation of external genitalia and clitoris. WGA-HRP was carried via primary sensory afferents and it labeled terminal projections in the spinal cord. The reaction product in these afferent fibers appeared as strings of fine granules. The pattern of spinal labeling for all injections directed and contained in the clitoris was identical, although the size of the injection site was positively related to the density of fibers labeled in the spinal cord. Figure 3 and Figure 4 show injection sites and representative spinal cord labeling for the largest and smallest injections, respectively. Figure 5 shows camera lucida drawings of the entire rostrocaudal range of labeling in each animal sampled. The smallest and the largest WGA-HRP injections resulted in a pattern of exclusively afferent labeling, consisting of labeled fibers and large, diffusely distributed granules of reaction product characteristic of terminal labeling. The distribution extended from S1 to L2 with no labeling seen in the L3 spinal cord.
FIG. 3.
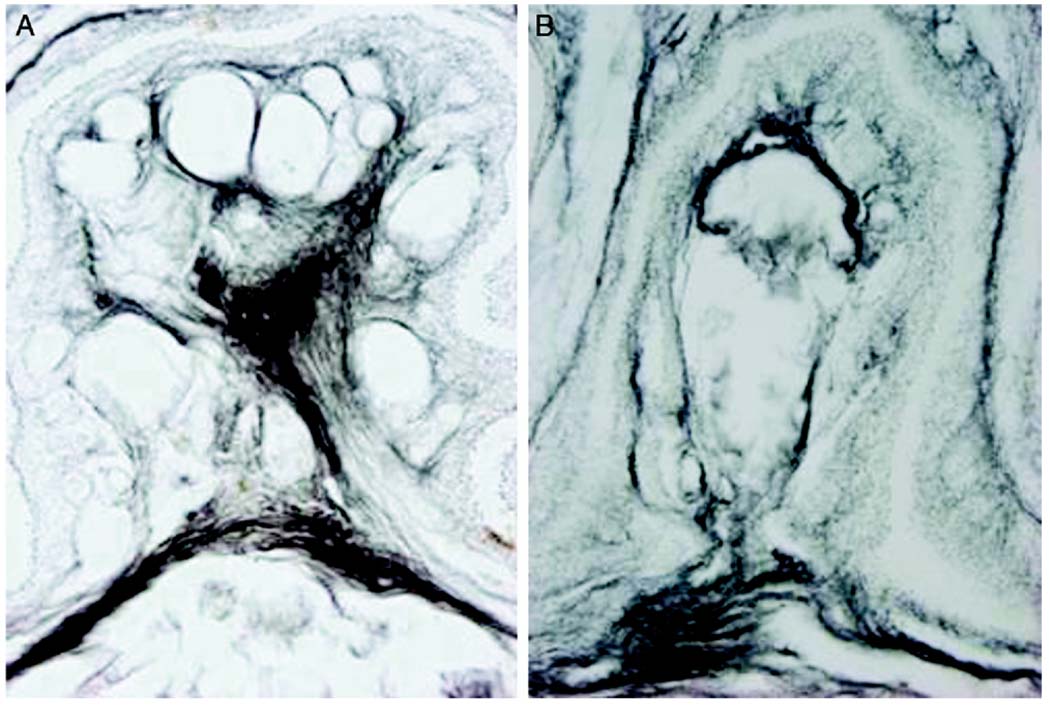
Dark field digital micrographs show labeling following large injection volume of 3 µl WGA-HRP into clitoris of 1 mouse. WGA-HRP was present bilaterally in external epithelium and clitoral sheath, that is labia (L) (A), ventral clitoris and tissue surrounding preputial glands and urethra (U), vaginal canal and gastrointestinal tract (B). Transverse sections through L2 (C) and L6 (D and E) spinal cord segments reveal resultant afferent labeling (arrowheads) of medial and lateral dorsal horns, and dorsal gray commissure. Scale bars represent 100 µm. BC, clitoral body.
FIG. 4.
Dark field digital micrographs of labeling following small injection volume of 1 µl WGA-HRP into clitoris of 1 mouse. No WGA-HRP was present in clitoral sheath, that is labia (L) (A). Injection site was restricted to epithelium ventral to clitoris, clitoris and limited tissue surrounding preputial glands (PG) (B). Transverse sections through L2 (C) and L6 (D and E) spinal cord segments of same mouse demonstrate resultant afferent labeling (arrowheads) of medial and lateral dorsal horns, and dorsal gray commissure. BC, clitoral body. Scale bars represent 100 µm.
FIG. 5.
Distribution of afferent labeling through lumbosacral spinal cord following WGA-HRP injection into cervix in 2 cases shows labeling pattern after largest (A) and smallest (B) injections in mice 15 and 18, respectively. Segmental boundaries, determined as midpoints between dorsal root entrances and cytoarchitectonic subdivisions, follow conventions of Molander et al.20 Sections were drawn at 800 µm intervals. Scale bars represent 500 µm.
The pattern of spinal cord labeling in the lumbosacral spinal cord differed from that in the upper lumbar region. In the S1 to L4 spinal levels afferent fibers entered the spinal cord bilaterally through Lissauer’s tract and coursed along the superficial dorsal horn medial and lateral. In agreement with pudendal nerve studies in the rat,16 collaterals coursed ventral, labeled lamina I–V, and were restricted to the medial portion of the dorsal horn (fig. 3 and fig. 4, C). The medial pathway followed the dorsal horn into the central gray commissure, where a dense aggregation of fibers formed around the midline toward and into lamina X. The lateral pathway followed the dorsal horn toward and into the lateral intermediate gray at the lateral edge of lamina V. In contrast to this labeling pattern, in the upper lumbar spinal cord (L2) bilateral labeling was restricted to the medial aspect of the dorsal horn adjacent to Lissauer’s tract (fig. 3 and fig. 4, D). Labeling from the large injection coursed ventromedial along the edge of the dorsal funiculus (fig. 3, E), while the resultant labeling from the smallest injection was restricted to the area adjacent to the medial zone of Lissauer’s tract (fig. 4, E). The largest injection also resulted in a column of labeled fibers in the medial portion of the L2/L3 gracile fasciculus (fig. 5).
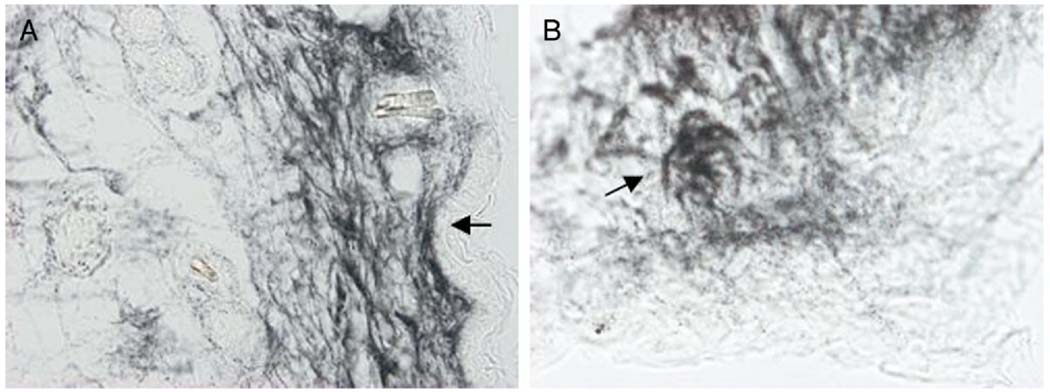
Silver staining demonstrated the fine pattern of innervation in the clitoris and the external genitalia. In the clitoral skin there was a dense collection of nerve endings and nerve corpuscles (fig. 6). Bundles of nerve fibers were present superficial to the tunica albuginea (fig. 7). In the clitoris there was a dense distribution of nerves in 2 regions. Fibers of the dorsal nerve of the clitoris were located at the dorsal side of clitoris and the ventral wall of the urethra (fig. 7, A). A dense collection of corpus cavernosus nerve fibers was located in the mid part of the clitoris with spreading fibers to all of the surrounding cavernous tissue (fig. 7, A). There were communicating branches between the corpus cavernosus and the dorsal clitoris nerves, mainly at the distal part of the clitoris (fig. 7). Nerve fibers from the skin of the perineal region passed to the dorsal clitoral nerve and from there to the pudendal nerve.
FIG. 6.
Female mouse external genitalia skin with neural mesh shows nerve endings in stroma under epithelium (arrows) (A) and sensorial corpuscle (arrow) in genital skin stroma (B). Bielschowsky silver stain, scale bar represents 50 (A) and 10 (B) µm.
FIG. 7.
Mouse distal (A) and proximal (B) clitoris. Bielschowsky silver stain, scale bars represent 50 µm
DISCUSSION
The study of clitoral neuroanatomy, including afferent pathways, as it relates to sexual arousal is germane to the strategic design of female reconstructive surgery, the medical diagnosis of and treatment for sexual arousal disorders, and the understanding of sexual function related to neural pathologies and trauma. A murine model may be a valuable tool for studies related to these situations. Species specific differences between murine and human models are apparent. However, in many aspects the gross anatomy of murine genitalia is similar to that in the human females.6 The mouse perineal urethra is surrounded by erectile tissue, forming the clitoral bulbs. In the mouse, as in human females, tissue organization in the corpora cavernosa of the clitoris is essentially similar to that of the penis except for the absence of a subalbugineal layer interposed between the tunica albuginea and the erectile tissue.10,22 The existence of smooth muscle in the cavernous tissue of the clitoral body suggests that the clitoral vasculature is involved in the process of sexual arousal and the corporeal smooth muscle of the cavernous tissue also assumes an important role. This has been found to be important during penile erection.23 Smooth muscle cells of the corpus cavernosum of the penis are connected by gap junctions, which may have an important role during the activation of erection.24 It will be of interest in further studies to identify the existence of gap junctions in clitoral tissue.
The general pattern of peripheral innervation of the genitals is comparable in males and females in animal and human models.9,25 Neural pathways controlling penile erection26 are similar to those controlling clitoral sexual arousal (fig. 2). As in the penis, nerve fibers are abundantly distributed in the corporeal bodies vs the clitoral body. The glans clitoris is similar to its male counterpart, in that it is highly innervated by the dorsal and cavernous nerves. The mouse female external genitalia and clitoris are also richly innervated, as shown in this study. There is extensive innervation of the dorsal aspect of the glans clitoris. Nerve endings and sensory-like corpuscles were identified in the skin of the mouse external genitalia. Large sensory corpuscles have been recognized morphologically in the human external genitalia for more than a century.27 More recent studies showed genital end bulbs throughout the penis.28,29 In the human penis the most numerous afferent terminations were found to be free nerve endings but corpuscular receptors were also noted, including pacinian and Ruffini’s, and so-called genital end bulbs. Free nerve ending and similar corpuscular receptors, including Pacini’s, Ruffini’s and Merkel’s corpuscles, were found in the clitoris30–32 and on the inner surface of the labia up to the beginning of the true vagina (which does not include the introitus) but not in the vagina.31 Specialized nerve endings in the genital sites send afferent impulses to create spinal reflexes that influence genital motility and blood flow, but also ascend the spinal cord spinothalamic and spinoreticular tracts to the brain. Here they are decoded and interpreted as sexual arousal/pleasure, and efferent outputs to the genitals and various organs become activated.33
The tissue organization of the corpus cavernosus of the clitoris has been described as similar to that of the penis10,22,34,35 There is an important difference, that is the absence of the subalbugineal layer between the erectile tissue and the tunica albuginea, as noted in the human clitoris. 10 In our study of the murine clitoris we confirmed these results.22 In males the subalbuginea layer has an important role during the full erection phase of the penis.23 Absence of the venous plexus of the subalbugineal layer in the clitoris suggests that the process of erection lacks at least 2 phases, including the full and the rigid erection phases during sexual arousal. This process needs further investigation in the clitoris.
Central projections of the genitals course through the pudendal and the hypogastric nerves.13,16,36,37 In general our findings about the sensorial innervation of the mouse clitoris correspond with those in studies of the human clitoris. 38 In our study in which WGA-HRP was injected directly into the mouse clitoris and surrounding epithelium the pattern of spinal cord labeling was consistent across subjects regardless of injection volume, lending confidence to the accuracy of our neuronal tracing techniques. WGA-HRP labeling was seen caudal in levels S1 through L4 and rostral in L2. Previous investigations indicate that the caudal regions are associated with the pudendal nerve13,16,37 and the rostral region is associated with the hypogastric nerve.36
Somatic sensory pathways originate from the clitoral skin, where there exists a dense collection of nerve endings. These sensory afferents pass from the dorsal clitoral nerve to the pudendal nerve. Various receptive fields in the perineum are carried to the central nervous system primarily by pudendal nerve afferents and the responsiveness to stimulation is estrogen influenced.22,39–41 Sensitivity to tactile stimulation of the perineal skin, particularly influenced by estrogen, supports sexual arousal physiology and motor responses. In rats and mice this includes the lordosis reflex, which induces contraction of the iliococcygeus and pubococcygeus muscles, causing increased intravaginal pressure. This may stimulate the penis during copulation. In human females perineal skin stimulation results in the reflex contraction of the bulbocavernosus and ischiocavernosus muscles, which are important for clitoral erection during intercourse. 9
Central projections of the cutaneous branches of the pudendal nerve (the dorsal nerve of the clitoris and perineal nerve but not the muscular branch) also ascend through the dorsal column nuclear complex in rats.16,37 Labeling in the L2 gracile fasciculus of the mouse with the largest injection is particularly interesting since this injection was deposited in the clitoral sheath and external epithelium adjacent to the clitoris (fig. 3, A), while the smaller injection was restricted to the clitoris and no dorsal column labeling was seen in this subject. Afferent axons in the gracile fasciculus transmit sensory information about fine touch from an area in the lower truck up the dorsal columns to the gracile nucleus. The laminar arrangement of the columns in the dorsal funiculus indicates that the medial portion of the gracile fasciculus is associated with the lumbosacral spinal cord. Smith and Bennett observed that dorsal column labeling in rats was usually associated with dorsal root ganglia 2 or 3 segments caudal to the dorsal column labeling,42 suggesting that these axons likely passed through the L5 dorsal root ganglia.
In female rats pudendal nerve afferent fibers and their terminal endings were HRP labeled, predominately in the L6/S1 spinal cord.16 In mice we report the greatest density of labeling from the clitoris in the L5/L6 spinal cord with sparse labeling caudal in the sacral spinal cord and rostral in L2. To our knowledge it is not known whether the difference in results is related to species anatomical differences or to methodological differences. In the previous study in rats HRP was applied to the cut end of the pudendal nerve, whereas in our study WGA-HRP was injected into the clitoris and then transported centrally via the pudendal nerve.
The autonomic outflow responsible for the command of the genital sexual response originates in the spinal cord. The afferent limb of the sexual reflex is mainly conveyed by the pudendal, hypogastric and pelvic nerves. Genital sensation remains in some women with complete spinal cord injury, suggesting that the transmission of sensory genital information to the brain is partly spared.43,44 This is mediated by the vagal nerve and it provides a spinal cord bypass pathway for vaginal-cervical sensibility in rats as well as in women with complete spinal cord injury above the entry level into the spinal cord of the known genitospinal nerves.44,45 The brain regions that show activation during female orgasms are the hypothalamic paraventricular nucleus, the medial amygdala, the anterior cingulate, the frontal, parietal and insular cortices, and the cerebellum.44 Stimulating pudendal sensory fibers leads to the activation of pudendal motoneurons and the subsequent contraction of striated perineal muscles in men and women.46 Intravaginal electrical stimulation has induced the inhibition of bladder contractions and urethral closure has been noted in women and in cats, presumably to promote urinary continence during intercourse. 47–49
CONCLUSIONS
In the mouse we report the greatest density of labeling for nerve fibers from the clitoris in the L5/L6 spinal cord with sparse labeling caudal in the sacral spinal cord and rostral in L2. Understanding the neuroanatomy of the clitoris using a murine model may provide a valuable tool for the study of sexual arousal disorders and the further understanding of sexual function related to neural pathologies and trauma.
ACKNOWLEDGMENTS
Colored resin was provided by Dr. Robert Henry, University of Tennessee. Susan Strider assisted with illustration and Pedro Aranda Espinosa provided technical support.
Study received Rockefeller University Animal Care and Use Committee approval.
Abbreviations and Acronyms
- HRP
horseradish peroxidase
- WGA
wheat germ agglutinin
REFERENCES
- 1.Brown AG. The dorsal horn of the spinal cord. Q J Exp Physiol. 1982;67:193. doi: 10.1113/expphysiol.1982.sp002630. [DOI] [PubMed] [Google Scholar]
- 2.Pascual JI, Insausti R, Gonzalo LM. Pudendal nerve topography in the rat spinal cord projections studied with the axonal tracer wheat germ agglutinin conjugated-horseradish peroxidase. J Urol. 1992;147:718. doi: 10.1016/s0022-5347(17)37365-2. [DOI] [PubMed] [Google Scholar]
- 3.Marson L, Murphy AZ. Identification of neural circuits involved in female genital responses in the rat: a dual virus and anterograde tracing study. Am J Physiol Regul Integr Comp Physiol. 2006;291:R419. doi: 10.1152/ajpregu.00864.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marson L, Foley KA. Identification of neural pathways involved in genital reflexes in the female: a combined anterograde and retrograde tracing study. Neuroscience. 2004;127:723. doi: 10.1016/j.neuroscience.2004.04.063. [DOI] [PubMed] [Google Scholar]
- 5.Marson L, Cai R, Makhanova N. Identification of spinal neurons involved in the urethrogenital reflex in the female rat. J Comp Neurol. 2003;462:355. doi: 10.1002/cne.10732. [DOI] [PubMed] [Google Scholar]
- 6.O’Connell HE, Hutson JM, Anderson CR, Plenter RJ. Anatomical relationship between urethra and clitoris. J Urol. 1998;159:1892. doi: 10.1016/S0022-5347(01)63188-4. [DOI] [PubMed] [Google Scholar]
- 7.Yucel S, De Souza A, Jr, Baskin LS. Neuroanatomy of the human female lower urogenital tract. J Urol. 2004;172:191. doi: 10.1097/01.ju.0000128704.51870.87. [DOI] [PubMed] [Google Scholar]
- 8.O’Connell HE, DeLancey JO. Clitoral anatomy in nulliparous, healthy, premenopausal volunteers using unenhanced magnetic resonance imaging. J Urol. 2005;173:2060. doi: 10.1097/01.ju.0000158446.21396.c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cruz Y, Zempoalteca R, Angelica Lucio R, Pacheco P, Hudson R, Martínez-Gómez M. Pattern of sensory innervation of the perineal skin in the female rat. Brain Res. 2004;1024:97. doi: 10.1016/j.brainres.2004.07.046. [DOI] [PubMed] [Google Scholar]
- 10.Toesca A, Stolfi VM, Cocchia D. Immunohistochemical study of the corpora cavernosa of the human clitoris. J Anat. 1996;188:513. [PMC free article] [PubMed] [Google Scholar]
- 11.Baskin LS, Erol A, Li YW, Liu WH, Kurzrock E, Cunha GR. Anatomical studies of the human clitoris. J Urol. 1999;162:1015. doi: 10.1016/S0022-5347(01)68052-2. [DOI] [PubMed] [Google Scholar]
- 12.Schraffordt SE, Tjandra JJ, Eizenberg N, Dwyer PL. Anatomy of the pudendal nerve and its terminal branches: a cadaver study. ANZ J Surg. 2004;74:23. doi: 10.1046/j.1445-1433.2003.02885.x. [DOI] [PubMed] [Google Scholar]
- 13.Langworthy OR. Innervation of the pelvic organs of the rat. Invest Urol. 1965;2:491. [PubMed] [Google Scholar]
- 14.Baljet B, Drukker J. The extrinsic innervation of the pelvic organs in the female rat. Acta Anat. 1980;107:241. doi: 10.1159/000145249. [DOI] [PubMed] [Google Scholar]
- 15.Reiner P, Woolsey J, Adler N, Morrinson A. A gross anatomical study of the peripheral nerves associated with reproductive function in the female albino rat. In: Adler NT, editor. Neuroendocrinology of Reproduction. New York: Plenum Press; 1981. pp. 545–549. [Google Scholar]
- 16.McKenna KE, Nadelhaft I. The organization of the pudendal nerve in the male and female rat. J Comp Neurol. 1986;248:532. doi: 10.1002/cne.902480406. [DOI] [PubMed] [Google Scholar]
- 17.Carson KA, Mesulam MM. Ultrastructural evidence in mice that transganglionically transported horseradish per-oxidase-wheat germ agglutinin conjugate reaches the intraspinal terminations of sensory neurons. Neurosci Lett. 1982;29:201. doi: 10.1016/0304-3940(82)90317-2. [DOI] [PubMed] [Google Scholar]
- 18.Kurz EM, Sengelaub DR, Arnold AP. Androgens regulate the dendritic length of mammalian motoneurons in adulthood. Science. 1986;232:395. doi: 10.1126/science.3961488. [DOI] [PubMed] [Google Scholar]
- 19.Sengelaub DR, Arnold AP. Development and loss of early projections in a sexually dimorphic rat spinal nucleus. J Neurosci. 1986;6:1613. doi: 10.1523/JNEUROSCI.06-06-01613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molander C, Xu Q, Grant G. The cytoarchitectonic organization of the spinal cord in the rat. I. The lower thoracic and lumbosacral cord. J Comp Neurol. 1984;230:133. doi: 10.1002/cne.902300112. [DOI] [PubMed] [Google Scholar]
- 21.Humason GL. Animal Tissue Techniques. San Francisco: WH Freeman; 1962. p. 210. [Google Scholar]
- 22.Martin-Alguacil N, Pfaff D, Shelley DN, Schober J. Clitoral sexual arousal: An immunocytochemical and innervation study of the clitoris. BJU Int. 2008;101:1407. doi: 10.1111/j.1464-410X.2008.07625.x. [DOI] [PubMed] [Google Scholar]
- 23.Andersson KE, Wagner G. Physiology of penile erection. Physiol Rev. 1995;75:191. doi: 10.1152/physrev.1995.75.1.191. [DOI] [PubMed] [Google Scholar]
- 24.Christ GJ, Brink PR, Melman A, Spray DC. The role of gap junctions and ion channels in the modulation of electrical and chemical signals in human corpus cavernosum smooth muscle. Int J Impot Res. 1993;5:77. [PubMed] [Google Scholar]
- 25.Hashimoto J, Murakami G, Tsugane MH, Chisaka O, Capecchi MR, Ogino T. Lumbosacral plexus in Hoxa9 knockout mice with special reference to their nerve variations identified according to whether they were interphenotypic or intergenotypic differences. Kaibogaku Zasshi. 1999;74:609. [PubMed] [Google Scholar]
- 26.De Groat WC, Steers WD. Neuroanatomy and neurophysiology of penile erection. In: Tanagho EA, Lue TF, McClure RD, editors. Contemporary Management of Impotence and Infertility. Baltimore: Williams & Wilkins; 1988. pp. 3–27. [Google Scholar]
- 27.Krause W. Ueber die Nervenendigungen in den Geschlechtsorganen. Z Nat Medizin. 1866;XXVII [Google Scholar]
- 28.Sommerova J. Pre- and postnatal development of sensory nerve endings in penis in man. Scr Med (Brno) 1976;49:267. [PubMed] [Google Scholar]
- 29.Yang CC, Bradley WE. Innervation of the human glans penis. J Urol. 1999;161:97. [PubMed] [Google Scholar]
- 30.Kantner M. Studies on the sensory system of the glans clitoridis. I. The senile clitoris. Z Mikrosk Anat Forsch. 1954;60:388. [PubMed] [Google Scholar]
- 31.Winkelman RK. In: Nerve Ending in Normal and Pathologic Skin: Contributions to the Anatomy of Sensation. Curtis AC, editor. Springfield, Illinois: CC Thomas; 1960. [Google Scholar]
- 32.Polácek P, Malinovský L. Fine structure of sensory nerve endings in the clitoris. Z Mikrosk Anat Forsch. 1971;84:293. [PubMed] [Google Scholar]
- 33.Hauser-Kronberger C, Cheung A, Hacker GW, Graf AH, Dietze O, Frick J. Peptidergic innervation of the human clitoris. Peptides. 1999;20:539. doi: 10.1016/s0196-9781(99)00005-4. [DOI] [PubMed] [Google Scholar]
- 34.Levin RJ. The physiology of sexual arousal in the human female: a recreational and procreational synthesis. Arch Sex Behav. 2002;31:405. doi: 10.1023/a:1019836007416. [DOI] [PubMed] [Google Scholar]
- 35.Sevely JL. The male clitoris. Am J Obstet Gynecol. 1988;159:533. doi: 10.1016/s0002-9378(88)80128-5. [DOI] [PubMed] [Google Scholar]
- 36.Williams-Ashman HG, Reddi AH. Differentiation of mesenchymal tissues during phallic morphogenesis with emphasis on the os penis: roles of androgens and other regulatory agents. J Steroid Biochem Mol Biol. 1991;39:873. doi: 10.1016/0960-0760(91)90344-5. [DOI] [PubMed] [Google Scholar]
- 37.Nadelhaft I, McKenna KE. Sexual dimorphism in sympathetic preganglionic neurons of the rat hypogastric nerve. J Comp Neurol. 1987;256:308. doi: 10.1002/cne.902560210. [DOI] [PubMed] [Google Scholar]
- 38.Ueyama T, Arakawa H, Mizuno N. Central distribution of efferent and afferent components of the pudendal nerve in rat. Anat Embryol (Berl) 1987;177:37. doi: 10.1007/BF00325288. [DOI] [PubMed] [Google Scholar]
- 39.Komisaruk BR, Adler NT, Hutchison J. Genital sensory field: enlargement by estrogen treatment in female rats. Science. 1972;178:1295. doi: 10.1126/science.178.4067.1295. [DOI] [PubMed] [Google Scholar]
- 40.Kow LM, Pfaff DW. Effects of estrogen treatment on the size of receptive field and response threshold of pudendal nerve in the female rat. Neuroendocrinology. 1973–1974;13:299. doi: 10.1159/000122214. [DOI] [PubMed] [Google Scholar]
- 41.Adler NT, Davis PG, Komisaruk BR. Variation in the size and sensitivity of a genital sensory field in relation to the estrous cycle in rats. Horm Behav. 1977;9:334. doi: 10.1016/0018-506x(77)90068-x. [DOI] [PubMed] [Google Scholar]
- 42.Smith KJ, Bennett BJ. Topographic and quantitative description of rat dorsal column fibres arising from the lumbar dorsal roots. J Anat. 1987;153:203. [PMC free article] [PubMed] [Google Scholar]
- 43.Komisaruk BR, Gerdes CA, Whipple B. ‘Complete’ spinal cord injury does not block perceptual responses to genital self-stimulation in women. Arch Neurol. 1997;54:1513. doi: 10.1001/archneur.1997.00550240063014. [DOI] [PubMed] [Google Scholar]
- 44.Komisaruk BR, Whipple B, Crawford A, Liu WC, Kalnin A, Mosier K. Brain activation during vaginocervical self-stimulation and orgasm in women with complete spinal cord injury: fMRI evidence of mediation by the vagus nerves. Brain Res. 2004;1024:77. doi: 10.1016/j.brainres.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 45.Komisaruk BR, Sansone G. Neural pathways mediating vaginal function: the vagus nerves and spinal cord oxytocin. Scand J Psychol. 2003;44:241. doi: 10.1111/1467-9450.00341. [DOI] [PubMed] [Google Scholar]
- 46.Vodusek DB. Pudendal SEP and bulbocavernosus reflex in women. Electroencephalogr Clin Neurophysiol. 1990;77:134. doi: 10.1016/0168-5597(90)90027-b. [DOI] [PubMed] [Google Scholar]
- 47.Erlandson BE, Fall M, Carlsson CA, Linder LE. Mechanisms for closure of the human urethra during intravaginal electrical stimulation. Scand J Urol Nephrol. 1977;44 suppl.:49. [PubMed] [Google Scholar]
- 48.Fall M, Erlandson BE, Sundin T, Waagstein F. Intravaginal electrical stimulation. Clinical experiments on bladder inhibition. Scand J Urol Nephrol. 1977;44 suppl.:41. [PubMed] [Google Scholar]
- 49.Ohlsson B, Lindström S, Erlandson BE, Fall M. Effects of some different pulse parameters on bladder inhibition and urethral closure during intravaginal electrical stimulation: an experimental study in the cat. Med Biol Eng Comput. 1986;24:27. doi: 10.1007/BF02441602. [DOI] [PubMed] [Google Scholar]