the plasma membrane separates the external and internal cellular environments. Many processes that injure the plasma membrane are a prelude to cell death (i.e., necrosis, apoptosis, and autophagy). Therefore, to maintain homeostasis and survival, cells must have mechanisms of membrane repair that limit intrusion of the external environment to the interior of the cell. At the heart of this repair machinery are highly regulated and coordinated endocytic and exocytic processes.
The article published in American Journal of Physiology-Cell Physiology by Bernatchez et al. (5) focuses on the role of myoferlin, caveolin-1, and dynamin in receptor-mediated and injury-induced endocytosis. Myoferlin is a 230-kDa transmembrane protein that is expressed primarily in cardiac and skeletal muscle. The study by Bernatchez et al. shows that myoferlin regulates caveolae/lipid raft and clathrin-mediated endocytosis but the greater effect is on the former process. Though a role for these two endocytic processes has been established for receptor trafficking, the intriguing interplay of myoferlin, caveolin-1, and dynamin in endocytosis-induced membrane repair is of note and worth highlighting for readers.
Membrane repair following injury was initially thought to be a passive event that was mediated by resealing of the lipid bilayer (15). However, this idea was later expanded to suggest that large disruptions (>1 μm) of the plasma membrane undergo “patch repair” where Ca2+ influx through membrane lesions triggers exocytosis of cytoplasmic vesicles that fuse with the injured membrane (6). Akin to synaptic vesicle fusion that releases neurotransmitters, the early insight that calcium-regulated exocytosis was involved in membrane repair provided a useful working hypothesis. Subsequent investigations then turned to identifying which intracellular vesicles were used to repair the damaged plasma membrane. These vesicles required three characteristics: 1) regulation by Ca2+, 2) capability of assembly to create a large area of patch, and 3) ability to fuse with the plasma membrane. Lysosomes were shown to fit all three criteria. Lysosomal membranes contain synaptotagmin VII (Syt VII), a calcium sensor (8), and, notably, mice deficient in Syt VII have defective membrane repair and show muscle fiber invasion by leukocytes, which together result in myopathy (7). The Ca2+ trigger induces fusion of lysosomes to form large membrane regions (3) that can then fuse with the plasma membrane (22). Inhibition of lysosome exocytosis, by modulating Syt VII activity or causing lysosomal aggregation, interferes with membrane resealing (21). The process of sensing injury, activating lysosomes, and mediating membrane resealing is rapid (occurring within seconds) and involves lysosomes in close proximity to the plasma membrane (17). Collectively, these observations suggest that exocytosis is highly regulated and critical to membrane repair.
Later studies established that endocytosis is of equal importance and may work in concert with exocytosis for membrane repair. It was initially shown that membrane injury not due to mechanical injury (i.e., exposure to toxins that create pores) results in Ca2+-dependent repair of membranes (16); however, although exocytosis was induced, an equally rapid and robust endocytic response that internalized the pore to maintain membrane integrity was also observed. The important implication of the current work is the identification of a molecular complex consisting of myoferlin, caveolin-1, and dynamin that may be integral to membrane repair (5).
Myoferlin is akin to other ferlin proteins in having C2 domains (which may serve as Ca2+ sensors) (10, 11) and a COOH-terminal membrane-spanning domain, but most of its structure resides in the cytoplasm. Myoferlin associates with both the plasma membrane and the nuclear membrane and can also be found in the nucleoplasm. Myoferlin is present in high amounts in regenerating muscle; mice deficient in myoferlin experience defective myogenesis and muscle regeneration (10, 12). Myoferlin was initially thought to be involved in plasma membrane dynamics because proteins in the ferlin family are homologous to fer-1, a spermatogenesis factor in Caenorhabditis elegans that mediates spermatid vesicle/plasma membrane fusion (2, 4). Bernatchez et al. confirm the interaction of myoferlin with the plasma membrane (5), but, in addition, they show interaction of myoferlin with caveolin-1 and localization in caveolae. This interaction and localization are necessary for membrane repair because small interfering RNA knockdown of either myoferlin or caveolin-1 leads to an equal degree of loss of membrane resealing following injury.
Caveolin-1 is a structural component of caveolae, which are specialized, lipid-rich microdomains that coordinate a variety of functional events (20). The budding (i.e., endocytosis) of caveolae from the plasma membrane requires dynamins, which are GTPases that are involved in various cellular processes. Dynamins self-assemble and oligomerize at the necks of plasma membrane caveolae, thereby resulting in caveolar budding and retention of dynamin in the membrane (9, 19). The trigger for this budding has remained elusive; however, on the basis of the molecular interactions proposed in the current study, we speculate that cellular stress, as sensed by myoferlin via Ca2+ influx, may be key to localized regulation of caveolin-dynamin dynamics.
Mutation or knockdown of caveolin-3, a muscle-specific caveolin, results in myopathies (1, 13, 25). Dysferlin (a member of the ferlin family with a function similar to myoferlin) is dependent on caveolin-3 expression for its retention in the membrane; knockdown of caveolin-3 results in mislocalized dysferlin and its rapid internalization (14). Perhaps the retention of dysferlin in the plasma membrane via caveolin-3 is a means to localize and anchor this “sensor of injury” to membranes and to facilitate rapid protective response. In this regard, it is interesting to note that cardiac myocyte-specific overexpression of caveolin-3 protects the heart from ischemia-reperfusion injury (which is known to disrupt membranes and lead to intracellular influx of Ca2+) (18, 24). Importantly, overexpression of caveolin-3 leads to the preservation of the ultrastructure of sarcolemmal membranes and intracellular organelles, mimicking the protection induced by sublethal ischemia before lethal hypoxic stress (24). Although the mechanism is unknown, multiple cycles of sublethal ischemia have been shown to preserve myocardial membrane and intracellular ultrastructure (18). With respect to membrane repair, a similar observation has been made: a second membrane disruption at the same site of original injury repairs more rapidly, an effect that occurs via endocytosis (23). Such results suggest that multiple exposures to injury enhance the efficiency of endocytosis and perhaps the maintenance or repair of membrane integrity. The study by Bernatchez et al. shows that increased myoferlin in a reconstituted system is sufficient to increase endocytosis independent of injury. The findings, however, lead to several questions. For example, does caveolin expression represent a control point for regulating the efficiency of endocytosis? Do membranes that have greater expression of caveolins and caveolae have increased expression and activity of ferlins and dynamins at the cell membrane? Are budded caveolae the “raw material” for sealing damaged plasma membranes? Can ferlins, caveolins, and dynamins be targeted as possible therapeutics for myopathic disease processes?
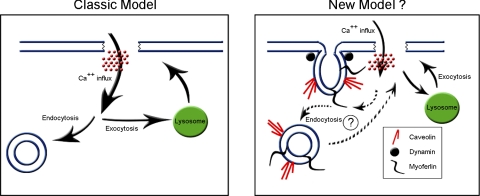
The current study defines three components (i.e., myoferlin, caveolin, and dynamin) of a molecular bandage that may be critical to the integrity of cellular membrane and may provide a means to regulate a variety of disease processes. Involvement of other elements, such as membrane tension and the cytoskeleton, may also contribute to membrane repair. A challenge for the future is to define the temporal nature of endocytic and exocytic processes and if the interaction of myoferlin, caveolins, and dynamins and their localization in caveolae represents a refinement or a paradigm shift (Fig. 1) in terms of membrane repair following injury.
Fig. 1.
Schematic of the classic model and a potential new model of membrane repair. A: in the classic model of plasma membrane repair, membrane injury leads to Ca2+ influx, which induces exocytic and endocytic repair pathways. In the exocytic pathway, lysosomes contribute membrane to patch the injury site. In the endocytic pathway, vesicles bud and remove the wound site from the plasma membrane. B: in the theorized new model, the exocytic repair pathway remains intact but the endocytic repair pathway takes a more prevalent role. In this model, injury-induced Ca2+ influx is sensed by myoferlin localized at the plasma membrane in caveolae and initiates dynamin-dependent endocytosis. The budded caveolae, due to their close proximity to the plasma membrane, may then patch the injured area, possibly by entering into an exocytic repair mode.
GRANTS
This work was supported by grants from the American Heart Association (060039N) and National Institutes of Health (HL-091071 and HL-066941).
REFERENCES
- 1.Aboumousa A, Hoogendijk J, Charlton R, Barresi R, Herrmann R, Voit T, Hudson J, Roberts M, Hilton-Jones D, Eagle M, Bushby K, Straub V. Caveolinopathy–new mutations and additional symptoms. Neuromuscul Disord 18: 572–578, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Achanzar WE, Ward S. A nematode gene required for sperm vesicle fusion. J Cell Sci 110: 1073–1081, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Bakker AC, Webster P, Jacob WA, Andrews NW. Homotypic fusion between aggregated lysosomes triggered by elevated [Ca2+]i in fibroblasts. J Cell Sci 110: 2227–2238, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Bashir R, Britton S, Strachan T, Keers S, Vafiadaki E, Lako M, Richard I, Marchand S, Bourg N, Argov Z, Sadeh M, Mahjneh I, Marconi G, Passos-Bueno MR, Moreira Ede S, Zatz M, Beckmann JS, Bushby K. A gene related to Caenorhabditis elegans spermatogenesis factor fer-1 is mutated in limb-girdle muscular dystrophy type 2B. Nat Genet 20: 37–42, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Bernatchez PN, Sharma A, Kodaman P, Sessa WC. Myoferlin is critical for endocytosis in endothelial cells. Am J Physiol Cell Physiol (June3, 2009). doi: 10.1152/ajpcell.00498.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bi GQ, Alderton JM, Steinhardt RA. Calcium-regulated exocytosis is required for cell membrane resealing. J Cell Biol 131: 1747–1758, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakrabarti S, Kobayashi KS, Flavell RA, Marks CB, Miyake K, Liston DR, Fowler KT, Gorelick FS, Andrews NW. Impaired membrane resealing and autoimmune myositis in synaptotagmin VII-deficient mice. J Cell Biol 162: 543–549, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czibener C, Sherer NM, Becker SM, Pypaert M, Hui E, Chapman ER, Mothes W, Andrews NW. Ca2+ and synaptotagmin VII-dependent delivery of lysosomal membrane to nascent phagosomes. J Cell Biol 174: 997–1007, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danino D, Hinshaw JE. Dynamin family of mechanoenzymes. Curr Opin Cell Biol 13: 454–460, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Davis DB, Delmonte AJ, Ly CT, McNally EM. Myoferlin, a candidate gene and potential modifier of muscular dystrophy. Hum Mol Genet 9: 217–226, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Davis DB, Doherty KR, Delmonte AJ, McNally EM. Calcium-sensitive phospholipid binding properties of normal and mutant ferlin C2 domains. J Biol Chem 277: 22883–22888, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Doherty KR, Cave A, Davis DB, Delmonte AJ, Posey A, Earley JU, Hadhazy M, McNally EM. Normal myoblast fusion requires myoferlin. Development 132: 5565–5575, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hagiwara Y, Sasaoka T, Araishi K, Imamura M, Yorifuji H, Nonaka I, Ozawa E, Kikuchi T. Caveolin-3 deficiency causes muscle degeneration in mice. Hum Mol Genet 9: 3047–3054, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Hernandez-Deviez DJ, Howes MT, Laval SH, Bushby K, Hancock JF, Parton RG. Caveolin regulates endocytosis of the muscle repair protein, dysferlin. J Biol Chem 283: 6476–6488, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Hoffman JF. On red blood cells, hemolysis and resealed ghosts. Adv Exp Med Biol 326: 1–15, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Idone V, Tam C, Goss JW, Toomre D, Pypaert M, Andrews NW. Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J Cell Biol 180: 905–914, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaiswal JK, Andrews NW, Simon SM. Membrane proximal lysosomes are the major vesicles responsible for calcium-dependent exocytosis in nonsecretory cells. J Cell Biol 159: 625–635, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murry CE, Richard VJ, Reimer KA, Jennings RB. Ischemic preconditioning slows energy metabolism and delays ultrastructural damage during a sustained ischemic episode. Circ Res 66: 913–931, 1990 [DOI] [PubMed] [Google Scholar]
- 19.Oh P, McIntosh DP, Schnitzer JE. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium. J Cell Biol 141: 101–114, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel HH, Murray F, Insel PA. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu Rev Pharmacol Toxicol 48: 359–391, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reddy A, Caler EV, Andrews NW. Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell 106: 157–169, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez A, Webster P, Ortego J, Andrews NW. Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells. J Cell Biol 137: 93–104, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Togo T, Alderton J, Bi G, Steinhardt R. The mechanism of facilitated cell membrane resealing. J Cell Sci 112: 719–731, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Tsutsumi YM, Horikawa YT, Jennings MM, Kidd MW, Niesman IR, Yokoyama U, Head BP, Hagiwara Y, Ishikawa Y, Miyanohara A, Patel PM, Insel PA, Patel HH, Roth DM. Cardiac-specific overexpression of caveolin-3 induces endogenous cardiac protection by mimicking ischemic preconditioning. Circulation 118: 1979–1988, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woodman SE, Park DS, Cohen AW, Cheung MW, Chandra M, Shirani J, Tang B, Jelicks LA, Kitsis RN, Christ GJ, Factor SM, Tanowitz HB, Lisanti MP. Caveolin-3 knock-out mice develop a progressive cardiomyopathy and show hyperactivation of the p42/44 MAPK cascade. J Biol Chem 277: 38988–38997, 2002 [DOI] [PubMed] [Google Scholar]