Abstract
Myoferlin is a member of the ferlin family of proteins that promotes endomembrane fusion with the plasma membrane in muscle cells and endothelial cells. In addition, myoferlin is necessary for the surface expression of vascular endothelial growth factor receptor 2 through the formation of a protein complex with dynamin-2 (Dyn-2). Since Dyn-2 is necessary for the fission of endocytic vesicles from the plasma membrane, we tested the hypothesis that myoferlin may regulates aspects of receptor-dependent endocytosis. Here we show that myoferlin gene silencing decreases both clathrin and caveolae/raft-dependent endocytosis, whereas ectopic myoferlin expression in COS-7 cells increases endocytosis by up to 125%. Interestingly, we have observed that inhibition of Dyn-2 activity or caveolin-1 (Cav-1) expression impairs endocytosis as well as membrane resealing after injury, indicating that Dyn-2 and Cav-1 also participate in both membrane fission and fusion processes. Mechanistically, myoferlin partially colocalizes with Dyn-2 and Cav-1 and forms a protein complex with Cav-1 solubilized from tissue extracts. Together, these data describe a new role for myoferlin in receptor-dependent endocytosis and an overlapping role for myoferlin-Dyn-2-Cav-1 protein complexes in membrane fusion and fission events.
Keywords: signaling, caveolin
internalization of extracellular cargo at the plasma membrane is a highly dynamic biological process regulated by various clathrin-dependent and -independent pathways. Receptor-dependent endocytosis, characterized by the fission of endocytic vesicles from the plasma membrane, requires the coat proteins clathrin, caveolins, and flotillins. Plasma membrane fission into endocytic vesicle is furthermore facilitated by the dynamin (Dyn) GTPase family of proteins, as well as cell-specific signaling molecules, leading to vesicle fusion with intracellular acceptor membranes.
Another, less characterized plasma membrane event in all cells is membrane repair after physical injury. In contrast to membrane endocytosis, membrane repair consists in the regulated fusion of subplasmalemmal vesicles (endomembranes) with plasma membranes to reseal damaged areas of membrane (36). Muscular dystrophy, characterized by generalized muscle weakness, is a disease potentially involving defective membrane resealing of myocytes resulting in impaired cellular regeneration and death (6). The molecular machinery responsible for this basic cellular function is beginning to be elucidated and includes members of the SNARE (5, 35, 36), annexins (31, 34), caveolin (29), ferlins (6), and perhaps additional cell-specific regulatory proteins.
The close relationships between membrane fission and fusion suggests that an active, regulated interplay may exist between these two events (40). Both processes involve significant actin/myosin dynamics (36), calcium (6, 44, 49), and Dyn-like proteins. For example, Vps1p, a Dyn-like GTPase protein involved in vesicle formation at the Golgi apparatus, was shown also to promote vacuole fusion in yeast (41). More recently, a member of the ADP-ribosylation factor (ARF) family of proteins, ARF1, has been described as a shared mediator of both endocytosis and exocytosis (30), evidence supporting cross-talk between membrane fission and fusion events.
All known mammalian ferlin gene products (myoferlin, dysferlin, and otoferlin) have been described to mediate endomembrane fusion events. Loss of dysferlin or myoferlin activity causes limb-girdle muscular dystrophy (10, 16, 17, 19) through impaired skeletal muscle repair after physical injury. Moreover, deficient synaptic vesicle fusion is a result of otoferlin gene disruption in mice, causing profound deafness (42). We and others have found myoferlin in endothelial cell (EC) plasma membranes (21) and lipid rafts/caveolae (24) where it participates in membrane resealing events and prevents Dyn and CBL-dependent ubiquitination and degradation of vascular endothelial growth factor (VEGF) receptor 2 (VEGFR2) (7). However, the role of myoferlin in modulating receptor-dependent endocytosis or the role of Dyn in membrane resealing events have not been explored. Herein, we provide evidence for a role for myoferlin in fission-based endocytosis and show that both Dyn-2 activity and Cav-1 are required for efficient EC membrane resealing after laser injury. Mechanistically, myoferlin colocalizes with Dyn-2 and interacts biochemically with Cav-1 thereby rationalizing the role of myoferlin in transferrin and caveolae-dependent endocytosis. Taken together, these data suggest that myoferlin and perhaps other ferlins are novel regulators of bidirectional membrane turnover events.
MATERIALS AND METHODS
Cell culture.
Bovine aortic EC (BAEC), EA.hy.926, human embryonic kidney (HEK), and COS-7 cells were grown in high glucose Dulbecco's modified Eagle's medium (DMEM, Invitrogen) supplemented with 10% FBS (Hyclone) and penicillin-streptomycin (Sigma).
Small interfering RNA treatment.
Myoferlin DNA target sequences (Qiagen) were (5′-3′): AACCCTGTCTGGAATGAGATT (for both bovine and human myoferlin; bhMyof), and AATCAGGACTGGCTTGATAAA (for human myoferlin; hMyof) as well as their respective scrambled controls, AATTCTCCGAACGTGTGAAGT [bh-scrambled nonsilencing siRNA (NS)] and AAAAATTCAAGGTGTACCGTG (hNS). Cell transfections were performed as previously published (7). Cav-1 knockdown was performed using the nearly identical AACGAGAAGCAAGTGTACGAC (human) or AGCGAGAAGCAAGTGTACGAC (bovine) small interfering RNA (siRNA) sequences as published (43) and a scrambled control GTGAAGCAAAGCGAAATGCAC siRNA.
In vitro endocytosis assay.
Following siRNA treatment (72 h), subconfluent BAEC were preincubated for 30 min at 4°C followed by an 8-min incubation at 37°C with Cy5-transferrin or Cy5-cholera toxin in DMEM + 0.25% FBS to minimize recycling of endocytosis markers. After incubation, nonspecific binding was removed by acid stripping and cells were fixed and visualized as described in Immunofluorescence staining. Fluorescence intensity of individual cells of similar sizes was quantified with Photoshop (Adobe) set at an Image Cache value of 1.
Plasmids and cell transfection.
Transfection of myoferlin-hemagglutinin (HA), heat shock protein 90 (HSP90)-HA, or β-galactosidase (β-Gal) in COS-7 cells was performed as published (7).
Western blot analysis and immunoprecipitation.
The myoferlin antibody was generated in rabbits using the synthetic peptide acetyl-CTEFTDEVYQNESRYPGGD-cooh coupled to KLH (Rockland) and affinity purified as described previously (7). β-COP and HA anti-sera were from ABR and Roche, respectively. Anti-CD71, Dyn-2, and HSP90 antibodies were from BD. Anti-V5 serum was from Invitrogen. Cav-1 antibody was from Santa Cruz. Immunoprecipitation was performed with the mentioned antibody and with protein-G-coated beads (Sigma).
Membrane repair assay.
EA.hy.926 were used because of their relative bulkiness and high membrane content. Following SU-1498 (10−5 M, 1 h, Calbiochem) or siRNA treatment (72 h) in a glass-bottom Petri plate, cells were incubated in DMEM with FM1-43 (25 μM, Molecular Probes) for 5 min. In the meantime, the Petri plate was mounted in a heated Plexiglas chamber (Zeiss) and incubated at 37°C, 5% CO2, and 95% humidity. Cells were visualized with Axiovert (Zeiss) or Leica AOBS TCS confocal microscopes, and damage was induced and analyzed as described previously (7). Experiments were performed on at least six EA.hy.926 per group in triplicate.
Immunofluorescence staining.
EC were grown on glass coverslips, fixed in 2% paraformaldehyde, and permeabilized with 0.1% Triton X-100 (TX100). Samples were blocked with 2.5% normal goat serum, treated for 1h with primary antibodies, washed and treated with secondary antibodies coupled to Alexa-488, −554 or Cy5. For lipid staining, cells were pretreated for 1 h with FM1-43FX, washed and fixed as described above. Cy5 was used in conjunction with FM1-43FX to minimize bleedthrough between the two channels. All samples were mounted on a glass slide with Gelvatol and DAPI (Sigma) and analyzed by using an Axiovert epifluorescent microscope (Zeiss). In each experiment, all photomicrographs were exposed and processed identically for a given fluorophore and corrected for background fluorescence using unlabeled specimens. For co-localization studies, z-plane images of typical cells were taken at 0.2 μm intervals, and images were deconvoluted by using the Openlab software.
Adenovirus constructs.
Dynamin-2 and control adenoviruses were generated as previously described (7) and amplified by the University of Iowa gene transfer core facility. Each virus was used at a multiplicity of infection of 25.
Surface biotinylation.
Surface biotinylation of BAEC following siRNA treatment was performed as previously described (7) with a cell-impermeable cross-linking kit (Pierce).
Statistical analysis.
Data were analyzed by ANOVA followed by Dunnett's t-test. Values were considered significantly different when P < 0.05 was observed.
RESULTS
Myoferlin participates in receptor-mediated endocytosis.
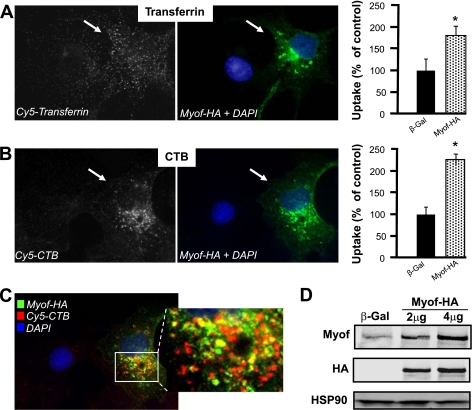
To test the role of myoferlin in receptor-dependent endocytosis, we examined whether overexpression of myoferlin increases endocytosis of fluorescently labeled transferrin and cholera toxin-B (CTB) chain, two well-established markers for receptor-dependent, clathrin- and caveolae/lipid raft-mediated endocytosis, respectively, using COS-7 cells, which have low endogenous myoferlin levels. COS-7 cells were transiently transfected with a plasmid encoding myoferlin-HA for 72 h, and the uptake of labeled transferrin and CTB was examined. As seen in Fig. 1, A and B (left and center), cells expressing myoferlin-HA (green) had greater uptake of transferrin and CTB compared with β-Gal-transfected cells in an 8-min uptake assay, the earliest time point that endocytosed tracers could be accurately quantified while minimizing intracellular recycling. Quantification of total cell fluorescence reveals that transfection of myoferlin-HA increases transferrin and CTB internalization by 82% and 125%, respectively (Fig. 1, A and B, right). Since myoferlin is a luminal surface protein enriched in caveolae/lipid rafts (7, 24), we investigated the localization of myoferlin-HA and CTB during an 8-min endocytosis assay. Deconvolution fluorescence microscopy (Fig. 1C) reveals significant colocalization (yellow) of myoferlin-HA (green) with CTB (red) in transfected COS-7 cells. Western blot analyses confirm that transfection of COS-7 cells with myoferlin-HA plasmid (2 and 4 μg) dose dependently increases myoferlin overexpression by up to 8.5-fold compared with β-Gal plasmid transfection (Fig. 1D, top).
Fig. 1.
Myoferlin overexpression increases endocytosis in vitro, whereas vessels with disrupted myoferlin gene show decreased endocytosis ex vivo. A and B: COS-7 cells expressing hemagglutinin-tagged myoferlin (Myof-HA, green, center) show increased endocytosis compared with control cells (black; blue nucleus). Data are means ± SE (n ≥ 10 cells in duplicate per group). Right: represents average data from the quantification of individual cells. * P < 0.05 compared with control-treated cells. C: total Myof-HA (green) exhibits partial colocalization (yellow) with cholera toxin-B (CTB, red), a marker of endocytosed caveolae, by deconvoluted fluorescence microscopy. Nucleus is shown in blue. D: transfection of COS-7 cells with Myof-HA plasmids (2 and 4 μg) increases myoferlin expression in a dose-dependent manner compared with β-galactosidase (β-Gal). Western blot analysis confirms plasmid-specific expression of HA tag. Heat shock protein 90 (HSP90) was used as loading control. This experiment was performed in duplicate, and typical data are shown.
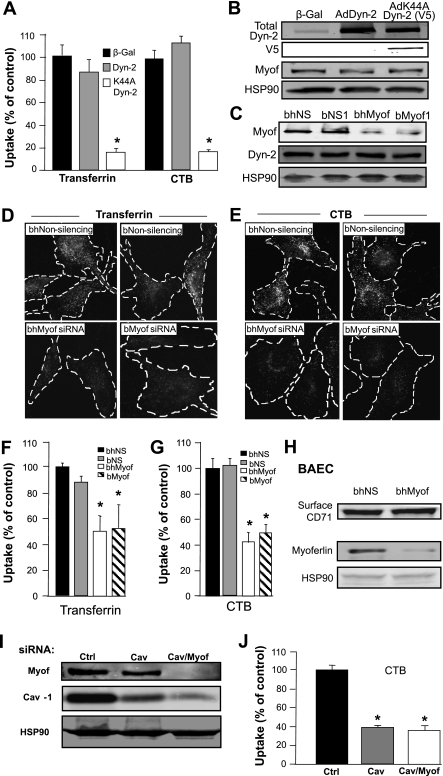
To examine whether the loss of myoferlin attenuates endocytosis in vitro, we used cultured BAEC that endogenously express high levels of myoferlin (7) and studied the effects of myoferlin gene silencing on transferrin and CTB endocytosis as described above in an 8-min assay. To validate the correlation between fluorescence labeling and receptor-dependent endocytosis in EC, we first examined the effects of overexpressed wild-type (WT) and dominant-negative Dyn-2 (V5 epitope-tagged K44A mutant) on endocytosis since clathrin and caveolae/raft-mediated endocytosis are both partially dependent on the GTPase activity of Dyn-2 (3, 14). Adenoviral expression of WT Dyn-2 did not influence transferrin or CTB uptake in EC at 8 min compared with a β-galactosidase-expressing virus (β-Gal), whereas adenoviral expression of K44A Dyn-2 reduces uptake of both markers by >80% (Fig. 2A). Overexpression of both WT and K44A Dyn-2 proteins as well as unmodulated endogenous myoferlin levels were confirmed by Western blotting (Fig. 2B) thereby confirming the known role of Dyn-2 in endocytosis in EC (47).
Fig. 2.
Attenuated dynamin-2 (Dyn-2) activity or myoferlin knockdown with small interfering RNA (siRNA) disrupts endocytosis. A: bovine aortic endothelial cells (BAEC) were transduced with adenoviruses (Ad) expressing wild-type (WT) Dyn-2, dominant-negative Dyn-2 (K44A Dyn-2), or β-Gal (as a control) for 48 h, and in vitro endocytosis assays were performed as described in Fig. 1B. *P < 0.05 compared with β-Gal-treated cells (n = at least 20 individual cells in duplicate). B: Western blot analysis confirms overexpression of WT Dyn-2 and K44A Dyn-2 (V5 epitope) without affecting endogenous myoferlin expression. C: pretreatment of BAEC with scrambled nonsilencing (NS) siRNA [bovine-human NS (bhNS) and bovine NS (bNS)] has no effect on myoferlin protein levels, whereas homologous bhMyof and bMyof siRNA attenuate myoferlin protein expression (75 nM). HSP90 is used as a loading control, and Dyn-2 expression is unaffected by myoferlin gene knockdown. D and E: myoferlin gene silencing reduces BAEC endocytosis. Following siRNA treatment, clathrin-dependent and caveolae/raft-dependent endocytosis was evaluated by treating EC with Cy5-transferrin or Cy5-CTB for 30 min at 4°C followed by an 8-min chase at 37°C. Noninternalized markers were stripped from the plasma membrane by acid washing. Identical experiments were conducted in cells treated with two different myoferlin siRNAs and their respective controls and were visualized by epifluorescence microscopy. F and G: histograms represent quantification of individual cells. Data are means ± SE (n ≥ 25 cells per group in duplicate). *P < 0.05 compared with respective scrambled siRNA-treated cells labeled with same dyes. H: surface biotinylation confirms the ample surface expression of CD71 (transferrin receptor 1) following myoferlin knockdown. I: pretreatment (72 h) of BAEC with Myof and caveolin-1 (Cav-1) siRNA (75 nM total) decreases both Cav-1 and Myof protein expression. Cells were treated with two scrambled sequences [75 nM combined; control (Ctrl)], Cav-1 siRNA/scrambled (75 nM combined; Cav), or Cav-1/Myof siRNA sequences (75 nM combined; Cav/Myof). HSP90 is used as loading control. J: double Cav-1/Myof knockdown does not further decrease CTB endocytosis compared with single Cav-1 gene silencing. BAEC were pretreated for 72 h as described in I and treated with Cy5-CTB as described in D and E. Data are means ± SE (n ≥ 20 cells per group in triplicate). *P < 0.05 compared with scrambled siRNA-treated cells.
To silence endogenous myoferlin gene expression, EC were treated with two different siRNAs (bovine-human myoferlin siRNA and bovine myoferlin siRNA; bhMyof and bMyof) compared with scrambled nonsilencing siRNA (bhNS and bNS), and both sequences caused a reduction in myoferlin levels by >95% (Fig. 2C, top) as previously published (7). As seen in Fig. 2, D and E, left, myoferlin silencing reduces the uptake of transferrin and CTB as visualized by epifluorescence microscopy. Total fluorescence quantification of individual cells reveals that myoferlin gene knockdown decreases endocytosis of transferrin and CTB by 49% and 58%, respectively, in BAEC compared with control siRNA sequences, demonstrating that myoferlin is necessary, but not sufficient, for endocytosis in cultured EC. Quantification of total cell fluorescence compared with nonsilencing siRNA-treated cells (% of uptake) is illustrated in Fig. 2, F and G. A potential explanation for the loss of transferrin uptake in EC following myoferlin silencing could be attributable to a decrease in transferrin receptor 1 (CD71) expression, the receptor responsible for the endocytosis of transferrin. Cell surface biotinylation of EC membranes was performed followed by avidin purification (7). Immunoblotting reveals that CD71 expression is unaffected by myoferlin siRNA sequences (Fig. 2H). Moreover, no obvious differences in basal transferrin and CTB staining (time 0) were observed (data not shown), furthermore suggesting that the defect in endocytosis observed is not likely due to a defect in the surface expression of transferrin and CTB receptors. Hence, these data document that myoferlin is necessary for EC endocytosis in vitro.
To test whether myoferlin and Cav-1 mediate their actions on caveolae-dependent endocytosis synergistically, we performed endocytosis assays in cells treated with both myoferlin and Cav-1 siRNA sequences. Pretreatment of BAEC with Cav-1 and myoferlin siRNA sequences causes a 92% and 95% decrease in their target protein expression, respectively (Fig. 2I, right lane), whereas selective Cav-1 knockdown causes an 84% decrease in Cav-1 expression (Fig. 2I, middle lane) compared with scrambled siRNA pretreatment. Interestingly, additional loss of myoferlin did not significantly decrease CTB endocytosis (63%) compared with selective Cav-1 gene silencing (60%) (Fig. 2J), supporting the concept that myoferlin and Cav-1 act in a similar rather than parallel fashion to mediate caveolae-dependent endocytosis.
Dynamin-2 participates in membrane resealing events.
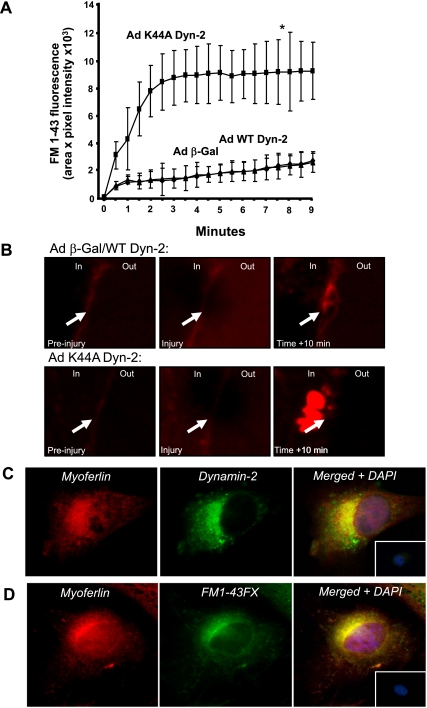
Since Dyn-2 is recognized as a molecular machine necessary for endocytosis of transferrin and CTB (Fig. 2A) and the idea that membrane resealing is an energy-dependent process, we next examined whether Dyn-2 influences membrane resealing after laser injury, a known function of ferlins (2, 7, 19, 48). The human EC line, EA.hy.926 cells were infected with either WT or K44A Dyn-2, cells incubated with FM1-43 and local membrane damaged achieved by direct laser irradiation (7). FM1-43 is soluble in water and lipid but fluoresces only in a lipidic environment such as biological membranes (8). After membrane damage, FM1-43 accumulates in intracellular vesicles reminiscent of membrane patches at sites of resealing (35). Increases in local fluorescence at injury sites (due to increased entry and fluorescence of FM1-43 when incorporated into endomembrane structures) were analyzed and quantified by time-lapsed imaging. Following initial membrane injury (time 0, Fig. 3A), a gradual but weak increase in local membrane fluorescence can be observed in control EC infected with β-Gal or WT Dyn-2 adenoviruses reflecting membrane resealing. However, in EC infected with Ad K44A Dyn-2, a more sustained increase in local fluorescent “patches” occurs following injury (Fig. 3A), revealing less efficient membrane resealing in cells expressing K44A Dyn-2. Detailed analysis of the active resealing process shows membrane repair leads to the outward clearance of endomembrane structures to the cell periphery (10 min; Fig. 3B, top), whereas cells infected with Ad K44A Dyn-2 display marked internal fluorescence, a sign of defective endomembrane fusion to the plasma membrane (Fig. 3B, bottom). These data suggest that Dyn-2 activity, similar to ferlins, is necessary for membrane fusion events after laser damage.
Fig. 3.
Inhibition of Dyn-2 activity impairs membrane resealing after laser injury. A: EA.hy.926 infected with adenoviruses as described above were incubated with FM1-43 (25 μM) and locally irradiated with a laser to induce membrane damage. Local increase in fluorescence was determined by 30-s time-lapse imaging for 10 min with a confocal microscope and analysis software. All AdK44A Dynamin-2 values, with the exception of origin, were significantly different (*) when compared with Adβ-Gal- or AdWT Dyn-2-infected cells. Experiments were performed on 6 EA.hy.926 per group in triplicate. B: representative images of a control (top) and an Ad K44A Dyn-2-treated (bottom) EA.hy.926 before, during, and 10 min after injury. Arrows show site of damage. Control cells show efficient resealing and expulsion of torn membrane structures outside of the plasma membrane. Deficient Dyn-2 activity decreases resealing after injury and causes patch accumulation close to injury site without efficient resealing. C and D: endogenous myoferlin colocalizes with Dyn-2 and lipid structures in EC by deconvolution fluorescence microscopy. Cultured EA.hy.926 were treated with a fixable FM1-43FX, which fluoresces in lipid structures (green), or a control solution for 1 h, rinsed, fixed and standard immunofluorescence (IF) against myoferlin (red) and Dyn-2 (green) was performed. Nuclei are shown in blue. Insets: typical merged nonimmune/control staining.
Since we have shown that knockdown of myoferlin (7) and reduced Dyn-2 activity (Fig. 3A) both impair membrane resealing, we performed deconvolution immunofluorescence microscopy to determine whether myoferlin and Dyn-2 colocalize in EC. As shown in Fig. 3C, in fixed cells, endogenous myoferlin (red) was found in a reticular pattern around the nucleus, in the cytoplasm, and in numerous puncta throughout the cells. Similarly, endogenous Dyn-2 staining (Fig. 3C, green) revealed perinuclear and cytoplasmic patterns. Merging of the images show significant colocalization between myoferlin and Dyn-2 (yellow) compared with cells labeled with nonimmune antisera (Fig. 3C, inset, right). Moreover, pretreatment of EC with a fixable analog of FM1-43 (FM1-43FX) for 1 h reveals a high degree of colocalization between lipidic structures (in which FM1-43 dyes fluoresce; green) and endogenous myoferlin (red) (Fig. 3D) compared with control conditions (Fig. 3D, inset, right).
Cav-1 and VEGFR2 signaling are critical for efficient membrane repair after laser injury.
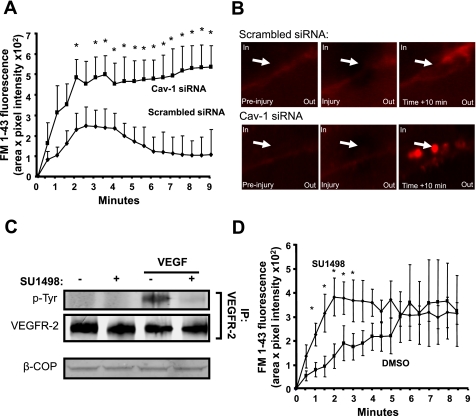
Since our past and current data show a close association between myoferlin and caveolae microdomains (Fig. 1) (7) and that aberrant caveolin activity is linked to the pathogenesis of muscular dystrophy (37, 45), we sought to determine whether the presence of Cav-1 is important for membrane resealing following physical injury in endothelial cells. Consequently, EA.hy.926 were pretreated with Cav-1 siRNA sequences and subjected to laser damage as previously described (7). As shown in Fig. 4A, Cav-1 gene silencing causes a marked increase in FM1-43 internal fluorescence compared with control siRNA sequences, a direct indication of aberrant membrane resealing. Similarly to Fig. 3B, resealing in control cells is characterized by the outward expulsion of damaged membrane structures (Fig. 4B, top), whereas Cav-1-silenced EA.hy.926 showed greater internal fluorescence (Fig. 4B, bottom). To our knowledge, these data provide the first direct evidence of the role of Cav-1 to membrane resealing.
Fig. 4.
Cav-1 and VEGF receptor 2 (VEGFR2) are required for optimal membrane resealing after injury. A: Cav-1 gene silencing decreases rate of resealing following laser injury. Cells were treated with a scrambled or Cav-1 siRNA sequences as described in Fig. 2I and challenged as described in Fig. 3A. *P < 0.05 compared with control treated cells (n = at least 6 individual cells in triplicate). B: representative images of a scrambled siRNA-treated (top) and a Cav-1 siRNA-treated (bottom) EA.hy.926 before, during, and 10 min after injury. Arrows show site of damage. Control cells show efficient resealing and expulsion of torn membrane structures outside of the plasma membrane. Deficient Cav-1 expression decreases resealing after injury and causes patch accumulation close to injury site without efficient resealing. C: 1-h pretreatment with VEGFR2 inhibitor SU-1498 (10−5 M) blocks VEGF-induced VEGFR2 phosphorylation. Following VEGF (10−9 M; 7.5 min) stimulation, VEGFR2 was immunoprecipitated (IP) and its expression and phosphorylation (phosphotyrosine; p-Tyr) levels were determined by immunoblotting. D: in BAEC treated as described in C, the initial rate of resealing was significantly impeded following laser injury, although there were no significant differences in the extent of membrane damage at later time points. *P < 0.05 compared with control treated cells (n = at least 5 individual cells in triplicate).
Previously published observations from our group and others reveal that both myoferlin and Dyn-2 are essential for VEGFR2 expression (7, 9). Since our current experiments show that these proteins are also necessary for the normal membrane repair after laser injury (Fig. 3A), we examined whether endogenous VEGFR2 signaling contributes to injury evoked membrane repair. Treatment with the VEGFR2-selective tyrosine kinase inhibitor SU-1498 (46) abolishes VEGF-stimulated autophosphorylation of VEGFR2 (Fig. 4C) and also delays the initial rate of membrane repair after injury (Fig. 4D) but not the magnitude, compared with DMSO alone (the vehicle for SU-1498). Collectively, these data suggest that membrane resealing and growth factor signaling may use molecules in common with those required for endocytic vesicle formation.
Cav-1 forms a protein complex with myoferlin-HA and colocalizes with endogenous Cav-1 in native EC.
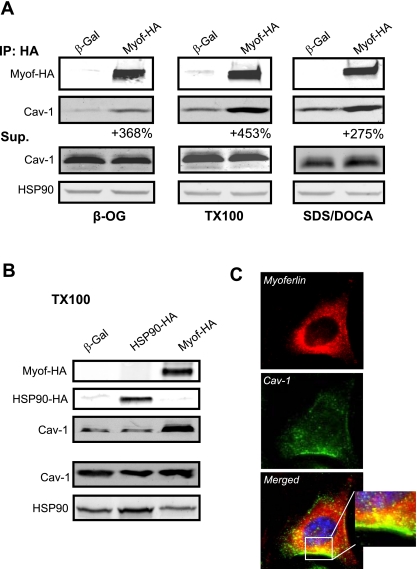
Since past and current data from our group show that myoferlin influences the rate of caveolae-dependent endocytosis (Figs. 1 and 2), is enriched in cholesterol-rich lipid microdomains (7), and partially colocalizes with active caveolae endocytosis (Fig. 1), we sought to determine whether myoferlin-HA can physically interact with Cav-1 in a protein complex in a cell-free system. HEK cells were transfected with myoferlin-HA (Myof-HA or β-Gal as a negative control) and the HA-epitope-tagged protein or control proteins purified and immobilized on an anti-HA column. Immunoisolated myoferlin was then incubated with mouse heart and lung protein extracts prepared using various detergents. Incubation of myoferlin-HA-coated resin with protein extracts prepared in β-octylglucopyranoside, TX100, or sodium dodecyl sulfate/deoxycholic acid-based buffers increases Cav-1 recovery by 368%, 453%, and 275%, respectively, compared with the nonspecific interactions with β-Gal (Fig. 5A). In contrast, immunoisolation of transfected HSP90-HA did not lead to increased Cav-1 coisolation in a TX100-based buffer (Fig. 5B), suggesting that Cav-1 forms a specific protein complex with myoferlin but not other HA tagged proteins. To further support the interaction of myoferlin with Cav-1, immunofluorescence experiments were performedon cultured BAEC to visualize the spatial location of myoferlin and Cav-1. Deconvolution microscopy reveals reticular-like staining for Cav-1 (green channel) and myoferlin (red channel) in permeabilized BAEC as previously described by our group (1, 7) as well as absence of significant nuclear staining (blue channel) for both myoferlin and Cav-1. As expected, partial colocalization was observed between myoferlin and Cav-1 (yellow color) in the form of puncta throughout the cytoplasm and also in close proximity to the plasma membrane (inset). Nonpermeabilized BAEC or incubation with nonimmune IgGs show little or no staining (data not shown). Together, these data rationalize the partial enrichment of myoferlin in caveolae/lipid rafts (7) and support its role in caveolae-dependent activity, such as endocytosis and membrane resealing.
Fig. 5.
Cav-1 forms a protein complex with myoferlin-HA. A: immobilized Myof-HA-coated or -uncoated matrix was allowed to interact with protein extracts isolated from mouse heart and lungs in β-octylglucopyranoside (β-OG), Triton X-100 (TX100), or sodium dodecyl sulfate/deoxycholic acid-based buffers. Supernatant (Sup) was used to confirm equal expression of Cav-1 and HSP90 (loading control). B: similar experiments as described in A were performed by using a HSP90-HA plasmid as a control and show that HA alone does not increase Cav-1 recovery. Experiments were performed in duplicate and typical results are shown. C: endogenous myoferlin partly colocalizes with Cav-1 in EC by deconvolution fluorescence microscopy. Cultured BAEC were fixed and permeabilized, and standard IF against myoferlin (red) and Cav-1 (green) was performed. Nuclei are shown in blue (merged images). Representative staining is shown. Inset: ×63 magnification.
DISCUSSION
The major findings of the present study are that myoferlin regulates endocytosis (caveolae/rafts to a greater extant than clathrin-mediated endocytosis) and that Dyn-2, Cav-1, and VEGFR2 signaling influences mechanisms of membrane resealing. Myoferlin overexpression increases endocytosis of both markers in a reconstituted system, whereas knockdown and knockout of myoferlin in native cells reduces endocytosis of both markers. These data, in conjunction with recent work showing a direct role for myoferlin in membrane resealing (7), demonstrates that receptor-dependent endocytosis and injury-triggered membrane resealing in EC may rely on similar molecules and mechanisms.
Endocytosis is a sophisticated, well-described biological process involving membrane trafficking that is characterized by the internalization of molecules from the cell surface into internal membrane compartments and the recycling of membrane components back to the plasma membrane. Our observation that diminution of myoferlin levels reduces the time-dependent uptake of transferrin and CTB was unexpected and suggests a role for this protein in both clathrin and caveolae/lipid raft-mediated endocytosis in cultured cells, respectively. Interestingly, myoferlin has six C2 domains important for binding to calcium, phospholipids, for protein-protein interactions, and several C2 domain-containing proteins were previously shown to take part in receptor-mediated endocytosis (32). By analogy to C2 containing proteins involved with synaptic vesicle fission, synaptotagmins are membrane proteins that contain two C2 domains that are considered necessary for synaptic vesicle endocytosis, in part through different lipid and protein-binding properties (12, 13, 18, 22). In a previous report, we have shown that silencing myoferlin also reduces membrane repair (7), thus suggesting conservation of mechanisms linking endocytosis with membrane repair.
Mechanistically, myoferlin may interact with Dyn-2 via its SH3 domain and also interact with Cav-1, the major Cav isoform in EC (7). Despite the idea that many signaling molecules can be localized to or isolated from endothelial caveolae/lipid rafts (1, 7, 21), very few are known to interact with Cav-1. The ferlin family member dysferlin, a protein involved in the repair of the sarcolemma of skeletal muscle myocytes, has been shown to form protein complexes with muscle specific Cav-3 (4), and this interaction may regulate the post-Golgi trafficking of dysferlin (27). Indeed, recent evidence illustrates the interplay between Cav-1, Cav-3, endocytosis, and the stability of dysferlin (28). Our data support the concept that myoferlin (and perhaps dysferlin) exists in a protein complex with Cav-1 and Dyn-2 which rationalizes the observations that the loss of myoferlin predominantly reduces the endocytosis of CTB, a ligand for GM1 in caveolae/lipid rafts compared with the endocytosis of transferrin which requires CD71 localized in clathrin-coated pits (39). Previously, we have shown using immunopurified myoferlin that Dyn-2 and VEGFR2 may exist in a protein complex and in the present study show that tagged myoferlin can interact with Cav-1, too, suggesting the existence of a multiprotein complex. However, direct biochemical evidence for these protein-protein interactions and the interaction of these components under native conditions with conventional immunoprecipitation techniques are lacking, raising the possibility that the interactions between myoferlin, Cav-1, and Dyn-2 may be indirect.
The current study also provides evidence of a link between Dyn-2 activity, presence of Cav-1, and membrane fusion events. In regard to Dyn-2, it has recently been suggested that there is an unexpected role for a fission factor, likely Dyn-2 dependent, in membrane fusion (40, 41). According to the “patch” hypothesis of membrane resealing, membrane disruption causes accumulation of endomembrane vesicles beneath the disrupted site, brought there by specialized transport systems, and the subsequent fusing of patches to form larger patch vesicles (36). Since Dyn-1 and -2 localize to sites of actin remodeling and actin-driven vesicle transport (11, 38), they may participate in active transport of vesicles along cytoskeletal tracks toward sites of injury. A corollary to this interpretation is that endosomal/lysosomal membranes created from Dyn-2-sensitive endocytosis could help provide a pool of endomembranes for resealing purposes. This raises the hypothesis that impaired cell growth and chemotaxis (7) in cells lacking myoferlin may be attributable to defective endocytosis. Interestingly, dominant-negative Dyn-2 also blocks endocytosis, cell growth (9, 23), and migration (38), suggesting a convergence of functions for myoferlin and Dyn-2. However, it is possible that K44A Dyn-2 may nonspecifically interfere with cellular processes due to its binding to other signaling proteins containing SH3 domains. Our current observation documenting the accumulation of endomembranes underneath the plasma membrane and defective resealing in cells expressing K44A Dyn-2 suggests that Dyn-2 mediates either vesicle fusion needed for patch formation, patch fusion to the damaged site, or both. On the other hand, Cav-3 has previously been described as a key player in plasma membrane resealing. Specific mutations or alterations of the Cav-3 gene, the muscle-specific isoform of caveolin, interferes with the physiological role of caveolae; loss of Cav-3 leads to a form of limb-girdle muscular dystrophy, whereas upregulation of Cav-3 leads to Duchenne muscular dystrophy (25, 26, 37). Although no clear link has been established between Cav-3 and direct membrane resealing, an explanation for the role of Cav-3 in these settings is its potential association with the dystrophin-glycoprotein complex (33), a well-established regulator of membrane integrity and resealing. These data lend credence to our current observation that Cav-1 can associate with myoferlin to mediate membrane resealing. However, one cannot rule out the fact that Cav-1 silencing can decrease caveolae numbers and compartmentalization of the resealing machinery in these specialized membrane microdomains.
Although our experiments are the first to document a role for myoferlin in receptor-dependent endocytosis, a recent report showed the importance of myoferlin interacting with EHD2 for efficient endocytic recycling (20), whereas our assays reflect early endocytosis and not recycling. Interestingly, EHD2 is an ATPase with a high degree of similarity with GTPase Dyn-2 (15) capable of regulating membrane remodeling, supporting the concept that ferlins and Dyn-2 regulate endocytic entry, recycling, and endomembrane docking and fusion at sites of injury and growth factor signaling. Hence, these data provide mounting evidence supporting a role for myoferlin in early and delayed endocytosis-related events at the plasma membrane and cytosol.
In summary, our study shows that impairment of endocytosis or resealing results in overlapping profound effects in EC, such as reduced VEGFR2 protein levels and growth arrest (7, 9), lending credence to concept that myoferlin, Dyn-2, and VEGFR2 may regulate overlapping, yet distinct, cellular functions and coordinate pathways for endocytosis and signaling versus membrane repair in response to an injurious stimuli.
GRANTS
This work was supported by National Institutes of Health Grants HL-064793, HL-061371, HL-081190, and P01-HL-70295, National Heart, Lung, and Blood Institute-Yale Proteomics Contract N01-HV-28186 (to W. C. Sessa), and Canadian Institutes of Health Research (CIHR) Operating Grant 178510 (to P. N. Bernatchez). P. N. Bernatchez is supported by fellowships from the CIHR and the American Heart Association and is Michael Smith Foundation for Health Research and CIHR Research Scholar.
ACKNOWLEDGMENTS
The authors thank Drs. Vijay Shah for the dynamin adenoviruses.
REFERENCES
- 1.Acevedo L, Yu J, Erdjument-Bromage H, Miao RQ, Kim JE, Fulton D, Tempst P, Strittmatter SM, Sessa WC. A new role for Nogo as a regulator of vascular remodeling. Nat Med 10: 382–388, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Achanzar WE, Ward S. A nematode gene required for sperm vesicle fusion. J Cell Sci 110: 1073–1081, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Altschuler Y, Barbas SM, Terlecky LJ, Tang K, Hardy S, Mostov KE, Schmid SL. Redundant and distinct functions for dynamin-1 and dynamin-2 isoforms. J Cell Biol 143: 1871–1881, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ampong BN, Imamura M, Matsumiya T, Yoshida M, Takeda S. Intracellular localization of dysferlin and its association with the dihydropyridine receptor. Acta Myol 24: 134–144, 2005 [PubMed] [Google Scholar]
- 5.Andrews NW, Chakrabarti S. There's more to life than neurotransmission: the regulation of exocytosis by synaptotagmin VII. Trends Cell Biol 15: 626–631, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Bansal D, Campbell KP. Dysferlin and the plasma membrane repair in muscular dystrophy. Trends Cell Biol 14: 206–213, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Bernatchez PN, Acevedo L, Fernandez-Hernando C, Murata T, Chalouni C, Kim J, Erdjument-Bromage H, Shah V, Gratton JP, McNally EM, Tempst P, Sessa WC. Myoferlin regulates vascular endothelial growth factor receptor-2 stability and function. J Biol Chem 282: 30745–30753, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Betz WJ, Bewick GS. Optical analysis of synaptic vesicle recycling at the frog neuromuscular junction. Science 255: 200–203, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharya R, Kang-Decker N, Hughes DA, Mukherjee P, Shah V, McNiven MA, Mukhopadhyay D. Regulatory role of dynamin-2 in VEGFR-2/KDR-mediated endothelial signaling. FASEB J 19: 1692–1694, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Britton S, Freeman T, Vafiadaki E, Keers S, Harrison R, Bushby K, Bashir R. The third human FER-1-like protein is highly similar to dysferlin. Genomics 68: 313–321, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Cao H, Garcia F, McNiven MA. Differential distribution of dynamin isoforms in mammalian cells. Mol Biol Cell 9: 2595–2609, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapman ER, Hanson PI, An S, Jahn R. Ca2+ regulates the interaction between synaptotagmin and syntaxin 1. J Biol Chem 270: 23667–23671, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Chapman ER, Jahn R. Calcium-dependent interaction of the cytoplasmic region of synaptotagmin with membranes. Autonomous function of a single C2-homologous domain. J Biol Chem 269: 5735–5741, 1994 [PubMed] [Google Scholar]
- 14.Chatterjee S, Cao S, Peterson TE, Simari RD, Shah V. Inhibition of GTP-dependent vesicle trafficking impairs internalization of plasmalemmal eNOS and cellular nitric oxide production. J Cell Sci 116: 3645–3655, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Daumke O, Lundmark R, Vallis Y, Martens S, Butler PJ, McMahon HT. Architectural and mechanistic insights into an EHD ATPase involved in membrane remodelling. Nature 449: 923–927, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Davis DB, Delmonte AJ, Ly CT, McNally EM. Myoferlin, a candidate gene and potential modifier of muscular dystrophy. Hum Mol Genet 9: 217–226, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Davis DB, Doherty KR, Delmonte AJ, McNally EM. Calcium-sensitive phospholipid binding properties of normal and mutant ferlin C2 domains. J Biol Chem 277: 22883–22888, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Davletov BA, Sudhof TC. A single C2 domain from synaptotagmin I is sufficient for high affinity Ca2+/phospholipid binding. J Biol Chem 268: 26386–26390, 1993 [PubMed] [Google Scholar]
- 19.Doherty KR, Cave A, Davis DB, Delmonte AJ, Posey A, Earley JU, Hadhazy M, McNally EM. Normal myoblast fusion requires myoferlin. Development 132: 5565–5575, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doherty KR, Demonbreun AR, Wallace GQ, Cave A, Posey AD, Heretis K, Pytel P, McNally EM. The endocytic recycling protein EHD2 interacts with myoferlin to regulate myoblast fusion. J Biol Chem 283: 20252–20260, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Durr E, Yu J, Krasinska KM, Carver LA, Yates JR, Testa JE, Oh P, Schnitzer JE. Direct proteomic mapping of the lung microvascular endothelial cell surface in vivo and in cell culture. Nat Biotechnol 22: 985–992, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Fernandez I, Arac D, Ubach J, Gerber SH, Shin O, Gao Y, Anderson RG, Sudhof TC, Rizo J. Three-dimensional structure of the synaptotagmin 1 C2B-domain: synaptotagmin 1 as a phospholipid binding machine. Neuron 32: 1057–1069, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Fish KN, Schmid SL, Damke H. Evidence that dynamin-2 functions as a signal-transducing GTPase. J Cell Biol 150: 145–154, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foster LJ, De Hoog CL, Mann M. Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc Natl Acad Sci USA 100: 5813–5818, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galbiati F, Volonte D, Chu JB, Li M, Fine SW, Fu M, Bermudez J, Pedemonte M, Weidenheim KM, Pestell RG, Minetti C, Lisanti MP. Transgenic overexpression of caveolin-3 in skeletal muscle fibers induces a Duchenne-like muscular dystrophy phenotype. Proc Natl Acad Sci USA 97: 9689–9694, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galbiati F, Volonte D, Minetti C, Bregman DB, Lisanti MP. Limb-girdle muscular dystrophy (LGMD-1C) mutants of caveolin-3 undergo ubiquitination and proteasomal degradation. Treatment with proteasomal inhibitors blocks the dominant negative effect of LGMD-1C mutant and rescues wild-type caveolin-3. J Biol Chem 275: 37702–37711, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Glover L, Brown RH., Jr Dysferlin in membrane trafficking and patch repair. Traffic 8: 785–794, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Hernández-Deviez DJ, Howes MT, Laval SH, Bushby K, Hancock JF, Parton RG. Caveolin regulates endocytosis of the muscle repair protein, dysferlin. J Biol Chem 283: 6476–6488, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Jaiswal JK, Marlow G, Summerill G, Mahjneh I, Mueller S, Hill M, Miyake K, Haase H, Anderson LV, Richard I, Kiuru-Enari S, McNeil PL, Simon SM, Bashir R. Patients with a non-dysferlin Miyoshi myopathy have a novel membrane repair defect. Traffic 8: 77–88, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Kumari S, Mayor S. ARF1 is directly involved in dynamin-independent endocytosis. Nat Cell Biol 10: 30–41, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Lennon NJ, Kho A, Bacskai BJ, Perlmutter SL, Hyman BT, Brown RH., Jr Dysferlin interacts with annexins A1 and A2 and mediates sarcolemmal wound-healing. J Biol Chem 278: 50466–50473, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Madziva MT, Bai J, Bhalla A, Chapman ER, Edwardson JM. Effects of synaptotagmin reveal two distinct mechanisms of agonist-stimulated internalization of the M4 muscarinic acetylcholine receptor. Br J Pharmacol 144: 761–771, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McNally EM, de Sa Moreira E, Duggan DJ, Bonnemann CG, Lisanti MP, Lidov HG, Vainzof M, Passos-Bueno MR, Hoffman EP, Zatz M, Kunkel LM. Caveolin-3 in muscular dystrophy. Hum Mol Genet 7: 871–877, 1998 [DOI] [PubMed] [Google Scholar]
- 34.McNeil AK, Rescher U, Gerke V, McNeil PL. Requirement for annexin A1 in plasma membrane repair. J Biol Chem 281: 35202–35207, 2006 [DOI] [PubMed] [Google Scholar]
- 35.McNeil PL, Kirchhausen T. An emergency response team for membrane repair. Nat Rev Mol Cell Biol 6: 499–505, 2005 [DOI] [PubMed] [Google Scholar]
- 36.McNeil PL, Steinhardt RA. Plasma membrane disruption: repair, prevention, adaptation. Annu Rev Cell Dev Biol 19: 697–731, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Minetti C, Sotgia F, Bruno C, Scartezzini P, Broda P, Bado M, Masetti E, Mazzocco M, Egeo A, Donati MA, Volonte D, Galbiati F, Cordone G, Bricarelli FD, Lisanti MP, Zara F. Mutations in the caveolin-3 gene cause autosomal dominant limb-girdle muscular dystrophy. Nat Genet 18: 365–368, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Orth JD, Krueger EW, Cao H, McNiven MA. The large GTPase dynamin regulates actin comet formation and movement in living cells. Proc Natl Acad Sci USA 99: 167–172, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parton RG. Ultrastructural localization of gangliosides; GM1 is concentrated in caveolae. J Histochem Cytochem 42: 155–166, 1994 [DOI] [PubMed] [Google Scholar]
- 40.Peplowska K, Ungermann C. Expanding dynamin: from fission to fusion. Nat Cell Biol 7: 103–104, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Peters C, Baars TL, Buhler S, Mayer A. Mutual control of membrane fission and fusion proteins. Cell 119: 667–678, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Roux I, Safieddine S, Nouvian R, Grati M, Simmler MC, Bahloul A, Perfettini I, Le Gall M, Rostaing P, Hamard G, Triller A, Avan P, Moser T, Petit C. Otoferlin, defective in a human deafness form, is essential for exocytosis at the auditory ribbon synapse. Cell 127: 277–289, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Saliez J, Bouzin C, Rath G, Ghisdal P, Desjardins F, Rezzani R, Rodella LF, Vriens J, Nilius B, Feron O, Balligand JL, Dessy C. Role of caveolar compartmentation in endothelium-derived hyperpolarizing factor-mediated relaxation: Ca2+ signals and gap junction function are regulated by caveolin in endothelial cells. Circulation 117: 1065–1074, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Smillie KJ, Evans GJ, Cousin MA. Developmental change in the calcium sensor for synaptic vesicle endocytosis in central nerve terminals. J Neurochem 94: 452–458, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sotgia F, Lee JK, Das K, Bedford M, Petrucci TC, Macioce P, Sargiacomo M, Bricarelli FD, Minetti C, Sudol M, Lisanti MP. Caveolin-3 directly interacts with the C-terminal tail of beta-dystroglycan. Identification of a central WW-like domain within caveolin family members. J Biol Chem 275: 38048–38058, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Strawn LM, McMahon G, App H, Schreck R, Kuchler WR, Longhi MP, Hui TH, Tang C, Levitzki A, Gazit A, Chen I, Keri G, Orfi L, Risau W, Flamme I, Ullrich A, Hirth KP, Shawver LK. Flk-1 as a target for tumor growth inhibition. Cancer Res 56: 3540–3545, 1996 [PubMed] [Google Scholar]
- 47.Van der Bliek AM, Meyerowitz EM. Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature 351: 411–414, 1991 [DOI] [PubMed] [Google Scholar]
- 48.Washington NL, Ward S. FER-1 regulates Ca2+-mediated membrane fusion during C. elegans spermatogenesis. J Cell Sci 119: 2552–2562, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Zefirov AL, Abdrakhmanov MM, Mukhamedyarov MA, Grigoryev PN. The role of extracellular calcium in exo- and endocytosis of synaptic vesicles at the frog motor nerve terminals. Neuroscience 143: 905–910, 2006 [DOI] [PubMed] [Google Scholar]