Abstract
We recently identified a myelinated vagal afferent subpopulation (Ah type) far more prevalent in female than male rats and showed that this difference extends to functionally specific visceral sensory afferents, baroreceptors of the aortic arch. Excitability of myelinated Ah-type afferents is markedly reduced after ovariectomy (OVX). Here we tested the hypothesis that 17β-estradiol can selectively restore excitability of these sex-specific vagal afferents. Acutely isolated vagal afferent neurons (VGN) from intact and OVX adult female rats were used with patch-clamp technique and current-clamp protocols to assess the effect of acute application of 17β-estradiol on neuronal excitability. At over physiologically relevant 17β-estradiol concentrations for rat (1–10 nM) excitability of myelinated Ah-type vagal afferents is restored to discharge frequencies comparable to those in intact females, albeit with some interesting differences related to burst and sustained patterns of neuronal discharge. Restoration of excitability occurs within 3 min of hormone application and is stereo specific, because 1,000 nM 17α-estradiol fails to alter excitability. Furthermore, activation of G protein-coupled estrogen receptor GPR30 with highly selective agonist G-1 similarly restores excitability of Ah-type afferents. The effectiveness of 17β-estradiol and G-1 is completely eliminated by application of high-affinity estrogen receptor ligand ICI-182,780. 17β-Estradiol conjugated with BSA is ∼70% as effective as 17β-estradiol alone in restoring Ah-type VGN excitability. These data support our conclusions that the cellular mechanisms leading to rapid restoration of neuronal excitability of myelinated Ah-type VGN after OVX occur, at least in part, via membrane-bound estrogen receptors. We contend that recovery of high-frequency discharge at physiologically relevant 17β-estradiol concentrations implies that this unique subtype of low-threshold myelinated vagal afferent may account for some of the sex-related differences in visceral organ system function. Sex differences in cardiovascular and gastrointestinal function and the potential role of GPR30 in modulation of sex-specific myelinated Ah-type vagal afferents are discussed.
Keywords: sensory afferent, visceral afferent, estrogen receptor, GPR30, ovariectomy
visceral afferents with cell bodies in the vagal ganglia innervate a functionally diverse range of organs associated with the cardiovascular, gastrointestinal, and pulmonary systems. Vagal afferent mediation of the reflexogenic properties of the autonomic nervous system (ANS) in addition to visceral sensation is well recognized (for review see Ref. 55). Because the peripheral sensory terminals can be influenced by a wide range of endogenous chemicals released as a consequence of ischemia, injury, and inflammation, the compendium of pathologies, related symptoms, and patient populations that are associated with vagal afferent function or dysfunction is quite extensive (21, 43, 54). Interestingly, across both the clinical and basic science literature there is considerable evidence for sex-related differences in vagally mediated visceral sensation, ANS reflex function, and dysfunction (6, 11).
Perhaps the most widely recognized visceral organs exhibiting sexual dimorphism across diverse measures of health and disease are the cardiovascular and gastrointestinal systems. Numerous studies have demonstrated a greater incidence of hypertension in men and postmenopausal women compared with premenopausal women (14, 39), an observation that has led to the widely held presumption of a “cardioprotective” effect associated with female sex hormones (11). The most compelling evidence for this can be demonstrated through measures of cardiovascular system-related ANS reflex function such as baroreceptor reflex sensitivity (BRS), which has been shown to be markedly reduced in hypertensive compared with normotensive women while no such or a lesser reduction occurs in hypertensive men (48). This issue, however, remains somewhat controversial, because there are studies that have shown no sex-related differences or even a greater BRS (9, 18, 52). There is far more agreement in the literature that functional gastrointestinal disorders (FGID) are more prevalent in women than in men (6, 37). For example, women present more frequently with irritable bowel syndrome (IBS) and dyspepsia than men, and the severity of symptoms has been shown to be closely associated with hormone levels across the estrous cycle (1, 17, 29, 37). Despite such compelling evidence for reflexogenic differences in ANS function between males and females and the potential influence of sex hormones on vagally mediated visceral sensation, there has yet to be a comprehensive investigation as to whether these differences arise, at least in part, because of fundamental sex-related differences in vagal afferent function.
Recently, we reported (30) that female rats exhibit nearly 50% more myelinated vagal afferents than age-matched males. Electrophysiological and pharmacological studies revealed that this was a consequence of a unique and functionally distinct subtype of low-threshold myelinated vagal afferent (Ah type) rarely found in males. The relative distribution of Ah-type afferents was essentially the same across intact and ovariectomized (OVX) female rats. The loss of sex hormones appeared to dramatically lessen the excitability of Ah-type vagal afferent neurons (VGN), but this observation was not thoroughly investigated. The main objectives of this study were to quantify the change in Ah-type VGN excitability in OVX rats and to determine whether acute application of estrogen receptor (ER) ligands 17α-estradiol and 17β-estradiol could restore the excitability of this unique subtype of myelinated vagal afferent. Our results suggest that 17β-estradiol acting, at least in part, by way of nongenomic signaling mechanisms consistent with membrane-bound isoforms of ERs can restore the excitability of myelinated Ah-type afferents in a concentration-dependent manner. For OVX rats, physiologically relevant concentrations of 17β-estradiol rapidly (<3 min) and selectively increased the excitability of Ah-type VGN to levels comparable to those observed in intact female rats, albeit with durations of action potential (AP) burst discharge that varied in a concentration-dependent manner. In a subset of experiments it was documented that this effect was mediated, at least in part, through activation of the recently discovered G protein-coupled ER GPR30.
MATERIALS AND METHODS
Preparation of all isolated VGN was carried out with nonbreeding adult female Sprague-Dawley rats with subgroups of intact (n = 12) and OVX (n = 10) animals of approximately the same age (12–16 wk) and weight (200–300 g). For the population of OVX rats, an ovariectomy was performed 1 wk before weaning but at not less than 3 wk of age. There are two essential reasons for utilizing a sexually immature animal model. First, we previously demonstrated (30) that this unique subset of myelinated afferents does not require the presence of estrogen and related sex hormones in order to bring about neurobiological development. Second, this was necessary to maintain methodological consistency with our earlier studies. The surgical procedures for the ovariectomy were consistent with those previously described in the literature (19). Anesthesia consisted of a 10-ml ketamine (100 mg/ml) and 1.4-ml xylazine (100 mg/ml) cocktail administered intraperitoneally at 0.1 ml/100 g body wt. On lack of reflex motor and sensory responses the surgical procedure was initiated under aseptic conditions. Bilateral flank incisions provided access for blunt dissection for removal of both ovaries and the majority of the oviducts. The surgical wounds were sutured closed, and a topical antibiotic was applied. The animals were allowed to recover for at least 10 days before removal of the stitches and then returned to normal animal housing for a period of at least 8 wk. Before study, confirmation that the estrous cycle was absent was carried out through successive examination of vaginal samples for at least 4 days by light microscopy (57). No attempt was made to quantify the phase of the estrous cycle of intact females. All animals were obtained from the Chinese Academy Shanghai SLAC Laboratory Animal Corporation. All experimental protocols were approved by the School of Medical Science Institutional Animal Care and Use Committee, Harbin Medical University.
Preparation of isolated adult vagal afferent neurons.
The technical procedures for bilateral dissection of adult rat vagal ganglia and enzymatic dispersion and plating of VGN have been previously described in detail (32, 33). Briefly, surgical dissection of the vagal ganglia was carried out under stereomicroscopy (×40), and ganglia were immediately placed in a chilled support medium consisting of 90 ml of DME-F-12 medium (Sigma, St. Louis, MO), 5 ml of fetal bovine serum (Hyclone, Logan, UT), 1.0 ml of penicillin-streptomycin (Invitrogen, Grand Island, NY), and 100 μM MITO + Serum Extender (Collaborative Biomedical Products, Bedford, MA). The ganglia were then enzymatically treated in the same solution but with the addition of 10 U/ml of papain (Sigma) at 37°C for 20 min. The ganglia were then transferred to support medium that contained 1.0 mg/ml of type II collagenase and 2.5 mg/ml of Dispase (Worthington Biochemical, Lakewood, NJ) at 37°C for an additional 30 min. Mechanical dispersion of the VGN, plating, and incubation in support medium were carried out as previously described (32, 33).
Electrophysiological techniques.
All electrophysiological and pharmacological studies were carried out at room temperature with isolated VGN that had been in culture for at least 4 but not more than 12 h. The whole cell current-clamp technique was carried out with a 200B patch-clamp amplifier (Axon Instruments). The pipettes were pulled from borosilicate glass (Sutter Instrument, Novato, CA) and heat polished down to a resistance of 1–2 MΩ in normal recording solution (see below). After offset adjustment and formation of a gigaohm seal the pipette capacitance was compensated. On going to the whole cell the neuronal capacitance (40–50 pF) and electrode access resistance (∼3 MΩ) were also compensated (60–80%). Single somatic APs were elicited with a 500-μs current pulse, and repetitive AP discharge was evoked with a 1,000-ms current step, both with magnitudes ∼1.5 that required for threshold discharge unless otherwise specified. Raw data were low-pass filtered to 10 kHz and digitized at 50 kHz. All experimental protocols, data collection, and preliminary analysis were carried out with pCLAMP 9 and the Digidata 1322A (Axon Instruments) operating on a PC platform.
Recording solutions.
For all experiments the extracellular solution consisted of (in mM) 137 NaCl, 5.4 KCl, 1.0 MgCl2, 2.0 CaCl2, 10 glucose, and 10 HEPES, and the pipette solution contained (in mM) 10 NaCl, 50 KCl, 50 K2SO4, and 5.0 MgCl2. pH of the extracellular and pipette solutions was adjusted to 7.35 and 7.25 with NaOH and KOH, respectively. Osmolarities of the extracellular and intracellular solutions were adjusted with d-mannitol to 310–315 and 290–295 mosM, respectively. Just before recording, 2.0 mM Mg-ATP and 2.0 mM Na-GTP were added to the pipette solution in addition to 4.0 mM BAPTA-K and 0.25 mM CaCl2 for a final buffered intracellular Ca2+ concentration of 10 nM. 17β-Estradiol was diluted to concentrations of 0.1, 0.3, 1, 3, 10, 30, 100, 300, and 1,000 nM from a 100 μM stock solution using extracellular recording solution. Test concentrations of 17β-estradiol were applied in an increasing manner as a bath perfusion with multiple multichannel perfusion systems (MP-8, Warner Instruments) and an exchange rate of 1.0 ml/min. Each test concentration was perfused for at least 3 min to ensure complete exchange of the bathing medium from the 1.5-ml recording chamber (RC-27, Warner Instruments) and to ensure that any observed effect of 17β-estradiol on AP discharge had reached steady state. Only those cells exposed to a complete set of test concentrations were included for analysis. After the last AP discharge test with 1,000 nM 17β-estradiol remaining in the bath, the high-affinity estrogen receptor ligand ICI-182,780 (10 μM) was continuously perfused for 3 min and the AP discharge test was then repeated. To determine whether the agonist effect was associated with a membrane-bound form of ER, membrane-impermeant bovine serum albumin-conjugated 17β-estradiol (BSA/E2) was tested in an identical manner, albeit at 10 times the concentration profiles used for 17β-estradiol on account of the lower affinity of this conjugated agonist for ER (12, 50). Because 17β-estradiol has approximately the same affinity for both the α- and β-isoforms of the ER, i.e., ERα and ERβ, respectively, the less active enantiomer 17α-estradiol was tested across the same concentrations as 17β-estradiol to assess the relative affinity this stereoisomer may have for any ER-mediated effect. The concentration-response relations were established based on the sustained AP discharge frequency to a 150-pA step depolarizing current. Discharge frequency was normalized to that observed at the highest concentration of 17β-estradiol, from which half-effective doses (EC50) were calculated. Because 17β-estradiol also has approximately the same affinity for the membrane-bound G protein-coupled ER 30 (GPR30) the high-affinity agonist 1-(4-(6-bromobenzo[1,3]dioxol-5-yl)-3a,4,5,9b-tetrahydro-3H- cyclopenta[c]quinolin-8-yl)-ethanone (G-1) was utilized in a subset of current-clamp studies. G-1 was diluted to 100 nM with AP recording solution from a 10 μM stock solution prepared with 100% dimethyl sulfoxide (DMSO; final concentration of 0.4% DMSO alone had no measurable effects on the cells).
Identification of neuronal cell types and data analysis.
Enzymatic dispersion removes the axon from the cell body, and thus cell type cannot be classified based on measurements of afferent fiber conduction velocity (CV). Recently, using an intact ganglion preparation for measurement of CV, we developed a reliable and robust methodology for classification of an isolated neuron as either a myelinated A- or Ah-type VGN or an unmyelinated C-type VGN through cluster analysis of a select set of AP waveshape characteristics (33). In addition to other measurements tabulated for statistical comparison the following three measurements were used for classification of the particular cell under study as an A-, Ah-, or C-type VGN: AP firing threshold (APFT), AP upstroke velocity measured at 50% peak-to-peak excursion (UVAPD50), and AP downstroke velocity (DVAPD50) (see Ref. 33 for details concerning all AP waveform measurements and the methodology behind and validation of the cluster analysis). All electrophysiological recordings were analyzed with Clampfit (V9, Axon Instruments), and pooled statistics were calculated with Excel. Average data are expressed as means ± SD, with significance assessed by two-way Student's t-test, χ2-test, or ANOVA where appropriate. A P value of <0.05 was required for compared measures to be considered significantly different.
RESULTS
Neuronal excitability of VGN classified as either myelinated A or Ah type or unmyelinated C type was carried out with acutely isolated cells from both intact and OVX female rats. For each cell, after measure of AP discharge with pulse and step depolarizing currents under control conditions, the effect of 17β-estradiol, ICI-182,780 + 17β-estradiol (ICI + E2), BSA-conjugated 17β-estradiol (BSA/E2), or 17α-estradiol on AP discharge frequency was carried out as previously described. Multiple measures of AP waveshape characteristics (see below) in response to a single current pulse and measure of repetitive AP firing frequency (APFF) in response to step depolarizing currents provided a quantitative basis for comparison of the effect each pharmacological agent had on cell excitability. Interestingly, none of the drug combinations or tested concentrations measurably altered the AP waveshape or APFF of myelinated A- or Ah-type or unmyelinated C-type VGN from intact female rats. Therefore, these data are not reported here, but the lack of an acute effect of estradiol on VGN from intact females is addressed in discussion.
Action potential waveshapes and discharge patterns of vagal afferent neurons.
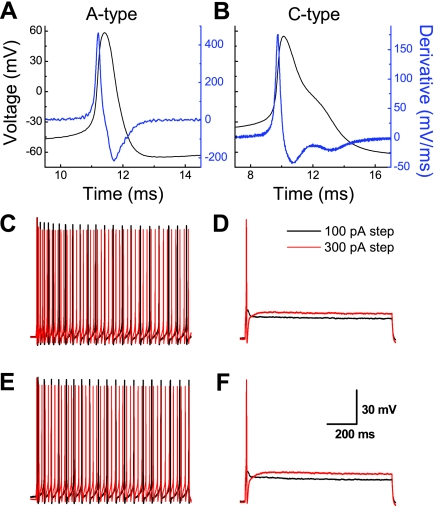
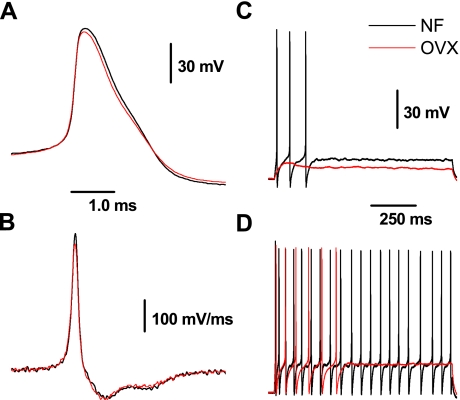
Both the waveshapes and patterns of repetitive AP discharge could be reliably classified as either myelinated A or Ah or unmyelinated C type with a previously reported cluster analysis technique (33). By far the most contrasting excitability profiles were exhibited by myelinated A-type and unmyelinated C-type VGN. We have reported extensively on the electrophysiological profiles of these two distinct classes of vagal afferents in male rats (25, 30, 31, 33, 45, 46). Here, we demonstrate that the measures of AP waveshape and neuronal excitability of myelinated A-type and unmyelinated C-type VGN are markedly similar across populations of adult intact and OVX female rats (Figs. 1 and 2A and Table 1). Furthermore, application of 1 μM 17β-estradiol did not alter the excitability or action potential profile of A- and C-type VGN afferents in intact and OVX rats (Fig. 1, E and F). This latter observation was investigated in greater detail through additional experiments (see below).
Fig. 1.
Lack of effect of 17β-estradiol (E2) on the excitability of identified myelinated A-type and unmyelinated C-type vagal afferent neurons (VGN) from adult ovariectomized (OVX) rats. A and B: representative action potential (AP) recordings from VGN classified as myelinated A type and unmyelinated C type, respectively (see materials and methods). C: for myelinated A-type VGN, repetitive discharge elicited by 100- and 150-pA step currents. E: application of 1 μM E2 failed to significantly alter discharge frequency at the same current magnitudes. D: for unmyelinated C-type VGN, 100- and 300-pA step current depolarizations elicited subthreshold and threshold response of a single AP, respectively. F: application of 1 μM E2 failed to significantly alter the subthreshold and threshold discharge at the same current magnitudes.
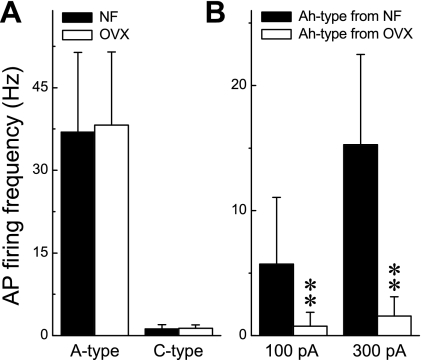
Fig. 2.
Contrasting excitability of VGN identified as myelinated A and Ah type and unmyelinated C type between intact and OVX rats. APs were elicited by a step depolarizing current, and neuronal excitability was quantified as average action potential firing frequency (APFF) over the 1,000-ms step. A: for 200- and 600-pA step currents applied to myelinated A-type and unmyelinated C-type VGN, respectively, there was no significant difference in the APFF between intact (NF) and OVX females. B: APFF of myelinated Ah-type VGN from OVX females were markedly reduced compared with APFF from intact females. Data are means ± SD. **P < 0.01 vs. intact females.
Table 1.
Comparison of action potential discharge characteristics across populations of myelinated A- and Ah-type and unmyelinated C-type VGN for intact and ovariectomized female rats
| Parameter | A Type |
Ah Type |
C Type |
|||
|---|---|---|---|---|---|---|
| Intact | OVX | Intact | OVX | Intact | OVX | |
| RMP | −63.4±2.37 | −61.5±1.69 | −63.8±2.42 | −62.0±1.17 | −63.3±2.68 | −61.6±2.77 |
| APFT | −43.9±2.25 | −41.6±2.93 | −36.7±3.74 | −35.6±2.79 | −25.8±3.56 | −27.5±4.56 |
| APpeak | 48.5±3.51 | 53.5±3.87 | 52.9±4.67 | 57.4±3.07 | 48.6±4.65 | 53.9±4.52 |
| APD50 | 0.82±0.18 | 0.78±1.3 | 1.91±0.20 | 1.64±0.11* | 2.69±0.64 | 2.50±0.44 |
| AHPpeak | −66.4±1.59 | −64.8±2.15 | −67.3±1.99 | −66.3±1.81 | −67.9±2.08 | −66.7±1.83 |
| AHP80 | 20.0±13.4 | 18.1±4.26 | 58.4±25.5 | 74.3±24.8 | 85.9±31.5 | 80.7±41.0 |
| UVAPD50 | 239±52 | 253±37 | 146±21 | 165±31 | 40.7±13.4 | 57.7±17.9 |
| DVAPD50 | −138±37 | −153±45 | −52.3±11.4 | −63.2±9.8* | −38.3±14.3 | −41.0±9.91 |
| n | 28 | 20 | 44 | 51 | 118 | 49 |
Average data are expressed as means ± SD for comparison of action potential (AP) discharge characteristics across populations of vagal afferent neurons (VGN) classified as myelinated A and Ah type and unmyelinated C type for both intact and ovariectomized (OVX) female rats. All APs were elicited by a 500-μs current pulse. RMP, resting membrane potential; APFT, AP firing threshold; APpeak, peak of AP waveform; APD50, AP duration at 50% peak-to-peak excursion of AP waveform; AHPpeak, peak of afterhyperpolarization; AHP80, time required to reach 80% recovery to RMP from AHPpeak; UVAPD50 and DVAPD50, upstroke and downstroke velocities, respectively, measured at APD50.
Significance (P < 0.05).
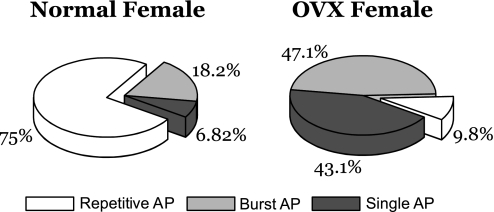
In a recent study (30) we demonstrated that loss of female sex hormones as a result of OVX markedly reduced the excitability of VGN classified as myelinated Ah-type afferents, an observation repeated in this follow-up study (Fig. 2B). An essential objective of this study was to first quantify and compare the relative excitability of Ah-type VGN from intact and OVX female rats across a large population of cells. This was critically important in order to determine whether the excitability profile of this unique cell type is homogeneous in response to current injections or exhibits a classifiable distribution of discharge patterns. After identification of a VGN as an Ah-type afferent with a brief 500-μs current pulse (Fig. 3A), a step current injection of 300 pA for 1,000 ms was applied to the cell under study to quantify the relative excitability of this unique, sex-specific subpopulation of VGN. For the population of VGN (n = 44) from intact female rats three distinct excitability profiles were observed, albeit with markedly different frequency distributions, namely, steady repetitive discharge throughout the 1,000-ms step depolarization (Fig. 3B), an AP burst of comparable frequency but terminating at variable time points before the end of the 1,000-ms step depolarization (Fig. 3C), and, in rare cases, only a single AP elicited in response to the 300-pA step current injection (Fig. 3D). A 75% majority (33/44) of Ah-type myelinated VGN from intact female rats fired repetitively (Fig. 4), with 18.2% (8/44) exhibiting burst discharge and only 6.8% (3/44) responding with a single AP. Interestingly, the averaged frequency of steady AP discharge and the instantaneous firing frequency (IFF) over the time course of the AP bursts were not significantly different even across varying step current magnitudes of 200 pA (22.6 ± 4.9 vs. 23.1 ± 3.7 Hz, respectively) and 300 pA (21.2 ± 3.4 vs. 21.7 ± 4.2 Hz, respectively). With the same investigative strategy for Ah-type VGN (n = 51) from OVX females it was clearly apparent that the excitability of these cells was markedly reduced. In response to the same 300-pA step current magnitude only 9.8% (5/51) exhibited sustained repetitive discharge, while the vast majority of Ah-type VGN from OVX rats now responded with either a short burst (47.1%, 24/51) of AP or only a single spike discharge (43.1%, 22/51) over the full 1,000-ms time course of the 300-pA step current depolarization (Fig. 4). A χ2-analysis (χ2 = 42.75) of these data demonstrated that myelinated Ah-type VGN from OVX rats were far less likely to exhibit sustained repetitive discharge than VGN from intact females (P < 0.001). Interestingly, as observed for the intact females, those few Ah-type VGN that exhibited sustained discharge did so at a frequency that was approximately the same as the IFF over the time course of the AP bursts.
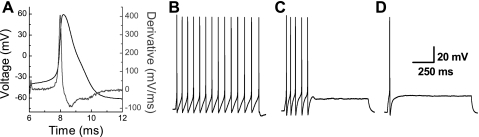
Fig. 3.
Representative AP waveforms of a VGN classified as a myelinated Ah-type afferent neuron from an adult intact female rat. A: Ah-type AP waveform in response to a 500-μs current pulse. Right y-axis presents the time derivative of membrane voltage to emphasize the combined upstroke and downstroke velocity profiles unique to Ah-type neurons. See materials and methods and Li and Schild (33) for additional details. Across the entire population of VGN classified as Ah type a suprathreshold 300-pA step depolarizing current was shown to elicit sustained repetitive discharge (B), burst discharge with a comparable instantaneous firing frequency as in B but only lasting a few hundred milliseconds (C), and, in very rare cases, discharge of only a single AP (D).
Fig. 4.
Contrasting excitability profiles of Ah-type VGN from intact and OVX adult female rats. Differential AP firing patterns of VGN classified as myelinated Ah type between intact (normal; n = 44) and OVX (n = 51) female rats are shown. A suprathreshold 300-pA step current depolarization of 1,000 ms elicited 1 of 3 patterns of excitability: repetitive, burst, and, in rare cases, only a single AP. The contrasting distributions of these 3 patterns of excitability emphasize the impact OVX has on reducing the excitability of Ah-type VGN in OVX rats.
Stark differences between action potential profiles and excitability to step current depolarization.
The dramatic reduction in capacity for repetitive discharge of myelinated Ah-type VGN (Figs. 2–4) may, at first, suggest that clues as to the potential ionic mechanism could be found through a careful analysis of the AP waveform. An overlay of representative Ah-type APs from intact and OVX rats revealed only a subtle difference in the duration of the action potential waveform. Indeed, there was a slight decrease in AP duration at 50% peak-to-peak excursion of AP waveform (APD50) and a concomitant increase in DVAPD50 of Ah-type VGN from OVX rats that proved to be significantly less than the same measures from intact female rats (P < 0.05; Fig. 5 and Table 1). Any potential explanation that may be associated with an underlying ion channel mechanism will require further study. No significant differences were noted across all other waveform measures from these two study populations. This latter point was further validated through a direct comparison of the time derivative of the Ah-type AP waveforms, which presented with nearly identical trajectories from these two study populations (Fig. 5B).
Fig. 5.
Subtle differences between the AP waveforms of VGN classified as Ah type from intact and OVX rats contradict the stark differences in excitability. A: overlay of Ah-type AP from intact and OVX rats elicited by a single 500-μs current pulse. B: overlay of Ah-type AP time derivatives from intact and OVX rats elicited by a single 500-μs current pulse. C: overlay of intact and OVX Ah-type VGN in response to a 100-pA step depolarizing current. D: overlay of intact and OVX Ah-type VGN in response to a 300-pA step depolarizing current.
17β-Estradiol restores excitability of Ah-type neurons from OVX rats.
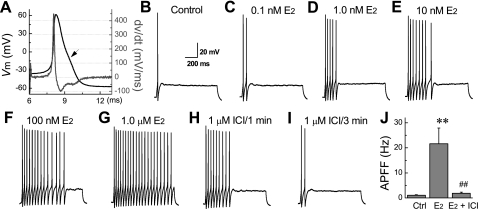
Following demonstration that OVX results in a dramatic reduction in current-evoked excitability of myelinated Ah-type VGN, an essential issue to be resolved is whether an acute application of 17β-estradiol can restore neuronal excitability to levels and patterns comparable to those exhibited by intact female rats. Classification as a myelinated Ah-type VGN was carried out through waveform analysis of a somatic AP elicited with a 500-μs current pulse (Fig. 6A). A near-threshold step current of 150 pA for 1,000 ms was then applied through the patch electrode. For the representative cell presented here, under control conditions this current magnitude elicited only a single spike at the start of the current step (Fig. 6B). Bath perfusion of 17β-estradiol was then carried out as described above for 3 min, and the 150-pA current step was repeated. Here the impact of increasing 17β-estradiol concentration from 0.1 through 1,000 nM on the excitability of myelinated Ah-type VGN from OVX rat is clearly demonstrated by the successive lengthening of the AP burst, up to sustained repetitive discharge at the highest concentration (Fig. 6, C–G). Interestingly, although the duration of the burst increased with increasing concentration of 17β-estradiol, IFF over the time course of the burst was approximately the same for all recordings. For example, the average (n = 5) IFF in response to a 150-pA step current at 1.0 nM 17β-estradiol was 20.9 ± 1.9 Hz, at 10 nM 17β-estradiol the IFF was 21.1 ± 1.6 Hz, and at 1,000 nM 17β-estradiol the IFF was 20.9 ± 1.5 Hz, with an ANOVA indicating no statistical difference in IFF of the burst across all tested concentrations. Within 3 min of inclusion of the high-affinity ER ligand ICI-182,780 (10 μM) in the perfusate along with 1,000 nM 17β-estradiol, cell excitability had essentially returned to baseline (Fig. 6, H and I). Application of 10 μM ICI-182,780 to VGN from intact and OVX females had no measurable effect on cell excitability (data not shown). A pooled comparison of IFF for all tested concentrations presented as a histogram emphasizes the dramatic restorative effectiveness of 17β-estradiol on the excitability of myelinated Ah-type VGN from OVX rat (Fig. 6J). A detailed examination of AP waveshape before and after the application of 17β-estradiol revealed a significant increase in APD50 and concomitant decrease in DVAPD50 to levels comparable to those observed in Ah-type VGN from intact female rats (Table 2). The only other significant effect was a prolongation of the afterhyperpolarization, presumably as a result of elevated recruitment of outward K+-channel currents in response to the increased APD. The maximum concentration of 1,000 nM 17β-estradiol had no effect on the AP waveshape of VGN classified as myelinated A type (Table 2). However, at this highest concentration there was a modest, albeit significant (P < 0.05) increase in APD50 and a concomitant decrease in DVAPD50 in VGN classified as unmyelinated C type.
Fig. 6.
Demonstration of the E2 concentration-dependent increase in excitability of Ah-type VGN and termination with application of ICI-182,780. A: validation that the neuron under study was an Ah-type VGN based on measures of AP firing threshold (APFT) and upstroke (UVAPD50) and downstroke (DVAPD50) velocity at 50% peak-to-peak excursion. Representative recording of cell excitability of an Ah-type VGN from an OVX rat with increasing concentration of E2, all in response to 150-pA step currents, is shown. Vm, membrane voltage; dv/dt, time derivative of membrane voltage. Arrow marks the delay or “hump” along the trajectory of repolarization. B: control recording showing no repetitive discharge at a 150-pA step current magnitude. C–G: demonstration of the effect of an increasing E2 concentration on the excitability of this Ah-type VGN to a consistent 150-pA step current. H and I: recordings from the same Ah-type neuron made after 1 (H) and 3 (I) min of bath perfusion with 10 μM ICI-182,780 (ICI) in the presence of 1,000 nM E2. J: pooled comparison of APFF to 150-pA step currents for control, 1,000 nM E2, and 1 μM E2 + 10 μM ICI. Data are means ± SD; n = 5 or 6. **P < 0.01 vs. control; ##P < 0.01 vs. E2. There was no statistical difference between APFF of the control and 1,000 nM E2 + 10 μM ICI recordings.
Table 2.
Comparison of AP discharge characteristics across populations of VGN classified as myelinated A and Ah type and unmyelinated C type from OVX rats under control conditions and in presence of 1.0 μM 17β-estradiol
| Parameter | A Type (n = 4) |
Ah Type (n = 6) |
C Type (n = 8) |
|||
|---|---|---|---|---|---|---|
| Control | E2 | Control | E2 | Control | E2 | |
| RMP | −62.0±1.80 | −62.2±2.11 | −64.0±1.23 | −63.4±3.08 | −62.5±1.68 | −62.3±2.67 |
| APFT | −43.8±3.0 | −44.4±2.21 | −45.5±3.06 | −44.4±3.08 | −28.5±2.27 | −27.0±2.88 |
| APpeak | 52.3±4.2 | 51.4±2.73 | 54.7±0.82 | 52.2±1.85 | 55.9±3.58 | 53.8±3.66 |
| APD50 | 0.75±0.15 | 0.78±0.15 | 1.56±0.07 | 1.77±0.09* | 2.26±0.33 | 2.55±0.21* |
| AHPpeak | −66.9±1.6 | −66.2±0.73 | −67.0±1.81 | −67.2±0.36 | −67.7±0.86 | −67.6±0.98 |
| AHP80 | 8.71±8.7 | 9.48±9.0 | 43.1±13.9 | 31.1±9.06* | 76.9±33.9 | 79.4±39.6 |
| UVAPD50 | 256±42 | 257±32 | 203±36.4 | 197±39.7 | 59.1±12.4 | 54.2±12.6 |
| DVAPD50 | −189±16 | −178±13 | −79.0±13.1 | −50.2±10.1* | −46.9±12.7 | −34.5±7.16* |
Average data are expressed as means ± SD for comparison of AP discharge characteristics across populations of VGN classified as myelinated A and Ah type and unmyelinated C type from OVX rats under control conditions and in the presence of 1.0 μM 17β-estradiol (E2). All APs were elicited by a 500-μs current pulse.
Significance (P < 0.05).
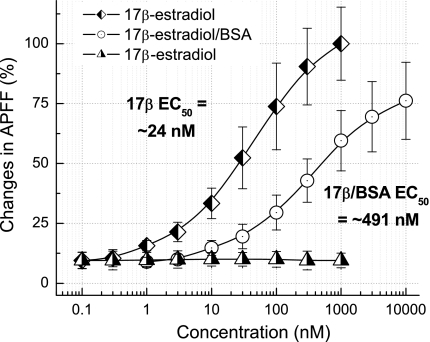
As a more rigorous quantitative assessment of the concentration-dependent effect of 17β-estradiol on restoring the population of Ah-type VGN from OVX rats toward the sustained excitability most often observed with intact females (Fig. 4), the IFF of the AP burst was averaged over the 1,000-ms time course of the 150-pA current step. In this manner, an average APFF could be calculated for each cell at each test concentration and then normalized to the sustained discharge rate of the cell under study at the highest concentration of 17β-estradiol (Fig. 6, D–G). Therefore, low concentrations of 17β-estradiol that brought about shorter-duration AP bursts would be expected to present with a lower normalized APFF, albeit with approximately the same IFF. Data (n = 6) acquired in the manner presented in Fig. 6 clearly showed a concentration-dependent increase in average APFF, with an effective EC50 of 24.2 nM (Fig. 7). Repeat experiments (n = 7) using the same protocol with BSA/E2 but at concentrations 10 times greater than those of 17β-estradiol on account of the presumed lower affinity of this ERβ agonist (12, 50) proved to closely mimic the general trend of 17β-estradiol on APFF, albeit with an EC50 approximately 20× larger. At the highest BSA/E2 concentration of 10 μM, the APFF was ∼25% lower than that observed for the 1 μM concentration of 17β-estradiol. Repeat experiments (n = 5) using the same protocol and concentrations of 17α-estradiol to assess the potential effectiveness of this stereoisomer in modulating the excitability of myelinated Ah-type VGN showed no differences in APFF across all concentrations from the average APFF recorded under control conditions.
Fig. 7.
Concentration-dependent effects of estradiol conjugate on normalized action potential firing frequency. For all conjugates, i.e., E2, BSA/E2, and 17α-estradiol, the average APFF over the time course of a 1,000-ms step current was measured at the concentrations specified along the x-axis. The general trends of the concentration-dependent effects of BSA/E2 (n = 7) were quite similar to those of E2 (n = 6), albeit at higher concentrations presumably because of a lower affinity of the BSA-conjugated form of E2 (12, 50). 17α-Estradiol (n = 5) did not significantly alter APFF across all concentrations tested.
Selective activation of GPR30 restores excitability of Ah-type neurons from OVX rats.
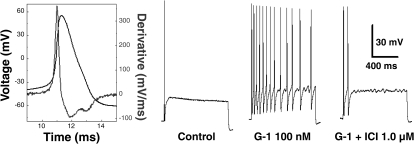
Because 17β-estradiol has approximately the same affinity for GPR30, the highly selective agonist G-1 was utilized to determine whether this novel membrane-bound ER was responsible, at least in part, for the estrogen-dependent elevation in excitability of myelinated Ah-type afferents from OVX rats. Again, classification as a myelinated Ah-type VGN was carried out through waveform analysis of a somatic AP elicited with a 500-μs current pulse (Fig. 8, left). A near-threshold step current of 150 pA for 1,000 ms was applied through the patch electrode, and for the representative cell presented here only a single spike was elicited at the start of the current step. Application of 100 nM G-1 immediately restored sustained repetitive discharge at this same step current magnitude. The average (n = 4) firing frequency significantly (P < 0.03) increased from 1.25 ± 0.5 Hz under control conditions to 8.75 ± 5.6 Hz in the presence of G-1. Application of ICI-182,780 in the presence of G-1, presumably terminating the activation of the GPR30, returned discharge characteristics that were not significantly different than control. As with 17β-estradiol (Fig. 1), G-1 had no effect on the excitability of myelinated A-type or unmyelinated C-type VGN (data not shown).
Fig. 8.
Selective activation of GPR30 restores the excitability of Ah-type VGN in OVX rats. In a subset of VGN identified as Ah type (n = 4), step depolarizing currents of 150 pA elicited only single APs under control conditions. Application of the GPR30-selective agonist G-1 (100 nM) restored repetitive discharge to levels comparable to those observed from Ah-type neurons from intact females (see. Figs. 2–4). Simultaneous application of the high-affinity ligand ICI-182,780 in the presence of G-1 eliminated the GPR30-mediated elevation in excitability.
DISCUSSION
We previously established (30) that female rats have at least 50% more myelinated vagal afferents than male rats and that this increase is largely comprised of a unique subpopulation of lightly myelinated (Ah type) fibers arising from VGN. These myelinated Ah-type afferents exhibited much the same pharmacological sensitivities and excitability characteristics to step current depolarizations as the faster-conducting A-type vagal afferents, albeit with lower rates of repetitive discharge. Interestingly, OVX selectively and dramatically lessens the excitability of myelinated Ah-type VGN but has no such effect on the repetitive discharge characteristics of A-type and unmyelinated C-type VGN (Figs. 1 and 2 and data not shown). This study set out to answer three specific questions: 1) Do the near-threshold discharge characteristics of myelinated Ah-type VGN in intact female rats present as a single homogeneous population or can distinct patterns be observed? 2) Is this distribution altered by OVX (Figs. 3 and 4)? 3) Can acute activation of presumably membrane-bound ER through application of physiologically relevant concentrations of 17β-estradiol restore the excitability of myelinated Ah-type VGN from OVX rats?
For intact female rats the estrous cycle is divided into four distinct phases, with each lasting ∼24 h. Over this time course concentrations of 17β-estradiol vary considerably, with stark differences between particular organ systems and general systemic blood flow. Not surprisingly, the highest concentrations of 17β-estradiol can be found in serum levels of ovarian venous plasma; proestrus averages ∼ 2,000 pg/ml or ∼7.4 nM, diestrus averages ∼525 pg/ml or ∼1.9 nM, estrus averages ∼150 pg/ml or ∼0.6 nM, and metestrus averages ∼400 pg/ml or ∼1.5 nM (49), while cycle-dependent systemic plasma concentrations of 17β-estradiol are approximately one-tenth of these concentrations (3). This is an important distinction, because vagal afferent terminals innervating visceral organ systems may experience 17β-estradiol concentrations that vary between these two extremes. Still, with the exception of estrus, all of these 17β-estradiol concentrations have been shown to restore the near-threshold discharge frequency of myelinated Ah-type VGN from OVX rats to levels comparable to those exhibited by Ah-type VGN from intact females (∼20 Hz for a 150-pA current step), albeit with burst durations that increase in a concentration-dependent manner up to sustained repetitive discharge at 1,000 nM 17β-estradiol (Fig. 6). Low-threshold myelinated vagal afferents have often been shown to be mechanosensitive, exhibiting patterns of phasic burst discharge in response to visceral organ function. Therefore, physiologically relevant systemic serum concentrations of 17β-estradiol in the range of 0.1–1.0 nM may be sufficient to both sustain and restore the low threshold excitability of this unique subset of sex-specific Ah-type myelinated vagal afferents. While higher concentrations were required to restore sustained repetitive discharge to low-magnitude, long-duration step current depolarizations (Figs. 6 and 7), greater-magnitude stimulation currents could likely have elicited longer burst durations at these lower physiological concentrations.
Interestingly, only 25% of myelinated Ah-type VGN from intact female rats exhibited burst or single AP discharge at suprathreshold step current magnitudes of up to 600 pA; the remaining 75% exhibited sustained repetitive discharge at 300 pA current intensities (n = 44, Fig. 4). It is unknown at this time whether the 25% of Ah-type VGN from randomly selected intact female rats that exhibited a reduced excitability were associated with the ∼24-h drop in 17β-estradiol concentration during the estrus phase of the 4-day rat estrous cycle. There is evidence in women that the severity of symptoms associated with FGID and other painful pathophysiologies involving vagal afferent pathways cycles with estrous phase (1, 17, 29, 37, 56). Comparable findings have also been observed in female rats (5, 23, 24, 56). Quantifying the serum levels of estradiol in the intact female rat just before electrophysiological study of acutely isolated myelinated Ah-type VGN may help determine whether the excitability of this unique subtype of vagal afferents from intact female rat is associated with the phase of the estrous cycle.
It is interesting to note that application of even the highest concentration of 1,000 nM 17β-estradiol had no effect upon the either the threshold or the frequency of sustained discharge of Ah-type afferents from intact female rats, despite the fact that dissociated VGN were incubated in an essentially estradiol-free medium for up to 12 h before electrophysiological study. It remains to be determined whether a longer culture time would result in diminished excitability comparable to that observed in OVX rats. However, it has been our experience that for incubation times much beyond 24 h cell excitability can be markedly influenced by the composition of the culture medium. Without proper controls it would therefore be difficult to associate any observed changes in baseline excitability as an ER-related effect. Since our experimental protocols were meant to specifically investigate the potential role of membrane-bound ER with acutely dissociated VGN, numerous plausible explanations exist. For example, it may be that the several-week sex hormone-deficient environment brought about by OVX results in trafficking of intracellular ER to the membrane surface, or perhaps the cyclical exposure of Ah-type VGN in intact females to estrogen and related hormones brings about some permanence in the as yet incompletely understood role of ER in modulating the excitability of this unique subtype of myelinated VGN. It would be best to coordinate the investigation of such possibilities with the previously recommended measurement of serum estradiol levels immediately before preparing the VGN for electrophysiological study.
17β-Estradiol modulation of myelinated Ah-type vagal afferent excitability in OVX rats.
Physiologically relevant concentrations of 17β-estradiol can acutely restore AP burst frequencies of myelinated Ah-type VGN that are comparable to those measured from intact females (Fig. 6), but these low concentrations are unable to acutely return this unique phenotype of vagal afferent to the more prevalent pattern of sustained repetitive discharge present in intact female rats (Fig. 4). To do so requires 17β-estradiol concentrations approaching 1,000 nM or 2–3 orders of magnitude greater than typical physiological concentrations for rats (3, 49). These data raise two important considerations. First, it appears that neuromodulatory capacity of ER receptors can acutely restore sustained repetitive discharge of myelinated Ah-type VGN to levels comparable to intact female rats but only at concentrations far in excess of normal physiological levels. Second, both the speed of response and effectiveness of the non-membrane-permeant ER receptor agonist BSA/E2 suggests that nongenomic mechanisms are responsible, at least in part, for the acute restoration of Ah-type VGN excitability (Fig. 7). This contention is strengthened by the fact that selective activation of the G protein-coupled ER GPR30 also restored excitability of Ah-type VGN (Fig. 8). Further study is required to determine the extent to which each ER subtype may contribute to the restoration of Ah-type VGN excitability.
It is unknown at this time whether an increased exposure time for these cells to physiologically relevant concentrations of 17β-estradiol could influence intracellular signaling pathways to transition the acutely restored patterns of burst discharge in OVX rats to the more prevalent sustained discharge exhibited from intact females (Fig. 4). Likewise, it is unknown whether an extended culture in medium lacking estradiol beyond the 12-h limit in this study would lessen the percentage of Ah-type VGN from intact female rats that exhibit sustained discharge. For these two scenarios, an increase or decrease, respectively, in cell excitability could implicate intracellular signaling or perhaps genomic mechanisms working by way of ER-mediated pathways to ultimately modulate cell sensitivity. Activation of membrane-bound ERα and ERβ has been shown to upregulate cAMP, protein kinase, and other intracellular signaling cascades that can influence downstream transcription factors (35, 58). It remains to be determined whether either the long-term transition toward reduced excitability of myelinated Ah-type VGN excitability following OVX or the possible restoration of excitability through long-term culture in physiological concentrations of estradiol involves such intracellular signaling pathways. Investigations along these lines would, as explained above, need to control for the noted changes in cell excitability as a result of the composition of the culture medium and duration of incubation.
Implications concerning health and disease in gastrointestinal system.
Vagal afferents are extensively distributed throughout the digestive tract, extending from the esophagus to the colon. Both basic science and clinical studies have confirmed that vagal afferents are involved in the reflex control of normal gastrointestinal function as well as reflexes associated with functional dyspepsia, gastroesophageal reflux, and other FGID that are more prevalent in women than in men (2, 6, 37). We contend that our previous demonstration (30) that intact female rats have at least 50% more low-threshold myelinated vagal afferents than age-matched males may represent a neuroanatomic foundation for the differential sensitivities to FGID between the sexes. Support for such a hypothesis is strengthened by our electrophysiological and pharmacological results documenting the changes in excitability of the unique phenotype of myelinated Ah-type VGN that not only represent the bulk of the increased numbers of low-threshold myelinated vagal afferents in female as compared to male rats but also demonstrate a unique sensitivity to physiologically relevant concentrations of ERβ agonists (Figs. 1–7). These data are consistent with at least two lines of evidence from the clinical and experimental animal literature. First, the increased incidence of functional esophageal disorders and dyspepsia and the prevalence of IBS in premenopausal women compared with men gradually decreases with transition into the postmenopausal stage of life (6). This issue remains somewhat controversial, because there are other studies suggesting that postmenopausal women show either no difference or even a higher prevalence of altered bowel function and more frequent IBS dysfunction than premenopausal women (29, 53). However, estrogen replacement therapies have been shown to be associated with a greater likelihood of FGID symptoms, which would be consistent with our observed increase in the sensitivity of myelinated Ah-type VGN with acute application of 17β-estradiol (22, 26). Second, in women there are multiple lines of evidence that the severity of symptoms associated with IBS and other FGID change throughout the estrous cycle (17, 29, 37). Indeed, in both rodent and human females pain threshold and pain tolerance vary in relationship to the stage of the estrous cycle with increased mechanosensation, presumably acting by way of low-threshold myelinated afferents, being implicated as a potential underlying mechanism.(56) These data are consistent with recent observations that GPR30 ER agonists can induce mechanical hyperalgesia and visceral hypersensitivity in the rat (27, 34). Collectively, these reports are consistent with our previous demonstration (30) that low-threshold myelinated Ah-type VGN can be sensitized through agonist activation of purinergic (P2x) receptors that have long been associated with afferent signaling of pain and inflammatory responses (51).
Implications concerning health and disease in cardiovascular system.
Our patch-clamp study (30) of fluorescently identified aortic baroreceptor (BR) neurons and morphometric analysis of aortic BR nerve fibers showed that, as with VGN of unknown sensory modality, females exhibit at least 50% more myelinated aortic BR than age-matched males. Electrophysiological and pharmacological studies further documented that this increase was the result of a unique and functionally distinct subtype of low-threshold myelinated Ah-type BR afferent rarely found in males. As in this study, OVX did not alter the elevated expression of myelinated Ah-type BR afferents but did lessen excitability (Fig. 4). However, it was not determined whether exogenous application of estrogen could restore the excitability of these sex-specific BR myelinated afferents. We contend that this unique phenotype of BR afferent represents a sex-specific neuroanatomic and neurobiological pathway associated with the recognized sexual dimorphism in neurocirculatory control that is known to be influenced by sex hormones associated with the estrous cycle (38, 41).
Sexual dimorphism in cardiovascular function is well recognized, and many clinical measures of ANS control of heart rate and blood pressure are markedly sexually dimorphic (8, 10, 13). For example, there is a preponderance of evidence in the literature that BRS is lower in healthy, premenopausal women compared with healthy age-matched men (4, 7), although a minority of studies have presented contrary observations (52). There is greater agreement in the literature that postmenopausal females present with BRS and hypertension that are more closely aligned with age-matched males, suggesting that ovarian hormones may play an important role in modulation of neurocirculatory control (15). However, the specific role that the vagal afferents may play in all these observations has yet to be adequately investigated, but several lines of supporting evidence can be found in the literature. A greater percentage of low-threshold Ah-type myelinated afferents may account for the predominant vagal tone in females compared with age-matched males (8). A recent study by Lavi et al. (28) has documented that numerous indexes of vagal cardiac tone in postmenopausal women were significantly decreased from those of premenopausal women. There is growing support in the literature for the suggestion that a transition from vagal parasympathetic to sympathetic control of neurocirculation may contribute to the increased cardiovascular morbidity of females with aging (28). OVX female rats have been shown to exhibit enhanced sympathetic activation and attenuated vagal tone, and it has been shown that acute injection of 17β-estradiol can enhance vagally mediated cardiovascular reflexes and autonomic tone (11, 44). Collectively, such observations are consistent with the recognized benefits to cardiovascular health of postmenopausal women using hormone replacement therapies (20). We contend that our results provide intriguing evidence that such benefits to cardiovascular health may be not only sex specific but also afferent specific.
Although both the rapid effect of 17β-estradiol and the similar effectiveness of BSA/E2 implicate a role for membrane-bound ER in mediating the excitability of myelinated Ah-type VGN, the particular subtype of receptor is unknown at this time. The most likely candidates for sensory and pelvic autonomic ganglia are the ERα and ERβ isoforms and perhaps the more recently identified estrogen G protein-coupled receptor GPR30 (40, 42). It is known that GPR30 mediates a variety of estrogen-dependent kinase activation pathways and transcriptional responses as well as exhibiting potential for modifying vascular-related signaling pathways.(47) Indeed, deletion of GPR30 in female mice has been shown to result in an elevated blood pressure (36). Mårtensson et al. (36) concluded that this was in response to an increased vascular resistance in response to an increased media-to-lumen ratio of the resistance arteries. Our data suggest that loss of GPR30, if indeed associated with the reduced excitability of BR afferents in OVX rats, could lessen the strength of the cardiovagal baroreflex, potentially contributing to the sustained elevation in arterial blood pressure observed in GPR30−/− mice, a supposition that is strengthened by recent evidence showing the expression of the ER GPR30 in vagal ganglia (16).
Summary and significance.
The major findings of our work are that 1) the neuronal excitability of myelinated Ah-type VGN is significantly reduced by OVX, 2) physiologically relevant concentrations of 17β-estradiol can selectively and rapidly restore the excitability of myelinated Ah-type VGN from OVX female rats while having no such effect on myelinated A-type and unmyelinated C-type VGN, 3) the acute effectiveness of BSA/E2 is highly suggestive of a role for membrane-bound ER receptors acting, at least in part, via nongenomic pathways, and 4) the G protein-coupled receptor GPR30 is responsible, at least in part, for the selective restoration of excitability in myelinated Ah-type VGN. Collectively, these observations help bring focus on the potential role of a unique and sex-specific subtype of myelinated vagal afferent in the clinical management of visceral pathophysiologies in women. The interpretation of clinical observations related to hormone replacement therapies could benefit from consideration of the potential impact that selective sensitization of low-threshold, presumably mechanosensory myelinated Ah-type vagal afferents may have on perception of visceral sensations as well as the potential reflexogenic impact on afferent-mediated ANS function associated with gastrointestinal and cardiovascular control.
GRANTS
This project was supported by Research Grants 1153111 from the State Education Department and 2007-474 from the State Health Department to G. F. Qiao and partially supported by National Heart, Lung, and Blood Institute Grant HL-072012 to J. H. Schild.
REFERENCES
- 1.Ahlawat SK, Cuddihy MT, Locke GR., III Gender-related differences in dyspepsia: a qualitative systematic review. Gend Med 3: 31–42, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Andrews PL, Sanger GJ. Abdominal vagal afferent neurones: an important target for the treatment of gastrointestinal dysfunction. Curr Opin Pharmacol 2: 650–656, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology 146: 1650–1673, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Beske SD, Alvarez GE, Ballard TP, Davy KP. Gender difference in cardiovagal baroreflex gain in humans. J Appl Physiol 91: 2088–2092, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Chadha HK, Hubscher CH. Convergence of nociceptive information in the forebrain of female rats: reproductive organ response variations with stage of estrus. Exp Neurol 210: 375–387, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Chang L, Toner BB, Fukudo S, Guthrie E, Locke GR, Norton NJ, Sperber AD. Gender, age, society, culture, and the patient's perspective in the functional gastrointestinal disorders. Gastroenterology 130: 1435–1446, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Christou DD, Jones PP, Jordan J, Diedrich A, Robertson D, Seals DR. Women have lower tonic autonomic support of arterial blood pressure and less effective baroreflex buffering than men. Circulation 111: 494–498, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Conte MR. Gender differences in the neurohumoral control of the cardiovascular system. Ital Heart J 4: 367–370, 2003 [PubMed] [Google Scholar]
- 9.Convertino VA. Gender differences in autonomic functions associated with blood pressure regulation. Am J Physiol Regul Integr Comp Physiol 275: R1909–R1920, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Curtis BM, O'Keefe JH., Jr Autonomic tone as a cardiovascular risk factor: the dangers of chronic fight or flight. Mayo Clin Proc 77: 45–54, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Dart AM, Du XJ, Kingwell BA. Gender, sex hormones and autonomic nervous control of the cardiovascular system. Cardiovasc Res 53: 678–687, 2002 [DOI] [PubMed] [Google Scholar]
- 12.De Potter CR, Eechaute W, Roels H, Leusen I. Comparative study between histochemical and biochemical estimation of estrogen receptors in tumors. J Recept Res 5: 245–265, 1985 [DOI] [PubMed] [Google Scholar]
- 13.Denton K, Baylis C. Physiological and molecular mechanisms governing sexual dimorphism of kidney, cardiac, and vascular function. Am J Physiol Regul Integr Comp Physiol 292: R697–R699, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Doyle MW, Bailey TW, Jin YH, Appleyard SM, Low MJ, Andresen MC. Strategies for cellular identification in nucleus tractus solitarius slices. J Neurosci Methods 137: 37–48, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Dubey RK, Oparil S, Imthurn B, Jackson EK. Sex hormones and hypertension. Cardiovasc Res 53: 688–708, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Dun SL, Brailoiu GC, Gao X, Brailoiu E, Arterburn JB, Prossnitz ER, Oprea TI, Dun NJ. Expression of estrogen receptor GPR30 in the rat spinal cord and in autonomic and sensory ganglia. J Neurosci Res 87: 1610–1619, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heitkemper MM, Jarrett ME. Update on irritable bowel syndrome and gender differences. Nutr Clin Pract 23: 275–283, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Hinojosa-Laborde C, Chapa I, Lange D, Haywood JR. Gender differences in sympathetic nervous system regulation. Clin Exp Pharmacol Physiol 26: 122–126, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Hinojosa-Laborde C, Lange DL, Haywood JR. Role of female sex hormones in the development and reversal of Dahl hypertension. Hypertension 35: 484–489, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Hodis HN. Assessing benefits and risks of hormone therapy in 2008: new evidence, especially with regard to the heart. Cleve Clin J Med 75, Suppl 4: S3–S12, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Holzer P. Efferent-like roles of afferent neurons in the gut: blood flow regulation and tissue protection. Auton Neurosci 125: 70–75, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobson BC, Moy B, Colditz GA, Fuchs CS. Postmenopausal hormone use and symptoms of gastroesophageal reflux. Arch Intern Med 168: 1798–1804, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji Y, Murphy AZ, Traub RJ. Estrogen modulation of morphine analgesia of visceral pain in female rats is supraspinally and peripherally mediated. J Pain 8: 494–502, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Ji Y, Tang B, Traub RJ. The visceromotor response to colorectal distention fluctuates with the estrous cycle in rats. Neuroscience 154: 1562–1567, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin YH, Bailey TW, Li BY, Schild JH, Andresen MC. Purinergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals in nucleus tractus solitarius. J Neurosci 24: 4709–4717, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khatibi EA, Samsioe G, Li C, Lidfeldbt J, Agardh CD, Nerbrand C. Does hormone therapy increase allergic reactions and upper gastrointestinal problems? Results from a population-based study of Swedish woman. The women's health in the Lund area (WHILA) study. Maturitas 48: 438–445, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Kuhn J, Dina OA, Goswami C, Suckow V, Levine JD, Hucho T. GPR30 estrogen receptor agonists induce mechanical hyperalgesia in the rat. Eur J Neurosci 27: 1700–1709, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Lavi S, Nevo O, Thaler I, Rosenfeld R, Dayan L, Hirshoren N, Gepstein L, Jacob G. Effect of aging on the cardiovascular regulatory systems in healthy women. Am J Physiol Regul Integr Comp Physiol 292: R788–R793, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Lee OY, Mayer EA, Schmulson M, Chang L, Naliboff B. Gender-related differences in IBS symptoms. Am J Gastroenterol 96: 2184–2193, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Li BY, Qiao GF, Feng B, Zhao RB, Lu YJ, Schild JH. Electrophysiological and neuroanatomical evidence of sexual dimorphism in aortic baroreceptor and vagal afferents in rat. Am J Physiol Regul Integr Comp Physiol 295: R1301–R1310, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li BY, Feng B, Tsu HY, Schild JH. Unmyelinated visceral afferents exhibit frequency dependent action potential broadening while myelinated visceral afferents do not. Neurosci Lett 421: 62–66, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Li BY, Schild JH. Comparisons of somatic action potentials from dispersed and intact rat nodose ganglia using patch-clamp technique. Acta Pharmacol Sin 23: 481–489, 2002 [PubMed] [Google Scholar]
- 33.Li BY, Schild JH. Electrophysiological and pharmacological validation of vagal afferent fiber type of neurons enzymatically isolated from rat nodose ganglia. J Neurosci Methods 164: 75–85, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu CL, Hsieh JC, Dun NJ, Oprea TI, Wang PS, Luo JC, Lin HC, Chang FY, Lee SD. Estrogen rapidly modulates 5-hydroxytrytophan-induced visceral hypersensitivity via GPR30 in rats. Gastroenterology (April1, 2009). doi: 10.1053/j.gastro.2009.03.047 [DOI] [PubMed] [Google Scholar]
- 35.Marino M, Galluzzo P, Ascenzi P. Estrogen signaling multiple pathways to impact gene transcription. Curr Genomics 7: 497–508, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mårtensson UE, Salehi SA, Windahl S, Gomez MF, Swärd K, Daszkiewicz-Nilsson J, Wendt A, Andersson N, Hellstrand P, Grände PO, Owman C, Rosen CJ, Adamo ML, Lundquist I, Rorsman P, Nilsson BO, Ohlsson C, Olde B, Leeb-Lundberg LM. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology 150: 687–698, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Mayer EA, Naliboff B, Lee O, Munakata J, Chang L. Gender-related differences in functional gastrointestinal disorders. Aliment Pharmacol Ther 13, Suppl 2: 65–69, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science 308: 1583–1587, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol 286: R233–R249, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Papka RE, Mowa CN. Estrogen receptors in the spinal cord, sensory ganglia, and pelvic autonomic ganglia. Int Rev Cytol 231: 91–127, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Pechere-Bertschi A, Burnier M. Female sex hormones, salt, and blood pressure regulation. Am J Hypertens 17: 994–1001, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol 70: 165–190, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Saleh TM. The role of neuropeptides and neurohormones in neurogenic cardiac arrhythmias. Curr Drug Targets Cardiovasc Haematol Disord 3: 240–253, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Saleh TM, Connell BJ, Saleh MC. Acute injection of 17beta-estradiol enhances cardiovascular reflexes and autonomic tone in ovariectomized female rats. Auton Neurosci 84: 78–88, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Schild JH, Alfrey KD, Li BY. Voltage-gated ion channels in vagal afferent neurons. In: Advances in Vagal Afferent Neurobiology, edited by Undem BJ, Weinreich D. Boca Raton, FL: CRC, 2005, p. 77–100 [Google Scholar]
- 46.Schild JH, Clark JW, Hay M, Mendelowitz D, Andresen MC, Kunze DL. A- and C-type rat nodose sensory neurons: model interpretations of dynamic discharge characteristics. J Neurophysiol 71: 2338–2358, 1994 [DOI] [PubMed] [Google Scholar]
- 47.Serock MR, Wells AK, Khalil RA. Modulators of vascular sex hormone receptors and their effects in estrogen-deficiency states associated with menopause. Recent Pat Cardiovasc Drug Discov 3: 165–186, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Sevre K, Lefrandt JD, Nordby G, Os I, Mulder M, Gans RO, Rostrup M, Smit AJ. Autonomic function in hypertensive and normotensive subjects: the importance of gender. Hypertension 37: 1351–1356, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Shaikh AA. Estrone and estradiol levels in the ovarian venous blood from rats during the estrous cycle and pregnancy. Biol Reprod 5: 297–307, 1971 [DOI] [PubMed] [Google Scholar]
- 50.Somjen D, Kohen F, Gayer B, Sharon O, Baz M, Limor R, Kulik T, Knoll E, Stern N. Role of putative membrane receptors in the effects of estradiol on human vascular cell growth. Am J Hypertens 17: 462–469, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Surprenant A, North RA. Signaling at purinergic P2X receptors. Annu Rev Physiol 71: 333–359, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Tank J, Diedrich A, Szczech E, Luft FC, Jordan J. Baroreflex regulation of heart rate and sympathetic vasomotor tone in women and men. Hypertension 45: 1159–1164, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Triadafilopoulos G, Finlayson M, Grellet C. Bowel dysfunction in postmenopausal women. Women Health 27: 55–66, 1998 [DOI] [PubMed] [Google Scholar]
- 54.Undem BJ, Kollarik M. The role of vagal afferent nerves in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2: 355–360, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Undem BJ, Weinreich D. Advances in Vagal Afferent Neurobiology Boca Raton, FL: CRC, 2005 [Google Scholar]
- 56.Wiesenfeld-Hallin Z. Sex differences in pain perception. Gend Med 2: 137–145, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Yener T, Turkkani TA, Aslan H, Aytan H, Cantug CA. Determination of oestrous cycle of the rats by direct examination: how reliable? Anat Histol Embryol 36: 75–77, 2007 [DOI] [PubMed] [Google Scholar]
- 58.Zhang D, Trudeau VL. Integration of membrane and nuclear estrogen receptor signaling. Comp Biochem Physiol A Mol Integr Physiol 144: 306–315, 2006 [DOI] [PubMed] [Google Scholar]