Abstract
Transient receptor potential melastatin (TRPM) is a subfamily of ion channels that are involved in sensing taste, ambient temperature, low pH, osmolarity, and chemical ligands. Melastatin 1/TRPM1, the founding member, was originally identified as melanoma metastasis suppressor based on its expression in normal pigment cells in the skin and the eye but not in aggressive, metastasis-competent melanomas. The role of TRPM1 and its regulation in normal melanocytes and in melanoma progression is not understood. Here, we studied the relationship of TRPM1 expression to growth and differentiation of human epidermal melanocytes. TRPM1 expression and intracellular Ca2+ levels are significantly lower in rapidly proliferating melanocytes compared to the slow growing, differentiated melanocytes. We show that lentiviral short hairpin RNA (shRNA)-mediated knockdown of TRPM1 results in reduced intracellular Ca2+ and decreased Ca2+ uptake suggesting a role for TRPM1 in Ca2+ homeostasis in melanocytes. TRPM1 knockdown also resulted in a decrease in tyrosinase activity and intracellular melanin pigment. Expression of the tumor suppressor p53 by transfection or induction of endogenous p53 by ultraviolet B radiation caused repression of TRPM1 expression accompanied by decrease in mobilization of intracellular Ca2+ and uptake of extracellular Ca2+. These data suggest a role for TRPM1-mediated Ca2+ homeostasis, which is also regulated by ultraviolet B, in melanogenesis.
Keywords: melanocytes, melanoma, melanogenesis, differentiation
transient receptor potential (TRP) family proteins function in diverse physiological processes ranging from vision, taste, olfaction, osmosensation, thermal, and nociception (26, 34). TRPM1, the founding member of the melastatin subfamily of TRPs, was originally discovered as a melanocyte-specific gene that is silenced in aggressive mouse melanoma cells (13) and as a differentially expressed gene in growth-arrested human metastatic melanoma cells (14, 44). TRPM1 mRNA shows uniform expression in benign nevi, variable expression in primary melanomas and regional or complete loss in melanoma metastasis (10). An inverse relationship between TRPM1 mRNA and increased metastatic risk of localized primary melanoma has also been reported, indicating that TRPM1 is a marker for aggressiveness of primary cutaneous melanoma (7, 12).
In normal tissues, TRPM1 is expressed primarily in the melanin pigment-producing cells melanocytes of the skin and the eye. Mammalian skin (and hair) pigmentation is a complex trait resulting from the interplay of genetic, hormonal, and environmental factors. Defects in the development of melanocyte or the biochemical and cell biological steps in melanogenesis or hormonal regulation lead to human pigmentation disorders (37). Interestingly, downregulation of TRPM1 in retina and skin has been reported to be associated with spotting pattern and congenital stationary night blindness in Appaloosa horses, suggesting a role for TRPM1 in melanogenesis (2). However, the exact function of TRPM1 in human epidermal melanocytes and its role in melanoma progression remains unknown. Following the discovery of TRPM1, additional TRPMs (M2-M8) have been identified and characterized (1, 11, 15, 19, 29, 31, 32, 38, 40). TRPM7, which is ubiquitously expressed and shown to facilitate cholinergic vesicle fusion with plasma membrane in neurons (4), also plays a role in detoxification of intermediates of melanin synthesis. In this role, TRPM7 was proposed to be critical for survival of melanophores in zebra fish (23). Recently, Yamamura et al. (43) reported that human melanoma cells express the cold-sensing menthol receptor TRPM8 and its activation can suppress melanoma cell viability.
Using heterologous expression in human embryonic kidney epithelial cells (HEK293), Xu et al. (42) showed that TRPM1 preferentially transports Ca2+ raising the possibility that in melanocytes, TRPM1 plays a role in Ca2+ homeostasis. Although a role for Ca2+ has been proposed in many aspects of melanocyte biology including their development, responses to mitogenic stimuli, melanogenesis, physiological adaption to light, photoprotection, and disease states such as vitiligo (6, 8, 20, 24, 35), mechanisms that mediate Ca2+ entry into melanocytes have not been defined.
From our observation that TRPM1 is upregulated in metastatic melanoma cells treated with a differentiation-inducing agent hexamethylene bisacetamide and its expression is associated with growth inhibition and increase in melanin pigmentation (14), we hypothesized that TRPM1 plays a role in the growth and differentiation of melanocytes by regulating Ca2+ homeostasis. In support of this, a similar role for TRPs in controlling cell proliferation by acting as molecular substrates for receptor-operated cation entry has been proposed earlier (9, 27). In this study, we show that in human melanocytes, 1) TRPM1 acts as a major regulator of Ca2+ uptake, 2) TRPM1 expression is related to melanocyte proliferation and differentiation in vitro, and 3) knockdown of TRPM1 decreases melanin accumulation. We also provide evidence for regulation of TRPM1 expression by ultraviolet B (UV-B) radiation via p53-mediated transcriptional repression. Our data raise the possibility that TRPM1-mediated Ca2+ homeostasis could play a role in regulation of melanogenesis.
MATERIALS AND METHODS
Cell culture.
Human melanocytes were cultured from neonatal foreskins as described previously (14) and passaged in Hams' F10 nutrient medium with 10% FBS, 85 nM 12-O- tetradecanoylphorbol-13-acetate (TPA), 0.1 mM 3-isobutyl-1-methylxanthine (IBMX), and 2.5 nM cholera toxin (CT). Confluent cells in TPA medium were trypsinized and replated in TPA medium or growth factor (GF) medium [Medium 254 containing bovine pituitary extract (0.2% vol/vol), fetal bovine serum (0.5% vol/vol), bovine insulin (5 μg/ml), basic fibroblast growth factor (2 ng/ml), hydrocortisone (0.18 μg/ml), heparin (3 μg/ml) and phorbol 12 myristate 13-acetate (10 ng/ml)]. All tissue culture media and supplements were purchased from Invitrogen (Carlsbad, CA). TPA, IBMX, and cholera toxin were from Sigma Aldrich (St. Louis, MO). Rabbit polyclonal anti-TRPM4 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and polyclonal rabbit antibodies to TRPM3, TRPM6, and polyclonal goat anti-TRPM7 antibodies were from Abcam (Cambridge, MA). All human subjects research described in this study were approved by the Institutional Review Board of University of Wisconsin.
RNA isolation and RT-PCR.
Total RNA was isolated using RNeasy mini kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Total RNA (1–3 μg) was reverse transcribed using the SuperScript III First-Strand Synthesis system for RT-PCR (Invitrogen), essentially according to manufacturer's instructions. Primers for TRPM1 were described previously (14). Primers for TRPM7 and other family members of TRPM were designed as described earlier (3, 23). All primers were obtained from Integrated DNA Technologies (San Diego, CA). PCR amplification was performed using 50–100 ng cDNA in a 25-μl reaction volume using the following cycling parameters: denaturation at 94°C for 5 min and then 30 cycles at 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s. For exons 19–27, the annealing temperature was 58°C for 1 min. Amplification of housekeeping genes β-actin or GAPDH was used to normalize RNA. Reactions without reverse transcriptase were performed to rule out genomic DNA contamination. PCR products were analyzed by agarose electrophoresis and visualized by ethidium bromide staining.
Real-time quantitative RT-PCR.
Total RNA was isolated and PCR was performed in triplicate with SYBR Green PCR core reagents (Applied Biosystems, Foster City, CA). Fifty to 150 ng of cDNA, 1 μM of forward and reverse primers for different 5′ or 3′ exons of TRPM1 (forward primer 5′-GAAGATAGAGCTGCTGAACTG-3′, reverse primer 5′-CTGGAGGAGCTTTATAAC-3′) and 3′ ends (forward primer 5′-ATGAACACCATGTCGCTGAA-3′ and reverse primer 5′-TCTTGGCCCATCCTAAACAC-3′) were used to produce amplicons of 154 and 371 bp, respectively. GAPDH was used as endogenous internal control (forward primer 5′-GAAGGTGAAGGTCGGAGTC-3′, reverse primer 5′-GAAGATGGTGATGGGATTTC-3′) with a product size of 236 bp. Real-time PCR was performed using ABI 7000 and cycle parameters: denaturaion at 95°C for 10 min, followed by 40 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s. The purity of product was checked by dissociation curve analysis as well as running the samples on 1% agarose gel. Data are shown as Ct values after normalization with GAPDH. The numerical value of Ct is inversely related to the amount of amplicon in the reaction, i.e., the lower the Ct, the greater is the amount of amplicon (36).
shRNA lentivirus.
For human TRPM1 shRNA lentivirus, short hairpin (sh) RNA plasmids targeting TRPM1 (NM_002420) and a negative control shRNA plasmid were purchased from Open Biosystems (Huntsville, AL). TRPM1 shRNA TRCN0000043974 (shTRPM1–74) targets nucleotides between 1055 and 1075 and shRNA TRCN0000043975 (shTRPM1–75) targets nucleotides between 2158 and 2178 (14). Efficiency of TRPM1 knockdown by these shRNAs was tested by transfecting HEK-293 cells with the long isoform of TRPM1 (TRPM1-L cDNAs) (44). We found that shTRPM1–74 was more effective (by RT-PCR and Western blotting) in knocking down TRPM1 and, therefore, we used shTRPM1–74 in this study.
We generated shTRPM1–74 and scrambled shRNA lentiviruses using transfection of HEK293 cells with the envelope plasmid pSVG and a second generation packaging plasmid (pCMVΔ8.2) obtained from Trono Lab (École polytechnique Fédérale de Lausanne, Switzerland). Detergent lysates (equivalent to 50 μg protein) of TRPM1 shRNA lentivirus-infected HEK293 cells were analyzed by Western blotting using TRPM1 antibody [diluted 1:250 in 5% nonfat dry milk in Tris-buffered saline with Tween-20 (TBST)]. Polyclonal antibody for TRPM1 was raised in rabbits by immunizing with GST (glutathione S-transferase)-TRPM1 fusion protein and purified, and the immune serum was precleared using GST-bound glutathione-Sepharose (44). Protein bands were detected by chemiluminescence (Amersham Biosciences, Piscataway, NJ).
UV irradiation.
A Daavlin Research Irradiator from Daavlin (Bryan, OH) was used for UVB exposure. The equipment contains four UVA and four UVB lamps that can be turned on and off independently and is periodically calibrated using International Light IL 1400, digital light meter (Daavlin). The exposure is controlled using two Daavlin Flex Control Integrating Dosimeters. The dose units are entered as mJ/cm2 (for UVB). Cells plated in 60-mm dishes were washed with PBS and exposed in PBS to UVB (10, 35, and 50 mJ/cm2) directly by removing the cover of the dishes. Immediately after UVB exposure, cells were washed with PBS, and fresh medium was added and incubated for 48 h in tissue culture incubator. Control cells were treated in similar way without UVB exposure. Two or three days later, cells were harvested for isolation of RNA and proteins for RT-PCR, real-time PCR, and Western blot analysis.
Measurements of intracellular Ca2+ transients.
Melanocytes in glass coverslips were placed in Tyrode solution supplemented with 1.8 mmol/l CaCl2. Cells were loaded with 10 μmol/l Fluo-3 AM (Molecular Probes) for 20 min at 37°C and then washed in the same solution without the fluorophore. Ca2+ transients were visualized in a Zeiss LSM 510 confocal microscope using the C-apochromat ×40 water immersion objective. Fluo-3 was excited with an argon laser at 488 nm and emitted fluorescence was measured at >505 nm. Images were acquired every 5 s in line-scan mode. A region of the cytoplasm was selected for each cell, and fluorescence values were analyzed using Zeiss LSM software. After correcting for the background, we normalized the fluorescence intensity values (F) to the basal fluorescence (F0). Experiments were carried out at room temperature (21–23°C).
Tyrosinase assay.
Tyrosine hydroxylase activity was measured as described previously (33). Briefly, the reaction mixture containing 10 μCi [3H]tyrosine/ml (specific activity 54.2 Ci/mmol; Perkin Elmer, Waltham, MA), 80 μM l-DOPA, and 100 μg lysate protein/ml, was incubated at 37°C for 60 min. The reaction was terminated by adding 0.5 ml of charcoal (10% wt/vol in 0.1 N HCl). The reaction mixtures were centrifuged and radioactivity in the supernatants was measured in a Packard 1900 CA liquid scintillation counter. All samples were tested in triplicate. Tyrosine hydroxylase activity is expressed as 103 cpm/h/mg protein.
Melanin measurement.
Total melanin content was determined as previously described (39). In brief, melanocytes were harvested, washed with PBS, counted, lysed in 0.5% Nonidet-P40/PBS, and melanins were solubilized in 0.1 M sodium hydroxide. Optical density of the clear supernatants was measured at 475 nm (Milton Roy 1001 plus spectrophotometer, Rochester, NY). Melanin content is expressed as nanograms per milligram protein or picogram per cell using a standard curve generated using synthetic melanin (Sigma).
Statistical analysis.
Fluorescence intensity data obtained from at least 10 cells in each field and real-time PCR data were analyzed by Student's t-test and are shown as mean ± SEM. Two-tailed P values <0.05 were considered statistically significant. Nonparametric, one way ANOVA and Bonferroni's multiple comparison test were used to compare TRPM1 expression. All statistical analyses were performed using GraphPad Prism (San Diego, CA).
RESULTS
Expression of TRPMs in human melanocytes.
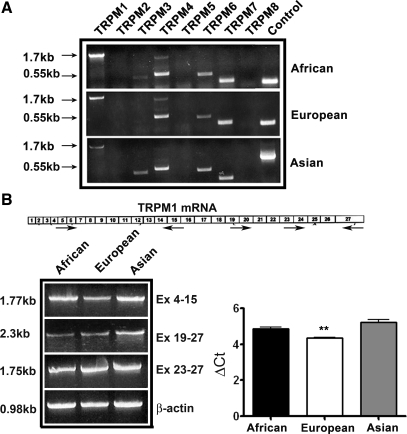
TRPM1, the founding member of the TRP melastatin family, was first identified in the mouse and human pigment cells of the skin and the iris. TRPM subfamily now consists of eight members (TRPM1–8). Expression of TRPM7 was recently shown in zebrafish melanophores and human melanocytes (23). Expression of other TRPMs in melanocytes has not been investigated. We first tested expression of TRPM1–8 in melanocytes isolated from neonatal foreskin of individuals with light and dark skin pigmentation. RT-PCR analysis of the expression pattern of TRPMs in representative melanocytes cultured from individuals of European, Asian (Korean), and African ancestry is shown in Fig. 1A. TRPM1 mRNA showed variable expression among the three melanocyte populations and mRNAs of TRPM-4, -6, and -7 showed nearly equal expression in all three melanocyte populations. Whereas a moderate to weak expression of TRPM3 was found, respectively, in melanocytes of Asian and African individuals, TRPM3 was undetectable in the melanocytes of European ancestry. TRPM-2, -5 and -8 were not detectable in melanocytes. These data show that in addition to TRPM1, TRPM-3, -4, -6, and 7 genes are also transcribed in melanocytes.
Fig. 1.
Expression of transient receptor potential melastatins (TRPMs) in human melanocytes. A: RT-PCR analysis of TRPM expression in primary melanocytes isolated from neonates of African, Asian (Korean), and European ancestry. PCR amplification of house keeping genes β-actin or GAPDH is shown in control lane. B: exon organization of TRPM1 (top) and RT-PCR analysis (bottom) of TRPM1 expression in melanocytes using primers spanning 5′ (Ex. 4–15) and 3′ ends (Ex. 19–27 and Ex. 23–27). Quantitation of TRPM1 mRNA by real-time PCR (bottom right). Data shown are ΔCt for African, European, and Asian melanocytes. **P < 0.05, Student's t-test.
The primers used for TRPM1 in Fig. 1A amplified a 1.7-kb region corresponding to the 5′ end of the 5.4-kb TRPM1 mRNA. Since TRPM1 produces at least three alternatively processed transcripts (14), we used three pairs of primers that amplify TRPM1 mRNA sequences that span exons 4–15, 19–23, and 23–27 to assess expression of different TRPM1 isoforms. In all melanocytes tested, TRPM1 mRNA sequences corresponding to these exons were amplified suggesting expression of all isoforms (Fig. 1B). Quantitation of TRPM1 mRNA expression by real-time PCR showed a significantly higher expression (lower ΔCt value) in melanocytes of European ancestry (Fig. 1B, P < 0.05).
Relationship of TRPM1 expression to growth and differentiation of melanocytes.
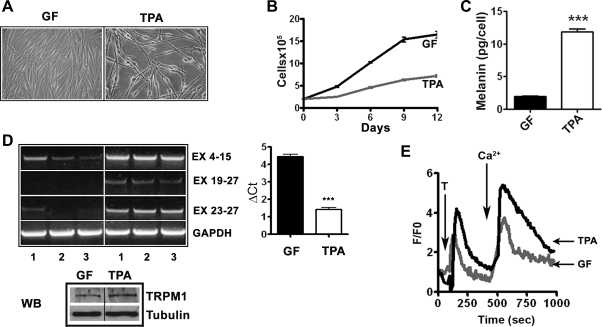
Since TRPM1 is expressed in normal melanocytes and silenced in rapidly proliferating malignant melanoma cells, we hypothesized that TRPM1 expression is inversely associated with melanocyte growth and/or directly correlates with melanocytic differentiation. To test this, we studied TRPM1 mRNA expression in genetically identical melanocytes (obtained from a single donor) grown under conditions that promote either proliferation or differentiation. Melanocytes cultured in chemically defined medium (TPA medium) showed characteristic bipolar, spindle, or dendritic morphology (28) and grew slowly (doubling time of >5 days) compared with the cells from the same donor cultured in GF-rich medium. Melanocytes in GF medium were epitheliod in shape and grew rapidly with a doubling time of 2–3 days (Fig. 2, A and B). Melanin content in fast-growing cells in GF medium was significantly less compared with well-differentiated slow-growing cells in TPA medium (Fig. 2C; 1.96 ± 0.27 pg/cell in GF-medium compared with 11.9 ± 0.94 pg/cell in TPA medium; P < 0.0001). In melanocytes cultured from three unrelated neonates of European ancestry, TRPM1 mRNA was more abundant in slower growing, more differentiated cells in TPA medium than in rapidly proliferating, less differentiated cells in GF medium (Fig. 2D). Quantitation by real-time PCR showed an eightfold higher TRPM1 mRNA expression in TPA medium compared with the cells grown in GF medium (Fig. 2D; ΔCt values 1.437 ± 0.08 for TPA compared with 4.458 ± 0.11 for GF medium; P < 0.0001). Western blot analysis using anti-TRPM1 antibody also showed that TRPM1 protein levels in cells cultured in TPA were higher compared with those in GF medium (Fig. 2D). These data suggest an inverse relationship between TRPM1 expression levels and proliferation of melanocytes and conversely a direct correlation between TRPM1 and melanogenesis, a differentiated function of melanocytes.
Fig. 2.
Relationship between TRPM1 expression and melanocyte growth and differentiation. A: morphology of melanocytes, from a single donor, cultured in 12-O-tetradecanoylphorbol-13-acetate (TPA) or growth factor (GF) media. Cells were photographed using ×40 objective. B: growth kinetics of melanocytes in TPA and GF media. Viable cells in culture were counted by trypan blue exclusion method (P < 0.01 by Student's t-test). C: melanin content in TPA and growth factor media (***P < 0.0001 by Student's t-test). D: RT-PCR analysis (as in Fig. 1B) of TRPM1 expression in three independent isolates (1, 2, and 3) of melanocytes from neonates of European ancestry grown in TPA and GF media (left). Quantitation of relative TRPM1 expression in GF and TPA media (right; P < 0.0001). Bottom, Western blot (WB) analysis of TRPM1 expression in TPA and GF media and tubulin levels show equal protein loading. E: cytosolic Ca2+ monitored by fluorescence intensity in Fluo 3-loaded melanocytes recorded following the addition of 3 μM thapsigargin (T) and 1.8 mmol/l Ca2+ to Tyrode solution. Change in fluorescence intensity was calculated in 15–20 cells in each experiment and change in intracellular Ca2+ in a representative cell is shown.
TRPM1 facilitates Ca2+ uptake by melanocytes.
TRPM1 belongs to the large TRP family of cation channel proteins, and in transfected heterologous HEK293 cells it has been shown to act primarily as Ca2+ channel. However, its Ca2+ channel function in melanocytes has not been investigated. We used fluorescence imaging of Fluo-3 AM-loaded melanocytes to measure changes in intracellular Ca2+ concentration in genetically identical human melanocytes cultured in TPA and GF media (Fig. 2E). The mean fluorescence intensity following addition of thapsigargin was nearly threefold higher in melanocytes in TPA medium than those cultured in growth factor supplemented medium (Fig. 2E, 6.19 ± 0.34 in TPA medium compared with 2.70 ± 0.28 in GF medium; P < 0.0001). Cells grown in TPA medium also showed a greater than twofold influx of extracellular Ca2+ into the cytosol upon addition of CaCl2 than cells grown in GF medium (9.35 ± 1.22 in TPA medium compared with 4.06 ± 0.45 in GF medium; P = 0.0002). Thus increased mobilization of intracellular Ca2+ and influx of extracellular Ca2+ show correlation with higher TRPM1 expression, slower growth, and differentiated phenotype of melanocytes.
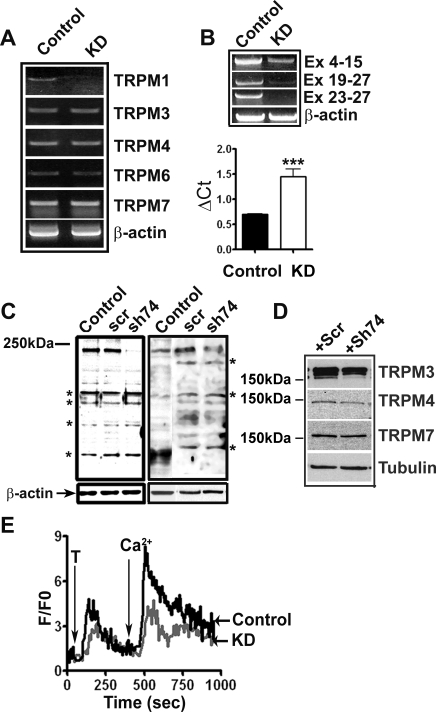
To investigate the contribution of TRPM1 to Ca2+ influx into melanocytes, we employed shRNA lentivirus to knockdown TRPM1 expression. The efficiency and specificity of TRPM1 knockdown was confirmed by RT-PCR using control and TRPM1 shRNA lentivirus-infected melanocytes (Fig. 3A). In TRPM1 shRNA lentivirus-infected cells, TRPM1 mRNA levels were reduced compared with the cells infected with control shRNA. There was no marked change in mRNA levels of other TRPM family members. Knockdown of TRPM1 was confirmed using the three sets of primers (see Fig. 1) that amplify 5′ and 3′ ends of TRPM1 mRNA (Fig. 3B). Expression of TRPM1 mRNA was nearly twofold lower in TRPM1 shRNA-transduced cells (Fig. 3B; ΔCt value 1.448 ± 0.16 in TRPM1 knockdown compared with 0.7045 ± 0.004 in control shRNA lentivirus-transduced cells; P < 0.05). Western blot analysis of shRNA lentivirus-infected HEK293 cells transfected with TRPM1-L cDNA (44) and primary melanocytes confirmed knockdown of TRPM1 protein (Fig. 3C). Western blot analysis showed only a modest change in the levels of other TRPMs (Fig. 3D).
Fig. 3.
TRPM1 knockdown by short hairpin RNA (shRNA) lentivirus. A: specificity of TRPM1 knockdown was analyzed by RT-PCR using RNA isolated from cells infected with either a scrambled (control) or TRPM1 shRNA lentivirus (KD) for 48 h. B: confirmation of TRPM1 knockdown using primers spanning different TRPM1 exons (top) and real-time PCR for TRPM1 mRNA expression in control and TRPM1 knockdown cells (bottom, P < 0.05). C: knockdown of TRPM1 protein expression by shRNA. Left: HEK293 cells transfected with TRPM1-L expression plasmid infected with either a scrambled (scr) shRNA or TRPM1shRNA (sh74) lentiviruses, control without any transduction, analyzed by Western blotting with anti-TRPM1 antibodies. Right: knockdown of endogenous TRPM1 in melanocytes. Control: nontransduced; Scr: scrambled shRNA; sh74: TRPM1 shRNA. *Major nonspecific bands. D: expression of TRPMs in control and TRPM1 KD melanocytes. Numbers show the relative migration of a 150-kDa prestained molecular weight marker. E: TRPM1 knockdown decreases Ca2+ influx in neonatal foreskin melanocytes. Cells grown on glass coverslips were infected with control scrambled and TRPM1 shRNA lentiviruses loaded with Fluo-3 dye and imaged as described in materials and methods.
Calcium imaging studies showed that the mean increase in intracellular fluorescence intensity upon addition of thapsigargin and then extracellular Ca2+ was significantly smaller in TRPM1 shRNA-transduced melanocytes than in control cells infected with a scrambled shRNA lentivirus (Fig. 3E; 3.72 ± 0.19 in knockdown vs. 4.36 ± 0.21 in control for thapsigargin; P = 0.04; and 6.07 ± 0.21 in knockdown vs. 7.11 ± 0.22 in control for Ca2+, P = 0.01). These data suggest that TRPM1 channel function contributes significantly to intracellular Ca2+ levels in melanocytes.
Effect of TRPM1 knockdown on melanocyte growth and differentiation.
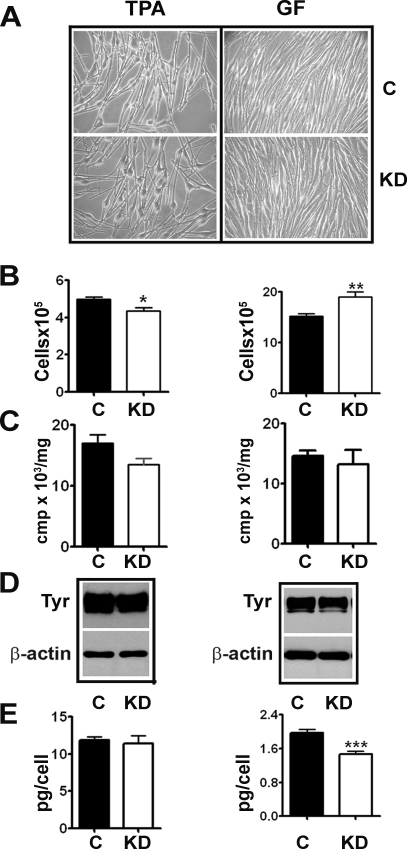
We then studied the effect of TRPM1 knockdown on melanocyte growth and differentiation. Melanocytes obtained from neonates of European ancestry and cultured in TPA and GF medium were transduced with control and TRPM1 shRNA lentiviruses and cultured for 2 wk (replenishing with additional virus). Transduction with either control or TRPM1 shRNA did not result in any marked changes in the gross morphology of melanocytes (Fig. 4A). Interestingly, whereas TRPM1 knockdown in melanocytes cultured in TPA medium resulted in a small but measurable decrease in cell growth, a further decrease in TRPM1 in GF medium resulted in growth stimulation (Fig. 4B). Although TRPM1 knockdown caused modest decrease (10–20%) in tyrosinase activity and protein (Fig. 4, C and D), but not in tyrosinase mRNA (data not shown), there was a significant decrease (>25%) in total cellular melanin only in cells in GF medium (Fig. 4E; 1.964 ± 0.09 in control compared with 1.461 ± 0.07 in knockdown; P = 0.0006). These data suggest a role for TRPM1 in maintaining intracellular tyrosinase activity.
Fig. 4.
Effect of TRPM1 knockdown on melanocyte growth and melanogenesis. Morphology (A), growth (B), tyrosinase activity (C), Western blot analysis of tyrosinase (Tyr) protein levels (D), and melanin content in control (c) and TRPM1-knockdown (KD) melanocytes (E). *P = 0.01, **P = 0.008, ***P = 0.0006.
Regulation of TRPM1 expression in melanocytes.
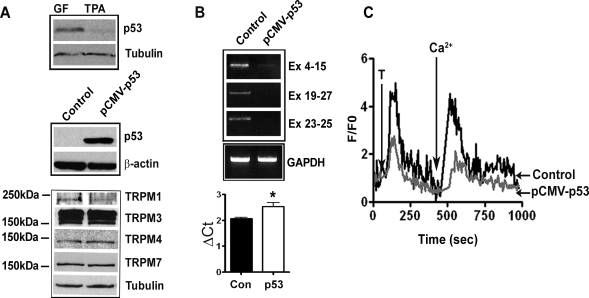
In a genome-wide analysis of p53-regulated genes, Wei et al. (41) identified TRPM1 among 542 potential p53 target genes. They identified a putative p53-binding motif at 25 kb upstream of TRPM1 transcription start site. Since there are no other expressed genes between this enhancer element and TRPM1 gene, and based on its distal location, it was predicted that p53 downregulates TRPM1. To verify this experimentally, we studied the effect of p53 on TRPM1 expression by transfecting primary melanocytes with wild-type p53 expression plasmid. First, we tested expression of endogenous p53 (Fig. 5A). In rapidly growing and less differentiated melanocytes cultured in GF medium, there was a higher level of p53 expression compared with the cells growing in TPA medium (Fig. 5A). We then expressed p53 in cells grown in TPA medium by transfecting with pCMV-p53 plasmid (Fig. 5A). RT-PCR analysis showed that in p53 overexpressing melanocytes, TRPM1 transcripts representing all exons were downregulated compared with vector-transfected cells (Fig. 5B). This was confirmed and quantitated by real-time PCR that showed 1.5-fold decrease in mRNA levels (Fig. 5B; ΔCt value for pCMV-p53 transfected 2.532 ± 0.16 compared with 2.069 ± 0.05 for control; P < 0.05). Western blot analysis showed a decrease in TRPM1 protein levels in p53-transfected cells. There was modest or no change in the expression of other TRPMs (Fig. 5A, bottom).
Fig. 5.
Inhibition of TRPM1 expression and Ca2+ influx by p53. A: Western blot analysis of p53 expression in melanocytes cultured in TPA and GF medium (top), melanocytes transfected with vector control or pCMV-p53 plasmid (middle). Expression of members of TRPM family (bottom) in melanocytes transfected with vector control or pCMV-p53. B: TRPM1 mRNA expression by RT-PCR in melanocytes transfected with vector control and pCMV-p53 plasmid (top) and quantitation of TRPM1 mRNA by real time PCR (bottom; *P < 0.05). C: cells transfected with the vector and pCMV-p53 were analyzed by Ca2+ imaging as described in materials and methods and in Fig. 2E.
Melanocytes expressing p53 showed significantly attenuated Ca2+ mobilization both from internal stores as well as from extracellular medium. Addition of thapsigargin and Ca2+ to the medium produced a smaller increase in cytoplasmic fluorescence intensity compared with cells transfected with the control vector (Fig. 5C; 4.78 ± 0.22 compared with 1.58 ± 0.18; P < 0.0001 for thapsigargin and 4.26 ± 0.30 vs. 1.38 ± 0.18; P < 0.0001 for extracellular Ca2+). These data raise the possibility that p53 plays a role in regulation of melanocyte Ca2+ homeostasis via regulation of expression of TRPM1 channel.
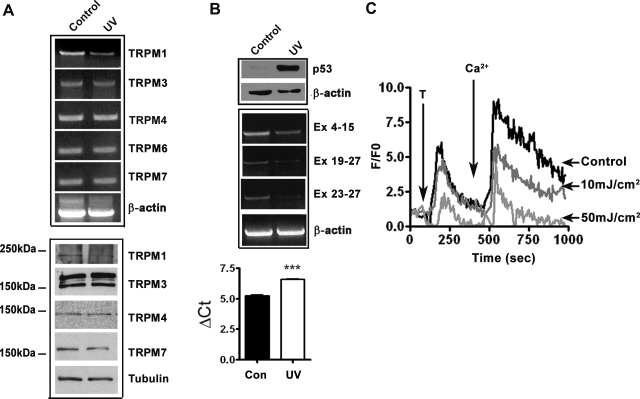
Since endogenous p53 can be induced in melanocytes by UVB irradiation, we asked whether exposure to UVB also results in downregulation of TRPM1 expression and attenuation of Ca2+ mobilization in melanocytes. Solar UV radiation consists of the long wavelength UVA (320–400 nm), short wavelength UVB (280–320 nm), and shorter wavelength UVC (200–280 nm). UVC does not reach the earth's surface since it is absorbed by earth's atmosphere. Therefore, nearly all studies on the effects of UV on human skin have been focused on UVA and UVB. Although UVA constitutes much of the solar UV radiation, UVB accounts for many of the physiological responses of skin to solar UV radiation (5, 17, 22). Therefore, we exposed cultured melanocytes to UVB radiation (35 mJ/cm2) and analyzed p53 (by Western blot analysis) and TRPM1 (by RT-PCR and real-time PCR) expression. As expected, exposure to UVB produced a significant upregulation of p53 (Fig. 6B). Consistent with the results of the transfection experiments, upregulation of endogenous p53 by UVB also resulted in downregulation of TRPM1 expression, with minimal or no significant change in expression levels of other TRPM family members expressed in melanocytes (Fig. 6, A and B). Real-time PCR data also showed 2.55-fold decrease in TRPM1 mRNA in cells exposed to UV radiation (Fig. 6B; Ct value after UV 6.603 ± 0.05 compared with ΔCt value 5.249 ± 0.09, P < 0.05). Fluorescence imaging studies showed that UVB exposure caused a dose-dependent decrease in mobilization of intracellular Ca2+ as well as uptake of extracellular Ca2+ (Fig. 6C, one-way ANOVA, P < 0.001). These data show that UVB radiation through p53-mediated transcriptional repression of TRPM1 influences Ca2+ homeostasis in melanocytes.
Fig. 6.
Ultraviolet B (UVB) treatment inhibits TRPM1 expression and Ca2+ influx. A: RT-PCR analysis of expression of TRPMs in control and UV-treated melanocytes (top). Effect of UV on the expression of other TRPMs analyzed by Western blotting (bottom). B: UV induced upregulation of p53 analyzed by Western blotting (top) and downregulation of TRPM1 mRNA expression (middle). β-Actin and tubulin are used as protein loading and RNA quality control, respectively. Relative quantification of TRPM1 mRNA expression in melanocytes after UV exposure (bottom ***P = 0.002) C: dose-dependent decrease in release of Ca2+ from intracellular stores and influx from extracellular medium by UVB radiation. Change in intracellular Ca2+ concentration was measured as described in materials and methods.
DISCUSSION
The TRPM family proteins have a wide range of physiological functions that include sensing taste, ambient temperature, and osmolarity (16). It is, therefore, of interest to note that melanocytes, which are thought to respond to physiological and environmental stimuli, show expression of TRPMs 1, 3, 4, 6, and 7 mRNAs, suggesting that multiple TRPM genes are transcribed in melanocytes and may be involved in melanocyte function and skin pigmentation. For example, TRPM 7, the cation-permeable channel and key regulator of Mg+2 homeostasis, has recently been shown to be involved in detoxification of melanin synthesis intermediates in zebrafish melanophores and also expressed in human melanoma cells (23).
Although TRPM1, the founding member of the TRPM family, was identified as melanocyte-selective protein, the functions of this protein are not known. Our data show that TRPM1 acts as an active Ca2+ channel in melanocytes. Calcium is involved in a wide range of vital cell functions including cell growth, attachment, motility, tissue morphology, signal transduction, and electrochemical responses by specialized excitable cells such as muscle cells and neurons. Although a role for Ca2+ has been proposed in many aspects of melanocyte biology, the mechanisms that regulate Ca2+ entry and Ca2+ homeostasis in melanocytes have not been investigated (6, 8, 20, 24, 35). Investigation of ryanodine receptor RyR1 (an intracellular Ca2+ channels responsible for releasing Ca2+ from the endoplasmic reticulum) in melanocytes showed that addition of the plant alkaloid ryanodine (which binds to RyR1) to human melanocytes in culture inhibited proliferation and stimulated pigmentation suggesting involvement of Ca2+ in both proliferation and differentiation of melanocytes (18). While this manuscript was being revised, Oancea et al. reported a role for TRPM1 in normal melanocyte pigmentation (30).
In this study, we show that higher levels of TRPM1 mRNA expression and intracellular Ca2+ concentrations correlate with slow proliferation and a more differentiated phenotype, and culture conditions that downregulated TRPM1 also stimulated proliferation of melanocytes. Knockdown studies using melanocytes from a single donor (hence, genetically identical) in different culture conditions suggested that TRPM1 activity is associated with both growth and differentiation. This is consistent with the downregulation or loss of TRPM1 expression in rapidly growing, less differentiated cutaneous melanoma cells. In human skin in situ, where epidermal melanocytes proliferate slowly, if at all, TRPM1 activity may be involved in mechanisms that contribute to maintain or promote differentiated function (i.e., melanin pigment synthesis). However, additional experiments are required to establish a physiological causal relationship between TRPM1 expression and melanocyte growth and/or differentiation.
Consistent with its melanocyte-restricted expression, we and others have shown that TRPM1 is regulated by the melanocyte transcription factor MITF (25, 44). Since MITF is expressed in almost all melanomas and also known to act as an oncogene (21), downregulation of TRPM1 in melanoma suggests that transcriptional factors other than MITF and other regulatory mechanisms are involved in regulation of TRPM1. Using chromatin immunoprecipitation method Wei et al. (41) recently identified 98 novel p53 target genes including TRPM1, that were suggested to represent novel aspects of p53 functions. In the TRPM1 gene, the consensus p53-binding two half sites were located 25-kb upstream of its transcription start site. Based on its distal location, it was predicted that p53 represses TRPM1 gene. Our data showing that p53 expression in melanocytes either by transfection or UV exposure leads to downregulation of TRPM1 transcription with concomitant decrease in internal store of Ca2+ as well as Ca2+ influx, implicate TRPM1 in UV-induced melanocyte proliferation and differentiation, hence, melanin pigmentation. This represents a novel function for p53 in melanocytes.
Members of TRPM family have been implicated in various human diseases, although the exact functional link between channel activity and the disease process is not clearly known. There is an inverse relationship between the TRPM1 mRNA levels and metastatic potential of cutaneous neoplastic lesions (12), suggesting that mechanisms that silence/downregulate TRPM1 transcription are activated in malignant melanocytes. A detailed biochemical and physiological studies on the functions of TRPM1, regulation of its expression and the intracellular signaling mechanisms activated by TRPM1-mediated Ca2+ influx will, therefore, help in understanding not only regulation of skin pigmentation but also melanoma tumor progression.
GRANTS
This study was supported in part by National Institutes of Health Grants R01 AR-056087 (to V. Setaluri) and RO1 HL-55438 (H. Valdivia).
REFERENCES
- 1.Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448: 204–208, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Bellone RR, Brooks SA, Sandmeyer L, Murphy BA, Forsyth G, Archer S, Bailey E, Grahn B. Differential gene expression of TRPM1, the potential cause of congenital stationary night blindness and coat spotting patterns (LP) in the Appaloosa horse (Equus caballus). Genetics 179: 1861–1870, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bollimuntha S, Singh BB, Shavali S, Sharma SK, Ebadi M. TRPC1-mediated inhibition of 1-methyl-4-phenylpyridinium ion neurotoxicity in human SH-SY5Y neuroblastoma cells. J Biol Chem 280: 2132–2140, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brauchi S, Krapivinsky G, Krapivinsky L, Clapham DE. TRPM7 facilitates cholinergic vesicle fusion with the plasma membrane. Proc Natl Acad Sci USA 105: 8304–8308, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brozyna A, Zbytek B, Granese J, Carlson JA, Ross J, Slominski A. Mechanism of UV-related carcinogenesis and its contribution to nevi/melanoma. Expert Rev Dermatol 2: 451–469, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buffey JA, Hill SE, Bleehen SS, Thody AJ, MacNeil S. Evidence for a calcium/calmodulin involvement in density-dependent melanogenesis in murine B16 melanoma cells. Pigment Cell Res 4: 112–119, 1991 [DOI] [PubMed] [Google Scholar]
- 7.Carlson JA, Ross JS, Slominski A, Linette G, Mysliborski J, Hill J, Mihm M., Jr Molecular diagnostics in melanoma. J Am Acad Dermatol 52: 743–775, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Carsberg CJ, Jones KT, Sharpe GR, Friedmann PS. Intracellular calcium modulates the responses of human melanocytes to melanogenic stimuli. J Dermatol Sci 9: 157–164, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Clapham DE. TRP channels as cellular sensors. Nature 426: 517–524, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Deeds J, Cronin F, Duncan LM. Patterns of melastatin mRNA expression in melanocytic tumors. Hum Pathol 31: 1346–1356, 2000 [PubMed] [Google Scholar]
- 11.Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron 54: 371–378, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Duncan LM, Deeds J, Cronin FE, Donovan M, Sober AJ, Kauffman M, McCarthy JJ. Melastatin expression and prognosis in cutaneous malignant melanoma. J Clin Oncol 19: 568–576, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Duncan LM, Deeds J, Hunter J, Shao J, Holmgren LM, Woolf EA, Tepper RI, Shyjan AW. Down-regulation of the novel gene melastatin correlates with potential for melanoma metastasis. Cancer Res 58: 1515–1520, 1998 [PubMed] [Google Scholar]
- 14.Fang D, Setaluri V. Expression and Up-regulation of alternatively spliced transcripts of melastatin, a melanoma metastasis-related gene, in human melanoma cells. Biochem Biophys Res Commun 279: 53–61, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Grimm C, Kraft R, Schultz G, Harteneck C. Activation of the melastatin-related cation channel TRPM3 by D-erythro-sphingosine. Mol Pharmacol 67: 798–805, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Harteneck C. Function and pharmacology of TRPM cation channels. Naunyn Schmiedebergs Arch Pharmacol 371: 307–314, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Kadekaro A, Kavanagh R, Wakamatsu K, Ito S, Pipitone M, Abdel-Malek Cutaneous photobiology Z. The melanocyte vs. the Sun: who will win the final round? Pigment Cell Res 16: 434–447, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Kang HY, Kim NS, Lee CO, Lee JY, Kang WH. Expression and function of ryanodine receptors in human melanocytes. J Cell Physiol 185: 200–206, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Launay P, Cheng H, Srivatsan S, Penner R, Fleig A, Kinet JP. TRPM4 regulates calcium oscillations after T cell activation. Science 306: 1374–1377, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Lavallee CR, Chalifoux JR, Moosally AJ, Balkema GW. Elevated free calcium levels in the subretinal space elevate the absolute dark-adapted threshold in hypopigmented mice. J Neurophysiol 90: 3654–3662, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Levy C, Khaled M, Fisher DE. MITF: master regulator of melanocyte development and melanoma oncogene. Trends Mol Med 12: 406–414, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Maddodi N, Setaluri V. Role of UV in cutaneous melanoma. Photochem Photobiol 84: 528–536, 2008 [DOI] [PubMed] [Google Scholar]
- 23.McNeill MS, Paulsen J, Bonde G, Burnight E, Hsu MY, Cornell RA. Cell death of melanophores in zebrafish trpm7 mutant embryos depends on melanin synthesis. J Invest Dermatol 127: 2020–2030, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Meyer zum Gottesberge A. Calcium dependent intercellular interaction of the neural crest derivate-melanocytes and the epithelial cells of the vestibular organ. Acta Otolaryngol Suppl (Stockh) 520: 360–361, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Miller AJ, Du J, Rowan S, Hershey CL, Widlund HR, Fisher DE. Transcriptional regulation of the melanoma prognostic marker melastatin (TRPM1) by MITF in melanocytes and melanoma. Cancer Res 64: 509–516, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Minke B, Wu CF, Pak WL. Induction of photoreceptor voltage noise in the dark in Drosophila mutant. Nature 258: 84–87, 1975 [DOI] [PubMed] [Google Scholar]
- 27.Montell C. The TRP superfamily of cation channels. Sci STKE 2005: re3, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Nakazawa K, Damour O, Collombel C. Modulation of normal human melanocyte dendricity by growth-promoting agents. Pigment Cell Res 6: 406–416, 1993 [DOI] [PubMed] [Google Scholar]
- 29.Numata T, Okada Y. Molecular determinants of sensitivity and conductivity of human TRPM7 to Mg2+ and Ca2+. Channels (Austin) 2: 283–286, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Oancea E, Vriens J, Brauchi S, Jun J, Splawski I, Clapham DE. TRPM1 forms ion channels associated with melanin content in melanocytes. Sci Signal 2: ra21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, Max M, Margolskee RF. A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci 5: 1169–1176, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, Stokes AJ, Zhu Q, Bessman MJ, Penner R, Kinet JP, Scharenberg AM. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 411: 595–599, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Pomerantz SH. L-tyrosine-3,5–3H assay for tyrosinase development in skin of newborn hamsters. Science 164: 838–839, 1969 [DOI] [PubMed] [Google Scholar]
- 34.Ramsey I, Delling M, Clapham D. An introduction to TRP channels. Ann Rev Physiol 68: 619–647, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Schallreuter-Wood KU, Pittelkow MR, Swanson NN. Defective calcium transport in vitiliginous melanocytes. Arch Dermatol Res 288: 11–13, 1996 [DOI] [PubMed] [Google Scholar]
- 36.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3: 1101–1108, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev 84: 1155–1228, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Talavera K, Yasumatsu K, Voets T, Droogmans G, Shigemura N, Ninomiya Y, Margolskee RF, Nilius B. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature 438: 1022–1025, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Tsuboi T, Kondoh H, Hiratsuka J, Mishima Y. Enhanced melanogenesis induced by tyrosinase gene-transfer increases boron-uptake and killing effect of boron neutron capture therapy for amelanotic melanoma. Pigment Cell Res 11: 275–282, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Vennekens R, Olausson J, Meissner M, Bloch W, Mathar I, Philipp SE, Schmitz F, Weissgerber P, Nilius B, Flockerzi V, Freichel M. Increased IgE-dependent mast cell activation and anaphylactic responses in mice lacking the calcium-activated nonselective cation channel TRPM4. Nat Immunol 8: 312–320, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Wei CL, Wu Q, Vega VB, Chiu KP, Ng P, Zhang T, Shahab A, Yong HC, Fu Y, Weng Z, Liu J, Zhao XD, Chew JL, Lee YL, Kuznetsov VA, Sung WK, Miller LD, Lim B, Liu ET, Yu Q, Ng HH, Ruan Y. A global map of p53 transcription-factor binding sites in the human genome. Cell 124: 207–219, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Xu XZ, Moebius F, Gill DL, Montell C. Regulation of melastatin, a TRP-related protein, through interaction with a cytoplasmic isoform. Proc Natl Acad Sci USA 98: 10692–10697, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamamura H, Ugawa S, Ueda T, Morita A, Shimada S. TRPM8 activation suppresses cellular viability in human melanoma. Am J Physiol Cell Physiol 295: C296–C301, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Zhiqi S, Soltani MH, Bhat KM, Sangha N, Fang D, Hunter JJ, Setaluri V. Human melastatin 1 (TRPM1) is regulated by MITF and produces multiple polypeptide isoforms in melanocytes and melanoma. Melanoma Res 14: 509–516, 2004 [DOI] [PubMed] [Google Scholar]