Abstract
Enhanced expression of the facilitative glucose transporter, GLUT1, has been shown to inhibit apoptosis in several cell systems including vascular smooth muscle cells (VSMCs). A decrease in apoptosis could lead to increased VSMC numbers in neointimal and medial arterial layers under several pathologic conditions. The hypothesis underlying these studies is that GLUT1 induces expression of antiapoptotic and prosurvival genes that increase VSMC survival. Transcriptomic analysis of A7r5 VSMCs, in which GLUT1 was acutely overexpressed, showed a 2.14-fold increase in c-FLICE inhibitory protein (cFLIP), which promotes cellular growth and prevents apoptosis through caspase 8 binding. We confirmed that overexpression of GLUT1 induced mRNA and protein expression of both the long and short isoforms of cFLIP (cFLIPL and cFLIPS) in primary and stable immortalized VSMC lines as well as in aortas from GLUT1 transgenic mice. Increased GLUT1 reduced VSMC death by more than twofold after serum withdrawal, as evidenced by decreased caspase 3 activity and Trypan blue exclusion studies. GLUT1 overexpression resulted in a greater than twofold increase in proliferating cell nuclear antigen expression and live cell numbers, consistent with augmented VSMC proliferation. Lentiviral knockdown of cFLIPL showed that cFLIPL was necessary for the proproliferative and antiapoptotic effects of GLUT1 overexpression. Taken together, these data suggest that GLUT1 induction of cFLIPL expression augments proliferation and prevents apoptosis in VSMCs.
Keywords: metabolism, glucose, neointimal hyperplasia, c-FLICE inhibitory protein
there are multiple interactions between the cellular machinery involved in glucose uptake and metabolism and the cellular mechanisms of programmed cell death, or apoptosis. Many investigators have found that glucose deprivation can promote apoptosis in a variety of cells (18). The converse also appears to be true, that induction of glucose uptake and metabolism can prevent or reduce apoptosis (7, 25). Several groups, including our own, have focused on the role of the facilitative glucose transporter, GLUT1, in reducing apoptosis in vascular smooth muscle cells (VSMCs) (5, 6, 13, 14). Additionally, GLUT1 mRNA and polypeptide are expressed at increased levels in hypoxic and ischemic VSMCs (13, 14).
While VSMCs normally exist in the tunica media in a quiescent nonproliferative state, known as the contractile state (9), vascular injury can induce the transformation of VSMCs to a proliferative, or synthetic state, as well as promote migration of VSMCs to the intimal region of the vessel leading to thickening of the neointima (9, 20, 23, 24). VSMCs that accumulate in the neointima after endothelial injury demonstrate increased GLUT1 expression (5).
Maintained or increased GLUT1 expression has been found to reduce VSMC apoptosis under a variety of conditions, including hypoxia (13, 14) and serum withdrawal (5). Enhanced GLUT1 expression has been shown to inhibit cytochrome c release and downstream caspase activation during hypoxia (13, 14). Similar studies in various cell types have suggested that glucose metabolism is critical for prevention of apoptosis due to other causes, such as growth factor withdrawal. Thompson's laboratory found that withdrawal of lymphocyte growth and survival factors led to cellular atrophy and apoptosis accompanied by reduction in GLUT1 expression, glucose uptake, ATP levels, and mitochondrial potential (25). In this system, apoptosis could be accelerated by glucose depletion (25) and could be prevented by Akt-stimulated increases in glucose uptake, GLUT1 expression, and glucose metabolism (21). Reduced activation of GSK-3 (5, 14) and the JNK pathway (13) also appear to play important roles in GLUT1's antiapoptotic effects in VSMCs.
While enhanced GLUT1 expression prevents apoptosis due to acute cell stress, as noted above, it is less certain that a sustained increase in GLUT1 expression has persistent antiapoptotic effects in VSMCs. In addition, downstream gene expression changes that may be critical for sustained antiapoptotic effects of enhanced GLUT1 expression have not been identified. Therefore, we wished to determine the effects of GLUT1 on gene expression in VSMCs, to identify other potential mediators of the antiapoptotic effects of GLUT1.
We now report that increased GLUT1 expression in cultured VSMCs leads to altered expression of a number of genes, including enhanced expression of c-FLICE inhibitory protein (cFLIP). cFLIP is a molecule that competitively inhibits caspase 8 activation by binding to the death receptor complex at the plasma membrane (3). We found that induction of cFLIP is essential for the antiapoptotic and cell proliferative effects in GLUT1-overexpressing VSMCs subjected to serum withdrawal.
MATERIALS AND METHODS
Antibodies.
Goat anti-rabbit and goat anti-mouse horseradish peroxidase-tagged antibodies (catalog no. sc-2030 and sc-2031, respectively) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The antibody for β-tubulin (05-661) was purchased from Upstate Biotechnology (Lake Placid, NY). The antibody for proliferating cell nuclear antigen (PCNA; P-8825) was purchased from Sigma Aldrich (St. Louis, MO). An antibody that recognizes the long and short isoforms of cFLIP (cFLIPL and cFLIPS) (NT no. 1159) was purchased from ProSci (Poway, CA).
Cell culture.
Whole body GLUT1 transgenic mice (GT1S) and control littermates (C57BL/6) are being reported in a separate study (Y. Wang, K. Heilig, T. Saunders, A. Minto, D. K. Deb, A. Chang, F. Brosius, and C. W. Heilig, unpublished observation). The GT1S GLUT1 expression construct for these mice was assembled with the LK440 vector, human β-actin promoter, and full-length GLUT1 cDNA as described in abstract form (4, 12) and more fully in the same manuscript. Conventional transgenic mice were generated on a C57BL/6J background. Primary VSMCs were isolated from these mice and their wild-type littermates, using a procedure outlined by Benson et al. (22). Aortas from 8- to 10-wk-old male mice were excised and cleaned of adventitia under sterile conditions. Aortas were cut into sections and digested with 1.4 mg/ml collagenase, type II (LS004174 ) from Worthington Biochemical (Lakewood, NJ), in DMEM. Free VSMCs were then collected between 4 and 6 h after collagenase addition and incubated for several days at 37°C in 5% CO2. Cells were maintained in DMEM containing 5.5 mM glucose and 10% fetal bovine serum. VSMCs were used between passages 3 and 10.
The procedures used in this study were approved by the University of Michigan Committee on the Use and Care of Animals. Veterinary care was provided by the University of Michigan Unit for Laboratory Animal Medicine. The University of Michigan is accredited by the American Association of Laboratory Animal Care. The animal care and use program conformed to the standards in Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 85-23, Revised 1996).
A7r5 rat embryonic VSMC cells (CRL-1444, American Type Culture Collection) were cultured in the same medium and conditions. Cells were plated at a density of 0.5 × 104 cells/100-mm plate, before all treatments, allowing for 70% confluency at the time of harvest. Cells were placed in serum-free medium for 14 h before quantitative RT-PCR (q-RT-PCR) experiments and 24 or 48 h before immunoblot, cell counting, and caspase 3 activity analyses.
GLUT1 infection.
Cells were infected with a GLUT1-containing adenoviral vector, or control vector, as previously described (17), for 2 h, in OPTI-MEM I, reduced-serum media (no. 31985, Gibco), at a multiplicity of infection of 50 (MOI 1 = 100 particles/cell). Two hours postinfection, OptiMEM media was replaced with fresh media (5.5 mM glucose containing DMEM + 10% fetal bovine serum). Serum was removed 24 h postinfection. The human GLUT1 cDNA adenoviral vector was a gift from Dr. Arno Kumagai (University of Michigan).
cFLIP knockdown.
Cells were infected with lentiviral vectors (2 μg per 100-mm plate) encoding short hairpin RNA (shRNA) for cFLIPL or scrambled sequence for 48 h, in DMEM + 10% fetal bovine serum with 5.5 mM glucose. Serum was withdrawn for either 24 or 48 h after infection. The mouse cFLIPL shRNA lentivirus (catalog no. RMM4431–98762272, clone ID V2LMM_26360) and the scrambled negative control (catalog no. RHS 4346) lentivirus were purchased from Open Biosystems (Huntsville, AL). Both were placed into pGIPz lentiviral vectors and expanded by the Life Sciences Institute at the University of Michigan.
Immunoblot analysis.
At harvest, media were collected from each plate and cells were removed using 0.25% Trypsin/EDTA (no. 25200, Invitrogen) for 3 min. Trypsinized cells were added to the media and centrifuged at 1,500 rpm and 4°C for 3 min. Pellets were washed with PBS and centrifuged, and cell pellets were stored at −80°C, overnight. Pellets were resuspended in 50 μl lysis buffer (10 mM Tris·HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 1% SDS, 1 mM Na3VO4 and PMSF, 1 uM okadaic acid, and 10 μg/ml leupeptin, aprotinin, and pepstatin) and sonicated for 4 s at 40% followed by centrifugation in a microfuge at high speed for 5 min. A BCA Assay Kit (Pierce no. 23227, Thermo Scientific, Rockford, IL) was used to determine the concentration of protein. Immunoblot analysis was conducted using 10% Bis/acrylimide gels, 10–25 μg protein per sample, and run for 1.5 h, at 70–150 V. Lumi-Light Western Blotting Substrate (12 015 200 001) was obtained from Roche (Indianapolis, IN). ECL-Plus Western Blotting Detection Reagent (RPN2132) was purchased from Amersham Biosciences (Pittsburgh, PA).
RNA isolation and microarray analysis.
RNA was isolated from cells using the standard TRIzol protocol (Invitrogen Life Technologies, Carlsbad, CA). This was followed by extraction with phenol/chloroform until the interface was clear. Total RNA was further purified using a Qiagen RNeasy Total RNA Isolation Kit (RNeasy mini protocol for RNA clean-up no. 74104) and an RNase-Free DNase Set (no. 79254, Qiagen) to digest unwanted genomic DNA. Fluorescently tagged RNA was hybridized to U230A Affymetrix Rat Gene Chips (accession no. GPL 341) in the University of Michigan Diabetes Research and Training Center-National Institute of Diabetes and Digestive and Kidney Diseases Core. Microarray data were submitted to the National Center for Biotechnology Information Gene Expression Omnibus (series ID GSE 15713).
q-RT-PCR.
RNA was isolated as previously described (2), and total RNA was reverse transcribed to cDNA using Taqman Reverse Transcription Reagents (Roche N808-0234). The murine cFLIPL-specific primer/probe set (Mm01255579_m1, fluorescence-labeled probe) was obtained from Applied Biosystems (Foster City, CA), and the murine glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-specific primer/ probe set was a gift from Dr. Matthias Kretzler (University of Michigan) (sense primer 5′-CATGGCCTTC CGTGTTCCTA-3′, antisense primer 5′-ATGCCTGCTTCACCACCTTCT-3′, and fluorescence-labeled probe 5′-CCCAATGTGTCCGTCGTGGATCTGA-3′). For q-RT-PCR with mouse-specific cFLIPL primer probe sets, TaqMan Fast Universal PCR Master Mix (4352042, Applied Biosystems) and 25 ng cDNA per PCR reaction was used. cFLIP rat primers were obtained from Integrated DNA Technologies (IDT, Coralville, IA). Rat primers recognized both cFLIP isoforms (sense primer 5′-CCAAACCTGACTTCTGCGGT-3′ and antisense primer 5′-ACCTGGTGGA TGACCTCAGC-3′), cFLIPL only (sense primer 5′-AGTCCAGCCAAGAAGCAA GA-3′ and antisense primer 5′-TGTAGCTCT CTTCATGTATG-3′), or cFLIPS only (sense primer 5′- GTCCAGCCAAGAAGC AAGA-3′ and antisense primer 5′-TTCTTTACCAAACACACGC-3′). Rat-specific GAPDH primers were purchased from IDT (sense primer 5′-ACAAGATGGTGA AGGTCGGT GTGA-3′ and antisense primer 5′-AGCTTC CCATTCTCAGCCTTGACT-3′). For q-RT-PCR using rat primers, we used SYBR green PCR master mix (no. 4324018, Roche) and 50 ng cDNA per PCR reaction. A Bio-Rad i-cycler was used for sequence detection, and gene expression was normalized to that of GAPDH.
Caspase 3 activity.
Caspase 3 activity assays were conducted using a caspase 3 Cellular Activity Assay Kit (235419, VWR International, West Chester, PA). The protocol was followed as outlined by the manufacturer. Briefly, cell pellets were isolated and frozen as outlined for immunoblot analysis, and cells were resuspended in lysis buffer + 1% Triton (2 × 107 cells/ml lysis buffer). Lysates were vortexed several times while incubating on ice for 30 min, then centrifuged at 14,000 rpm (20,800 g) and 4°C for 10 min. Protein lysates, 96-well plate, assay buffer, and caspase 3 substrate were warmed to 37°C for 15 min and added to appropriate wells. The reaction was started by adding caspase 3 substrate to each well. The 96-well plate was maintained at 37°C and read at 405 nm every 30 min for 4 h.
Statistical analysis.
Data are expressed as means ± SE, unless otherwise indicated in the figures, and for multiple groups were analyzed using one-way analysis of variance, followed by Newman-Keuls post hoc analysis or, for two groups, by Student's t-test. Data were considered statistically significant at P < 0.05.
RESULTS
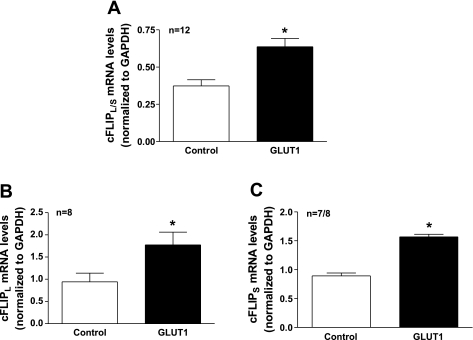
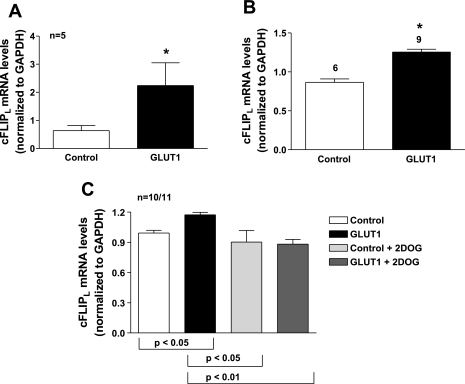
GLUT1 induced cFLIP mRNA and protein expression in A7r5 immortalized and primary VSMCs. A7r5 cells were infected with GLUT1-containing adenoviral vectors or empty vector controls. We confirmed that GLUT1 protein levels were 2-fold greater in GLUT1-infected VSMCs compared with control cells by immunoblot analysis (data not shown). RNA was isolated and hybridized to U230A Affymetrix Rat Gene Chips to determine changes in transcription. Of the 15,650 cDNAs represented on the chip, 95 genes showed >2-fold increase in expression and 80 genes showed a >2-fold reduction in expression in the GLUT1-overexpressing A7r5 cells (supplemental data table; supplemental data for this article can be found on the American Journal of Physiology-Cell Physiology website). Enhanced GLUT1 expression increased the expression of cFLIP, a prosurvival molecule that inhibits caspase activity, by 2.14-fold. The increase in cFLIP mRNA expression was confirmed by q-RT-PCR using rat-specific primers that recognized both the long and short isoforms of cFLIP (Fig. 1A). Expression of both cFLIPL and cFLIPS isoforms was increased in response to GLUT1 expression (Fig. 1, B and C). Similarly, cFLIPL mRNA expression was increased 3.54 ± 0.36-fold in aortas (Fig. 2A) and 1.5 ± 0.01-fold in primary VSMCs cultured from aortas of GLUT1 transgenic mice when compared with control littermates (Fig. 2B). The induction of cFLIPL mRNA expression was abrogated by incubation of A7r5 cells with the nonmetabolized glucose analog and glycolytic inhibitor, 2-deoxyglucose (Fig. 2C).
Fig. 1.
Glucose transporter (GLUT) 1 increases c-FLICE inhibitory protein, long isoform (cFLIPL) mRNA expression in A7r5 cells. Quantitative RT-PCR (q-RT-PCR) experiments with primers that detected both cFLIP long and cFLIP short isoforms (cFLIPL/S) confirmed enhanced cFLIP expression in GLUT1-infected A7r5 cells compared with control cells (A). Both the long (cFLIPL; B) and the short (cFLIPS; C) cFLIP isoforms were increased in response to enhanced GLUT1 levels. cFLIP mRNA levels were normalized to those of GAPDH. *P < 0.05.
Fig. 2.
GLUT1 increases cFLIP mRNA expression in primary vascular smooth muscle cells (VSMCs). GLUT1 enhanced cFLIPL mRNA expression based on q-RT-PCR experiments in excised aortas (A) and in primary VSMCs grown from aortas (B) from GLUT1 transgenic mice and control littermates. The increase in cFLIPL expression was abrogated by treatment with the glycolytic inhibitor 2-deoxyglucose (2DOG; C). cFLIP mRNA levels were normalized to those of GAPDH. *P < 0.05.
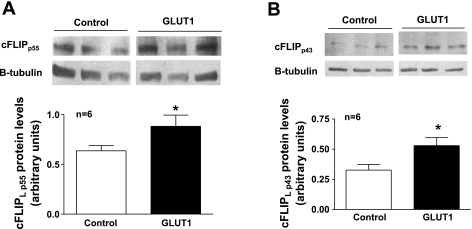
Furthermore, there was an increase in cFLIPL (1.88 ± 0.1) and cFLIPS (1.7 ± 0.04) protein expression in GLUT1-overexpressing A7R5 rat cells (data not shown) and primary GLUT1 transgenic mouse VSMCs (GLUT1 cells) (Fig. 3). Caspase 8 can cleave cFLIPL to produce a protein product known as p43FLIP, which continues to inhibit caspase 8 and is more efficient for recruitment and binding of receptor interacting protein 1 (RIP1) to the caspase 8/cFLIP complex than the full-length (p55) cFLIPL protein (14, 15). This cleavage event and RIP1 recruitment have been shown to stimulate the NF-κB pathway and proliferation (14, 15). There was a modest yet significant increase in the full-length cFLIP p55 protein (1.4 ± 0.11, Fig. 3A) with an even greater increase in the cleaved cFLIP p43 protein (1.6 ± 0.07, Fig. 3B), in GLUT1 cells.
Fig. 3.
GLUT1 increases cFLIP protein expression in primary VSMCs. GLUT1 enhanced cFLIPL protein expression based on immunoblot analysis of primary VSMCs grown from aortas from GLUT1 transgenic mice compared with those from control littermates. Immunoblot analysis confirmed increased cFLIPL protein expression in the full-length p55 protein (A) and the cleaved p43 protein (B). cFLIP protein levels were normalized to those of β-tubulin. *P < 0.05.
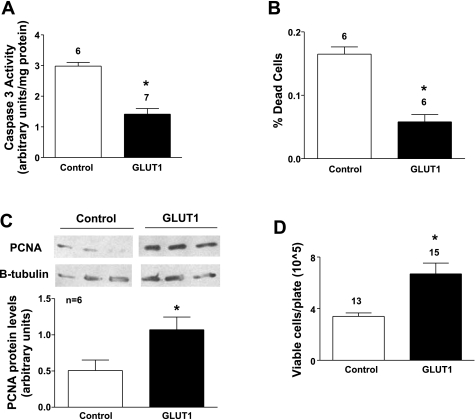
GLUT1 protects primary VSMCs from apoptosis and promotes proliferation. There was a significant decrease in caspase 3 activity in GLUT1 cells compared with control cells after serum withdrawal of 48 h (Fig. 4A). In addition, there were fewer dead cells as determined by Trypan blue exclusion (Fig. 4B). Together, these results demonstrate that GLUT1 is associated with decreased apoptosis in primary VSMCs subjected to serum withdrawal. To determine whether there were concomitant changes in cell proliferation, immunoblot analysis using an antibody to PCNA was performed. PCNA expression, an indicator of cells in early G1 phase and S phase of the cell cycle, was significantly higher in GLUT1 cells compared with controls in both A7r5 cells (data not shown), and primary VSMCs (Fig. 4D), showing that GLUT1 increased DNA synthesis and growth. Cell counts were higher in GLUT1 cells than in control VSMCs 48 h after serum withdrawal (Fig. 4C), consistent with increased proliferation and/or decreased apoptosis in GLUT1 cells.
Fig. 4.
GLUT1 prevents VSMC apoptosis and induces proliferation in primary VSMCs. Caspase 3 activity was decreased in GLUT1 cells grown from aortas of GLUT1-overexpressing mice compared with controls (A). Increased GLUT1 expression reduced the number of dead cells based on Trypan blue staining (B). PCNA immunoblotting demonstrated increased PCNA protein levels in GLUT1-overexpressing cells (C). Cellular counts confirmed an increase in proliferation in GLUT1 cells compared with controls (D). PCNA protein levels were normalized to those of β-tubulin. *P < 0.05.
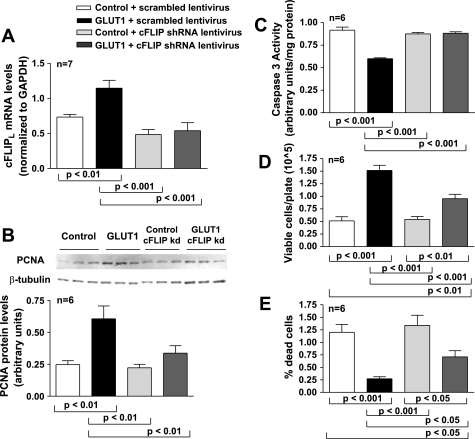
cFLIPL is necessary for GLUT1-mediated prevention of apoptosis and induction of proliferation. To determine whether cFLIP was necessary to prevent apoptosis and induce proliferation, GLUT1 cells and control primary VSMCs were infected with shRNA lentivirus to knock down cFLIPL expression. cFLIPL expression was decreased in GLUT1 cells by ∼60%. While less than in many knockdown studies, this degree of knockdown was desired because it returned cFLIP levels to approximately that of control cells (Fig. 5A). Knockdown of cFLIPL decreased PCNA expression (Fig. 5B) and increased caspase 3 activity in GLUT1 cells (Fig. 5C) to levels of control cells. Total cell counts were reduced and dead cells were increased by cFLIPL knockdown in GLUT1-overexpressing cells (Fig. 5, D and E). These data suggest that cFLIPL plays an important role in GLUT1-induced prevention of VSMC apoptosis and induction of proliferation.
Fig. 5.
cFLIPL is necessary for GLUT1-mediated prevention of apoptosis and induction of proliferation. Knockdown (kd) of cFLIPL expression in GLUT1 cells (A) resulted in decreased PCNA expression (B) and increased caspase 3 activity (C) and had little effect in control cells. Knockdown of cFLIPL expression resulted in a decrease in the number of live (D) and an increase in the number of dead (E) GLUT1 cells, again with little effect on control cells. cFLIP mRNA levels were normalized to those of GAPDH, and PCNA protein levels were normalized to those of β-tubulin. shRNA, short hairpin RNA.
DISCUSSION
This study demonstrates for the first time that increased GLUT1 levels induce expression of the antiapoptotic protein cFLIP and that cFLIP mediates much of the antiapoptotic and proproliferative effects of enhanced VSMC GLUT1 expression in a cultured cell system. In these studies, we used two cultured cell models, A7r5 VSMCs, an immortalized rat embryonic VSMC line infected with either a GLUT1 or control adenovirus, and primary murine VSMCs, cultured from GLUT1 transgenic or wild-type littermate mice, for the majority of our experiments. Transcriptomic analysis was conducted on GLUT1-overexpressing and control A7r5 cells because previous studies on GLUT1's antiapoptotic effects were performed in this cell line (13). However, because signaling and regulation in this immortalized cell line may not necessarily reproduce those found in primary VSMCs and in VSMCs in vivo, we obtained confirmatory results in primary VSMCs and aortas to ensure that changes presented were physiologically relevant. GLUT1 effects on cFLIP were also confirmed in both primary VSMCs and in aortas excised from GLUT1 transgenic and control mice, establishing that these relationships exist in vivo as well as in tissue culture models.
We have previously shown that GLUT1 prevents hypoxia-induced apoptosis in VSMCs and cardiac myocytes largely via a mitochondrial, caspase 9-dependent pathway (8, 13, 14, 17). However, in this study, we did not test whether cFLIP acted via a mitochondrial pathway or a death receptor pathway, or both. Serum withdrawal has usually been associated with activation of the mitochondrial pathway, but there are no definitive studies that have dissected the mechanisms of serum withdrawal- induced apoptosis in VSMCs. Certainly, the known effects of cFLIP on death receptor-mediated pathways, via inhibition of caspase 8 (3), suggest that a death receptor pathway may play a role in VSMC apoptosis in our studies.
The downstream changes in apoptosis and cell survival seen with increased expression of GLUT1 in our current system were relatively modest. However, these are likely to be biologically significant since chronic induction of GLUT1 and cFLIP expression sensitizes the cell to external stimuli and therefore, over time, is associated with long-term effects on growth and apoptosis. The even more substantial effect of chronic GLUT1 expression on cFLIP levels in aorta in vivo compared with the changes induced in cultured cells also supports the hypothesis that long-term chronic effects of enhanced GLUT1 expression are substantial.
While cFLIP induction was critical for GLUT1-mediated prevention of VSMC apoptosis and induction of VSMC proliferation, the exact mechanism or mechanisms by which cFLIP mediates these effects remain unidentified. cFLIPL has been shown to associate in lymphocytes with TNF receptor-associated factor 1 and 2 (TRAF-1 and TRAF-2), and receptor interacting protein (RIP1), which together promote NF-κB activation (3, 10, 16), and such primary NF-κB activation could account for some of cFLIP's antiapoptotic and proproliferative effects in VSMCs. However, we saw little NF-κB activation in our cultured cell system in response to TNF-α (unpublished observation), so it is unlikely that this is the main pathway in our system. cFLIP also enhances a number of additional antiapoptotic responses in a variety of cell types. Its best described antiapoptotic function is as a dominant-negative inhibitor of caspase 8 in which it binds FADD and caspase 8 to block further recruitment of caspase 8 to the death-inducing signaling complex (3). cFLIP has also been shown to associate with RAF1, which activates ERK through MAPK/ERK kinase 1 (MEK1) and MEK2, which could explain some of its proproliferative effects in VSMCs (10).
The phosphatidylinositol 3-kinase (PI3K)/Akt/GSK-3 pathway has been shown to influence changes in apoptosis and proliferation, and we have evidence that induction of GLUT1 expression is associated with increased phosphorylation of Akt (S473 and T308) and GSK-3 (S 21/9) (14), which can be abrogated in the presence of glycolytic inhibitors (unpublished observation). These data suggest that the PI3K/Akt/GSK-3 pathway and perhaps PI3K may play a role in GLUT1-induced proliferation and prevention of apoptosis. Interestingly, the PI3K/Akt pathway has been shown to upregulate cFLIP expression (19). Therefore, it will be important to determine whether GLUT1-induced cFLIP expression is dependent on activation of PI3K/Akt and whether the PI3K/Akt pathway is necessary for cFLIP to exert its effects on known apoptotic and proliferative pathways.
Overexpression of GLUT1 may occur in several pathological conditions. Restenosis after angioplasty is associated with increased vascular smooth muscle proliferation (11) and, in some studies, a reduction in apoptosis (1). Since GLUT1 expression is chronically enhanced in neointimal cells (18), it could potentiate an increase in proliferation and/or reduction in apoptosis after angioplasty, leading to more exuberant restenosis. Moreover, it has been well established that cancer cells overexpress GLUT1 and demonstrate enhanced glucose uptake and metabolism. These changes are associated with cellular transformation and survival of malignant cells (15). On the basis of the data presented here, it is interesting to speculate that increased cFLIP expression, in response to enhanced GLUT1 transporter expression and increased metabolism, may play a role in cancer progression.
While many of the interactions remain to be elucidated, understanding the link between glucose uptake and metabolism and the signaling mechanisms that contribute to abnormal proliferation in neointimal or malignant cells could lead to the identification of additional therapeutic agents to block restenosis and treat cancers. On the basis of our findings, it is plausible that cFLIP could be a potential target for treatment of these disease processes.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants RO1-HL-60156 and HL-65567 (to F. C. Brosius), an American Heart Association Greater Midwest Affiliate Predoctoral Fellowship award 0515483Z (to E. D. Vesely), the University of Michigan Systems and Integrative Biology National Research Service Award Training Grant (to E. D. Vesely), and Michigan Diabetes Research and Training Center Cell and Molecular Biology Core, NIH Grant P60 DK020572.
ACKNOWLEDGMENTS
We thank Alicia Jackson, Hongyu Zhang, Anna Henger, and Julia Coppola for technical support.
REFERENCES
- 1.Bauriedel G, Schluckebier S, Hutter R, Welsch U, Kandolf R, Luderitz B, Prescott MF. Apoptosis in restenosis versus stable-angina atherosclerosis: implications for the pathogenesis of restenosis. Arterioscler Thromb Vasc Biol 18: 1132–1139, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Berthier CC, Zhang H, Schin M, Henger A, Nelson RG, Yee B, Boucherot A, Neusser MA, Cohen CD, Carter-Su C, Argetsinger LS, Rastaldi MP, Brosius FC, Kretzler M. Enhanced expression of Janus kinase-signal transducer and activator of transcription pathway members in human diabetic nephropathy. Diabetes 58: 469–477, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Budd RC, Yeh WC, Tschopp J. cFLIP regulation of lymphocyte activation and development. Nat Rev Immunol 6: 196–204, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Chen SHK, Brosius FC, III, Heilig CW. Diabetes increases glomerular GLUT1 and antisense-GLUT1 protects against diabetic glomerulosclerosis (Abstract). J Am Soc Nephrol 14: 581A, 2003 [Google Scholar]
- 5.Hall JL, Chatham JC, Eldar-Finkelman H, Gibbons GH. Upregulation of glucose metabolism during intimal lesion formation is coupled to the inhibition of vascular smooth muscle cell apoptosis. Role of GSK3beta. Diabetes 50: 1171–1179, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Hall JL, Gibbons GH, Chatham JC. IGF-I promotes a shift in metabolic flux in vascular smooth muscle cells. Am J Physiol Endocrinol Metab 283: E465–E471, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Hammerman PS, Fox CJ, Thompson CB. Beginnings of a signal-transduction pathway for bioenergetic control of cell survival. Trends Biochem Sci 29: 586–592, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Heilig C, Brosius F, Siu B, Concepcion L, Mortensen R, Heilig K, Zhu M, Weldon R, Wu G, Conner D. Implications of glucose transporter protein type 1 (GLUT1)-haplodeficiency in embryonic stem cells for their survival in response to hypoxic stress. Am J Pathol 163: 1873–1885, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.House SJ, Potier M, Bisaillon J, Singer HA, Trebak M. The non-excitable smooth muscle: calcium signaling and phenotypic switching during vascular disease. Pflügers Arch 456: 769–785, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kataoka T, Budd RC, Holler N, Thome M, Martinon F, Irmler M, Burns K, Hahne M, Kennedy N, Kovacsovics M, Tschopp J. The caspase-8 inhibitor FLIP promotes activation of NF-kappaB and Erk signaling pathways. Curr Biol 10: 640–648, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Kearney M, Pieczek A, Haley L, Losordo DW, Andres V, Schainfeld R, Rosenfield K, Isner JM. Histopathology of in-stent restenosis in patients with peripheral artery disease. Circulation 95: 1998–2002, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Kirkpatrick JN, Collins KA, Heilig K, Wang YL, Lammertin G, Schlimme M, Hall J, Quigg R, Lang RM, Heilig C. Cardiac hypertrophy and renal disease in GLUT-1 overexpressing mice (Abstract). In: 55th Annual Scientific Session of the American College of Cardiology, Atlanta, GA: Elsevier Science, 2006, p. 38A–38A [Google Scholar]
- 13.Lin Z, Weinberg JM, Malhotra R, Merritt SE, Holzman LB, Brosius FC 3rd. GLUT-1 reduces hypoxia-induced apoptosis and JNK pathway activation. Am J Physiol Endocrinol Metab 278: E958–E966, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Loberg RD, Vesely E, Brosius FC., 3rd Enhanced glycogen synthase kinase-3beta activity mediates hypoxia-induced apoptosis of vascular smooth muscle cells and is prevented by glucose transport and metabolism. J Biol Chem 277: 41667–41673, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Macheda ML, Rogers S, Best JD. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol 202: 654–662, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Maelfait J, Beyaert R. Non-apoptotic functions of caspase-8. Biochem Pharmacol 76: 1365–1373, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Malhotra R, Tyson DG, Sone H, Aoki K, Kumagai AK, Brosius FC., 3rd Glucose uptake and adenoviral mediated GLUT1 infection decrease hypoxia-induced HIF-1alpha levels in cardiac myocytes. J Mol Cell Cardiol 34: 1063–1073, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Moley KH, Mueckler MM. Glucose transport and apoptosis. Apoptosis 5: 99–105, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Okano H, Shiraki K, Inoue H, Kawakita T, Yamanaka T, Deguchi M, Sugimoto K, Sakai T, Ohmori S, Fujikawa K, Murata K, Nakano T. Cellular FLICE/caspase-8-inhibitory protein as a principal regulator of cell death and survival in human hepatocellular carcinoma. Lab Invest 83: 1033–1043, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev 75: 487–517, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Rathmell JC, Fox CJ, Plas DR, Hammerman PS, Cinalli RM, Thompson CB. Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Mol Cell Biol 23: 7315–7328, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ray JL, Leach R, Herbert JM, Benson M. Isolation of vascular smooth muscle cells from a single murine aorta. Methods Cell Sci 23: 185–188, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Thyberg J. Phenotypic modulation of smooth muscle cells during formation of neointimal thickenings following vascular injury. Histol Histopathol 13: 871–891, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Thyberg J, Blomgren K, Hedin U, Dryjski M. Phenotypic modulation of smooth muscle cells during the formation of neointimal thickenings in the rat carotid artery after balloon injury: an electron-microscopic and stereological study. Cell Tissue Res 281: 421–433, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Vander Heiden MG, Plas DR, Rathmell JC, Fox CJ, Harris MH, Thompson CB. Growth factors can influence cell growth and survival through effects on glucose metabolism. Mol Cell Biol 21: 5899–5912, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]