Abstract
Gonadotropin-releasing hormone (GnRH) acts via seven transmembrane receptors to stimulate gonadotropin secretion. Sustained stimulation desensitizes GnRH receptor (GnRHR)-mediated gonadotropin secretion, and this underlies agonist use in hormone-dependent cancers. Since type I mammalian GnRHR do not desensitize, agonist-induced internalization and downregulation may underlie desensitization of GnRH-stimulated gonadotropin secretion; however, research focus has recently shifted to anterograde trafficking, with the finding that human (h)GnRHR are mostly intracellular. Moreover, there is little direct evidence for agonist-induced trafficking of hGnRHR, and whether or not type I mammalian GnRHR show agonist-induced internalization is controversial. Here we use automated imaging to monitor expression and internalization of hemagglutinin (HA)-tagged hGnRHRs, mouse (m) GnRHR, Xenopus (X) GnRHRs, and chimeric receptors (hGnRHR with added XGnRHR COOH tails, h.XGnRHR) expressed by adenoviral transduction in HeLa cells. We find that agonists stimulate downregulation and/or internalization of mGnRHR and XGnRHR, that GnRH stimulates trafficking of hGnRHR and can stimulate internalization or downregulation of hGnRHR when steps are taken to increase cell surface expression (addition of the XGnRHR COOH tail or pretreatment with pharmacological chaperone). Agonist effects on internalization (of h.XGnRHR) and downregulation (of hGnRHR and h.XGnRHR) were not mimicked by a peptide antagonist and were prevented by a mutation that prevents GnRHR signaling, demonstrating dependence on receptor signaling as well as agonist occupancy. Thus agonist-induced internalization and downregulation of type I mammalian GnRHR occurs in HeLa cells, and we suggest that the high throughput imaging systems described here will facilitate study of the molecular mechanisms involved.
Keywords: seven transmembrane receptor, trafficking, G protein-coupled receptor
seven transmembrane (7TM) receptors are characteristically stimulated at the cell surface to activate their heterotrimeric G proteins but they are actually found in many cellular compartments. Newly synthesized receptors undergoing anterograde transport to the plasma membrane (PM) are found within the endoplasmic reticulum (ER), the Golgi stack and in transport vesicles (14, 15, 41). 7TM receptors also undergo retrograde transport from the cell surface via endosomes, from which they may be targeted to sorting compartments and then to lysosomes or back to the PM (15, 18, 41). Some 7TM receptors are located primarily at the cell surface, whereas others are largely trapped within the ER unless coexpressed with chaperone proteins that facilitate ER exit (1, 10, 14, 15, 41). Indeed, significant ER retention is increasingly seen as the norm because as much as 50% of all newly synthesized protein fails to meet ER exit quality control criteria (16). For example, only 40% of the δ-opioid receptors exit the ER after appropriate folding and glycosylation (35, 36). Much of the work in this field has highlighted the importance of COOH-terminal tails that can influence the efficiency of ER exit as well as the internalization and endosomal sorting of 7TM receptors (18, 41). For internalization, the key role of COOH tails relates to their phosphorylation and subsequent β-arrestin binding. This not only desensitizes the receptor but also targets it for internalization (often via clathrin-coated vesicles). Since the phosphorylation most often occurs within the COOH tail (26, 37) these structures are important for agonist-induced desensitization and internalization.
The cloned gonadotropin-releasing hormone (GnRH) receptor (GnRHR) can be divided into structurally and functionally distinct groups. Type I mammalian GnRHR are selectively activated by GnRH I (termed GnRH hereinafter for simplicity) and are unique among known 7TM receptors in that they lack COOH tails. This apparently underlies their failure to undergo agonist-induced phosphorylation, bind β-arrestins, rapidly desensitize and their slow internalization from the PM (2, 5, 13, 20–22, 27, 28, 31, 34, 43, 44). In contrast, type II mammalian GnRHR and all cloned nonmammalian GnRHR have COOH-terminal tails. Where investigated they have been found to be selectively activated by GnRH II [(His5, Trp7, Tyr8)GnRH] and to undergo agonist-induced phosphorylation leading to β-arrestin binding with consequent receptor desensitization and agonist-induced internalization. GnRH mediates central control of reproduction by stimulating the synthesis and secretion of luteinizing hormone and follicle-stimulating hormone. It acts via Gαq-coupled 7TM receptors to stimulate phospholipase C, causing Ca2+ mobilization and protein kinase C activation (5, 6, 31, 40), which mediate GnRH effects on gonadotropin synthesis and secretion. GnRH-stimulated gonadotropin secretion can be blocked by antagonists and mimicked by agonists, but sustained stimulation causes desensitization so both types of ligand ultimately reduce gonadal steroid levels, which underlies the use of GnRH analogs to treat various forms of steroid-dependent cancers. Since type I mammalian GnRHR do not desensitize, the regulation of cell surface GnRHR number (rather than coupling) is seen as the key determinant of gonadotrope responsiveness. It has long been known that agonists can stimulate internalization and downregulation of cell surface GnRHR (6), but recent work has focused on anterograde GnRHR trafficking, spurred largely by the observation that cell surface expression of human (h)GnRHR is very low (compared to other GnRHRs) in heterologous expression systems. This is attributed to the presence of a primate-specific Lys191 (9, 42), the lack of a second glycosylation site near the NH2 terminus (27) as well as the absence of any COOH tail (2, 20, 25, 34, 43). These all reduce cell surface GnRHR levels, and although the mechanisms are largely unknown, the key issue appears to be localization. The importance of GnRHR compartmentalization is best illustrated by point mutants that cause hypogonadotropic hypogonadism. Here, an important observation is that exposure to a membrane-permeant nonpeptide GnRHR antagonist (IN3) “rescues” signaling via most of these mutants (7, 9, 23). This nonpeptide antagonist (NPA) is thought to aid folding into a conformation needed for ER exit and subsequent trafficking to the PM. The NPA also increases signaling via the wild-type GnRHR, suggesting that a large proportion of hGnRHRs do not traffic to the PM (7). We have found that, when expressed in MCF7 breast cancer cells, <1% of hemagglutinin (HA)-tagged hGnRHRs are at the PM, whereas most Xenopus (X)GnRHRs (>50%) are at the cell surface (17, 39). The intracellular HA-GnRHR were largely colocalized with calreticulin (ER marker), and IN3 increased the proportion of HA-hGnRHRs at the cell surface 10- to 20-fold, consistent with the idea that a large proportion of intracellular HA-hGnRHRs are located within the ER as a potentially functional intracellular receptor reserve.
The intracellular localization of hGnRHR also has important implications for understanding retrograde transport: it is often assumed that agonist-induced internalization and downregulation contribute to the efficacy of GnRH agonists in cancer therapy, but we know of no direct evidence for such regulation of hGnRHR. Extrapolation from the early studies performed primarily with rodent GnRHR (6, 19, 24, 38) is less compelling in light of the known differences between rodent and hGnRHR compartmentalization [and hence trafficking (9, 42)], and a recent study (monitoring uptake of radiolabeled antibodies targeting tagged GnRHRs) revealed that type I mammalian GnRHR undergo constitutive but not agonist-induced internalization in COS-7 or human embryonic kidney (HEK)293 cells (33). Here we have used novel automated cell imaging methods to monitor cell surface expression and internalization of HA-tagged hGnRHRs, XGnRHRs, and chimeric receptors (hGnRHR with an added XGnRHR COOH-terminal tail) expressed by adenoviral transduction in HeLa cells. Our data show that trafficking and internalization of hGnRHRs can, indeed, be stimulated by receptor activation and suggest that our high-throughput imaging models will be useful for further work to determine the mechanisms involved.
MATERIALS AND METHODS
Materials and cell culture.
Peptides were from Sigma (Poole, UK) except for Buserelin [(T-BuSer6, Pro9 NH ethylamide)GnRH], which was provided by Prof. J. Sandow (Aventis Pharma, Frankfurt, Germany). A membrane-permeant indole-based GnRH antagonist IN3 [(2S)-2-[5-[2-(2-azabicyclo[2.2.2]oct-2-yl)-1,1-dimethy-2-oxoethyl]-2-(3,5-dimethylphenyl)-1H-indol-3-yl]-N-(2-pyridin-4-ylethyl)propan-1-amine] was provided by Dr. Ashton Wallace (Merck, Rahway, NJ). Culture media were from Gibco BRL (Paisley, UK) and plasticware was from Corning (supplied by Appleton Woods, Birmingham, UK) or Nunc (supplied by Fisher, Loughborough, UK). Sera were from First Link (Brierly Hill, UK), and antibodies (Abs) were from Invitrogen (Paisley, UK) or Cambridge Biosciences (Cambridge, UK). HeLa cells were cultured in DMEM with 10% fetal calf serum, 2 mM l-glutamine, 50 IU/ml penicillin, and 50 μg/ml streptomycin (culture medium). For imaging experiments the cells were plated in black-sided and clear-bottomed 96-well plates from Corning (Appleton Woods). GnRHRs with NH2-terminal (exofacial) HA-tags were expressed in these cells using recombinant, E1 deleted adenovirus (Ad)-expressing HA-hGnRHRs, HA-mGnRHRs, HA-XGnRHRs, or HA-h.XGnRHRs (a chimera consisting of the HA-hGnRHR with an added XGnRHR COOH-terminal tail) prepared as described (3, 4, 17, 22, 39). For some experiments, receptors with an A261K mutation that prevents G protein activation (32) were also expressed (Ad HA-A261K.hGnRHR or HA-A261K.h.XGnRHR, prepared as above). In all cases, cells were transduced with Ad (1–3 plaque-forming units/nl, 6 h incubation) the day after plating and assays were performed the following day.
Quantification of receptor expression.
Cell surface and whole cell HA-GnRHR expression were measured by fluorescence microscopy using a semiautomated system for image acquisition (IN Cell Analyzer 1000, GE Healthcare UK, Little Chalfont, UK) and validated algorithms for image segmentation and quantification (IN Cell Analyzer version 1.0 software) as described (17). The cells were cultured in 96-well plates at 2,500–5,000 cells/well, infected with Ad HA-GnRHRs, and left for 16–24 h before staining. For cell surface receptor staining, they were incubated for 1 h at 4°C with mouse primary Ab (mouse monoclonal anti-HA-11 clone 16B12, stock at 5–7 mg/ml diluted 1:200 in DMEM with 1% BSA) and then washed with ice-cold PBS, fixed (30 min in 2% paraformaldehyde/PBS), and permeabilized (10 min in PBS-0.1% Triton X-100). The cells were then washed (3×), blocked (1 h in PBS-0.1% Triton X-100–1% BSA), and incubated 1 h with the secondary Ab (Alexa Fluor 488-conjugated goat anti-mouse IgG at 1:500 in PBS-0.1% Triton X-100–1% BSA). They were then washed with PBS (2×), incubated with 0.3 μM 4′,6-diamidino-2-phenyindole (DAPI, 15 min), and washed before imaging. For whole cell staining, cells were washed with PBS, fixed, permeabilized, and blocked before exposure to the anti-HA primary Ab. They were then washed with PBS (3×) and incubated with the secondary Ab and DAPI, as above. Digital images were acquired, collecting one to four fields per well with a ×10 objective (Plan Apochromat, numerical aperture 0.45) providing images of 100–1,000 cells (per well) in a total imaged area of 0.6–2.4 mm2. These were segmented and quantified using the IN Cell 1000 Analyzer software (Dual Area Analysis Algorithm version 1.0, GE Healthcare). After subtraction of background fluorescence (measured in cell-free regions), this gave fluorescence intensity in arbitrary fluorescence units (AFU) per cell and per well. In some experiments we also defined the proportion of imaged cells expressing measurable HA-GnRHRs (cells in which fluorescence was >20% above background) and compounded these values [% positively stained (+ve) cells × AFU in +ve cells] as an expression index (EI). In several experiments, the cell surface EI was expressed as a percentage of the whole cell EI to calculate the proportional cell surface expression (PCSE). Nonspecific labeling was negligible with these protocols as revealed by the low fluorescence intensity in control cells receiving no Ad or by omission of primary or secondary Ab (not shown).
Quantification of HA-GnRHR turnover and internalization.
To monitor receptor trafficking, intact HA-GnRHR transduced HeLa cells were incubated 0–60 min at 37°C with mouse anti-HA (1:200) in DMEM with 2% fetal calf serum with or without 10−7 M GnRH, and the incubations were terminated by washing the cells (1×) in ice-cold PBS. They were then fixed, permeabilized, and stained with secondary Ab and DAPI as above. Alternatively, HA-GnRHR-expressing cells were incubated 60 min with anti-HA (1:200) in DMEM with 2% BSA at ∼21°C, then washed and incubated 0–60 min at 37°C in DMEM with 2% BSA with treatments described in the figure legends. The cells were then washed in ice-cold PBS after which they were fixed, permeabilized, stained with secondary Ab and DAPI, and then used for image capture and quantification of whole cell HA-GnRHR expression as above. These experiments revealed a time-, temperature-, and agonist-dependent increase in HA-GnRHR staining within small punctuate regions that were often concentrated around the nucleus. In preliminary experiments, fluorophore-labeled transferrin was included (during the 60-min incubation with primary Ab), and the bright HA-GnRHR stained points were often colocalized with transferrin, consistent with redistribution of the HA-tagged GnRHR from the cell surface to endosomes (supplemental data Fig. 1; supplemental data for this article can be found at the American Journal of Physiology-Cell Physiology Web site). To quantify this redistribution, we used automated software (Dual Area Analysis Algorithm version 1.0) to define the perimeters of the nuclei, and then to add a collar of 2 μm around the nucleus and identify the small intensely stained regions “inclusions.” For each cell we determined the number of inclusions in the area defined by the nucleus and collar and the mean value for each well (supplemental data Fig. 2).
Statistical analysis and data presentation.
The figures show the data (means ± SE) of three or four wells in experiments that are representative of at least two similar experiments or show data pooled from at least three independent experiments. Where data are normalized for pooling, this was as described in the figure legends. Statistical analysis was by one- or two-way ANOVA and post hoc tests (as detailed in the figure legends) accepting P < 0.05 as statistically significant.
RESULTS
Imaging of HA-tagged GnRHRs in HeLa cells.
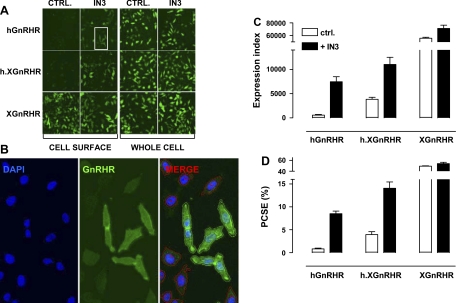
We have previously described an automated imaging system for GnRHR quantification in hormone-dependent cancer cells (17) and here, our initial aim was to develop a similar assay for HeLa cells, a model used to explore GnRHR signaling and trafficking (3, 4, 21). We used recombinant adenovirus to express HA-tagged human and Xenopus laevis GnRHR, and a chimera consisting of the hGnRHR sequence in tandem with the COOH tail of the XGnRHR (h.XGnRHR). The cells were incubated for 18 h with or without the NPA IN3 before measuring GnRHR expression by fluorescence immunohistochemistry with automated image acquisition. This revealed that whole cell staining was comparable for all three receptors but that cell surface staining was much lower with the hGnRHR and h.XGnRHR than with the XGnRHR and that cell surface expression of hGnRHR and h.XGnRHR (but not XGnRHR) was increased by IN3 (Fig. 1A). We then used automated algorithms to segment the images and measure stain intensity (in AFU) in each cell (Fig. 1B). We also determined the proportion of cells that were positively stained (AFU >20% above background) and calculated an EI by compounding these values. Cell surface and whole cell EI values were then used to determine PCSE. This confirmed that cell surface expression of XGnRHRs is much higher than that of hGnRHRs or h.XGnRHRs and that cell surface expression of hGnRHR and h.XGnRHR (but not XGnRHR) was greatly increased by IN3. Moreover, the PCSE values (with and without IN3) mirrored the EI data (compare Fig. 1, C and D) because whole cell expression levels were comparable for all three receptors (not shown). These data demonstrate that in HeLa cells [as in hormone-dependent cancer cell lines (17)] the proportion of GnRHRs expressed at the cell surface is dependent on receptor structure, is subject to pharmacological manipulation, and can be readily measured by automated imaging.
Fig. 1.
Quantification of hemagglutinin (HA)-tagged gonadotropin-releasing hormone (GnRH) receptors in HeLa cells by fluorescence immunohistochemistry with semiquantitative image acquisition and analysis. HeLa cells were transduced with the adenovirus (Ad)-expressing HA-tagged hGnRHRs, human Xenopus GnRHRs (h.XGnRHRs), or XGnRHRs [1 plaque-forming unit (pfu)/nl] and then incubated 18 h in medium with 0 or 10−7 M IN3. They were then stained for cell surface receptors [incubation of intact cells with primary antibody (Ab)], and nuclei were stained with 4′,6-diamidino-2-phenyindole (DAPI), before image acquisition and analysis as described in materials and methods. To measure whole cell receptor expression, permeabilized cells were exposed to primary and secondary Ab before imaging as above. A: thumbnail images from individual wells stained for cell surface and whole cell receptor expression after transduction with Ad GnRHR. CTRL, control. B: higher magnification for a region of cells (IN3-treated cell surface HA-hGnRHRs from the white-boxed thumbnail region in A) stained for nuclei (DAPI, blue) or GnRHR (green). The merged image illustrates the perimeters of the nuclei and cells determined using IN Cell 1000 Analyzer software and the application of a filter to define positively (+ve) stained cells (HA-hGnRHR staining >20% above background - green perimeter traces) and negatively stained (−ve) cells (HA-hGnRHR staining <20% above background - red perimeter traces). C: expression index (EI) calculated by compounding the percentage of +ve cells by the mean receptor fluorescence intensity (arbitrary units). D: proportional cell surface expression (PCSE) calculated by expressing the cell surface EI as a percentage of the whole cell EI. These data are all from the same representative experiment, and bar charts show means ± SE for 4–8 replicate wells (i.e., data derived from >5,000 individual cells).
Peptide agonist and NPA effects on cell surface HA-GnRHR expression.
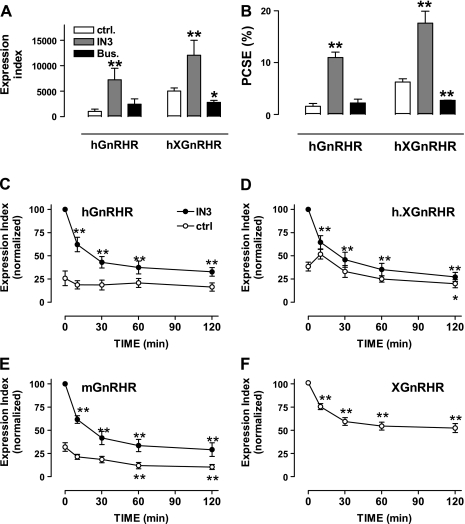
We next measured effects of IN3 and the peptide agonist Buserelin and found that the NPA increased the proportion of hGnRHRs at the cell surface (PCSE increased almost 7-fold, from 1.6% to 11%), whereas the agonist had no measurable effect on hGnRHR PCSE (Fig. 2, A and B). IN3 also increased the proportion of h.XGnRHRs at the cell surface (PCSE increased from 6.3% to 17.6%), whereas the agonist reduced the h.XGnRHR PCSE (Fig. 2, A and B). These compounds had no measurable effect on whole cell expression of either receptor (not shown), so the PCSE values were mirrored by measures of cell surface expression (compare Fig. 2, A and B). In time course experiments, the IN3 effects on HA-hGnRHR and h.XGnRHR expression and PCSE were measurable within 2–4 h and maximal after 4–24 h (not shown). To explore the time dependence of agonist effects, cells expressing HA-GnRHR were incubated for 18 h with 0 or 10−7 M IN3, then washed and incubated for various periods with or without 10−7 M GnRH before determining the cell surface EI. For these experiments we included mGnRHR and XGnRHR because rodent and XGnRHRs are better expressed at the cell surface than hGnRHR (using GnRH II as the agonist for XGnRHR). As shown (Fig. 2F), GnRH II caused a rapid reduction in cell surface expression of XGnRHRs (significant reduction at 10 min, maximal effect at 120 min). GnRH also reduced expression of mGnRHR and h.XGnRHR (within 60–120 min) but did not measurably alter cell surface expression of the hGnRHR (Fig. 2, C–E, open symbols). Preincubation with IN3 increased cell surface expression of the hGnRHR (4- to 5-fold), h.XGnRHR (2- to 3-fold), and mGnRHR (2- to 3-fold) but did not alter expression of the XGnRHR (not shown), and in IN3-pretreated cells, GnRH caused a pronounced reduction in cell surface expression of each of the mammalian GnRHRs. In similar experiments, addition of a peptide antagonist (after 18 h with IN3) did not reduce hGnRHR, h.XGnRHR, or mGnRHR expression, demonstrating the effect to be agonist specific (not shown).
Fig. 2.
Agonist and antagonist effects on GnRHR expression at the cell surface. A and B: HeLa cells transduced with HA-hGnRHR or HA-h.XGnRHR were treated as described for Fig. 1 except that they were incubated for 18 h with 10−7 M IN3, 10−7 M Buserelin (Bus), or with no addition (CTRL) before staining for HA-tagged GnRHRs in intact cells (cell surface) and permeabilized cells (whole cell) as above. Data shown are the surface expression index (arbitrary units) and PCSE determined as described above. C–F: HeLa cells transduced with HA-hGnRHR, HA-h.XGnRHR, HA-mouse (m) GnRHR, or HA XGnRHR were incubated for 18 h with 10−7 M IN3 (●) or with no addition (○) before being washed and incubated for the indicated periods with 10−7 M GnRH (C–E) or GnRH II (F) at 37°C. These incubations were terminated by washing at 4°C, before staining for cell surface HA-tagged GnRHRs. Data shown are the surface expression index (arbitrary units) determined as above and normalized to the internal control (IN3 treated for C–E). All values are means ± SE (n = 3) from 3 separate experiments each with 3–6 replicate wells. In F, the data obtained with and without IN3 pretreatment were indistinguishable and have therefore been pooled. *P < 0.05, **P < 0.01 compared with CTRL.
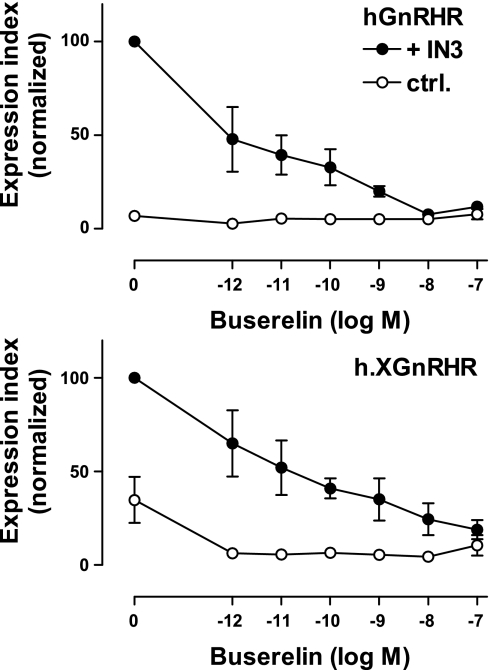
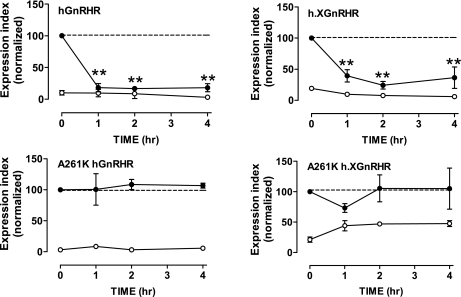
Dose-response curves (Fig. 3) revealed comparable potencies for the Buserelin effects on hGnRH and h.XGnRHR expression (IC50 values of ∼10−10 M in IN3-pretreated cells), and the pIC50 value for the Buserelin effect on h.XGnRHR expression was lower in control cells than in IN3-treated cells (pIC50 values of 10.5 ± 0.4 and <12, respectively, in control and IN3-pretreated cells), consistent with the presence of the competitive antagonist. We also used a similar protocol to determine whether signaling was necessary for the agonist-induced reduction in cell surface GnRHR expression. As expected, the agonist had no measurable effect on cell surface hGnRHR expression in control cells, but when cells had been pretreated with IN3 to increase cell surface expression, it caused a pronounced reduction that was evident at 1 h and was maintained for 4 h of incubation. Similarly, Buserelin caused only a modest reduction in cell surface expression of h.XGnRHRs but had a more pronounced effect in cells pretreated with IN3. In similar experiments with receptors bearing an A261K mutation that prevents G protein activation (32), IN3 caused a pronounced increase in cell surface expression of the A261K.hGnRHR and A261K.h.XGnRHR, but the agonist failed to reduce cell surface expression of these receptors, even after IN3 pretreatment (Fig. 4).
Fig. 3.
Dose dependence of agonist effects on cell surface GnRHR expression. HeLa cells transduced with HA-hGnRHR or HA-h.XGnRHR (1 pfu/ml) were incubated for 18 h with 10−7 M IN3 (●) or with no addition (○) before being washed and incubated for 2 h with the indicated concentration of Buserelin at 37°C. These incubations were terminated by washing at 4°C, before staining for cell surface HA-tagged GnRHRs. Data shown are the surface expression index (arbitrary units) determined and normalized as above and are means ± SE (n = 3) from 3 separate experiments each with 3 replicate wells.
Fig. 4.
Signal dependence of agonist effects on cell surface GnRHR expression. HeLa cells transduced with HA-hGnRHR or HA-h.XGnRHR with or without the A261K mutation (1 pfu/nl) were incubated for 18 h with 10−7 M IN3 (●) or with no addition (○) before being washed and incubated for the indicated periods with 10−7 M Buserelin at 37°C. These incubations were terminated by washing at 4°C, before staining for cell surface HA-tagged GnRHRs. Data shown are the surface expression index (arbitrary units) determined as above and are means ± SE (n = 3) from 3 separate experiments (each with 3 replicate wells per treatment) and are normalized to the internal control value. **P < 0.01 compared with CTRL.
Trafficking of HA-GnRHR from the cell surface.
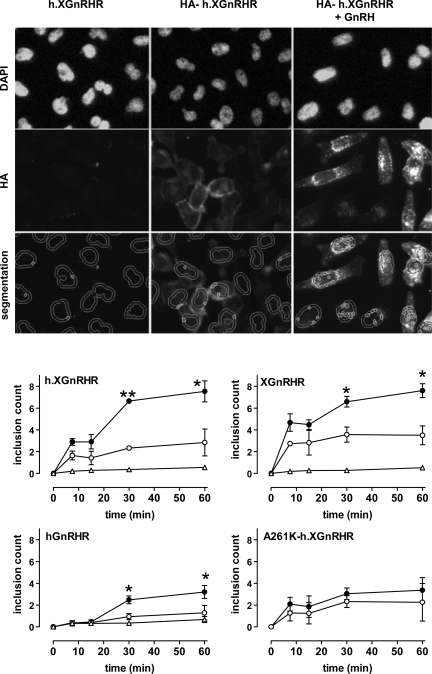
We also developed automated imaging assays to monitor ligand effects after labeling of cell surface receptors with anti-HA in intact cells. In the first experiments we simply transduced cells with Ad GnRHR (with or without exofacial HA tag), then incubated them with a primary anti-HA for various periods at 37°C (with or without agonist) before fixation, permeabilization, and staining. Nonspecific staining (HA detection in cells expressing nontagged h.XGnRHR) was negligible, but clear HA-h.XGnRHR staining was observed after 7.5–60 min of incubation with anti-HA (Fig. 5 and data not shown). In unstimulated cells, HA-h.XGnRHR was relatively evenly distributed over the cells, with more intense staining evident at the perimeter of many cells. Addition of GnRH reduced this perimeter staining and caused a pronounced redistribution of HA-h.XGnRHR to intense punctuate regions that was often prevalent around the nucleus, and similar staining was seen in cells expressing HA-hGnRHR or HA-XGnRHR (Fig. 5 and data not shown). These staining patterns are suggestive of agonist-induced redistribution to endosomes, and this was confirmed by monitoring uptake of fluorophore-labeled transferrin (which undergoes receptor-mediated uptake into endosomes and is then trafficked to postendosomal sorting compartments) under similar conditions. In control cells, there was little colocalization of HA-GnRHR and transferrin, but in GnRH stimulated cells, the fluorophores were often colocalized in punctuate regions that were often prevalent around the nucleus (supplemental data Fig. 1), supporting the notion that GnRH stimulates the redistribution of HA-GnRHR from the cell surface to endosomes. This redistribution was quantified using an automated image segmentation algorithm to determine the number of small intensely stained inclusions in the area defined by adding a 2-μm collar to the nuclear perimeter. Using this “inclusion count” as a measure of GnRHR trafficking from the PM to endosomes, we found that this was relatively slow for each of the receptors used but was increased by stimulation with GnRH (HA-hGnRHR and HA-h.XGnRHR) or GnRH II (HA-XGnRHR), with maximal inclusion counts after 30–60 min of activation in each case (Fig. 5). Very few inclusions were seen in cells expressing nontagged GnRHRs, and GnRH had no measurable effect on distribution of HA-A261K-h.XGnRHR (Fig. 5).
Fig. 5.
Anti-HA loading: time and agonist dependence. Top rows: representative images from HeLa cells transduced with h.XGnRHR (no HA tag) or from cells expressing HA-tagged h.XGnRHR before being washed and incubated 30 min at 37°C with primary Ab (mouse anti-HA at 1:200) with 0 or 10−7 M GnRH as shown. These incubations were terminated by washing at 4°C, before fixation, permeabilization, and staining (DAPI and Alexa Fluor 488-conjugated anti-mouse IgG), followed by image acquisition and analysis. Bottom row: illustration of the use of automated algorithms to segment the images, defining the perimeters of the nuclei (from the DAPI stain), adding a 2-μm collar, and identifying the bright punctuate regions of HA-GnRHR stain within the cross-sectional area defined by nucleus and collar. This number (“inclusion count,” the mean number of the small circles in the segmented images) provides a measure of receptors that have trafficked from the plasma membrane to putative endosomes. In the bottom panels, HeLa cells transduced with HA-tagged hGnRHR, h.XGnRHR, A261K-h.XGnRHR, or XGnRHR (1 pfu/nl) were incubated for 0–60 min at 37°C in medium with primary antibody and 0 (○) or 10−7 M GnRH (●, GnRH II for the XGnRHR) as indicated. Nontagged versions of three of the GnRHRs were also included as negative controls (▵). The cells were washed, fixed, permeabilized, and stained before image acquisition and analysis, as above. The data shown are inclusion counts as means ± SE (n = 3) pooled from 4 separate experiments (each with 4 replicate wells per treatment). *P < 0.05, **P < 0.01 compared with corresponding control value without GnRH.
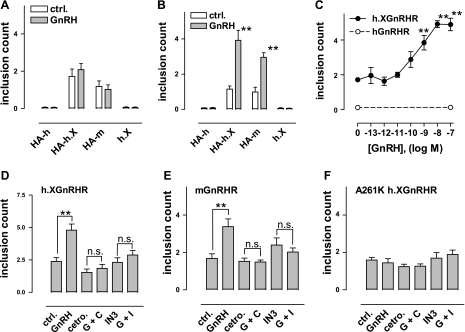
The data above reveal that the trafficking of hGnRHR, h.XGnRHR, and XGnRHR is agonist stimulated, but they do not necessarily equate to agonist-induced internalization. This is because the assay is dependent on antibody (Ab) binding to cell surface receptors and redistribution to inclusions, so agonists could conceivably be increasing trafficking to the PM rather than internalization. To monitor internalization more directly, cell surface HA-GnRHR were Ab loaded by incubation for 60 min at 21°C, before washing and incubation for varied times at 37°C in the presence or absence of agonist. HA-GnRHR-stained inclusions were then quantified by imaging as above. As shown (Fig. 6, A and B), GnRH increased internalization of the HA-h.XGnRHR and HA-mGnRHR at 60 min but not at 15 min (data obtained at 0 min were similar to those at 15 min, and data obtained at 30 min were intermediate between the 15 and 60 min data, not shown). Very few inclusions were seen with nontagged h.XGnRHR (demonstrating that the Ab redistribution is receptor mediated). The inclusion count was also very low (and not measurably altered by GnRH) in cells expressing HA-hGnRHR, which is consistent with the lower hGnRHR number at the cell surface during primary Ab loading. We also used this assay to determine the dose dependence of GnRH on h.XGnRHR internalization using only the 60-min time point. As shown (Fig. 6C), GnRH caused a dose-dependent increase in inclusion number (pEC50 9.5 ± 0.2). Finally, this assay was used to determine effects of agonists and antagonists on internalization. As shown (Fig. 6, D–F), GnRH increased the internalization of h.XGnRHR and mGnRHR but did not increase internalization of the A261K-h.XGnRHR. Moreover, cetrorelix and IN3 both blocked the stimulatory effects of GnRH on internalization of h.XGnRHR and mGnRHR. Thus, the internalization of these receptors (Fig. 6), like the downregulation of cell surface receptor number (Figs. 2–4), is stimulated by agonist but is not increased by a peptide antagonist and is dependent on receptor signaling.
Fig. 6.
Agonist-induced redistribution of HA-GnRHR. A and B: HeLa cells transduced with HA-tagged hGnRHR, h.XGnRHR, or mGnRHR or with nontagged h.XGnRHR as a negative control (HA-h, HA-h.X, HA-m, and h.X, respectively) were washed and incubated for 60 min at room temperature (∼21°C) in medium with anti-HA (1:200). They were then washed and incubated for 15 min (A) or 60 min (B) at 37°C in medium with 0 or 10−7 M GnRH as indicated before being processed for determination of inclusion count, as above. GnRH effects were only statistically significant (**P < 0.01 compared with corresponding control) after 60 min in cells expressing HA-h.XGnRHR or HA-mGnRHR. C: HeLa cells transduced with HA-h.XGnRHR (●) or HA-hGnRHR (○) were loaded with primary Ab as above, then washed and incubated for 60 min at 37°C in medium with the indicated concentration of GnRH. D–F: HeLa cells transduced with HA-h.XGnRHR, HA-mGnRHR, or HA-A261K h.XGnRHR were prepared and loaded with anti-HA as above, before being washed and incubated for 60 min at 37°C in medium with no addition (CTRL), GnRH (10−9 M), cetrorelix (Cetro; 10−7 M), IN3 (10−7 M), or with GnRH plus cetrorelix (G + C) or GnRH plus IN3 (G + I) as indicated. In all cases, incubations were terminated by washing at 4°C and cells were then processed to determine the inclusion count, as described in materials and methods. The data shown are means ± SE (n = 3 or 4) pooled from 3 or 4 separate experiments (each with 2–4 replicate wells per treatment). ANOVAs revealed “treatment” as a significant variable in A–E (but not in F). NS, not significant. **P < 0.01 compared with corresponding control without GnRH.
DISCUSSION
Sustained stimulation with GnRH agonists desensitizes GnRH-stimulated gonadotropin secretion, and although this effect underlies therapeutic applications of GnRH agonists, the mechanisms remain poorly understood. Reduction in gonadotropin and GnRHR synthesis is important in vivo, but it is also assumed that agonist-induced GnRHR internalization and consequent downregulation of cell surface GnRHR play a role. This assumption is made despite the paucity of direct data on retrograde trafficking of hGnRHR. Indeed, the low cell surface expression of hGnRHR has precluded extensive study of their internalization or downregulation, and extrapolation from early work with rodent models is difficult in light of the finding that their localization (and therefore their trafficking) differs markedly from that of hGnRHR (9, 42). Moreover, type I mammalian GnRHR are structurally and functionally unique, in that they lack COOH-terminal tails and are thought not to undergo agonist-induced arrestin binding or rapid homologous desensitization (2, 5, 13, 20–22, 27, 28, 31, 34, 43, 44). Since arrestin typically targets desensitized 7TM receptors for internalization, the lack of arrestin binding begs the question of how GnRHR could be targeted for internalization, and a recent study revealed that, in COS-7 and HEK293 cells, epitope-tagged rat GnRHR and hGnRHR underwent constitutive, but not agonist-induced, internalization (33). This underlines the fact that most work on hGnRHR internalization has actually followed the receptor-mediated uptake of radiolabeled agonists and therefore does not distinguish between agonist-induced and constitutive internalization. It also begs the fundamental question of whether or not hGnRHR undergo agonist-induced internalization and consequent downregulation.
To address these issues, we have developed automated imaging models to monitor the compartmentalization and trafficking of GnRHR with exofacial HA tags. We show that only a small proportion of GnRHRs are located at the cell surface after Ad-mediated transduction in HeLa cells and that this proportion is dependent on receptor structure (i.e., is much higher for XGnRHRs that for hGnRHRs and increased by addition of the XGnRHR tail to the hGnRHR) and can be increased in a dose- and time-dependent manner by the NPA IN3. These data (Fig. 1) are in accord with the notion that most hGnRHRs are actually intracellular and that NPAs can act as pharmacological chaperones that increase the efficiency of GnRHR exit from the ER and thereby increase anterograde GnRHR trafficking to the PM (7, 9, 23, 42). We also found that agonists reduce the cell surface expression of mGnRHR, XGnRHR, or h.XGnRHR but did not measurably reduce hGnRHR expression (Fig. 2). However, when cells were pretreated with IN3, agonists did cause a pronounced time- and dose-dependent reduction in cell surface expression of hGnRHR, as well as the h.XGnRHR and XGnRHR (Fig. 2).
An alternative possible explanation of these data is that the agonist reduced cell surface hGnRHR expression in IN3-pretreated cells by inhibiting IN3 binding, but this is unlikely because the membrane-impermeant peptide agonists act at the cell surface, whereas IN3 is thought to act intracellularly. Moreover, a peptide antagonist (cetrorelix) failed to reduce hGnRHR expression in IN3-pretreated cells (not shown), and the agonist failed to reduce cell surface expression of hGnRHR or h.XGnRHR mutated to prevent G protein activation [A261K (32)], demonstrating dependence of this effect on signaling rather than just receptor occupancy. Thus, our data reveal that, in HeLa cells, there is no measurable agonist-induced downregulation of cell surface hGnRHR but when steps are taken to first increase cell surface expression (i.e., by addition of the XGnRHR COOH tail or by pretreatment with NPA) there is a clear agonist-stimulated and receptor activation-dependent downregulation of cell surface hGnRHR (Fig. 2).
An obvious question raised by our data is why agonists downregulate cell surface hGnRHR expression in IN3-pretreated cells but not in control cells. This could clearly just reflect the low hGnRHR at the cell surface and the detection limits of the assay, but an alternative possibility is that GnRH stimulates both anterograde and retrograde trafficking and therefore causes no net change in hGnRHR expression at the PM. This would be consistent with other 7TM receptors such as δ-opioid peptide receptors that are largely stored in intracellular transport vesicles that are delivered to the cell surface in response to activation of a small subpopulation of receptors at the cell surface (45). In radioligand binding studies we have shown that equilibrium binding of 125I-labeled Buserelin to cell surface hGnRHRs (in MCF7 cells) is extremely low and can be increased by raising the temperature to 37°C, suggesting the existence of cryptic receptors that move to the PM at temperatures permissive for trafficking (39). Since no such temperature-dependent increase was seen with A261K-hGnRHR, we suggested that the cell surface receptor activation may stimulate anterograde GnRHR trafficking (39). This effect was relatively rapid (evident within 5 min and maximal within 30 min at 37°C), and any such effect occurring in HeLa cells could clearly oppose effects of agonist-induced internalization on cell surface receptor number.
We also monitored GnRHR trafficking and found that anti-HA bound to cell surface GnRHR and then trafficked to bright points that were prevalent around the nucleus. In preliminary experiments we found that this redistribution was time, temperature, and agonist dependent. Moreover, no such redistribution was seen in control cells expressing nontagged GnRHR, and fluorophore-labeled transferrin colocalized with the anti-HA in the bright points of HA-GnRHR-expressing cells. These data are entirely consistent with previous confocal microscopy studies showing agonist-induced redistribution of HA-XGnRHR to endocytic vesicles where they colocalize with arrestins and transferrin (21 and data not shown), so we developed an automated system to quantify these puncta (supplemental data). This system has much higher throughput than conventional confocal microscopy approaches, and we routinely acquired digital images from over >10,000 individual cells (i.e., from a 96-well plate with one field per well) within 20 min, and image analysis was completed within a similar time frame.
In the first experiments we incubated cells with anti-HA with 0 or 10−7 M GnRH I (hGnRHR or h.XGnRHR) or GnRH II (XGnRHR) and found that the agonists increased the inclusion count for all three receptors (at 30 and/or 60 min). To our knowledge, this experiment (Fig. 5) provides the first direct demonstration of agonist-stimulated hGnRHR trafficking, but in this assay (where primary Ab and agonist are present together) the agonist effect could reflect stimulation of hGnRHR trafficking to and/or from the cell surface. To more directly monitor internalization, we first labeled cell surface HA-GnRHR with primary Ab, then washed the cells and incubated them for varied periods at 37°C with test compounds. Receptor-independent Ab uptake was very low (as seen in cells expressing a nontagged h.XGnRHR), but there was a clear agonist-dependent internalization of h.XGnRHR and mGnRHR that was measurable at 30 min and maximal at 60 min (Fig. 6). With this assay, HA-hGnRHR staining was very weak and we were unable to measure either basal or GnRHR-stimulated hGnRHR internalization. However, the GnRH effect on h.XGnRHR internalization was found to be dose dependent and (like the effect on mGnRHR internalization) was blocked by a peptide and nonpeptide antagonists. Interestingly, GnRH failed to alter trafficking of the signal-dead A261K-h.XGnRHR in either assay (Figs. 5 and 6). Thus, G protein activation is not needed for the NPA to increase GnRHR trafficking to the cell surface (Fig. 4) but is necessary for agonist-induced GnRHR downregulation (Fig. 4) and for agonist-induced internalization (Fig. 6). These data are consistent with work showing that the A261K mutation slows GnRHR-mediated uptake of radiolabeled agonist (32) and the notion that agonist-induced internalization underlies the observed agonist-induced downregulation.
Our data reveal agonist-induced downregulation, trafficking, and/or internalization of type I mammalian GnRHR. As such they are consistent with early work showing agonist-induced downregulation of cell surface GnRHR in primary rat pituitary cell cultures (30) and in a murine gonadotroph lineage cell line (27). They are also consistent with work on heterologously expressed rat GnRHR (20, 43), showing agonist-induced (but not antagonist-induced) downregulation of cell surface GnRHR. Similarly, rat GnRHR mediated uptake of radiolabeled agonists was faster than antagonists (43), suggesting agonist-induced internalization, although the uncoupling of ligands from receptors after internalization (11) and the relatively slow dissociation (of antagonists as compared with agonists) complicate interpretation of such experiments. In contrast, a recent study following uptake of antibodies (targeted to epitope-tagged GnRHR) concluded that internalization of type I mammalian GnRHR was constitutive, rather than agonist stimulated (33). The reason for this difference is unknown but it could relate to the different cell types used (HeLa herein and COS-7 or HEK293 cells in the earlier work), and this highlights the need for similar studies in gonadotropes. An additional obvious concern with the Ab-loading techniques used here is that the primary Ab could itself affect receptor function, particularly in light of early experiments showing that Ab targeting GnRHR bound ligands could do so (8). However, we have found no measurable effect of NH2-terminal HA tags on GnRHR affinity, specificity, or signaling (17, 39 and data not shown) and that the primary Ab used here does not stimulate GnRHR or alter the ability of GnRH to do so (functional assays using 10−11 to 10−6 M GnRH). Similarly, Pawson et al. (33) observed no effect of their primary Ab (anti-HA) on HA-GnRHR function (33). It is also of interest that the agonist-induced trafficking of HA-GnRHR seen here is similar to that observed with chimeric GnRHR-green fluorescent protein (GFP) reporter constructs (11), allaying concern that the inclusion of spacer sequences and GFP at the COOH-terminal was necessary for the agonist-induced trafficking of these chimeras.
In summary, we have found that agonists (but not antagonists) stimulate the internalization and/or downregulation of cell surface mGnRHR and XGnRHR and that GnRH stimulates trafficking of hGnRHR. We were also able to demonstrate GnRH-stimulated internalization or downregulation of cell surface hGnRHR but only when steps were taken to increase cell surface expression (addition of the XGnRHR COOH tail or pretreatment with IN3). Agonist effects on internalization (of h.XGnRHR) and downregulation (of hGnRHR and h.XGnRHR in IN3 pretreated cells) were not mimicked by peptide antagonists and were prevented by A261K mutation, demonstrating dependence on receptor signaling as well as agonist occupancy. The lack of measurable agonist-induced hGnRHR downregulation may simply reflect the low number of hGnRHR at the cell surface and the limit of detection of the assays used, but it could also reflect concomitant stimulation of trafficking to and from the PM. We suggest that the high throughput automated imaging systems described here will be of value for study of the molecular mechanisms controlling GnRHR compartmentalization and trafficking in future studies.
GRANTS
This work was supported by the Wellcome Trust (Project Grant Awards 062918 and 076557 and Equipment Grant 078407, to C. A. McArdle).
ACKNOWLEDGMENTS
We are grateful to Dr. A. Wallace (Merck, Rahway, NJ) for providing the IN3 and to Dr. A. Pawson (MRC Human Reproductive Science Unit, Edinburgh, UK) and Drs. S. Struthers and T. Kohout (Neurocrine Biosciences, San Diego, CA) for helpful discussions throughout the work.
REFERENCES
- 1.Bernier V, Lagace M, Bichet DG, Bouvier M. Pharmacological chaperones: potential treatment for conformational diseases. Trends Endocrinol Metab 15: 222–228, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Blomenrohr M, Heding A, Sellar R, Leurs R, Bogerd J, Eidne KA, Willars GB. Pivotal role for the cytoplasmic carboxyl-terminal tail of a non-mammalian gonadotropin-releasing hormone receptor in cell surface expression, ligand binding, and receptor phosphorylation and internalization. Mol Pharmacol 56: 1229–1237, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Caunt CJ, Finch AR, Sedgley KR, Oakley L, Luttrell LM, McArdle CA. Arrestin-mediated ERK activation by gonadotropin-releasing hormone receptors: receptor-specific activation mechanisms and compartmentalization. J Biol Chem 281: 2701–2710, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Caunt CJ, Hislop JN, Kelly E, Matharu AL, Green LD, Sedgley KR, Finch AR, McArdle CA. Regulation of gonadotropin-releasing hormone receptors by protein kinase C: inside out signalling and evidence for multiple active conformations. Endocrinology 145: 3594–3602, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Cheng CK, Leung PC. Molecular biology of gonadotropin-releasing hormone (GnRH)-I, GnRH-II, and their receptors in humans. Endocr Rev 26: 283–306, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Conn PM, Huckle WR, Andrews WV, McArdle CA. The molecular mechanism of action of gonadotropin releasing hormone (GnRH) in the pituitary. Recent Prog Horm Res 43: 29–68, 1987 [DOI] [PubMed] [Google Scholar]
- 7.Conn PM, Knollman PE, Brothers SP, Janovick JA. Protein folding as posttranslational regulation: evolution of a mechanism for controlled plasma membrane expression of a G protein-coupled receptor. Mol Endocrinol 20: 3035–3041, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Conn PM, Rogers DC, McNeil R. Potency enhancement of GnRH agonists: GnRH receptor micro-aggregation stimulates gonadotropin release. Endocrinology 111: 335–337, 1982 [DOI] [PubMed] [Google Scholar]
- 9.Conn PM, Ulloa-Aguirre A, Ito J, Janovick JA. G protein-coupled receptor trafficking in health and disease: lessons learned to prepare for therapeutic mutant rescue in vivo. Pharmacol Rev 59: 225–250, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Cooray SN, Chan L, Webb TR, Metherell L, Clark AJ. Accessory proteins are vital for the functional expression of certain G protein-coupled receptors. Mol Cell Endocrinol 300: 17–24, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Cornea A, Janovick JA, Lin X, Conn PM. Simultaneous and independent visualization of the gonadotropin-releasing hormone receptor and its ligand: independent processing and recycling in living cells. Endocrinology 140: 4272–4280, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Davidson JS, Flanagan CA, Zhou W, Becker II, Elario R, Emeran W, Sealfon SC, Millar RP. Identification of N-glycosylation sites in the gonadotropin-releasing hormone receptor: role in receptor expression but not ligand binding. Mol Cell Endocrinol 107: 241–245, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Davidson JS, Wakefield IK, Millar RP. Absence of rapid desensitization of the mouse gonadotropin-releasing hormone receptor. Biochem J 300: 299–302, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong C, Filipeanu CM, Duvernay MT, Wu G. Regulation of G protein-coupled receptor export trafficking. Biochim Biophys Acta 1768: 853–870, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards SW, Tan CM, Limbird LE. Localization of G protein-coupled receptors in health and disease. Trends Pharmacol Sci 21: 304–308, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Ellgaard L, Molinari M, Helenius A. Setting the standards: quality control in the secretory pathway. Science 286: 1882–1888, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Finch AR, Sedgley KR, Caunt CJ, McArdle CA. Plasma membrane expression of GnRH receptors: regulation by antagonists in breast, prostate, and gonadotrope cell lines. J Endocrinol 196: 353–367, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanyaloglu AC, von Zastrow M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol 48: 537–568, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Hazum E, Cuatrecasas P, Marian J, Conn PM. Receptor-mediated internalization of fluorescent gonadotropin-releasing hormone by pituitary gonadotropes. Proc Natl Acad Sci USA 77: 6692–6695, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heding A, Vrecl M, Bogerd J, McGregor A, Sellar R, Taylor PL, Eidne KA. Gonadotropin-releasing hormone receptors with intracellular carboxyl-terminal tails undergo acute desensitization of total inositol phosphate production and exhibit accelerated internalization kinetics. J Biol Chem 273: 11472–11477, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Hislop JN, Everest HM, Flynn A, Harding T, Uney JB, Troskie BE, Millar RP, McArdle CA. Differential internalization of mammalian and non-mammalian gonadotropin-releasing hormone receptors. Uncoupling of dynamin-dependent internalization from mitogen-activated protein kinase signaling. J Biol Chem 276: 39685–39694, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Hislop JN, Madziva MT, Everest HM, Harding T, Uney JB, Willars GB, Millar RP, Troskie BE, Davidson JS, McArdle CA. Desensitization and internalization of human and Xenopus gonadotropin-releasing hormone receptors expressed in alphaT4 pituitary cells using recombinant adenovirus. Endocrinology 141: 4564–4575, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Janovick JA, Goulet M, Bush E, Greer J, Wettlaufer DG, Conn PM. Structure-activity relations of successful pharmacologic chaperones for rescue of naturally occurring and manufactured mutants of the gonadotropin-releasing hormone receptor. J Pharmacol Exp Ther 305: 608–614, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Jennes L, Coy D, Conn PM. Receptor-mediated uptake of GnRH agonist and antagonists by cultured gonadotropes: evidence for differential intracellular routing. Peptides 7: 459–463, 1986 [DOI] [PubMed] [Google Scholar]
- 25.Lin X, Janovick JA, Brothers S, Blomenrohr M, Bogerd J, Conn PM. Addition of catfish gonadotropin-releasing hormone (GnRH) receptor intracellular carboxyl-terminal tail to rat GnRH receptor alters receptor expression and regulation. Mol Endocrinol 12: 161–171, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Luttrell LM, Lefkowitz RJ. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci 115: 455–465, 2002 [DOI] [PubMed] [Google Scholar]
- 27.McArdle CA, Forrest-Owen W, Willars G, Davidson J, Poch A, Kratzmeier M. Desensitization of gonadotropin-releasing hormone action in the gonadotrope-derived alpha T3-1 cell line. Endocrinology 136: 4864–4871, 1995 [DOI] [PubMed] [Google Scholar]
- 28.McArdle CA, Franklin J, Green L, Hislop JN. Signaling, cycling and desensitization of gonadotropin-releasing hormone receptors. J Endocrinol 173: 1–11, 2002 [DOI] [PubMed] [Google Scholar]
- 30.McArdle CA, Willars GB, Fowkes RC, Nahorski SR, Davidson JS, Forrest-Owen W. Desensitization of gonadotropin-releasing hormone action in alphaT3-1 cells due to uncoupling of inositol 1,4,5-trisphosphate generation and Ca2+ mobilization. J Biol Chem 271: 23711–23717, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Millar RP, Lu ZL, Pawson AJ, Flanagan CA, Morgan K, Maudsley SR. Gonadotropin-releasing hormone receptors. Endocr Rev 25: 235–275, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Myburgh DB, Millar RP, Hapgood JP. Alanine-261 in intracellular loop III of the human gonadotropin-releasing hormone receptor is crucial for G-protein coupling and receptor internalization. Biochem J 331: 893–896, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pawson AJ, Faccenda E, Maudsley S, Lu ZL, Naor Z, Millar RP. Mammalian type I gonadotropin-releasing hormone receptors undergo slow, constitutive, agonist-independent internalization. Endocrinology 149: 1415–1422, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Pawson AJ, Katz A, Sun YM, Lopes J, Illing N, Millar RP, Davidson JS. Contrasting internalization kinetics of human and chicken gonadotropin-releasing hormone receptors mediated by C-terminal tail. J Endocrinol 156: R9–R12, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Petaja-Repo UE, Hogue M, Laperriere A, Bhalla S, Walker P, Bouvier M. Newly synthesized human delta opioid receptors retained in the endoplasmic reticulum are retrotranslocated to the cytosol, deglycosylated, ubiquitinated, and degraded by the proteasome. J Biol Chem 276: 4416–4423, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Petaja-Repo UE, Hogue M, Laperriere A, Walker P, Bouvier M. Export from the endoplasmic reticulum represents the limiting step in the maturation and cell surface expression of the human delta opioid receptor. J Biol Chem 275: 13727–13736, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Pierce KL, Premont RT, Lefkowitz RJ. Seven transmembrane receptors. Nat Rev Mol Cell Biol 3: 639–650, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Schvartz I, Hazum E. Internalization and recycling of receptor-bound gonadotropin-releasing hormone agonist in pituitary gonadotropes. J Biol Chem 262: 17046–17050, 1987 [PubMed] [Google Scholar]
- 39.Sedgley KR, Finch AR, Caunt CJ, McArdle CA. Intracellular gonadotropin-releasing hormone receptors in breast cancer and gonadotrope lineage cells. J Endocrinol 191: 625–636, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Stojilkovic SS, Catt KJ. Expression and signal transduction pathways of gonadotropin-releasing hormone receptors. Recent Prog Horm Res 50: 161–205, 1995 [DOI] [PubMed] [Google Scholar]
- 41.Tan CM, Brady AE, Nickols HH, Wang Q, Limbird LE. Membrane trafficking of G protein-coupled receptors. Annu Rev Pharmacol Toxicol 44: 559–609, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Ulloa-Aguirre A, Janovick JA, Brothers SP, Conn PM. Pharmacologic rescue of conformationally-defective proteins: implications for the treatment of human disease. Traffic 5: 821–837, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Vrecl M, Anderson L, Hanyaloglu A, McGregor AM, Groarke AD, Milligan G, Taylor PL, Eidne KA. Agonist-induced endocytosis and recycling of the gonadotropin-releasing hormone receptor: effect of beta-arrestin on internalization kinetics. Mol Endocrinol 12: 1818–1829, 1998 [DOI] [PubMed] [Google Scholar]
- 44.Willars GB, Royall JE, Williams B, El-Gehani F, Everest H, Nahorski SR, McArdle CA. Rapid downregulation of the type I Ins(1,4,5)P3 receptor and desensitisation of gonadotropin-releasing hormone-mediated Ca2+ responses in αT3–1 gonadotropes. J Biol Chem 276: 3123–3129, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Zhang X, Bao L, Guan JS. Role of delivery and trafficking of delta-opioid peptide receptors in opioid analgesia and tolerance. Trends Pharmacol Sci 27: 324–329, 2006 [DOI] [PubMed] [Google Scholar]