Abstract
Numerous cell types express functional connexin (Cx) hemichannels (HCs), and membrane depolarization and/or exposure to a divalent cation-free bathing solution (DCFS) have been shown to promote HC opening. However, little is known about conditions that can promote HC opening in the absence of strong depolarization and when extracellular divalent cation concentrations remain at physiological levels. Here the effects of metabolic inhibition (MI), an in vitro model of ischemia, on the activity of mouse Cx32 HCs were examined. In HeLa cells stably transfected with mouse Cx32 (HeLa-Cx32), MI induced an increase in cellular permeability to ethidium (Etd). The increase in Etd uptake was directly related to an increase in levels of Cx32 HCs present at the cell surface. Moreover, MI increased membrane currents in HeLa-Cx32 cells. Underlying these currents were channels exhibiting a unitary conductance of ∼90 pS, consistent with Cx32 HCs. These currents and Etd uptake were blocked by HC inhibitors. The increase in Cx32 HC activity was preceded by a rapid reduction in mitochondrial membrane potential and a rise in free intracellular Ca2+ concentration ([Ca2+]i). The increase in free [Ca2+]i was prevented by HC blockade or exposure to extracellular DCFS and was virtually absent in parental HeLa cells. Moreover, inhibition of Cx32 HCs expressed by HeLa cells in low-confluence cultures drastically reduced cell death induced by oxygen-glucose deprivation, which is a more physiological model of ischemia-reperfusion. Thus HC blockade could reduce the increase in free [Ca2+]i and cell death induced by ischemia-like conditions in cells expressing Cx32 HCs.
Keywords: ischemia, connexons, calcium, cell death
connexin (Cx) hemichannels (HCs) are integral membrane proteins that originally were thought of only as precursors to gap junction (cell-cell) channels. However, in the last several years, it has become clear that Cx HCs can function in the plasma membrane in the absence of docking (6, 14, 18, 21). Cx HCs are hexamers of Cx subunits and constitute large ion channels permeable to inorganic ions and signaling molecules. In the human and mouse genomes 21 and 20 Cxs, respectively, have been identified (13). Cx HCs are highly regulated, and it is becoming apparent that their opening can occur under physiological and pathophysiological conditions (33).
Induction of cell death triggered by the opening of the Cx HCs was originally observed in Xenopus laevis oocytes injected with rat Cx46 mRNA (34). Subsequently, it was reported that Cx43 HCs opened in ventricular myocytes (23), astrocytes (8, 27), and epithelial cells of the proximal renal tubule (47) when cells were subjected to metabolic inhibition (MI). Opening of Cx HCs could contribute to the loss of ionic gradients and metabolites across the membrane, which could accelerate cell death. Accordingly, HeLa cells transfected with the mouse Cx43 gene (HeLa-Cx43) exhibit functional Cx43 HCs and are more susceptible to cell death when subjected to MI than parental HeLa cells (HeLa-p) (10). We have also shown (8) that treatment with gap junction channel inhibitors blocks uptake of the permeability tracers Lucifer yellow and ethidium (Etd). However, it is unknown whether HCs formed by Cxs other than Cx43 and Cx46 act as mediators of cell death. The exogenous expression of Cx32 in the C6 cell line (derived from a rat astroglioma) placed in medium with a low Ca2+ concentration correlated with increased release of extracellular ATP to the medium, suggesting that Cx32 HCs are permeable to the nucleotide (3, 11). However, it remains unknown whether Cx32 HCs open under similar conditions.
The present study examines whether MI, a condition that mimics an ischemic episode, affects the activity of Cx32 HCs. In HeLa-Cx32 but not HeLa-p cells, MI induced cellular permeabilization and was accompanied by an increase in membrane current. These MI-induced effects could be blocked with HC inhibitors. Instances in which single-channel activity was visible revealed a unitary conductance of ∼90 pS, in agreement with that expected of a Cx32 HC. Activity of Cx32 HCs was directly related to increased levels of Cx32 HCs present at the cell surface. The rise in HC activity was preceded by a small, but rapid increase in intracellular Ca2+ concentration ([Ca2+]i), which was due to influx from the extracellular medium via basally active Cx32 HCs. This rise in [Ca2+]i, in turn, increased surface expression of HCs, leading to increased Ca2+ influx. Oleamide, a HC blocker, considerably increased cell viability in HeLa-Cx32 under oxygen-glucose deprivation (OGD).
METHODS
Cell cultures.
HeLa cell cultures transfected with mouse Cx32 (HeLa-Cx32) and parental HeLa (HeLa-p) cells were kindly provided by Dr. Klaus Willecke (University of Bonn, Bonn, Germany). Previously, our laboratory tested the null expression of Cx32 and other Cxs in HeLa-p cells (43). Cells were cultured in DMEM-10% fetal bovine serum (GIBCO, Invitrogen), 100 U/ml penicillin, 100 μg/ml streptomycin sulfate, and 1 μg/ml puromycin, the latter as a selector of transfected cells. The cells were seeded at a density of 50,000 cells/plate with 60-mm-diameter glass coverslips (no. 1, Hirschmann Laborgeräte, Eberstadt, Germany) and were used 24 h later to maximize the number of noncontacting cells, generally reaching <5% confluence (low-confluence cultures). Alternatively, the cells were seeded at a density of 500,000 cells per 60-mm-diameter dish with glass coverslips, fed 24 h later, and used 48 h after seeding. In this condition, the cells generally reached 50–60% confluence (subconfluent cultures). Because untransfected HeLa-p cells and HeLa-p cells transfected with the plasmid used to express Cxs showed similar responses as described previously (39), we used untransfected HeLa-p cells as controls in our experiments. Cultures of HeLa-Cx32 at the two levels of confluence used gave similar Cx32 levels (not shown) and thus were considered equivalent for the purposes described in the present work.
Metabolic inhibition and removal of oxygen and glucose.
Cell cultures were subjected to MI with chemical ischemia or application of compounds that block the pathways of ATP production. We used iodoacetic acid (0.3 mM), an inhibitor of the enzyme glyceraldehyde-3-phosphate dehydrogenase, and antimycin A (10 ng/ml, from a 2.5 mg/ml stock solution), an inhibitor of complex III of the mitochondrial respiratory chain (8, 19, 23). Both inhibitors (Sigma, St. Louis, MO) were dissolved in saline and applied to cultures.
In the OGD model, cell cultures were kept in saline solution without glucose, used in dye uptake assays (see below). Subsequently, the cultures were placed in a chamber with a controlled seal (Billups-Rothenberg, Del Mar) that had two valves communicating with the outside. One of these introduced nitrogen to 100%, maintaining a constant flow for 3 min. After different time periods, up to 6 h, the chamber was opened and the cultures were exposed to a saline solution with glucose (5 mM) in the presence of ambient oxygen. Some coverslips were subjected to dye uptake assays (see below), and others were exposed for 2 min to a saline solution with glucose (5 mM) and dextran conjugated with rhodamine (Dex-Rd, mol mass 10 kDa, 10 μM) and then rinsed twice carefully; the uptake incidence was used as an index of cell death.
Immunofluorescence.
The subcellular localization of Cxs was assessed by indirect immunofluorescence. HeLa cells seeded on glass coverslips (no. 1) were fixed and permeabilized with 70% ethanol at −20°C for 20 min and then incubated with blocking solution [rat serum, 50% in sterile PBS plus 0.05% (wt/vol) bovine serum albumin] for 30 min. Subsequently, the samples were incubated at 4°C overnight with an appropriate dilution of primary antibody (72-F, monoclonal anti-Cx32 antibody) dissolved in blocking solution. After six washes of 5 min each in PBS, the samples were incubated with a secondary fluorescein thiocyanate-conjugated antibody dissolved in blocking solution for 30 min in the dark at room temperature. After a second round of six washes, each 5 min in PBS, the coverslips were washed in distilled water, dried by runoff, and mounted with Fluoromount G or settlement (DABCO) for inspection under a Olympus BX51WI immersion microscope equipped with an image acquisition system.
Dye uptake assay.
Dye uptake was measured with ethidium bromide (Etd) as previously described (8, 37, 43). Briefly, coverslips with subconfluent HeLa cell cultures were transferred to a 30-mm dish coated with a thin layer of Vaseline to promote adhesion. Immediately, a phosphate-free buffer solution (in mM: 140 NaCl, 5.4 KCl, 1.8 CaCl2, and 10 HEPES, pH 7.4, containing 5 μM Etd) was exchanged for the culture medium. Recordings were made with an Olympus BX51WI microscope (Melville) equipped with an image acquisition system (Q-IMAGING 32-0086B-125 Retiga1300i, QImaging, Surrey, BC, Canada). Results were analyzed with MetaFluo (Universal Images, Molecular Devices) and transferred to a spreadsheet (Microsoft Excel) for plotting. For analysis of nonconfluent cultures, we calculated the average fluorescence obtained from a region of interest (ROI) whose shape corresponded approximately to the shape of the cell. For each value, the mean fluorescence obtained from a ROI located immediately adjacent to the cell and the same distance from the center of the field optical region was used as background. For analysis of subconfluent cultures, we calculated the average fluorescence obtained from a ROI whose shape corresponded approximately to the shape of the subconfluent cell, but the background fluorescence was taken as the average fluorescence obtained from five ROIs in various locations within the optical field that were devoid of cells. In cultures under MI, ROIs were adjusted at each time of data acquisition if they showed noticeable changes in volume.
Some of the uptake assays were conducted with DCFS (Ca2+ and Mg2+). To accomplish this, the corresponding salts were not included in the stock solution, and ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA, 5 mM Titriplex-VI, Merck) was added. In some assays, cells were preincubated for 1 h with the Ca2+ chelator BAPTA-AM (5 μM).
Biotinylation of plasma membrane proteins.
We used the protocol described by Musil and Goodenough (30, 31) and modified by Retamal et al. (37) and Schalper et al. (43). Briefly, subconfluent cell cultures (90-mm-diameter dishes) were washed three times with Hanks' phosphate buffer solution (HPBS, pH 8.0) and cooled on ice. Three milliliters of HPBS containing 0.5 mg/ml sulfo-NHS SS-biotin (Pierce, Rockford, IL) was added and incubated for 30 min at 4°C. The cultures were then washed three times with HPBS containing 15 mM glycine (pH 8.0) to neutralize the excess free biotin. Cells were harvested with a scraper (rubber policeman) in the same way as samples prepared for immunotransfer (described below). After the amount of protein was determined in each sample volumes containing the same amount of protein were taken, and to each was added an excess of NeutrAvidin (Pierce) (1 ml of NeutrAvidin per 3 g biotinylated protein, according to manufacturer's instructions). The mixture was incubated for 1 h at 4°C. One milliliter of wash buffer was added (HPBS pH 7.2 + 0.1% SDS and 1% Nonidet P-40) and centrifuged for 2 min at 14,000 rpm and 4°C, and the supernatant was discarded. The NeutrAvidin pellet was washed three times with the same buffer. After the last wash, 40 ml of HPBS containing 0.1 M glycine (pH 2.8) was added to the pellet to release proteins bound to biotin. The pellet was resuspended and spun at 14,000 rpm for 2 min at 4°C. The supernatant was transferred to an Eppendorf tube (1.5 ml), and the pH was adjusted by adding 10 μl of 1 M Tris buffer pH 7.5 and 40 μl of Laemmli buffer. The relative levels of Cx32 present in each sample were measured by immunoblotting as described below. The density of the bands was measured, and the results are expressed in arbitrary units (AU). The relative levels were compared with the levels of immunoreactivity present in samples containing known concentrations of total protein derived from sister cultures.
Western blot analysis.
Cell cultures were washed once with PBS solution (pH 7.4), and the cells were harvested with a scraper in 1 ml of a mixture of protease inhibitors (200 mg/ml soybean trypsin inhibitor, 1 mg/ml benzamidine, 1 mg/ml ε-aminocaproic acid, and 2 mM PMSF) and phosphatases (20 mM Na4P2O7 and 100 mM NaF). The suspension was centrifuged at 5,000 rpm for 1 min at 4°C. The pellet was resuspended in 50 ml of solution containing protease inhibitors and sonicated on ice (Ultrasonic disrupted cell, MICROSON). Protein levels were measured in aliquots of cell lysates with the Bio-Rad assay (Bio-Rad Laboratories, Hercules, CA). The samples were resuspended in Laemmli buffer and immediately loaded onto a 8% acrylamide gel. After electrophoresis, proteins were electrotransferred to a nitrocellulose membrane. The amount of protein loaded per wheal was checked again by staining the blots with Ponceau red, followed by washes with distilled water. Nonspecific protein binding was blocked by incubation of the nitrocellulose membrane in PBS-BLOTTO (5% skim milk in PBS solution) for 40 min. The membrane was then incubated overnight with the primary antibody previously described (Ref. 39, monoclonal anti-Cx32 antibody 72-F), followed by several washes with PBS, and incubated with antibody conjugated with horseradish peroxidase (Pierce). The immuno-enhanced chemiluminescence was detected with the SuperSignal kit (Pierce) according to manufacturer's instructions. The resulting immunoblots were scanned, and densitometric analyses were performed with Scion IMAGE software.
Estimate of intracellular free Ca2+ concentration.
We estimated the free [Ca2+]i with the Ca2+ indicator fura-2 (Molecular Probes, Eugene, OR) as described by Schalper et al. (43). In brief, cells were incubated in DMEM (serum free) culture medium containing 5 μM of the membrane-permeant AM ester form of fura-2 (fura-2 AM) dissolved in DMSO for 1 h. The cells were subsequently washed thoroughly with buffer and imaged. Fluorescence was measured at excitation wavelengths of 340 and 380 nm. The emission wavelength was 510 nm. Images were acquired at a rate of 1/min and analyzed with MetaFluo software (Universal Images, Molecular Devices). Ca2+ was estimated from the ratio of fluorescence (340/380) as described previously (43).
Estimate of mitochondrial membrane potential.
We used a modification of the method described by Ankarcrona et al. (1) using the probe JC-1 (5,5′,6,6′-tetrachloro-l,l′,3,3′-tetraethylbenzimidazolcarbocyanine iodide, Molecular Probes). Cells were incubated with 0.5 mg/ml JC-1 for 10 min. The cells were washed thoroughly with buffer and imaged. JC-1 is a liposoluble cation that enters the cell, where it incorporates into organelles that exhibit negative membrane potentials. JC-1 was detected in two forms. The multimeric form is specific to mitochondria because of the hyperpolarized potential of the mitochondrial matrix (Ψm) and detected at a wavelength of 590 nm (480 nm excitation). A monomer form is not associated with mitochondria and is detected at 527 nm (480 nm excitation). The 590-to-527 fluorescence ratio is directly proportional to Ψm and can be used as an index of Ψm (1, 22, 26). Alternating images of JC-1 and fura-2 fluorescence were acquired in the same cells at a rate of one image every 5 min.
Electrophysiology.
To examine opening of HCs in nonconfluent cells electrophysiologically, whole cell patch recordings were employed (3, 23), as modified by Contreras et al. (9). Nonconfluent cultures were used to record isolated cells. Briefly, cells were grown on coverslips, which were transferred to a recording chamber (SA-0LY/2, Warner) mounted on an Olympus IX70 inverted microscope (Melville). The chamber was superfused with a modified Krebs-Ringer solution (in mM: 140 NaCl, 1 MgCl2, 5.4 KCl, 1.8 CaCl2 and 10 HEPES, pH 7.3). The pipette solution contained (in mM) 10 Na aspartate, 1 MgCl2, 130 KCl, 0.26 CaCl2, 2 EGTA, 5 TEA Cl, and 5 HEPES, pH 7.3. In MI experiments, KCl was replaced by an equimolar amount of CsCl in the extracellular solution, and 2 mM BaCl2 was added to suppress Ca2+-activated K+ currents. The intracellular solution also was changed to (in mM) 130 CsCl, 10 Na aspartate, 0.1 EGTA, 7 TEA Cl, 1 MgCl2, and 10 HEPES, pH 7.3. Recordings were obtained with an Axopatch 200B amplifier (Axon Instruments) and acquired with an AT-MIO-16X board (National Instruments, Austin, TX) and our own acquisition software (written by Dr. E. B. Trexler).
Statistical analysis.
For each group of data, the results are expressed as means ± SE, and n is the number of independent experiments or the number of cells, as indicated. For statistical analysis of two data sets, we used a t-test. Three or more data sets were compared with each other by one-way analysis of variance (ANOVA) followed by a Newman-Keuls posttest. To compare data sets obtained from different groups of cells (HeLa-p vs. HeLa-Cx32) and under the same condition (control or MI), we used a one-way ANOVA followed by a Newman-Keuls posttest. Differences were considered significant at P ≤ 0.05. The analyses were performed with GraphPad Prism 5 software for Windows (1992–2007, GraphPad Software).
RESULTS
Metabolic inhibition induces permeabilization of Cx32 but not parental HeLa cells.
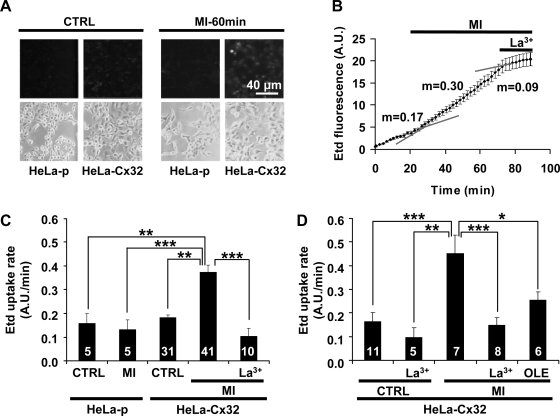
MI has been used as a simple model of ischemia by several groups, including ours (8, 23, 37, 47). Although MI occurs in normoxia, it shares several characteristics with hypoxia-reoxygenation, including the decline in ATP levels and the generation of free radicals (8, 37). In the time period analyzed (<90 min) in this study, MI did not affect the permeability of HeLa-p cells to Etd (Fig. 1). Basal uptake of Etd in HeLa-Cx32 and HeLa-p cells was similar, but MI induced chemically with iodoacetic acid and antimycin A (see methods) increased Etd uptake (∼100%) only in HeLa-Cx32 cells. In HeLa-p cells, Etd uptake was not affected by La3+ (not shown), whereas in HeLa-Cx32 cells under MI, uptake was reduced by La3+ to values comparable to those of HeLa-Cx32 under basal conditions treated with La3+ (Fig. 1, B–D). In low-confluence or subconfluent cultures, the increase in the Etd uptake induced by MI was similar and completely blocked by application of La3+ (100 μM) or oleamide (200 μM) (Fig. 1, C and D), which are known HC blockers (8, 23, 25), suggesting that the increase in Etd uptake was mediated by Cx32 HCs. In this study, Etd uptake that was resistant to La3+ was considered to be mediated by a process unrelated to Cx HCs; we did not undertake studies to identify this process.
Fig. 1.
Metabolic inhibition enhances ethidium (Etd) uptake in HeLa cells transfected with connexin32 (HeLa-Cx32) but not in HeLa-parental (HeLa-p) cells. Fluorescent and corresponding bright-field images of cells exposed to 100 μM Etd for 2 min are shown. A: HeLa-p and HeLa-Cx32 cells under control conditions (Ctrl) or after 60 min of metabolic inhibition (MI-60min). B: representative plot of intensity of Etd fluorescence [in arbitrary units (AU)] over time. At the start of the experiment, 5 μM Etd was added to the bath. At ∼20 min, cells were exposed to MI, and at ∼70 min 100 μM La3+ was applied. Each point is the average ± SE fluorescence of 9 cells. Gray lines correspond to extrapolations of the linear regressions computed for each condition; m, rate of Etd uptake. C and D: rates of Etd uptake (5 μM) measured in low-confluence and subconfluent cultures of HeLa-p and HeLa-Cx32, respectively. Shown are rates of Etd uptake under control conditions (CTRL) or under MI, MI + 100 μM La3+ (La3+), or MI + 200 μM oleamide (OLE). Number of experiments is indicated in each column. Each experiment monitored fluorescence in 15 (C) and 25 (D) cells. Each bar indicates mean ± SE. *P < 0.05, **P < 0.01, ***P < 0.001, 1-way ANOVA with Newman-Keuls posttest.
Increase in membrane permeability induced by MI is directly related to expression of Cx32 and levels of Cx32 HCs at cell surface.
The increase in Etd uptake induced by MI correlated well (r2 = 0.813) with an increase in overall Cx32 immunoreactivity detected by immunofluorescence, suggesting that MI may increase the number of HCs in the plasma membrane (Fig. 2, A–C). To demonstrate biochemically that HeLa-Cx32 cells contain Cx32 protein in the plasma membrane, we biotinylated cell surface proteins, followed by precipitation and immunoblotting. We found that HeLa-Cx32 cells contain Cx32 on the surface, that levels increased approximately twofold during the first 15 min of exposure to MI (Fig. 2D), and that increased levels remained high for 45–60 min, paralleling the magnitude and time course of the increase in Etd uptake (Fig. 2, E and F). Furthermore, by comparing the levels of biotinylated protein with the total amount of protein, we found that ∼4% and ∼8% of Cx32 form HCs in resting and metabolically inhibited cells, respectively (Fig. 2E).
Fig. 2.
Metabolic inhibition increases the number of hemichannels (HCs) at the surface of HeLa-Cx32 cells. HeLa-Cx32 cells were exposed to MI for ∼65 min in the presence of 10 μM Etd, and images were taken to evaluate dye uptake rate. The cells were then fixed and processed for Cx32 detection by immunofluorescence. A, left: Etd uptake induced by MI. Right: Cx32 immunoreactivity (Cx32-IR) in the same field shown on left. B: time lapse record showing Etd uptake over time in the cells (1–7) indicated in A. C: correlation analysis of rate of Etd uptake and level of Cx32-IR in cells under MI. Each point corresponds to Etd uptake and immunofluorescence measured in a single cell in a total of 3 separate experiments. D, left: immunoblot of Cx32 present in an aliquot of total rat liver homogenate (L; 50 μg of proteins) and at the cellular surface of subconfluent HeLa-Cx32 cells under control conditions (Ctrl) or exposed to MI for 15 or 60 min. In the last 3 lanes, surface proteins were biotinylated, precipitated, and subjected to immunoblot for Cx32. Right: samples of total HeLa-p homogenate and serial dilutions of total HeLa-Cx32 cell homogenates were analyzed by immunoblotting. L, aliquot of total liver homogenate used as a marker of the electrophoretic migration of the Cx32 monomer (27 kDa); dim indicates the mobility of the Cx32 dimer in the lanes loaded with a total liver homogenate aliquot for both panels. E: estimated amount of Cx32-forming HCs expressed as % of total amount of Cx32. Regression line was obtained from known quantities of proteins indicated in D. Percentages of Cx32-forming HCs under control conditions (Ctrl) or under MI for 60 min (MI-60) are indicated by dotted lines. F: results from 4 separate experiments. Each value is the average ± SE. **P < 0.01, t-test.
Inhibition of P2x or TRPV1 channels does not prevent MI-induced permeabilization of HeLa-Cx32 cells.
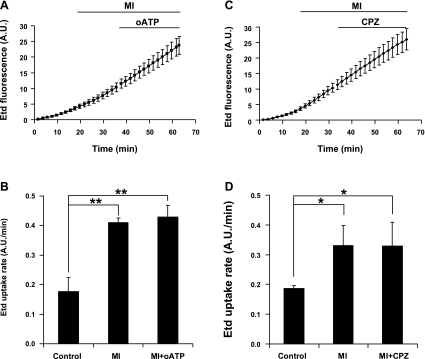
During ischemia, mechanisms that lead to cell depolarization and cell death are activated, including the activation of purinergic P2x receptors (16) and transient receptor potential (TRP)V channels (28). Therefore, it has been suggested that these other channels could mediate the uptake of fluorescent tracers (2, 32, 45). Thus MI experiments were conducted in the presence of 300 μM oxidized ATP (oATP), a P2x channel blocker, or 10 μM capsazepine, a TRPV1 channel blocker. We found that neither compound applied ∼10 min after the addition of the metabolic inhibitors could reverse the increase in Etd uptake (Fig. 3), indicating that P2x and TRPV1 channels do not mediate the increase in Etd uptake induced by MI.
Fig. 3.
Etd uptake induced by MI is not blocked by either oxidized ATP or capsazepine in HeLa-Cx32 cells. A and C: representative plots obtained from time lapse experiments of Etd uptake in HeLa-Cx32 cells. Cells were maintained under control conditions for the first 15 min and then treated to induce MI. Oxidized ATP (oATP, 300 μM; A) or capsazepine (CPZ, 10 μM; C) was added ∼20 min after initiation of MI. Each point corresponds to the average ± SE of 16 (A) and 9 (C) cells. B and D: mean rates of Etd uptake obtained from experiments illustrated in A and C, respectively (n = 3 experiments). *P < 0.05, **P < 0.01, 1-way ANOVA with Newman-Keuls posttest.
MI increases macroscopic membrane current but does not affect unitary conductance of Cx32 HCs.
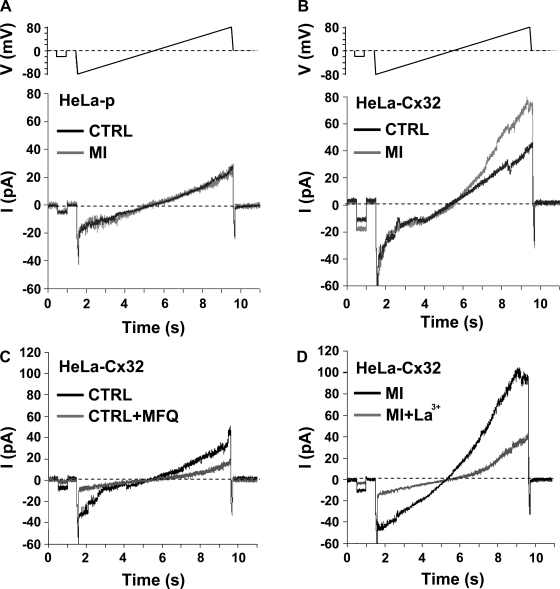
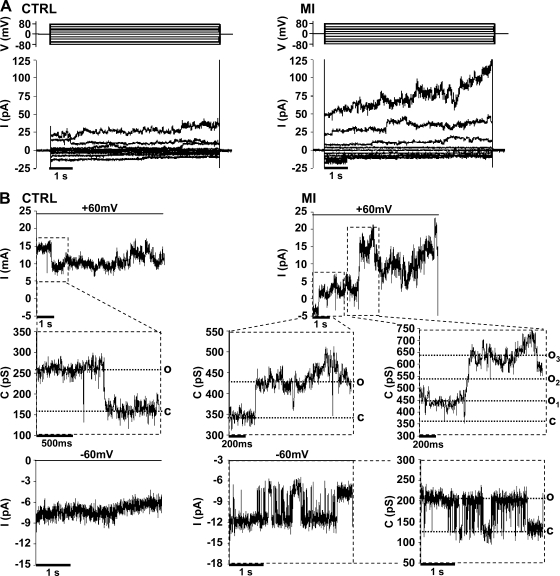
Gómez-Hernández et al. (17) recorded unitary currents of Cx32 HCs expressed in Xenopus oocytes, which in the absence of added extracellular divalent cations exhibited a unitary conductance of ∼87 pS. To assess Cx32 HC activity during MI, whole cell patch recordings were obtained in HeLa cells. A larger macroscopic current (∼2-fold) was present in HeLa-Cx32 cells compared with HeLa-p cells, particularly at positive membrane potentials (Fig. 4, A and B). Currents were larger still when cells were exposed to MI. Unitary current events were only occasionally observed near the resting membrane potential but were more evident on depolarization to positive potentials. Current transitions had a mean value of 95 ± 21 pS (n = 219 events) under control conditions and 95 ± 20 pS (n = 75 events) under MI (Fig. 4B and Fig. 5). These conductance values were independent of the applied voltage indicative of a lack of significant open channel rectification. We also applied voltage ramps from −80 to +80 mV (8 s) and found transitions of 98 ± 21 pS (n = 103 events) under control conditions and 95 ± 18 pS (n = 158 events) under MI. Both macroscopic currents (Fig. 4, C and D) and the associated unitary transitions (not shown) were inhibited by mefloquine or La3+ under control and MI conditions, indicating that there is basal Cx32 HC activity that increases during MI.
Fig. 4.
HeLa-Cx32 cells exhibit macroscopic currents attributable to Cx32 HCs. Low-confluence cell cultures were used to record from single cells. Currents were obtained with whole cell patch-clamp recordings under control conditions (CTRL, black lines) and after exposure to MI (gray lines). Examples of membrane currents (I) recorded in HeLa-p cells (A) and in HeLa-Cx32 cells (B) are shown. The voltage (V) protocol (shown above current records) consisted of ramps applied from −80 to +80 mV, preceded by a −20 mV prepulse. HeLa-Cx32 cells showed larger currents after MI, particularly at positive voltages. C and D: HC blockers reduce currents attributable to Cx32 HCs under control conditions and MI. Current responses were evoked by applying voltage ramps as described in A and B. Shown is an example with mefloquine (MFQ, 15 μM), which reduced currents in a control HeLa-Cx32 cell (C, gray line), and La3+ (100 μM), which completely abolished the large increase in current observed after 60 min under MI (D, gray line).
Fig. 5.
MI enhances the activity but not the unitary conductance of Cx32-HCs. Membrane currents were measured in a whole cell voltage-clamp recording configuration using low-density cultures of HeLa-Cx32 cells under control conditions (CTRL) or under MI. Recordings were obtained ∼40 min after transfer of cells on coverslips from culture medium to the recording extracellular solution. A: representative current traces elicited by 10-s voltages steps from −80 to +80 mV, in 20-mV increments. B: views of unitary current events recorded at +60 and −60 mV taken from the current trace shown in A under control conditions and MI, respectively. Despite a high level of background noise in the whole cell configuration, unitary current transitions were identifiable and were converted on a point-by-point basis to conductance values (C) as indicated in the expanded region of the records. Unitary conductances of transitions recorded under control or MI conditions were ∼90 pS. Open (O) and closed (C) hemichannel states are indicated.
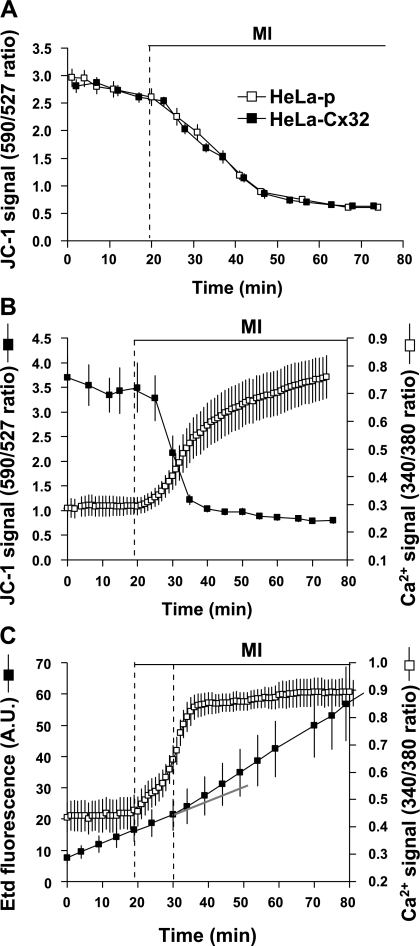
Both reduction in Ψm and rise in [Ca2+]i precede increase in membrane permeability of HeLa-Cx32 cells under MI.
In normal cells [Ca2+]i is closely regulated, and mitochondrial dysfunction has been attributed as an important cause of the rise in [Ca2+]i observed in ischemia-reperfusion (15). Thus we monitored Ψm and found that MI induced a rapid fall in Ψm that was similar in HeLa-p and HeLa-Cx32 cells, indicating that MI-induced changes in Ψm are independent of Cx expression (Fig. 6A). However, in HeLa-Cx32 cells, the fall in Ψm was accompanied by a concomitant increase in [Ca2+]i (Fig. 6B). To assess the possible relationship between the increase in [Ca2+]i and HC activity during MI, Etd uptake and changes in [Ca2+]i were measured simultaneously. The increase in [Ca2+]i during MI in HeLa-Cx32 cells was found to precede the increase in Etd uptake by ∼10 min (Fig. 6C), suggesting that HCs are present at the cell surface before the increase in Etd uptake (Figs. 1 and 6) and the increase in HC levels at the cell surface (Fig. 2) were sufficient to produce an increase in [Ca2+]i of ∼50%.
Fig. 6.
MI increases intracellular Ca2+ concentration ([Ca2+]i), which is inversely related to the decrease of mitochondrial membrane potential (Ψm) and precedes the increase in membrane permeability to Etd. A: representative plots of the fluorescence ratio (590 nm/527 nm) with the probe JC-1 obtained from HeLa-p and HeLa-Cx32 cells. This ratio reflects Ψm; a decrease in this ratio indicates a fall in Ψm. Dashed vertical line indicates the start of MI. B: time-lapse experiment showing changes in JC-1 and fura-2 fluorescence ratios to simultaneously monitor changes in Ψm and [Ca2+]i over time. Cells were maintained under control conditions for 20 min and then exposed to MI. Total recording time was 80 min. Dashed vertical line indicates the start of MI. C: time-lapse experiment showing Etd uptake simultaneously with changes in [Ca2+]i. As in B, MI was applied after 20 min in control conditions. First dashed vertical line indicates the start of MI, and second dashed vertical line indicates the time at which there was a change in the rate of Etd uptake after MI. Solid gray line is an extrapolation of the basal rate of Etd uptake under control conditions. Data points in B and C represent average ± SE fluorescence ratios obtained from 10 cells.
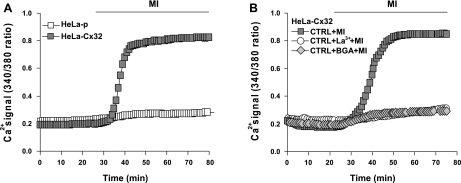
MI elevates [Ca2+]i of HeLa cells in a Cx32-dependent manner.
It has been suggested that [Ca2+]i is a key factor that controls the levels of Cx43 HCs in HeLa cells (43). As previously indicated, a rapid increase in [Ca2+]i occurred after application of metabolic inhibitors (Fig. 6, B and C). The increase in [Ca2+]i did not occur in HeLa-p cells, indicating that the rise in [Ca2+]i was linked to Cx32 expression (Fig. 7A). To identify the source of Ca2+ entry, HC blockers La3+ and 18β-glycyrrhetinic acid (BGA) were added 10 min before initiation of MI in HeLa-Cx32 cells. Both La3+ and BGA prevented the increase in [Ca2+]i (Fig. 7B), suggesting that Ca2+ enters the cells from the extracellular medium through basally active Cx32 HCs present in the surface membrane.
Fig. 7.
Increase in [Ca2+]i occurs with metabolic inhibition in HeLa-Cx32 but not HeLa-p cells and is prevented by HC blockers. A: representative plots of relative changes in [Ca2+]i over time during MI in HeLa-p cells (n = 11) and HeLa-Cx32 cells (n = 15). B: time course of relative change in [Ca2+]i in HeLa-Cx32 cells exposed to MI in the absence or presence of blockers. MI was induced after 20 min. HC blockers La3+ (100 μM) and 18β-glycyrrhetinic acid (BGA, 35 μM) were applied 10 min before induction of MI and maintained during the experiment. Each point represents the mean ± SE of 8 (CTRL+MI), 10 (CTRL+La3++MI), or 15 (CTRL+BGA+MI) cells.
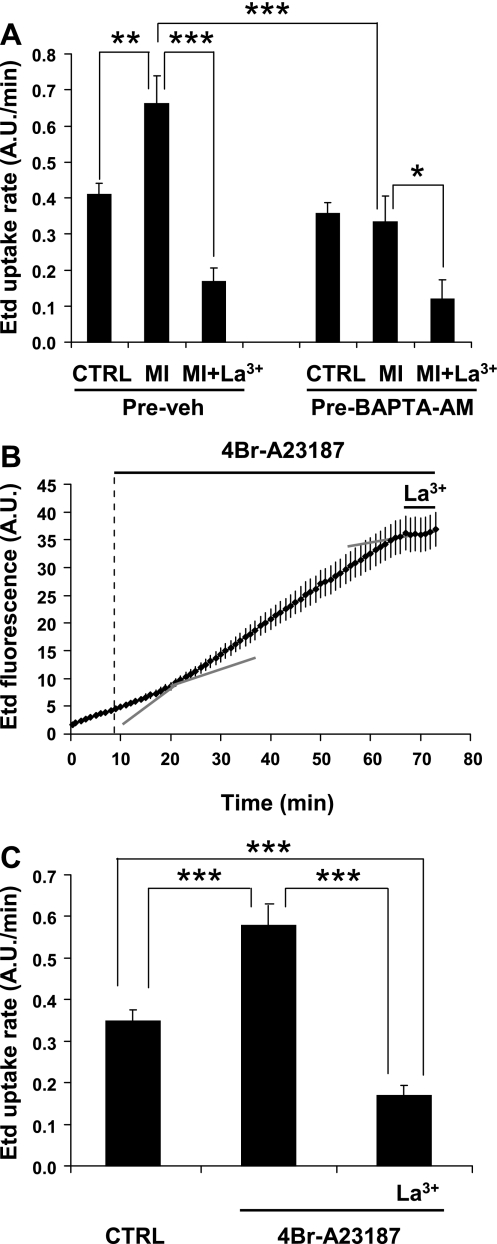
MI-induced increase in membrane permeability of HeLa-Cx32 cells requires rise in [Ca2+]i.
To ascertain whether the increase in Etd uptake required an increase in [Ca2+]i, cells were preloaded with BAPTA-AM, a chelator of intracellular free Ca2+. In the presence of BAPTA, the increase in Etd uptake induced by MI was completely abolished (Fig. 8A). Moreover, about two-thirds of the remaining Etd uptake was sensitive to La3+, suggesting that most of it was mediated by basally active Cx32 HCs (Fig. 8A). To assess whether an increase in [Ca2+]i is sufficient to enhance Etd uptake, HeLa-Cx32 were treated with 5 μM 4Br-A-23187, a Ca2+ ionophore, and Etd uptake was evaluated over time. After an ∼10-min lag period, Etd uptake increased and remained high throughout the ∼50-min recording. Subsequent application of 100 μM La3+ reduced the rate of Etd uptake to ∼0.1 AU/min (Fig. 8B). Notably, this value was comparable to that measured under control conditions in HeLa-p or HeLa-Cx32 cells (compare Fig. 8C with Fig. 1, C or D) or under MI in HeLa-Cx32 cells preloaded with BAPTA, suggesting that the increase in membrane permeability observed after 4Br-A-23187 was mediated by Cx32 HCs.
Fig. 8.
BAPTA-AM, a chelator of intracellular free Ca2+, prevents the increase in Etd uptake induced by MI, whereas 4Br-A-23187, a Ca2+ ionophore, increases Etd uptake in HeLa-Cx32 cells. A: rates of Etd uptake measured in subconfluent HeLa-Cx32 cell cultures preincubated for 1 h with DMSO [0.01% (vol/vol), Pre-veh] or with BAPTA-AM (5 μM, Pre-BAPTA-AM). Each bar is the average ± SE of 5 experiments. In each experiment, 20 cells were analyzed. At the end of each experiment 100 μM La3+ (MI+La3+) was added. *P < 0.05, **P < 0.01, ***P < 0.001, 1-way ANOVA with Newman-Keuls posttest. B: representative plot of Etd uptake over time obtained from HeLa-Cx32 cells in a subconfluent culture. Cells were exposed to 4-Br-A-23187 (5 μM) (indicated by vertical dashed line) and then to La3+ (100 μM) as indicated. C: rates of Etd uptake; each bar is the average ± SE of 10 cells. ***P < 0.001, 1-way ANOVA with Newman-Keuls posttest.
It should be noted that cells treated only with DMSO, the vehicle used to dissolve BAPTA-AM and 4Br-A-23187, showed a higher baseline uptake than cells under control conditions [0.4 AU/min (Fig. 8A) compared with 0.2 AU/min (Fig. 1, C and D, and Fig. 3, B and D)]. This could be explained by the fact that DMSO has been shown to increase [Ca2+]i (42), which could increase the activity of HCs without inducing MI. However, on exposure to MI an even greater increase in uptake occurred, concomitant with a larger increase in [Ca2+]i (Fig. 8A).
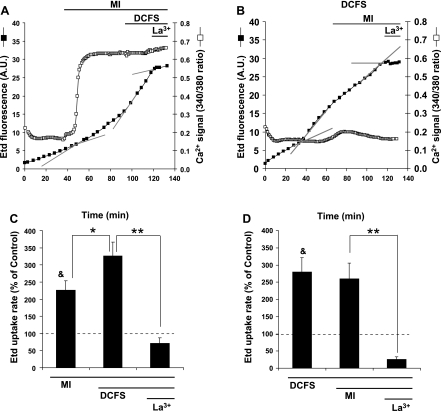
Increase in membrane permeability induced by MI can be potentiated by low extracellular concentration of divalent cations.
Exposure to DCFS has been shown to increase the activity of Cx32 HCs (17). To assess whether Cx32 HCs of cells under MI are sensitive to extracellular divalent cations, Etd uptake was measured in HeLa-Cx32 cells under MI and was followed by exposure to a DCFS. After ∼10 min of MI, Etd uptake increased approximately twofold. Subsequent exposure to a DCFS produced a further increase of ∼40% that occurred rapidly, with little delay (Fig. 9, A and B). The different time courses of the these responses suggests that MI increases HC activity by increasing the levels of HCs in the cell surface, whereas removal of divalent cations increases HC activity by a different mechanism, most likely by enhancing the open probability of existing HCs, as suggested by Gómez-Hernández et al. (17). When we reversed the order of these two treatments, i.e., removed divalent cations first and then exposed cells to MI while maintaining low divalent cations, we observed that Etd uptake rose rapidly on removal of extracellular divalent cations, but subsequent exposure to MI did not produce a further increase in Etd uptake (Fig. 9, C and D). Treatment with La3+ reduced Etd uptake induced by either MI followed by DCFS or DCFS followed by MI, indicating that under both conditions Etd uptake was mediated by Cx32 HCs (Fig. 9). When DCFS followed MI, the lack of a significant rise in [Ca2+]i was consistent with removal of the source of Ca2+, namely, the extracellular solution. Thus, the lack of effect of MI on the Etd uptake in cells bathed in DCFS (Fig. 9D) can be explained by the absence of Ca2+ influx that is required to induce Cx32 HC activity when cells are exposed to MI (Fig. 9D). As can be seen in Fig. 9, C and D, MI exposure following DCFS actually caused a small decrease in Etd uptake. Antimycin A, which we used as a metabolic inhibitor in these studies, has been proposed to be a gap junction blocker (27, 36). However, acute application of antimycin A, at the concentration used to induce MI in this study, caused only a small inhibition of Cx32 HCs evidenced by a small reduction in Etd uptake in HeLa-Cx32 cells that was not statistically significant (Supplemental Fig. S1).1 However, the tendency to inhibit Cx32 HCs might explain the smaller than expected (∼1.4-fold) increase in Etd uptake caused by DCFS in cells under MI (Fig. 9B) compared with the ∼2.8-fold increase induced under control conditions (Fig. 9D).
Fig. 9.
MI induces Ca2+ entry from the extracellular space but does not induce opening of all Cx32 HCs. A: plots of Etd uptake and changes in [Ca2+]i simultaneously in response to MI followed by exposure to a divalent cation-free solution (DCFS) under MI. La3+ (100 μM) was applied after exposure to DCFS. B: plots of Etd uptake and changes in [Ca2+]i simultaneously in response to DCFS followed by exposure to MI in DFCS. La3+ (100 μM) was applied after exposure to MI. C: average rates of Etd uptake obtained under MI alone, MI followed by DCFS, and after addition of La3+. Each bar represents the average ± SE of 8 experiments. D: average rates of Etd uptake rates obtained on exposure to DCFS alone, DCFS followed by MI, and after addition of La3+. Each bar represents the average ± SE of 10 experiments. Each experiment in B and D includes analysis of 15 cells. *P < 0.05, **P < 0.01, 1-way ANOVA with Newman-Keuls posttest. &P < 0.05 with respect to control.
Inhibition of Cx32 HCs reduces susceptibility of HeLa-Cx32 cells exposed to oxygen-glucose deprivation.
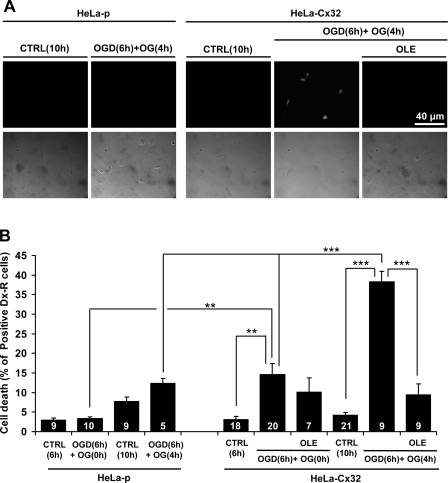
In HeLa-Cx43 and rat cortical astrocytes, death induced by MI was shown to be accelerated by enhanced Cx43 HC activity (8, 10). Moreover, OGD was shown to increase the membrane permeability of endothelial cells as well as cortical astrocytes, which express Cx43 HCs (33). We found that exposure to 6-h OGD followed by 1 h of reoxygenation caused a increase in the rate of Etd uptake in cultures of HeLa-Cx32 but not HeLa-p cells [0.47 ± 0.04 (n = 6) in HeLa-Cx32 vs. 0.21 (n = 1) in HeLa-p; each is average of Etd uptake rate for 15 cells; data not shown]. Therefore, we tested whether OGD followed by reoxygenation in glucose-containing saline induced death of HeLa-Cx32 cells. Exposure to 6-h hypoxia followed by 0-h reoxygenation increased the number of dead cells in HeLa-Cx32 but not HeLa-p cultures (Fig. 10). The increase in cell death was even more pronounced after 4-h reoxygenation, at which time the percentage of dead cells was ∼40% in HeLa-Cx32 and ∼12% in HeLa-p cultures, suggesting that Cx32 HCs reduce cell viability. Four hours of reoxygenation drastically reduced the viability of HeLa-Cx32 cells but caused only a small increase in cell death in cultures of HeLa-p cells (Fig. 10). Moreover, oleamide applied 2 h before reoxygenation completely prevented the increase in cell death observed in HeLa-Cx32 cultures but did not significantly affect cell viability in HeLa-p cultures (Fig. 10).
Fig. 10.
Necrosis induced by oxygen-glucose deprivation (OGD) in HeLa-Cx32 cells is prevented by oleamide. A: cultures of HeLa-p or HeLa-Cx32 cells were subjected to 6 h of OGD in the absence or presence of oleamide (Ole, 200 μM) applied 2 h before restoration of oxygen and glucose. Subsequently, cultures were exposed to solutions with glucose in the presence of ambient oxygen (OG) and incubated with dextran conjugated with rhodamine (Dex-Rd, 10 μM) for 5 min at time zero (0 h) or 4 h later (4 h) in the absence or presence of Ole (200 μM). The number of dead cells was quantified. Shown are examples of fluorescent (top) and corresponding bright-field (bottom) images taken under the conditions indicated. Cultures were kept under control conditions {saline solutions with glucose in presence of ambient oxygen for 6 h [CTRL (6h)] or 10 h [CTRL (10h)]}. B: % of cells stained with Dex-Rd (Dx-R, 10 μM) obtained in dissociated cultures of HeLa-p or HeLa-Cx32 cells. Each column represents the mean ± SE for the number of fields indicated, obtained from 5 independent experiments. **P < 0.01, ***P < 0.001, 1-way ANOVA with Newman-Keuls posttest.
DISCUSSION
In this work we show that MI, a simple model for in vitro ischemia-reperfusion, increases the activity of Cx32 HCs. In addition, we found that Cx32 HCs appear to be permeable to Ca2+ and that inhibitors of Cx32 HCs enhance the viability of cells subjected to OGD. Therefore, we suggest that ischemia-like conditions increase the activity of Cx32 HCs, which serve as a pathway for Ca2+ entry, thereby contributing to acceleration of cell death.
Under basal conditions, which includes physiological concentrations of extracellular divalent cations, Cx32 HCs appeared to remain predominantly closed because the rate of Etd uptake was low and not significantly reduced by La3+. In support of this, we rarely observed unitary events with conductance values corresponding to Cx32 HCs, particularly at hyperpolarized resting membrane potentials. Similar results were reported for Cx32 HCs comprised of human Cx32, which is highly homologous to mouse Cx32 (17). When we exposed HeLa-Cx32 cells to MI, cellular permeabilization mediated by Cx32 HCs increased despite the presence of extracellular divalent cations. This conclusion is supported by the following findings: 1) MI did not permeabilize HeLa-p cells, 2) the cellular levels of Cx32 protein and rate of Etd uptake in cells under MI showed a close positive correlation, 3) the MI-induced increase in Etd uptake was insensitive to blockers of P2x and TRPV1 channels, two other putative transmembrane pathways, 4) under MI, large unitary events consistent with those of Cx32 HCs were recorded in HeLa-Cx32 but not HeLa-p cells, 5) the rise in Cx32 levels at the cell surface (∼2-fold) was similar to the rise in Etd uptake and the increase in macroscopic membrane currents, and 6) the rise in free [Ca2+]i occurred in HeLa-Cx32 but not HeLa-p cells and was sensitive to HC blockers. Although macroscopic currents in HeLa-Cx32 cells under MI tended to increase on depolarization, consistent with opening of Cx32 HC, we often observed that macroscopic currents at hyperpolarized membrane potentials were increased compared with control HeLa-p cells (specifically during ramp protocols; Fig. 4D). These were always inhibited by La3+, whereas control cells displaying comparable leak currents were not. Thus, although Cx32 HCs tend to open with depolarization, they do function at negative voltages.
The amount of Cx32-forming HCs was ∼4% in cells under resting condition and was ∼8% after 60 min of MI. In rat cortical astrocytes, which express only Cx43 HCs, the amount of surface HCs was also reported to be low (∼15%) under control conditions and during MI increased to about twofold (37). The increase in Cx32 HC levels in the plasma membrane detected by biotinylation of surface proteins could have resulted from a reduced rate of internalization and/or enhanced insertion. Further studies will be required to elucidate which mechanism(s) is at play. The increase in Cx32 HC levels at the cell surface alone can account for the observed proportional increase in Etd uptake and in membrane currents, assuming no difference in the properties of the individual HCs under control and MI conditions. It is possible that the conductance and permeability properties of Cx32 HCs could have changed, perhaps as a consequence of posttranslational modification of Cx32-forming HCs during MI. However, this possibility is unlikely, given that the unitary conductance of Cx32 HCs in cells under MI was identical to that in cells under control conditions (∼90 pS). Moreover, the presence of unitary events characteristic of Cx32 HCs and their sensitivity to La3+, which does not block Pnx1 HCs (35), further supports our contention that Etd uptake was mediated by Cx32 HCs. We did observe that the increase in the rate of Etd uptake of HeLa-Cx32 cells exposed to extracellular DCFS under MI was lower (1.4-fold) than the 2.8-fold increase observed when HeLa-Cx32 cells were exposed to DCFS without MI. Although this inconsistency might be the result of differences in Cx HCs under control conditions and under MI, it could also be the result of a nonlinear relationship between levels of surface HCs and the fraction of HCs sensitive to DCFS. There is also an inhibitory effect of the metabolic inhibitor used, antimycin A. Nonetheless, further studies are needed to explain this finding.
During MI, we observed a decrease in the mitochondrial potential Ψm, which could explain the sharp decline in intracellular ATP levels in cells under MI (8). It has been proposed that dysfunctional mitochondria release Ca2+ (48). Thus MI induced by antimycin A, which blocks the respiratory chain, could produce a rise in [Ca2+]i via mechanisms related to mitochondrial dysfunction. Accordingly, we found that the decrease in Ψm was accompanied by an increase in [Ca2+]i. Additionally, the reduction in ATP levels, which could drastically reduce Ca2+ pump activity, could also contribute to the net rise in [Ca2+]i (46, 48). The lack of ATP can also inhibit the exchange of Na+ and K+ through ATPase, which can lead to cell depolarization. Therefore, activation of voltage-dependent Ca2+ channels might occur, and they may serve as a pathway for Ca2+ influx from the extracellular medium. However, we found that [Ca2+]i increased in HeLa-Cx32 cells, but not HeLa-p cells, and that the increase was prevented by inhibition of Cx32 HCs. Thus, although mechanisms related to Ca2+ extrusion from the cell interior and release from intracellular stores may contribute to the sustained rise in [Ca2+]i [as suggested by the very small increase in [Ca2+]i induced by MI in HeLa-p (Fig. 7A) or in HeLa Cx32 under MI in the absence of external Ca2+ (Fig. 9B)], our findings indicate that in HeLa-Cx32 cells under MI the main source of the increase in [Ca2+]i is Ca2+ influx through HCs. Under resting conditions Ca2+ influx is low, consistent with a low level of Cx32 HC activity and because the ATP-dependent extrusion mechanisms are functioning fully. Under MI, Ca2+ influx is increased because of an increase in HC activity and possibly because the extrusion mechanisms become partly compromised. During MI, we observed that [Ca2+]i reached a new steady state in ∼20 min, which might be explained by the involvement of Ca2+ binding sites (e.g., proteins and other molecules) with lower affinities compared with those operating under normal conditions and the possible existence of a lower Ca2+ level of extrusion activity maintained by the lower ATP levels. Clearly, in other cell types under MI additional membrane channels, including TRP channels, P2x, and NMDA receptors, could contribute to the Ca2+ influx (4, 12, 28, 41). However, in the HeLa cells used in this study, Cx32 HCs are the main route of Ca2+ entry. It is important to emphasize that levels of Cx32 HCs rise in the first 15 min of MI (Fig. 2D), just 5–10 min before the establishment of a new steady state of [Ca2+]i (Fig. 6C and 7), suggesting that Ca2+ influx into the cell is mediated by increasing HC numbers. After a critical time, the amount of Cx32 HCs is enough to induce a visible change in the rate of Etd uptake (Fig. 6C). Experiments with higher time resolution are needed to clarify this point. Cx32 HCs also might play a relevant role in several cell types that express Cx32 endogenously such as oligodendrocytes, hepatocytes, and lacrimal cells (40).
What could be the mechanism by which elevated [Ca2+]i increases Cx32 HC activity? One possibility involves protein kinases. It was reported that the activity of p38 MAP kinase is necessary for increased activity of Cx43 HCs induced by proinflammatory cytokines (38) and by FGF-1 or 4Br-A-23187 (43). In this work, preincubation with SB-203519 (an inhibitor of the p38 MAP kinase) partially prevented the increase in Etd uptake induced by MI (Supplemental Fig. S2), indicating that despite a fall in ATP levels during MI, p38 MAP kinase activity is needed to induce increased activity of Cx32 HCs, possibly by a Ca2+-dependent pathway (11, 43). The activity of p38 MAP kinase could be increased by [Ca2+]i increase (29) or as consequence of oxidant stress (20), which also occurs in cells under metabolic inhibition (37). It remains unknown whether p38 MAP kinase induces covalent changes in Cx32-forming HCs or whether it affects the vesicle trafficking pathway leading to an increase in the number of Cx32 HCs at the cell surface.
Cx32 forms poorly selective channels permeable to negatively charged molecules such as ATP (net charge: −3; Refs. 3, 7) as well as positively charged molecules, such as Etd (net charge: +1) and propidium (net charge: +2, Ref. 11). Thus it is conceivable that Cx32 HCs could be permeable to divalent cations such as Ca2+. Although extracellular Ca2+ regulates Cx32 HCs (17), the sensitivity is such that substantial HC activity can remain at physiological Ca2+ concentrations, thereby allowing for Ca2+ flux.
Agents that elevate [Ca2+]i have been proposed to favor ATP release to the extracellular environment through Cx32 HCs (11). This increase in ATP release might result from the rise in levels of surface Cx32 HCs and/or changes in biophysical properties of Cx32 HCs leading to higher ATP permeability. Here we show that BAPTA-AM, an intracellular Ca2+ chelator, drastically reduced MI-induced Etd uptake. This is consistent with the previously proposed importance of this second messenger in regulating activity of Cx32 HCs (11). Recently, it was shown that HeLa cells transfected with Cx43 or Cx45 show an increase in the levels of HCs in the cell surface after treatment with a Ca2+ ionophore (43). A similar mechanism might explain the increase in activity of Cx32 HCs (evaluated with Etd uptake) in response to 4Br-A-23187 since the response was similar to that induced by MI, where levels of Cx32 HCs increased about twofold.
What other factors could increase the activity of HCs during ischemia? As mentioned above, the levels of intracellular ATP drop sharply, and this occurs during the first 15 min of MI (8, 10), which is before a rise in Etd uptake takes place. ATP also drops during ischemia in various tissues, producing a lack of substrate for protein kinases. Protein subunits of HCs including Cx32 and Cx43 are phosphoproteins (39, 44), and the open probability of at least Cx43 HCs increases on dephosphorylation (5, 24). A similar mechanism might affect Cx32 HCs, but further studies will be required for its demonstration.
The protective effect of oleamide on the survival of cells exposed to OGD followed by reoxygenation reveals an important role of HCs in the process of cell death. Cell death is induced by the insult (OGD and reoxygenation), and an increased Cx32 HC activity could enhance the cells' susceptibility to the insult by disruption of the electrochemical gradients across the plasma membrane. Therefore, inhibition of Cx32 HCs in tissues expressing Cx32 that are affected by ischemia-reperfusion might increase cell viability. Inhibition of Cx32 HCs should prevent or reduce the influx of Ca2+, which activates intracellular pathways related to cell death including proteases, lipases, and nucleases (1, 4).
In conclusion, our results indicate that HeLa-Cx32 cells present functional Cx32 HCs under resting conditions, but during MI HC activity increases mainly because of an increase in HCs at the cell surface. This change in the number of functional HCs increases the permeability of the cell membrane and could accelerate cell death processes induced by episodes of hypoxia-reoxygenation.
GRANTS
This work was supported by FONDECYT Grant 1070591, FONDEF Grant D07I1086, and Núcleo Milenio de Inmunología e Inmunoterapia PO4/03-F (to J. C. Sáez) and National Institute of General Medical Sciences Grant GM-54179 (to V. K. Verselis). H. A. Sánchez received a Comisión Nacional de Investigación Científica y Tecnológica fellowship for graduate studies.
Supplementary Material
ACKNOWLEDGMENTS
Data presented in this work are from a thesis presented as partial fulfillment of the requirement for the degree of Doctor in Biological Sciences (H. A. Sánchez) in the Pontificia Universidad Católica de Chile.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Ankarcrona M, Dypbukt JM, Orrenius S, Nicotera P. Calcineurin and mitochondrial function in glutamate-induced neuronal cell death. FEBS Lett 394: 321–324, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Araya R, Riquelme MA, Brandan E, Sáez JC. The formation of skeletal muscle myotubes requires functional membrane receptors activated by extracellular ATP. Brain Res Rev 47: 174–188, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Arcuino G, Lin JH, Takano T, Liu C, Jiang L, Gao Q, Kang J, Nedergaard M. Intercellular calcium signaling mediated by point-source burst release of ATP. Proc Natl Acad Sci USA 99: 9840–9845, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bano D, Nicotera P. Ca2+ signals and neuronal death in brain ischemia. Stroke 38: 674–676, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Bao X, Lee SC, Reuss L, Altenberg GA. Change in permeant size selectivity by phosphorylation of connexin 43 gap-junctional hemichannels by PKC. Proc Natl Acad Sci USA 104: 4919–4924, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett MV, Contreras JE, Bukauskas FF, Sáez JC. New roles for astrocytes: gap junction hemichannels have something to communicate. Trends Neurosci 26: 610–617, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braet K, Aspeslagh S, VanDamme W, Willecke K, Martin PEM, Evans WH, Leybaert L. Pharmacological sensitivity of ATP release triggered by photoliberation of inositol-1,4,5-trisphosphate and zero extracellular calcium in brain endothelial cells. J Cell Physiol 197: 205–213, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Contreras JE, Sánchez HA, Eugenín EA, Speidel D, Theis M, Willecke K, Bukauskas FF, Bennett MVL, Sáez JC. Metabolic inhibition induces opening of unapposed connexin43 gap junctions hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc Natl Acad Sci USA 99: 495–500, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Contreras JE, Sáez JC, Bukauskas FF, Bennett MVL. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc Natl Acad Sci USA 100: 11388–11393, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Contreras JE, Sánchez HA, Véliz LP, Bukauskas FF, Bennett MVL, Sáez JC. Role of connexin-based gap junction channels and hemichannels in ischemia-induced cell death in nervous tissue. Brain Res Rev 47: 290–303, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Vuyst E, Decrock E, Cabooter L, Dubyak GR, Naus CC, Evans WH, Leybaert L. Intracellular calcium changes trigger connexin 32 hemichannel opening. EMBO J 25: 34–44, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donato R, Rodrigues RJ, Takahashi M, Tsai MC, Soto D, Miyagi K, Villafuertes RG, Cunha RA, Edwards FA. GABA release by basket cells onto Purkinje cells, in rat cerebellar slices, is directly controlled by presynaptic purinergic receptors, modulating Ca2+ influx. Cell Calcium 44: 521–532, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Dobrowolski R, Willecke K. Connexin-caused diseases and corresponding mouse models. Antioxid Redox Signal 11: 283–295, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Evans WH, De Vuyst E, Leybaert L. The gap junction cellular internet: connexin hemichannels enter the signalling limelight. Biochem J 397: 1–14, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiskum G, Danilov CA, Mehrabian Z, Bambrick LL, Kristian T, McKenna MC, Hopkins I, Richards EM, Rosenthal RE. Postischemic oxidative stress promotes mitochondrial metabolic failure in neurons and astrocytes. Ann NY Acad Sci 1147: 129–38, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franke H, Krugel U, Illes P. P2 receptors and neuronal injury. Pflügers Arch 452: 622–644, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Gómez-Hernández JM, De Miguel M, Larrosa B, González D, Barrio LC. Molecular basis of calcium regulation in connexin-32 hemichannels. Proc Natl Acad Sci USA 100: 16030–16035, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexons channels. Nat Rev Mol Cell Biol 4: 285–294, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Gores GJ, Flarsheim CE, Dawson TL, Nieminen AL, Herman B, Lemasters JJ. Swelling, reductive stress, and cell death during chemical hypoxia in hepatocytes. Am J Physiol Cell Physiol 257: C347–C354, 1989 [DOI] [PubMed] [Google Scholar]
- 20.Guyton KZ, Liu Y, Gorospe M, Xu Q, Holbook NJ. Activation of mitogen-activated protein kinase by H2O2. Role in cell survival following oxidant injury. J Biol Chem 271: 4138–4142, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Harris AL. Connexin channel permeability to cytoplasmic molecules. Prog Biophys Mol Biol 94: 120–143, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holtsberg FW, Steiner MR, Keller JN, Mark RJ, Mattson MP, Steiner SM. Lysophosphatidic acid induces necrosis and apoptosis in hippocampal neurons. J Neurochem 70: 66–76, 1998 [DOI] [PubMed] [Google Scholar]
- 23.John SA, Kondo R, Wang SY, Goldhaber JI, Weiss JN. Connexin-43 hemichannels opened by metabolic inhibition. J Biol Chem 274: 236–240, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Kim DY, Kam Y, Koo SK, Joe CO. Gating connexin 43 channels reconstituted in lipid vesicles by mitogen-activated protein kinase phosphorylation. J Biol Chem 274: 5581–5587, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Kondo RP, Wang SY, John SA, Weiss JN, Goldhaber JI. Metabolic inhibition activates a nonselective current through connexin hemichannels in isolated ventricular myocytes. J Mol Cell Cardiol 32: 1859–1872, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Kulkarni GV, Lee W, Seth A, McCulloch CAG. Role of mitochondrial membrane potential in concanavalin A-induced apoptosis in human fibroblasts. Exp Cell Res 245: 170–178, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Martínez AD, Sáez JC. Regulation of astrocyte gap junctions by hypoxia-reoxygenation. Brain Res Rev 32: 250–258, 2000 [DOI] [PubMed] [Google Scholar]
- 28.McNulty S, Fonfria E. The role of TRPM channels in cell death. Pflügers Arch 451: 235–242, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Mu D, Zhang W, Chu D, Liu T, Xie Y, Fu E, Jin F. The role of calcium, p38 MAPK in dihydroartemisinin-induced apoptosis of lung cancer PC-14 cells. Cancer Chemother Pharmacol 61: 639–645, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Musil LS, Goodenough DA. Biochemical analysis of connexin43 intracellular transport, phosphorylation, and assembly into gap junctional plaques. J Cell Biol 115: 1357–1374, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Musil LS, Goodenough DA. Multisubunit assembly of an integral plasma membrane channel protein, gap junction connexin43, occurs after exit from the ER. Cell 74: 1065–1077, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Myrdal SE, Steyger PS. TRPV1 regulators mediate gentamicin penetration of cultured kidney cells. Hear Res 204: 170–182, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orellana JA, Sáez PJ, Shoji KF, Schalper KA, Palacios-Prado N, Velarde V, Giaume C, Bennett MVL, Sáez JC. Modulation of brain hemichannels and gap junction channels by pro-inflammatory agents and their possible role in neurodegeneration. Antioxid Redox Signal 11: 369–399, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paul DL, Ebihara L, Takemoto LJ, Swenson KI, Goodenough DA. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J Cell Biol 115: 1077–1089, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J 25: 5071–5082, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plaisance I, Duthe F, Sarrouille D, Hervé JC. The metabolic inhibitor antimycin A can disrupt cell-to-cell communication by an ATP- and Ca2+-independent mechanism. Pflügers Arch 447: 181–194, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Retamal MA, Cortés CJ, Reuss L, Bennett MV, Sáez JC. S-nitrosylation and permeation through connexin 43 hemichannels in astrocytes: induction by oxidant stress and reversal by reducing agents. Proc Natl Acad Sci USA 103: 4475–4480, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Retamal MA, Froger N, Palacios-Prado N, Ezan P, Sáez PJ, Sáez JC, Giaume C. Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J Neurosci 27: 13781–13792, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sáez JC, Nairn AC, Czernik AJ, Spray DC, Hertzberg EL, Greengard P, Bennett MVL. Phosphorylation of connexin 32, a hepatocyte gap-junction protein, by cAMP-dependent protein kinase, protein kinase C and Ca2+/calmodulin-dependent protein kinase II. Eur J Biol Chem 271: 3779–3786, 1990 [DOI] [PubMed] [Google Scholar]
- 40.Sáez JC, Berthoud VM, Brañes MC, Martínez AD, Beyer EC. Plasma membrane channels formed by connexins, their regulation and functions. Physiol Rev 83: 1359–1400, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Samways DSK, Egan TA. Acidic amino acids impart enhanced Ca2+ permeability and flux in two members of the ATP-gated P2X receptor family. J Gen Physiol 129: 245–256, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santos NC, Figueira-Coelho J, Martin-Silva J, Saldanha C. Multidisciplinary utilization of dimethyl sulfoxide: pharmacological, cellular, and molecular aspects. Biochem Pharmacol 65: 1035–1041, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Schalper KA, Palacios-Prado N, Retamal MA, Shoji KF, Martinez AD, Sáez JC. Connexin hemichannel composition determines the FGF-1-induced membrane permeability and free [Ca2+]i responses. Mol Biol Cell 19: 3501–3513, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Solan JL, Lampe PD. Connexin phosphorylation as a regulatory event linked to gap junction channel assembly. Biochim Biophys Acta 1711: 154–163, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Suadicani SO, Brosnan CF, Scemes E. P2X7 receptors mediate ATP release astrocytic intercellular Ca2+ signaling. J Neurosci 26: 1378–1385, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanaka E, Niiyama S, Sato S, Yamada A, Higashi H. Arachidonic acid metabolites contribute to the irreversible depolarization induced by in vitro ischemia. J Neurophysiol 90: 3213–3223, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Vergara L, Bao X, Cooper M, Bello-Reuss E, Reuss L. Gap-junctional hemichannels are activated by ATP depletion in human renal proximal tubule cells. J Membr Biol 196: 173–184, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto S, Tanaka E, Shoji Y, Kudo Y, Inokuchi H, Higashi H. Factors that reverse the persistent depolarization produced by deprivation of oxygen and glucose in rat hippocampal CA1 neurons in vitro. J Neurophysiol 78: 903–911, 1997 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.