Abstract
Fibronectin plays important roles in erythropoiesis through the fibronectin receptors VLA-4 and VLA-5. However, the substantial role of these fibronectin receptors and their functional assignment in erythroid differentiation are not yet fully understood. Here, we investigated the effects of cell adhesion to fibronectin on erythroid differentiation using K562 human erythroid progenitor cells. Erythroid differentiation could be induced in K562 cells in suspension by stimulating with hemin. This hemin-stimulated erythroid differentiation was highly accelerated when cells were induced to adhere to fibronectin by treatment with TNIIIA2, a peptide derived from tenascin-C, which has recently been found to induce β1-integrin activation. Another integrin activator, Mn2+, also accelerated hemin-stimulated erythroid differentiation. Adhesive interaction with fibronectin via VLA-4 as well as VLA-5 was responsible for acceleration of the hemin-stimulated erythroid differentiation in response to TNIIIA2, although K562 cells should have been lacking in VLA-4. Adhesion to fibronectin forced by TNIIIA2 causally induced VLA-4 expression in K562 cells, and this was blocked by the RGD peptide, an antagonist for VLA-5. The resulting adhesive interaction with fibronectin via VLA-4 strongly enhanced the hemin-stimulated activation of p38 mitogen-activated protein kinase, which was shown to serve as a signaling molecule crucial for erythroid differentiation. Suppression of VLA-4 expression by RNA interference abrogated acceleration of hemin-stimulated erythroid differentiation in response to TNIIIA2. Thus, VLA-4 and VLA-5 may contribute to erythropoiesis at different stages of erythroid differentiation.
Hematopoietic stem and progenitor cells proliferate and differentiate in the bone marrow and fetal liver (1–6). Stromal cells of the bone marrow and fetal liver form a hematopoietic microenvironment called a “niche.” This microenvironment niche plays a crucial role in the regulation of the proliferation and differentiation of hematopoietic stem and progenitor cells. Besides humoral factors that include hematopoietic growth factors, adhesive interaction of hematopoietic stem and progenitor cells with stromal cells and/or the extracellular matrix (ECM)2 in the hematopoietic microenvironment is indispensable for hematopoietic development (1–6). The ECM in the hematopoietic microenvironment is composed of various macromolecules, such as fibronectin (FN), collagens, laminins, and proteoglycans. Among them, FN is one of the most important parts of the microenvironment niche (7–11). Also, in erythropoiesis, the importance of the adhesion of erythroid progenitors to FN via the FN receptors VLA-4 and VLA-5 has been reported (11–16). However, the substantial role of these FN receptors and their functional assignment in erythroid differentiation are not yet fully understood.
We previously found that FN, which provides scaffolding for the adhesion of various cell types, has an alternative functional site opposing cell adhesion (17). A 22-mer peptide derived from the 14th FN type III-like (FNIII) repeat of the FN molecule, termed FNIII14, strongly suppresses cell adhesion to FN by inhibiting the activation of β1-integrins including VLA-4 and VLA-5 (18, 19). Conversely, we have recently found that tenascin (TN)-C, which is an anti-adhesive ECM protein (20, 21), has a functional site for stimulating cell adhesion to FN (22). A 22-mer peptide derived from the FNIII repeat A2 in the TN-C molecule, termed TNIIIA2, can induce the conformational change necessary for functional activation of FN receptors through binding with syndecan-4 (22, 23). The active sites of FNIII14 and TNIIIA2 appear to be cryptic in the molecular structures of FN and TN-C but are exposed by conformational change through interaction with other ECM molecules or by processing with matrix metalloproteinase-2 (22, 24). Thus, these functional sites found in FN and TN-C molecules, which act in opposition to their parental ECM proteins, may act as a negative feedback loop for preventing excessive cellular responses to these ECM proteins in biological processes with ECM rearrangement. In any case, FNIII14 and TNIIIA2 enable us to control, either negatively or positively, the adhesion of various cell types to FN.
Various hematopoietic progenitor cell lines have been used in in vitro studies of hematopoietic differentiation. However, most hematopoietic progenitor cell lines are nonadherent, because their cell surface β1-integrins, including FN receptors, have impaired ligand-binding activity (25, 26). Therefore, in order to investigate the role of cell adhesion to FN in hematopoietic differentiation, their FN receptors must be activated. Since TNIIIA2 can induce activation of FN receptors in various hematopoietic progenitor cell lines (22), this peptide factor may be useful for investigating the substantial role of cell adhesion to FN in hematopoietic differentiation. Here, we investigate the effects of cell adhesion to FN on erythroid differentiation using TNIIIA2 and Mn2+ as the integrin activator and the human erythroid progenitor cell line K562, which only expresses VLA-5, as the FN receptor (27). As a result, we show that hemin-stimulated erythroid differentiation of K562 cells is strongly enhanced when K562 cells are forced to adhere to FN. Sustained adhesion to FN via VLA-5, which is induced by TNIIIA2 or Mn2+, causes induction of VLA-4 expression. The resulting adhesive interaction with FN via newly expressed VLA-4 then generates a conspicuous increase in the hemin-stimulated phosphorylation/activation of p38 MAP kinase, which is shown to serve as a signaling molecule crucial for erythroid differentiation of K562 cells.
EXPERIMENTAL PROCEDURES
Materials
Synthetic peptides, such as CS-1 (LHPGEILDVPST), RGD (GRGDSP), TNIIIA2 (RSTDLPGLKAATHYTITIRGVC), and its control TNIIIA2scr (RSTDLPGLKAATHTVYRIGIC) (22), and FNIII14 (19) and its inactive mutant FNIII14scr (19) were obtained from Operon Biotechnology (Tokyo, Japan). Antibodies (Abs) to ERK1/2, phospho-ERK1/2 (Tyr202/Tyr204), p38, and phospho-p38 were obtained from Cell Signaling Technology. Function-blocking monoclonal antibodies (mAbs) against integrin subunits α4 (P1H4) and α5 (P1D6) were purchased from Chemicon. A mAb recognizing the active conformation of integrin subunit β1, AG89 (28), was purchased from MBL (Nagoya, Japan). Specific inhibitors for MEK and p38, PD98059, and SB203580, respectively, were purchased from Calbiochem. A specific inhibitor of JNK, SP600125, was obtained from Cayman Chemical Co. Hemin and 3,3-dimethoxybenzidine (o-dianisidine) were purchased from Sigma.
Cell Culture
K562 cells were grown in RPMI 1640 medium containing 10% fetal bovine serum, glutamate (2 mm), kanamycin sulfate (60 μg/ml), amphotericin B (200 ng/ml), and NaHCO3 (10 mm). Cells were stimulated to induce erythroid differentiation by culture in RPMI 1640 medium containing 10% fetal bovine serum with hemin (50 μm) in the absence or presence of various factors to be tested. After culturing for the indicated periods, cells were washed and incubated with a solution containing o-dianisidine (0.04%) and hydrogen peroxide (0.3%) at room temperature for 1 h to stain hemoglobin-positive cells (29). Hemoglobin-positive cells were counted microscopically at ×100 magnification in four different areas as differentiated cells. The total number of cells in the same area was similarly counted. Data represent the percentage of the number of hemoglobin-positive cells relative to the total number of cells.
Cell Adhesion Assay
The cell adhesion assay was performed as described previously (22) with some modifications. Briefly, cells (1 × 104) suspended in serum-free medium with or without Mn2+ (0.5 mm), FNIII14 or its control FNIII14scr, TNIIIA2 or its control TNIIIA2scr, and their combination were seeded in a 96-well plate coated with FN in the absence or presence of antagonists for VLA-4 and VLA-5, as indicated. Cells were incubated for 1 h at 37 °C and then fixed with formalin. The total number of cells seeded into the well was counted microscopically at ×100 magnification in four different areas. After washing away the unadhered cells, the adhered cells in the same area were similarly counted. Data represent the percentage of the number of adhered cells relative to the total number of cells seeded into the well.
Flow Cytometric Analysis
The activation status of β1-integrins on the cell surface was evaluated by flow cytometric analysis, as described previously (22), using FITC-labeled anti-β1-integrin, AG89, which recognizes an active β1-integrin-specific epitope (28) at 4 °C for 30 min.
To determine the expression of integrin subunits during erythroid differentiation, cell suspension containing hemin (50 μm) with or without TNIIIA2 was cultured in the presence or absence of an antagonist for FN receptors. After culturing for the indicated period, cells were collected with PBS(−) containing 50 mm EDTA; washed; incubated with normal IgG, anti-integrin α4 mAb (P1H4), or α5 mAb (P1D6); and then incubated with FITC-labeled secondary Ab. The percentage of integrin-positive cells was determined by setting electronic gates to exclude 95% of positive cells (fluorescent) in the isotype controls.
Western Blotting
Cells were lysed in Laemmli buffer containing 2 mm EDTA, 1 mm phenylmethylsulfonyl fluoride, 50 mm sodium fluoride, 10 mm sodium orthovanadate, 5 μg/ml aprotinin, and 10 μg/ml leupeptin and analyzed by immunoblotting using enhanced chemiluminescence (GE Healthcare), as described previously (22).
Protein Knockdown by RNA Interference
siRNAs of integrin α4 subunit (5′-AACUAUCAACGAAAGAACUGGTT-3′; GenBankTM accession number NM000885.4) (Sigma), p38 (5′-GCCCAUAAGGCCAGAAACUTT-3′; GenBankTM accession number NM001315.2) (Cell Signaling Technologies), and the negative control siRNA (Sigma) were obtained as noted. The siRNAs were transfected into cells by using Transmessenger (Qiagen), and the cells were used for experiments 2 days after transfection.
RESULTS
Positive and Negative Regulation of K562 Cell Adhesion to FN
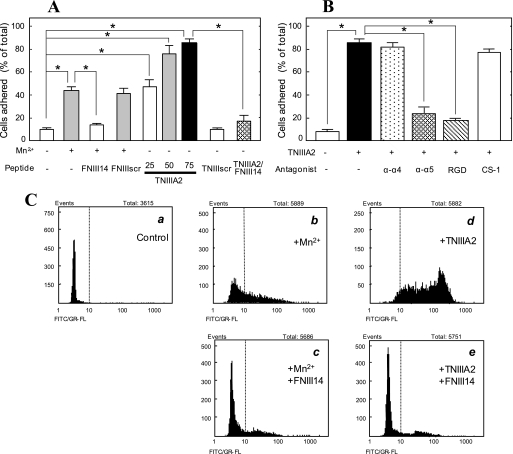
K562 cells are incapable of adhering to FN, because of their inactivated integrins (22, 25–27). When these cells were stimulated on the FN substrate with Mn2+, an integrin activator (25, 26), the cells adhered to FN (Fig. 1A). FNIII14 (19) inhibited this Mn2+-induced K562 cell adhesion to FN, whereas FNIII14scr, which has a shuffled FNIII14 amino acid sequence (19), did not (Fig. 1A). Conversely, TNIIIA2 induced the adhesion of K562 cells to FN without Mn2+ in a dose-dependent manner (Fig. 1A). TNIIIA2scr, in which the active amino acid sequence of TNIIIA2 is shuffled (22), had no proadhesive activity (Fig. 1A). These negative and positive modulations of K562 cell adhesion to FN by these peptides were due to their effects on the activation of β1-integrins. An active conformation-specific epitope of β1-integrin, which can be detected by mAb AG89 (28), was exposed by treating cells with TNIIIA2 (Fig. 1C, d) as well as Mn2+ (b) but buried by treatment with FNIII14 (c and e). TNIIIA2-induced adhesion of K562 cells to FN was reversed by the addition of VLA-5 antagonists, anti-α5 mAb, and RGD peptide (GRGDSP) but not by the addition of VLA-4 antagonists, anti-α4 mAb, and CS-1 peptide (Fig. 1B).
FIGURE 1.
Adhesion of K562 cells to FN through β1-integrin activation. A, K562 cell suspension with or without Mn2+ (0.5 mm), FNIII14 or its control FNIII14scr (100 μg/ml), TNIIIA2 (25–75 μg/ml) or its control TNIIIA2scr (100 μg/ml), or a combination of these was seeded in a 96-well plate coated with the FN (5 μg/ml). B, K562 cell suspension with or without TNIIIA2 (75 μg/ml) was seeded in the FN-coated plate in the presence of the function-blocking mAb to integrin α4 or α5 subunit (20 μg/ml) or RGD or CS-1 peptide (200 μg/ml). An adhesion assay was performed in A and B, as described under “Experimental Procedures.” The ordinates show the percentage of the number of adhered cells, relative to the total number of cells seeded into the well. Each point represents the mean ± S.E. of triplicate determinations. Data are representative of three individual experiments. *, p < 0.01 compared with control. C, flow cytometric analysis of β1-integrin activation in K562 cells. Cells suspended in serum-free medium were incubated at 37 °C for 30 min with occasional shaking under the following conditions. a, none; b, Mn2+ (0.5 mm); c, Mn2+ (0.5 mm) plus FNIII14 (100 μg/ml); d, TNIIIA2 (50 μg/ml); e, TNIIIA2 (50 μg/ml) plus FNIII14 (100 μg/ml). Flow cytometric analyses were performed using the FITC-labeled normal mouse IgG (a) or FITC-labeled anti-β1 integrin mAb, AG89 (b–e), as described under “Experimental Procedures.” Data shown are representative of three individual experiments.
Adhesion to FN Accelerates Hemin-stimulated Erythroid Differentiation of K562 Cells
Like most hematopoietic progenitor cell lines, K562 cells can grow in suspension without adhering to the ECM. To assess the effect of adhesion to FN on cellular differentiation of K562 cells, exponentially growing K562 cells were stimulated with hemin in the presence or absence of TNIIIA2. Erythroid differentiation was evaluated by counting the cells expressing hemoglobin. When K562 cells were cultured with hemin (50 μm) in the absence of TNIIIA2, nearly 40% of cells became hemoglobin-positive after culture for 3 days (Fig. 2, A (b) and B). Stimulation with hemin at higher concentrations did not cause a further increase in the proportion of hemoglobin-positive cells but reduced the viability of K562 cells (data not shown). Most cells remained unadhered throughout the stimulation with hemin.
FIGURE 2.
Effect of induced adhesion of K562 cells on their erythroid differentiation stimulated by hemin. Erythroid differentiation was stimulated by hemin (50 μm) in K562 cells in the presence or absence of TNIIIA2 (75 μg/ml). After culturing for 3 days (A) or the indicated periods (B), cells were stained with o-dianisidine, as described under “Experimental Procedures.” One of three individual experiments is shown in A. The percentage of the number of hemoglobinized cells, relative to the total number of cells, is shown in B. Data represent the mean ± S.E. of triplicate determinations. *, p < 0.05 compared with control.
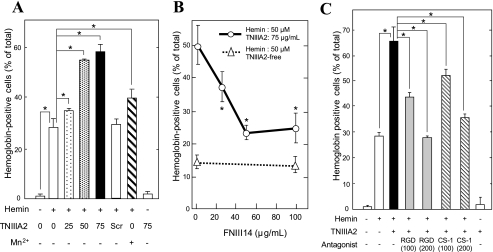
Next, erythroid differentiation of K562 cells was stimulated with hemin in the presence of TNIIIA2, in which cells were induced to adhere to the FN in response to TNIIIA2. As a result, a considerable number of hemoglobin-positive cells appeared after stimulation for only 1 day, and more than 70% of cells were hemoglobinized after culture for 3 days (Fig. 2, A (c) and B). Throughout the culture, most cells adhered to the FN substrate, indicating that TNIIIA2 is capable of maintaining the FN receptor activated for a long period. TNIIIA2 accelerated hemin-stimulated erythroid differentiation in a dose-dependent manner (Fig. 3A). TNIIIA2scr, which had no proadhesive activity (see Fig. 1A), could not accelerate hemin-stimulated differentiation (Fig. 3A). Mn2+, which induced K562 cell adhesion to FN (see Fig. 1A), was capable of accelerating hemin-stimulated erythroid differentiation to a lesser extent than TNIIIA2 (Fig. 3A). Stimulation of cells with TNIIIA2 alone, without hemin, induced adhesion to FN but not hemoglobinization in K562 cells (Fig. 3A).
FIGURE 3.
Adhesion-dependent acceleration of hemin-stimulated erythroid differentiation of K562 cells. A, cell suspension with or without hemin (50 μm) were seeded into a 96-well plate coated with FN (5 μg/ml) and cultured in the presence of TNIIIA2 (0–75 μg/ml), TNIIIA2scr (75 μg/ml), or Mn2+ (0.5 mm). B, erythroid differentiation was stimulated by hemin (50 μm) (open triangles with a dotted line) or by hemin plus TNIIIA2 (75 μg/ml) (open circles with a solid line) in the presence of FNIII14 at the indicated concentrations. C, effects of antagonists for VLA-5 (RGD peptide) and VLA-4 (CS-1 peptide) on the acceleration of hemin-stimulated erythroid differentiation in response to TNIIIA2. Erythroid differentiation was stimulated with hemin (50 μm) in the presence of TNIIIA2 (75 μg/ml) with or without the RGD peptide (100 and 200 μg/ml) or the CS-1 peptide (100 and 200 μg/ml). After culture for 3 days, hemoglobinized cells were stained with o-dianisidine as described under “Experimental Procedures.” Data represent the mean ± S.E. of triplicate determinations. One of three individual experiments is shown. *, p < 0.05 compared with control.
On the other hand, FNIII14, which was capable of reversing forced adhesion of K562 cells induced by Mn2+ or TNIIIA2 (see Fig. 1A), alone showed no effect on the hemin-stimulated erythroid differentiation of K562 cells in suspension (Fig. 3B). However, accelerated differentiation of K562 cells, which was stimulated by hemin in combination with TNIIIA2, was reversed by the addition of FNIII14, depending on its increased concentration (Fig. 3B). This decrease in erythroid differentiation by the addition of FNIII14 was accompanied by a decrease in the number of cells adhered to FN (data not shown). Thus, acceleration of hemin-stimulated erythroid differentiation in response to TNIIIA2 resulted from adhesion of K562 cells to FN.
Involvement of VLA-4 Expression in the Adhesion-dependent Acceleration of Hemin-stimulated Erythroid Differentiation
We next investigated whether VLA-5, the only FN receptor expressed on K562 cells, contributed to the acceleration of hemin-stimulated erythroid differentiation in response to TNIIIA2. As expected, acceleration of hemin-stimulated erythroid differentiation in response to TNIIIA2 was attenuated by the addition of the RGD peptide, a VLA-5 antagonist (Fig. 3C), in which TNIIIA2-induced adhesion of K562 cells was also attenuated (data not shown). Unexpectedly, acceleration of hemin-stimulated erythroid differentiation in response to TNIIIA2 was also reduced with the CS-1 peptide, a VLA-4 antagonist (Fig. 3C), although K562 cells should have been lacking in VLA-4 (27).
We therefore examined the expression of integrin subunits and their changes during erythroid differentiation of K562 cells by flow cytometric analysis (Fig. 4). Consistent with general knowledge (27), K562 cells highly expressed integrin α5 (Fig. 4n) and β1 (data not shown), but barely expressed integrin α4 (Fig. 4d). No remarkable changes in the expression of α4 (Fig. 4, e and h) and β1 were observed during the hemin-stimulated erythroid differentiation of K562 cells in suspension. When erythroid differentiation was stimulated by hemin under adhesive conditions (in the presence of TNIIIA2 or Mn2+) (see Figs. 1 and 3), α4 became detectable 1 day after stimulation (Fig. 4, f for Mn2+ and g for TNIIIA2) and further increased remarkably 3 days after the stimulation (Fig. 4, i for Mn2+ and j for TNIIIA2). Induction of the α4 expression was detectable in K562 cells stimulated with TNIIIA2 alone without hemin (m), whereas this was not accompanied by hemoglobinization (Fig. 3, A and C). Induction of the α4 expression was abolished when erythroid differentiation was stimulated by hemin and TNIIIA2 in the presence of the RGD peptide (k). In contrast, the CS-1 peptide did not influence induction of the α4 expression (l), although it abrogated acceleration of hemin-stimulated erythroid differentiation in response to TNIIIA2 (Fig. 3C). The integrin subunit β7 was not detected on K562 cells either before or after the stimulation with hemin and TNIIIA2 (data not shown), suggesting that integrin α4 is probably expressed as VLA-4 on K562 cell surfaces.
FIGURE 4.
Induction of VLA-4 expression in adhesion-dependent acceleration of hemin-stimulated erythroid differentiation in response to TNIIIA2. Expression of cell surface integrins on K562 cells was analyzed by flow cytometry. Cells were stimulated as follows: isotype control of cells untreated (a); isotype control of cells stimulated with hemin (50 μm) plus TNIIIA2 (75 μg/ml) (b and c); hemin alone (e, h, and o); hemin plus Mn2+ (0.5 mm) (f and i); hemin plus TNIIIA2 (g, j, and p); hemin plus TNIIIA2 and the RGD peptide (200 μg/ml) (k and q); hemin plus TNIIIA2 and the CS-1 peptide (200 μg/ml) (l); TNIIIA2 alone (m). After culture for the indicated number of days, expression of integrin α4 and α5 was analyzed using normal IgG (a–c), anti-α4 mAb (d–m), and anti-α5 mAb (n–q), and the proportion of integrin-positive cells was determined, as described under “Experimental Procedures.” Data shown are representative of three individual experiments.
To clarify the necessity of VLA-4 expression in the acceleration of hemin-stimulated erythroid differentiation in response to TNIIIA2, K562 cells transfected with control siRNA or integrin α4 siRNA were subjected to examination for erythroid differentiation. Hemin-stimulated erythroid differentiation was not influenced by transfection with α4 siRNA, whereas its acceleration in response to TNIIIA2 was markedly attenuated by transfection with α4 siRNA (Fig. 5A). In these cells, induction of α4 expression was strongly suppressed (Fig. 5B). K562 cells transfected with control siRNA, in which induction of the α4 expression was evident (Fig. 5B), normally underwent erythroid differentiation in response to hemin with or without TNIIIA2 (Fig. 5A). These results suggest that induction of VLA-4 expression is needed for the acceleration of hemin-stimulated erythroid differentiation of K562 cells.
FIGURE 5.
Suppression of VLA-4 expression by RNA interference abrogates acceleration of the hemin-stimulated erythroid differentiation in response to TNIIIA2. K562 cells were transfected with control siRNA or integrin α4 siRNA, as described under “Experimental Procedures.” Cells untransfected (none) or transfected with control siRNA or with integrin α4 siRNA were then stimulated for 2 days with hemin (50 μm) alone or hemin plus TNIIIA2 (50 μg/ml), as indicated. A, aliquots of cell suspensions were examined for erythroid differentiation, as described under “Experimental Procedures.” B, other aliquots were subjected to flow cytometric analysis for the detection of α4 expression. Data represent the mean ± S.E. of triplicate determinations. One of three individual experiments is shown. p < 0.01 compared with control.
Involvement of p38 Activation in Adhesion-dependent Acceleration of Hemin-stimulated Erythroid Differentiation
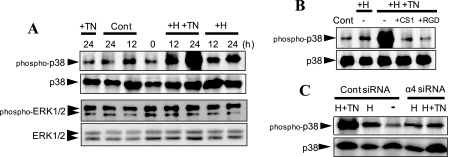
Members of the MAP kinase family, such as ERK1/2, p38, and JNK, are reportedly involved in signaling for erythropoiesis (30–32). To identify the MAP kinase involved in erythroid differentiation of K562 cells, we investigated phosphorylation of the MAP kinases during erythroid differentiation, as observed by immunoblotting using phospho-specific mAbs for MAP kinases. ERK1/2 showed no remarkable alteration in their phosphorylation during hemin-stimulated erythroid differentiation (Fig. 6A). Phosphorylated JNK was barely detected in K562 cells cultured under the same conditions (data not shown). In contrast, a significant increase in p38 phosphorylation was detected in cells stimulated with hemin (Fig. 6A).
FIGURE 6.
Implication of p38 MAP kinase activation in acceleration of hemin-stimulated erythroid differentiation in response to TNIIIA2. A, K562 cells were seeded on a culture plate coated with FN and stimulated with TNIIIA2 (75 μg/ml) alone (+TN), hemin (50 μm) alone (+H), or their combination (+H+TN) for the indicated times at 37 °C. B, cell suspension without (−) or with the CS-1 (+CS1) or RGD (+RGD) peptide (200 μg/ml) was seeded on a culture plate coated with FN and then stimulated for 24 h with hemin (50 μm) in the absence (+H) or presence (+H+TN) of TNIIIA2 (75 μg/ml). C, cells transfected with control siRNA or integrin α4 siRNA (see the legend to Fig. 5) were stimulated for 24 h without (−) or with hemin (50 μm) (H) or hemin plus TNIIIA2 (75 μg/ml) (H+TN). Cell lysates were subjected to Western blot analysis using anti-phospho-p38, anti-phospho-ERK1/2, or anti-p38 mAb, as described under “Experimental Procedures.” Data are representative of three individual experiments.
Stimulation with TNIIIA2 alone did not influence the phosphorylation of p38, whereas stimulation with hemin in combination with TNIIIA2 resulted in a conspicuous increase in p38 phosphorylation (Fig. 6A). This conspicuous increase in p38 phosphorylation was completely prevented by pretreating cells with the CS-1 peptide (Fig. 6B), suggesting the implication of VLA-4 signaling in the adhesion-dependent increase in p38 phosphorylation. Supporting this, siRNA-based suppression of integrin α4 expression also blocked the conspicuous increase in p38 phosphorylation (Fig. 6C). The RGD peptide also abolished the increased phosphorylation of p38 (Fig. 6B), probably due to prevention of VLA-4 expression (see Fig. 4k). Like TNIIIA2, Mn2+ increased the hemin-stimulated phosphorylation of p38, to a lesser extent than TNIIIA2 (data not shown).
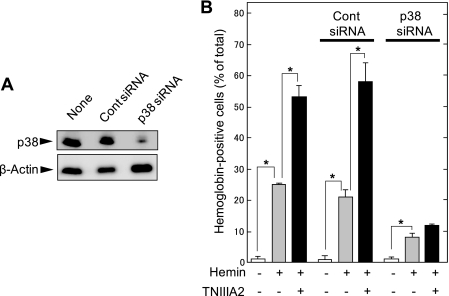
The necessity of p38 phosphorylation in hemin-stimulated erythroid differentiation and its adhesion-dependent acceleration was confirmed by siRNA-based down-regulation of p38. K562 cells were transfected with control siRNA or p38 siRNA. Expression of p38 was markedly down-regulated by p38 siRNA but hardly by control siRNA (Fig. 7A). Down-regulation of p38 expression by p38 siRNA resulted in abrogation of not only acceleration of the hemin-stimulated erythroid differentiation in response to TNIIIA2 but also the differentiation stimulated by hemin alone. Transfection with control siRNA did not influence erythroid differentiation of K562 cells stimulated by hemin with or without TNIIIA2. In agreement with these results, pretreatment of K562 cells with SB203580, a specific inhibitor of p38, but not with PD98059 and SP600125, inhibitors of MEK and JNK, respectively, reduced erythroid differentiation to the basal level (supplemental Fig. 1).
FIGURE 7.
Small interfering RNA-based down-regulation of p38 expression abrogates the hemin-stimulated erythroid differentiation of K562 cells. Cells were transfected with control siRNA or p38 siRNA, as described under “Experimental Procedures.” A, cell lysates were subjected to Western blot analysis using anti-p38 mAb or anti-β-actin. Data are representative of three individual experiments. B, cells untransfected (None) or transfected with control siRNA or p38 siRNA were stimulated for 2 days with hemin (50 μm) alone or hemin plus TNIIIA2 (75 μg/ml) and then examined for detection of hemoglobinized cells. Each point represents the mean ± S.E. of triplicate determinations. Data are representative of three individual experiments. *, p < 0.01 compared with control.
DISCUSSION
In the present study, we found that erythroid differentiation stimulated by hemin was greatly enhanced when K562 cells were induced to adhere to FN by activating VLA-5 with TNIIIA2 (Figs. 2 and 3). Since Mn2+, another integrin activator, was also capable of accelerating hemin-stimulated erythroid differentiation (Fig. 3A) and since acceleration of hemin-stimulated erythroid differentiation was inhibited by prevention of adhesion to FN (Fig. 3, B and C), it is conceivable that acceleration of the hemin-stimulated erythroid differentiation is dependent on adhesion of K562 cells to FN. This adhesion-dependent acceleration of erythroid differentiation was abrogated by not only a VLA-5 antagonist (RGD peptide) but also a VLA-4 antagonist (CS-1 peptide) (Fig. 3C), although K562 cells should have been lacking in VLA-4. This conflict was explained by results showing that sustained adhesion to FN via VLA-5 causally induced VLA-4 expression in K562 cells (Fig. 4). In fact, this induction of VLA-4 expression was prevented with the RGD peptide, a VLA-5 antagonist (Fig. 4). Since adhesion-dependent acceleration of erythroid differentiation was blocked with the CS-1 peptide (Fig. 3C) and also by siRNA-based suppression of α4 expression (Fig. 5), it is conceivable that the induced VLA-4 plays an indispensable role in the adhesion-dependent acceleration of erythroid differentiation. Furthermore, newly expressed VLA-4 was shown to be involved in a conspicuous increase in p38 phosphorylation (Fig. 6), as discussed below. Thus, VLA-5 and VLA-4 were shown to contribute to erythropoiesis at different stages of erythroid differentiation; VLA-5 triggers the induction of VLA-4 expression, and the resulting VLA-4 positively modulates the activation of p38, a critical signaling molecule for erythroid differentiation.
Several previous studies demonstrated that activation of p38 and/or JNK but not ERK is required for erythroid differentiation induced by butyrate (30), erythropoietin (32), hydroxyurea (33), or hemin (34). In the present study, siRNA-based down-regulation of p38 (Fig. 7) and experiments using the specific inhibitor of p38 (supplemental Fig. 1) demonstrated that the phosphorylation/activation of p38 plays a key role in the hemin-stimulated erythroid differentiation of K562 cells. Additionally, induced adhesion of K562 cells to FN with TNIIIA2 or Mn2+ conspicuously increased the hemin-generated phosphorylation of p38 (Fig. 6A), and this increased phosphorylation of p38 was reversed by suppression of α4 expression induction (Fig. 6C). The results suggest that VLA-4-mediated adhesion is responsible for a conspicuous increase in p38 phosphorylation/activation. However, since stimulation with TNIIIA2 or Mn2+ without hemin was incapable of generating p38 activation and also hemoglobinization, cell adhesion to FN is not sufficient per se for erythroid differentiation of K562 cells. Integrin-mediated adhesion appears to accelerate supportively, not induce directly, erythroid differentiation. Further study is required for an understanding of the molecular mechanism by which VLA-4-mediated adhesion to FN can generate a conspicuous activation of p38 in concert with hemin.
With regard to the induction of integrin expression during differentiation of K562 cells, Zutter et al. (35) reported that megakaryocytic differentiation is accompanied by expression of integrin α2β1, a collagen receptor. In contrast to the erythroid differentiation observed here, induction of α2 integrin expression contributes to the acquisition of the ability to adhere to collagen. Expression of the α2 gene requires signaling via the MAP kinase pathway to activate two tandem AP1-binding sites in the α2 integrin enhancer (36). Inhibition of the MAP kinase cascade with PD98059, a MEK inhibitor, prevented the expression of α2 integrin in cells induced to become megakaryocytic.
TN-C is highly expressed by the stromal cells of the hematopoietic system or lymphoid organs, particularly in the microenvironment surrounding the maturing hematopoietic cells (37–39). Interestingly, a study of TN-C gene-deficient mice showed that TN-C-deficient mice retain their capacity for hematopoiesis, whereas the extent of the colony-forming capacity of hematopoietic progenitor cells and production of hematopoietic cells in long term bone marrow cultures of TN-C-deficient mice are significantly lower than in those of TN-C-expressing control mice (40). Thus, TN-C is considered to play a supportive role in hematopoiesis, similar to the role of TNIIIA2 in erythroid differentiation.
On the other hand, there have been many studies indicating that the ECM proteins, including TN-C, have biologically active cryptic sites, so-called “matricryptic sites” (41). These matricryptic sites can be exposed through biological processes, such as conformational change in response to interaction with cells or other ECM components and processing with the ECM-degrading proteinases that are secreted by cells of hematopoietic origin, including hematopoietic stromal cells and mononuclear cells (22, 24, 42–45). It is important to ascertain if the TNIIIA2-related matricryptic site(s) is actually exposed in the microenvironment of the hematopoietic system.
Recently, Seki et al. (46) have identified TN-C as a key molecule promoting stromal cell-dependent erythropoiesis, as judged by hemoglobin-positive erythroid colony formation from fetal liver cells of mice. They demonstrated that siRNA-based down-regulation of TN-C suppresses the erythroid colony formation, and conversely, the addition of exogenous TN-C increases formation in stromal cell lines. Although the active site(s) of the TN-C molecule has not been determined in their study, it may be possible that the TNIIIA2-related functional site on the TN-C molecule is involved in erythroid colony formation. It is also important to ascertain if adhesion-dependent acceleration of erythroid differentiation observed here using K562 cells and hemin is valid for erythroid development in vivo. A study on the effect of TNIIIA2 on erythroid colony formation is now under way.
Supplementary Material
This work was supported, in part, by the “High-Tech Research Center” Project for Private Universities with a matching fund subsidy from MEXT (Ministry for Education, Culture, Sports, Science and Technology) of Japan, 2004–2008, and by the Vehicle Racing Commemorative Foundation.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
- ECM
- extracellular matrix
- FN
- fibronectin
- FNIII
- FN type III-like
- TN
- tenascin
- MAP
- mitogen-activated protein
- Ab
- antibody
- ERK
- extracellular signal-regulated kinase
- JNK
- c-Jun N-terminal kinase
- mAb
- monoclonal antibody
- MEK
- mitogen-activated protein kinase/extracellular signal-regulated kinase kinase
- o-dianisidine
- 3,3-dimethoxybenzidine
- FITC
- fluorescein isothiocyanate
- siRNA
- small interfering RNA.
REFERENCES
- 1.Williams D. A. (1994) Pediatr. Res. 36, 557–560 [DOI] [PubMed] [Google Scholar]
- 2.Clark B. R., Gallagher J. T., Dexter T. M. (1992) Baillieres Clin. Haematol. 5, 619–652 [DOI] [PubMed] [Google Scholar]
- 3.Coulombel L., Vuillet M. H., Leroy C., Tchernia G. (1988) Blood 71, 329–334 [PubMed] [Google Scholar]
- 4.Murti K. G., Brown P. S., Kumagai M., Campana D. (1996) Exp. Cell Res. 226, 47–58 [DOI] [PubMed] [Google Scholar]
- 5.Williams D. A., Rios M., Stephens C., Patel V. P. (1991) Nature 352, 438–441 [DOI] [PubMed] [Google Scholar]
- 6.Wilson A., Trumpp A. (2006) Nat. Rev. Immunol. 6, 93–106 [DOI] [PubMed] [Google Scholar]
- 7.Patel V. P., Lodish H. F. (1987) J. Cell Biol. 105, 3105–3118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vuillet-Gaugler M. H., Breton-Gorius J., Vainchenker W., Guichard J., Leroy C., Tchernia G., Coulombel L. (1990) Blood 75, 865–873 [PubMed] [Google Scholar]
- 9.Tada T., Widayati D. T., Fukuta K. (2006) Anat. Histol. Embryol. 35, 235–240 [DOI] [PubMed] [Google Scholar]
- 10.Weinstein R., Riordan M. A., Wenc K., Kreczko S., Zhou M., Dainiak N. (1989) Blood 73, 111–116 [PubMed] [Google Scholar]
- 11.Eshghi S., Vogelezang M. G., Hynes R. O., Griffith L. G., Lodish H. F. (2007) J. Cell Biol. 177, 871–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kapur R., Cooper R., Zhang L., Williams D. A. (2001) Blood 97, 1975–1981 [DOI] [PubMed] [Google Scholar]
- 13.Papayannopoulou T., Priestley G. V., Nakamoto B. (1998) Blood 91, 2231–2239 [PubMed] [Google Scholar]
- 14.van der Loo J. C., Xiao X., McMillin D., Hashino K., Kato I., Williams D. A. (1998) J. Clin. Invest. 102, 1051–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yanai N., Sekine C., Yagita H., Obinata M. (1994) Blood 83, 2844–2850 [PubMed] [Google Scholar]
- 16.Hamamura K., Matsuda H., Takeuchi Y., Habu S., Yagita H., Okumura K. (1996) Blood 87, 2513–2517 [PubMed] [Google Scholar]
- 17.Fukai F., Takahashi H., Habu Y., Kubushiro N., Katayama T. (1996) Biochem. Biophys. Res. Commun. 220, 394–398 [DOI] [PubMed] [Google Scholar]
- 18.Fukai F., Hasebe S., Ueki M., Mutoh M., Ohgi C., Takahashi H., Takeda K., Katayama T. (1997) J. Biochem. 121, 189–192 [PubMed] [Google Scholar]
- 19.Kamiya S., Kato R., Wakabayashi M., Tohyama T., Enami I., Ueki M., Yajima H., Ishii T., Nakamura H., Katayama T., Takagi J., Fukai F. (2002) Biochemistry 41, 3270–3277 [DOI] [PubMed] [Google Scholar]
- 20.Erickson H. P. (1993) Curr. Opin. Cell Biol. 5, 869–876 [DOI] [PubMed] [Google Scholar]
- 21.Fischer D., Brown-Lüdi M., Schulthess T., Chiquet-Ehrismann R. (1997) J. Cell Sci. 110, 1513–1522 [DOI] [PubMed] [Google Scholar]
- 22.Saito Y., Imazeki H., Miura S., Yoshimura T., Okutsu H., Harada Y., Ohwaki T., Nagao O., Kamiya S., Hayashi R., Kodama H., Handa H., Yoshida T., Fukai F. (2007) J. Biol. Chem. 282, 34929–34937 [DOI] [PubMed] [Google Scholar]
- 23.Saito Y., Shiota Y., Nishisaka M., Owaki T., Shimamura M., Fukai F. (2008) Biol. Pharm. Bull. 31, 1003–1007 [DOI] [PubMed] [Google Scholar]
- 24.Watanabe K., Takahashi H., Habu Y., Kamiya-Kubushiro N., Kamiya S., Nakamura H., Yajima H., Ishii T., Katayama T., Miyazaki K., Fukai F. (2000) Biochemistry 39, 7138–7144 [DOI] [PubMed] [Google Scholar]
- 25.Davis G. E., Camarillo C. W. (1993) J. Immunol. 151, 7138–7150 [PubMed] [Google Scholar]
- 26.Ni H., Li A., Simonsen N., Wilkins J. A. (1998) J. Biol. Chem. 273, 7981–7987 [DOI] [PubMed] [Google Scholar]
- 27.Elices M. J., Osborn L., Takada Y., Crouse C., Luhowskyj S., Hemler M. E., Lobb R. R. (1990) Cell 60, 577–584 [DOI] [PubMed] [Google Scholar]
- 28.Takagi J., Isobe T., Takada Y., Saito Y. (1997) J. Biochem. 121, 914–921 [DOI] [PubMed] [Google Scholar]
- 29.Minegishi N., Minegishi M., Tsuchiya S., Fujie H., Nagai T., Hayashi N., Yamamoto M., Konno T. (1994) J. Biol. Chem. 269, 27700–27704 [PubMed] [Google Scholar]
- 30.Witt O., Sand K., Pekrun A. (2000) Blood 95, 2391–2396 [PubMed] [Google Scholar]
- 31.Whalen A. M., Galasinski S. C., Shapiro P. S., Nahreini T. S., Ahn N. G. (1997) Mol. Cell. Biol. 17, 1947–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagata Y., Takahashi N., Davis R. J., Todokoro K. (1998) Blood 92, 1859–1869 [PubMed] [Google Scholar]
- 33.Park J. I., Choi H. S., Jeong J. S., Han J. Y., Kim I. H. (2001) Cell Growth & Differ. 12, 481–486 [PubMed] [Google Scholar]
- 34.Di Pietro R., di Giacomo V., Caravatta L., Sancilio S., Rana R. A., Cataldi A. (2007) J. Cell. Biochem. 100, 1070–1079 [DOI] [PubMed] [Google Scholar]
- 35.Zutter M. M., Fong A. M., Krigman H. R., Santoro S. A. (1992) J. Biol. Chem. 267, 20233–20238 [PubMed] [Google Scholar]
- 36.Zutter M. M., Painter A. D., Yang X. (1999) Blood 93, 1600–1611 [PubMed] [Google Scholar]
- 37.Chilosi M., Lestani M., Benedetti A., Montagna L., Pedron S., Scarpa A., Menestrina F., Hirohashi S., Pizzolo G., Semenzato G. (1993) Am. J. Pathol. 143, 1348–1355 [PMC free article] [PubMed] [Google Scholar]
- 38.Ocklind G., Talts J., Fässler R., Mattsson A., Ekblom P. (1993) J. Histochem. Cytochem. 41, 1163–1169 [DOI] [PubMed] [Google Scholar]
- 39.Klein G., Beck S., Müller C. A. (1993) J. Cell Biol. 123, 1027–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohta M., Sakai T., Saga Y., Aizawa S., Saito M. (1998) Blood 91, 4074–4083 [PubMed] [Google Scholar]
- 41.Davis G. E., Bayless K. J., Davis M. J., Meininger G. A. (2000) Am. J. Pathol. 156, 1489–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stetler-Stevenson W. G. (1996) Am. J. Pathol. 148, 1345–1350 [PMC free article] [PubMed] [Google Scholar]
- 43.Sage E. H. (1997) Trends Cell Biol. 7, 182–186 [DOI] [PubMed] [Google Scholar]
- 44.Janowska-Wieczorek A., Marquez L. A., Nabholtz J. M., Cabuhat M. L., Montaño J., Chang H., Rozmus J., Russell J. A., Edwards D. R., Turner A. R. (1999) Blood 93, 3379–3390 [PubMed] [Google Scholar]
- 45.Vu T. H., Werb Z. (1998) Gelatinase B: Structure, Regulation and Function: Matrix Metalloproteinases (Parks W. C., Mecham R. P. eds) pp. 115–148, Academic Press, Inc., San Diego [Google Scholar]
- 46.Seki M., Kameoka J., Takahashi S., Harigae H., Yanai N., Obinata M., Sasaki T. (2006) Exp. Hematol. 34, 519–527 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.