Abstract
Sepsis is a leading cause of death that is characterized by uncontrolled inflammatory response. In this study, we report that scavenger receptor BI (SR-BI), a high density lipoprotein receptor, is a critical survival factor of sepsis. We induced sepsis using an established septic animal model, cecal ligation and puncture (CLP). CLP induced 100% fatality in SR-BI-null mice but only 21% fatality in wild type littermates. SR-BI-null mice exhibited aberrant inflammatory responses with delayed inflammatory cytokine generation at the early stage of sepsis and highly elevated inflammatory cytokine production 20 h after CLP treatment. To understand the mechanisms underlying SR-BI protection, we elucidated the effect of mac ro phage SR-BI on inflammatory cytokine generation. Macrophages from SR-BI-null mice produced significantly higher levels of inflammatory cytokines than those of wild type controls in response to LPS. Importantly, transgenic mice overexpressing SR-BI were more resistant to CLP-induced septic death. Using an HEK-BlueTM cell system, we demonstrated that expression of SR-BI suppressed TLR4-mediated NF-κB activation. To understand why SR-BI-null mice had a delayed inflammatory response, we elucidated the effect of SR-BI on LPS clearance during sepsis. Compared with wild type controls, SR-BI-null mice had lower plasma LPS levels in the early stage of sepsis and elevated plasma LPS levels 20 h following CLP treatment. In conclusion, our findings demonstrate that SR-BI is a critical protective modulator of sepsis in mice. SR-BI exerts its protective function through its role in modulating inflammatory response in mac ro phages and facilitating LPS recruitment and clearance.
Sepsis is one of the major causes of death that claims over 215,000 lives and costs $16.7 billion per year in America alone (1–4). The death rate from sepsis is high, exceeding 50%, due to poor understanding of the disease (5). Identifying molecules involved in sepsis, especially endogenous protective modulators, is of great importance not only in understanding the mechanisms but also in providing new insights for efficient therapies.
Scavenger receptor BI (SR-BI2 or Scarb1) is a 75-kDa membrane protein expressed in the liver, endothelial cells, macrophages, and steroidogenic tissues (6, 7). It is a well established high density lipoprotein (HDL) receptor. It mediates intracellular uptake of cholesterol ester from HDL, which plays a key role in regulating plasma cholesterol levels and steroidogenesis (8–11). Mice deficient in SR-BI have a 2-fold increase in plasma cholesterol levels and develop cardiovascular diseases (10, 12–16). Recent studies reveal that SR-BI is a multifunctional protein. It activates endothelial nitric-oxide synthase in endothelial cells in the presence of HDL (17–20), induces apoptosis in the absence of HDL/endothelial nitric-oxide synthase (21), and protects against nitric oxide (NO)-induced oxidative damage (22). Emerging evidence indicates that specific expression of SR-BI in macrophages provides protection against the development of atherosclerosis, and importantly, it seems that SR-BI exerts this protection independently of its role in regulating lipoprotein metabolism or cholesterol efflux (23–25). The mechanisms underlying the protection of macrophage SR-BI against atherosclerosis are unclear.
We recently reported that SR-BI protects against endotoxin-induced animal death through suppression of NO-induced cytotoxicity (22). A recent study by Cai et al. (26) confirmed the protective role of SR-BI in LPS-induced animal death, and the authors demonstrated that SR-BI-mediated glucocorticoid synthesis contributes to its protective function. These studies suggest that SR-BI might play a role in sepsis. Interestingly, using in vitro cell culture, Vishnyakova et al. (27) reported recently that SR-BI enhances the uptake of Gram-negative bacteria, which raises a possibility that this SR-BI-mediated bacterial uptake may facilitate bacterial infection and therefore play a deleterious role in sepsis. Given the limitations of endotoxemia animal model (28), it is of importance to determine whether and how SR-BI plays a protective role in sepsis. In this study, we assessed the role of SR-BI in sepsis using an established septic animal model, cecal ligation and puncture (CLP). We demonstrated that SR-BI is a critical survival factor of sepsis in mice. In contrast to significant protection against LPS-induced animal death by SR-BI-mediated corticosterone generation, corticosterone did not provide protection against CLP-induced septic animal death, indicating that SR-BI has other functions than regulating corticosterone production in sepsis. Using a transgenic animal model, primary macrophages, and HEK-Blue cell system, we demonstrated that SR-BI modulates inflammatory response in macrophages via TLR4 signaling, which contributes to protection against septic death.
EXPERIMENTAL PROCEDURES
Materials
Anti-SR-BI serum was custom made by Sigma Genosys using a 15-amino acid peptide derived from the C-terminal of human SR-BI. LPS (E. coli serotype K12) was from InvivoGen. Endo-low fetal bovine serum was from Hyclone. Macrophage colony stimulate factor was from R&D Systems. Corticosterone was from Sigma. Blood agar plate (MacConkey II) was from BD Biosciences. Enzyme-linked immunosorbent assay (ELISA) kits for quantifying tumor necrosis factor-α (TNF-α) and IL-6 were from eBioscience. Competitive ELISA kit for quantifying mouse cortisol or corticosterone and nitrite/nitrate (NOx) kit were from Cayman Chemical. An ELISA kit for quantifying LPS was from Charles River.
SR-BI-null Mice
SR-BI+/− mice on C57BL/6 × 129 background were obtained from Jackson Laboratories. SR-BI−/−, SR-BI+/−, and SR-BI+/+ littermates were generated by breeding SR-BI+/− mice. Mouse tail DNA was used for PCR genotyping. The animals were fed with a standard laboratory chow diet. Animal care and experiments were approved by the Institutional Animal Care and Use Committee of the University of Kentucky.
Generation of SR-BIC323G Transgenic Mice
C323G mutated SR-BI cDNA was generated and subcloned into a pCAGGS vector as described previously (21). The transgenic mice in a C57BL/6xCBA background were generated by the Transgenic Core Facility of Mayo Clinic (Scottsdale, AZ). Transgenic mice were selected by PCR using tail DNA. The transgenic mice were crossed to SR-BI-null mice to generate transgenic and nontransgenic littermates in an SR-BI-null background. The breeders were fed with 0.1% probucol to correct female infertility.
CLP Septic Animal Model
CLP was performed as described by Wesche-Soldato et al. (29). Briefly, 10- to 12-week-old mice were anesthetized by inhalation of 2–5% isoflurane in 100% oxygen using anesthesia equipment. A midline incision (1.0 cm) was made below the diaphragm. The cecum was isolated, ligated, punctured twice with a 22-gauge needle, and gently compressed to extrude a small amount of cecal material. The cecum was returned to the abdomen, and the muscle and skin incisions were closed with 6–0 Ethilon suture material. The mice were subsequently resuscitated with 1 ml phosphate-buffered saline subcutaneously. Sham animals were similarly treated without ligation/puncture of the cecum. Survival was monitored for a 5-day period.
Biochemical Assays
10- to 12-week-old mice were euthanized by CO2 inhalation 2, 4, 8, and 20 h following CLP. The blood was obtained by cardiac puncture or tail bleed and stored at −80 °C. The serum alanine aminotransferase levels were quantified with the Analyst Chemistry system, which was used to determine the degree of liver damage; the serum nitrite/nitrate (NOx) levels were measured with a nitrite/nitrate kit, which was used to estimate the generation of NO in vivo; and the serum TNF-α, IL-6, corticosterone, or LPS levels were quantified with corresponding ELISA kits.
Supplementation of Corticosterone
Corticosterone was administered to 10- to 12-week-old SR-BI-null mice following the method described by Cai et al. (26). Then the CLP was performed, and survival was monitored for a 5-day period.
Quantification of Cytokine Generation in LPS-stimulated Macrophages
Bone marrow-derived macrophages were cultured as described previously (30). Briefly, bone marrow cells were cultured in a 6-well plate at 2 × 106 cells/well in RPMI 1640 medium containing 20% fetal bovine serum and 25 ng/ml of mouse macrophage colony stimulate factor for 6 days. The medium was changed daily. For measurement of cytokines, the cells were incubated in phosphate-buffered saline for 2 h and treated with LPS at 0, 0.25, 0.5, and 1 ng/ml for 2, 4, 8, and 20 h in RPMI 1640 medium containing 20% fetal bovine serum, and cytokines in the culture supernatant were quantified with the corresponding ELISA kit.
Generation of HEK-Blue Cells Stably Expressing Wild Type and C323G Mutant SR-BI
HEK-Blue cells, which stably express TLR4, CD14, MD2, and a NF-κB reporter, were from InvivoGen. The HEK-Blue cells were transfected with wild type SR-BI, C323G mutant SR-BI cDNA, or pLNCX2 vector and mixed clones stably expressing SR-BI, SR-BIC323G, or vector were obtained by G418 selection.
Analysis of TLR4-mediated NF-κB Activation
HEK-Blue cells stably expressing SR-BI, SR-BIC323G, or pLNCX2 vector were cultured in a 96-well plate in complete Dulbecco's modified Eagle's medium containing 0.5 mg/ml G418 and 10% Endo-low fetal bovine serum to 90% confluency and treated with LPS at 0, 0.5, 1, and 2 ng/ml for 16 h. Then, 100 μl of the culture supernatant was mixed with 100 μl HEK-Blue detection medium and incubated at 37 °C for 1 h. LPS activates TLR4, resulting in induction of NF-κB reporter expression, which catalyzes the HEK-Blue detection medium to blue. The blue color was quantified by measuring absorption at 650 nm.
Lipoprotein Profiling by Fast Protein Liquid Chromatography
Serum (50 μl) was resolved by gel filtration chromatography with fast protein liquid chromatography equipped with a Superose 6 column (GE Healthcare). The column was eluted at a flow rate of 0.5 ml/min in buffer containing 150 mm NaCl, 10 mm Tris/HCl, pH 7.4, 0.01% sodium azide, and 0.5 ml/fraction was collected. One hundred microliters of sample was mixed with 100 μl of 2× assay reagent to determine the cholesterol content of fractions (Wako Chemicals).
Analysis of Bacteremia
Blood was diluted (100 and 1,000 times with phosphate-buffered saline), and 200 μl of the diluted blood was plated on a MacConkey II agar plate. After a 24-h incubation at 37° C, the number of purple clones was counted. qPCR analysis was employed to quantify the number of bacteria in the liver following the method described by Steinman et al. (31). Total DNA was isolated from 30 mg of liver using a DNeasy kit (Qiagen). qPCR was performed with bacteria-specific primers (forward, 5′-GAGGAAGGIGIGGAIGACGT; reverse, 5′-AGGAGGTGATCCAACCGCA) using a knowing number of DH-5α bacteria as standard.
Statistical Analysis
The survival assay was analyzed by a Log-Rank x2 test using SAS software. Comparing two groups, significance in experiments was determined by 2-tailed Student's t test. Significance in experiments comparing more than two groups was evaluated by one-way analysis of variance, followed by post hoc analysis using Tukey's test. Means were considered different at p < 0.05.
RESULTS
SR-BI Is a Critical Survival Factor in CLP-induced Septic Death
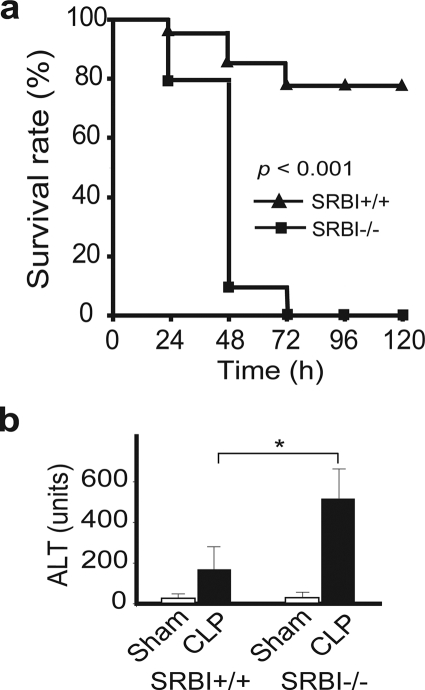
We employed a well established CLP sepsis model to determine whether SR-BI is required for protection against bacteria-induced septic death. As shown in Fig. 1a, mice deficient in SR-BI were significantly more susceptible to CLP-induced septic death. CLP treatment induced 19% fatality in SR-BI-null mice and 5% in wild type littermates at 24 h, 90% fatality in SR-BI-null mice and 10% in wild type littermates at 48 h, and 100% fatality in SR-BI-null mice but only 21% fatality in wild type littermates at 72 h. SR-BI-mediated protection against CLP-induced liver injury was assessed by measuring serum alanine aminotransferase levels. Compared with wild type controls, SR-BI-null mice had a significant increase in serum alanine aminotransferase levels (Fig. 1b) 20 h following CLP treatment. These findings indicate that SR-BI is a critical survival factor in sepsis.
FIGURE 1.
SR-BI protects against septic death in mice. a, survival analysis. 10- to 12-week-old SR-BI-null mice (solid square; n = 21) and wild type littermates (solid triangle; n = 19) were treated with CLP, and the survival was observed for 5 days. The data were expressed as the percentage of mice survived at indicated times (p < 0.001 versus wild type). b, CLP-induced liver injury. Twenty h following CLP treatment, SR-BI-null and wild type control mice (n = 6 per group) were sacrificed, and the liver injury was assessed by measuring serum alanine aminotransferase levels. Sham animals (n = 6 per group) were similarly treated without ligation/puncture of the cecum. Mean ± S.D. *, p < 0.05 versus wild type).
Aberrant Innate Immune Response in SR-BI-null Mice during Sepsis
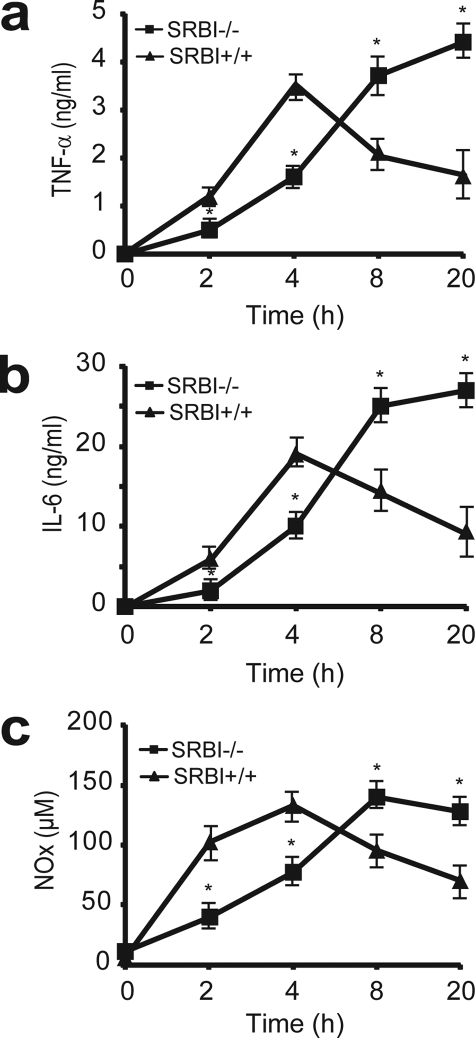
A rapid innate immune response that generates high levels of inflammatory cytokines constitutes the first line of the self-defense system against bacterial infections. However, uncontrolled production of cytokines is a major cause of septic death (32). To understand why SR-BI-null mice are susceptible to septic death, we assessed inflammatory cytokine production in CLP-treated SR-BI-null and wild type littermates. We treated the mice with CLP for 2, 4, 8, and 20 h and then determined the serum cytokine levels. As shown in Fig. 2, SR-BI-null and wild type mice exhibited distinct inflammatory responses during sepsis. Wild type mice exhibited a typical acute phase response with a rapid and strong induction of TNF-α and IL-6 in the early stage of sepsis (2–8 h) with significant decreases in TNF-α and IL-6 levels by 20 h. However, SR-BI-null mice exhibited an aberrant pattern of inflammatory cytokine generation during sepsis. Four h after CLP treatment, although serum TNF-α and IL-6 levels increased in SR-BI-null mice, the increases were significantly less than that of wild type control mice, indicating a delayed immune response in SR-BI-null mice. Interestingly, in contrast to the decrease in cytokine levels in wild type mice 20 h after CLP treatment, the serum TNF-α and IL-6 levels continued to increase in SR-BI-null mice, indicating a prolonged and uncontrolled cytokine generation in the late stage of sepsis in the absence of SR-BI (Fig. 2, a and b).
FIGURE 2.
Aberrant inflammatory response in SR-BI-null mice during sepsis. SR-BI-null mice and wild type littermates were treated with CLP for 2, 4, 8, and 20 h, and the serum concentrations of TNF-α (a), IL-6 (b), and NOx (c) were quantified (n = 6 per group with triplicate measurements). Mean ± S.D. *, p < 0.05 versus wild type.
Similar to cytokines, NO is another key molecule in innate immune response during sepsis (33). Upon bacterial infections, inducible nitric-oxide synthase expression in macrophages is induced to generate a high level of NO, which is used to kill bacteria and modulate immune reactions (33). However, too much NO is deleterious and can cause oxidative cell damage (34). We measured serum nitrite and nitrate (NOx) levels to determine whether SR-BI affects NO production in sepsis. As shown in Fig. 2c, SR-BI-null mice exhibited an aberrant pattern of NO generation during sepsis. In contrast to an increase in NO generation in the early stage of sepsis in wild type mice, the NO generation in SR-BI-null mice was significantly less than that of wild type controls, indicating a delayed NO generation in SR-BI-null mice. Furthermore, unlike the gradual decrease in NOx levels in the late stage of sepsis in wild type mice, the serum NOx levels in SR-BI-null mice reached the peak at 8 h and remained high at 20 h, indicating a prolonged NO generation in the late stage of sepsis in the absence of SR-BI.
Contribution of SR-BI-mediated Corticosterone Generation to Sepsis
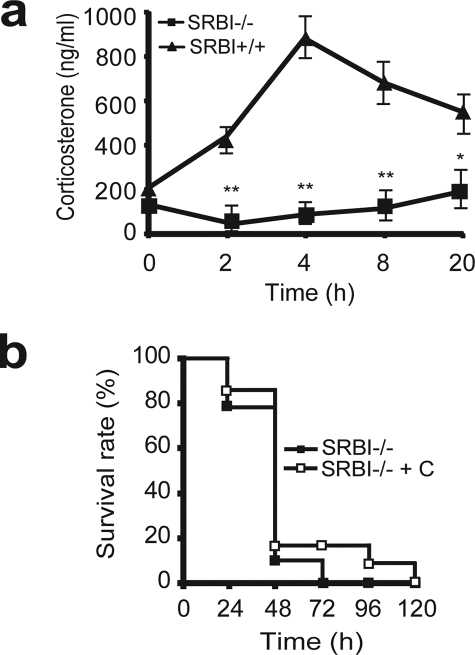
SR-BI is highly expressed in adrenal glands. It mediates the intracellular uptake of cholesterol from HDL in adrenal glands, thus providing substrate for corticosteroid synthesis (35). A recent study by Cai et al. (26) showed that SR-BI-null mice have a defect in inducible corticosterone generation in LPS-challenged mice, and supplementation of corticosterone to LPS-challenged SR-BI-null mice rescues the mice from endotoxic death, indicating that SR-BI-mediated corticosterone production contributes to its protection against endotoxic animal death. We elucidated the effect of SR-BI on corticosteroid generation during sepsis by measuring serum corticosterone levels. As expected, CLP induced a 4-fold increase in corticosterone generation in wild type mice 4 h following CLP treatment (SR-BI+/+ basal, 198 ± 55 ng/ml versus SR-BI+/+ CLP, 830 ± 189 ng/ml), but mice deficient in SR-BI failed to generate any inducible corticosterone during sepsis (SR-BI−/− basal, 165 ± 33 ng/ml versus SR-BI−/− CLP, 160 ± 67 ng/ml) (Fig. 3a), indicating that SR-BI is required for inducible corticosterone production during sepsis. To determine whether this SR-BI-mediated corticosterone production contributes to its protection against septic death, we administered corticosterone in drinking water to SR-BI-null mice 8 h prior to CLP treatment following the method described by Cai et al. (26). Unexpectedly, in contrast to significant protection against LPS-induced endotoxic animal death by corticosterone (26), corticosterone supplementation failed to protect SR-BI-null mice from CLP-induced septic death (Fig. 3b). The data clearly indicate that SR-BI is required for inducible corticosterone production during sepsis, but this SR-BI-mediated inducible corticosterone production is not responsible for protection against septic death.
FIGURE 3.
Contribution of SR-BI-mediated corticosterone generation to sepsis. a, effect of SR-BI on corticosterone production during sepsis. SR-BI-null mice and wild type littermates were treated with CLP for 2, 4, 8, and 20 h. The serum corticosterone concentration was quantified (n = 6 per group with triplicate measurements). Mean ± S.D. *, p < 0.05; **, p < 0.01 versus wild type. b, effects of corticosterone (C) supplementation on mouse survival. SR-BI-null mice were administered 100 μg/ml of corticosterone in drinking water 8 h prior to CLP treatment (n = 13). The mice were monitored for survival for a 5-day period. The data were expressed as the percentage of mice survived at indicated times. p = no significance versus no corticosterone treated SR-BI-null mice.
SR-BI Modulates Inflammatory Cytokine Generation in Macrophages
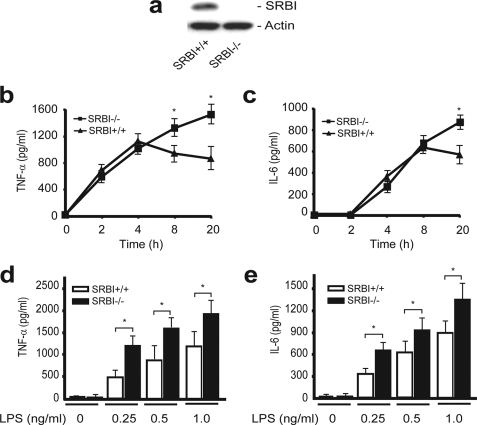
We then looked for an alternative explanation for SR-BI protection against septic death. Considering that SR-BI is expressed in macrophages and that macrophages are one of the major types of cells that generate inflammatory cytokines during sepsis (36, 37), we determined whether SR-BI directly affects cytokine production using bone marrow-derived macrophages. We treated the macrophages with 0.5 ng/ml LPS and quantified TNF-α and IL-6 production. Wild type and SR-BI-null macrophages exhibited distinct inflammatory responses to LPS stimuli (Fig. 4, b and c). For wild type macrophages, TNF-α production reached its maximum level by 4 h, and IL-6 production reached its maximum level by 8 h. However, for SR-BI-null macrophages, the TNF-α and IL-6 levels continued to rise and surpassed cytokine levels of wild type macrophages by 20 h, indicating uncontrolled TNF-α and IL-6 generation in macrophages deficient in SR-BI. We also treated the macrophages with various amounts of LPS. In the presence of pathological levels of LPS, LPS induced the generation of TNF-α and IL-6 in an LPS dose-dependent manner, and the presence of SR-BI effectively suppressed the generation of TNF-α and IL-6 (Fig. 4, d and e).
FIGURE 4.
SR-BI suppresses inflammatory cytokine generation in macrophages. a, Western blot analysis of SR-BI in bone marrow-derived macrophages (30 μg protein/lane). b to e, effect of SR-BI on inflammatory cytokine generation in macrophages. Macrophages from SR-BI-null (square) and wild type (triangle) were treated with 0.5 ng/ml of LPS for the indicated times. The concentrations of TNF-α (b) and IL-6 (c) in the culture supernatant were quantified. The macrophages were also treated with 0.25, 0.5, and 1.0 ng/ml of LPS for 20 h, and the concentrations of TNF-α (d) and IL-6 (e) were quantified (n = 4 per group with triplicate measurements). Mean ± S.D. *, p < 0.05 versus wild type.
Macrophage SR-BI Provides Protection against Septic Death
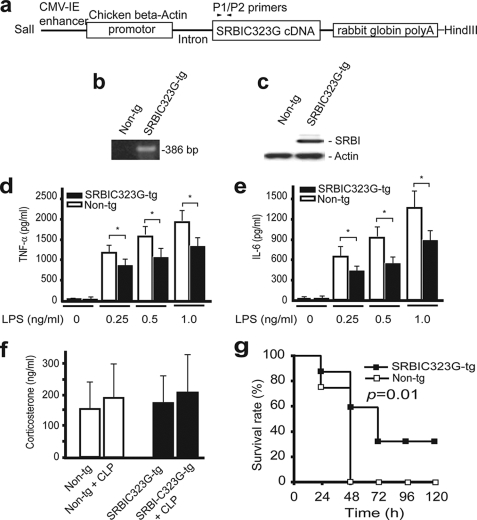
We used SR-BI transgenic mice to determine whether expression of SR-BI in macrophages contributes to protection against septic death. In using this approach, an important consideration is our ability to distinguish the effect of SR-BI on modulating inflammatory response in macrophages from its effects on inducing apoptosis, protecting NO-induced cytotoxicity, and regulating corticosterone generation. To address the problem, we used the C323G mutant SR-BI. Our early studies indicated that this mutated SR-BI loses its apoptotic activity and anti-NO activity completely (21, 22). Our recent data also indicated that this mutant loses its cholesterol uptake activity completely.3 Therefore, the C323G mutant SR-BI is a unique tool to determine the contribution of the macrophage SR-BI to sepsis. We generated the SR-BIC323G transgenic mice and bred the mice with SR-BI-null mice to generate SR-BIC323G transgenic mice in an SR-BI-null background (Fig. 5, a and b). We isolated bone marrow cells from the transgenic and nontransgenic littermates and confirmed the expression of SR-BIC323G in bone marrow-derived macrophages (Fig. 5c). Then, we determined the effect of SR-BIC323G on inflammatory cytokine generation using bone marrow-derived macrophages. As expected, the expression of the C323G mutant SR-BI in macrophages significantly suppressed the generation of TNF-α or IL-6 as compared with nontransgenic SR-BI-null mice (Fig. 5, d and e). Finally, we challenged the transgenic mice with CLP. As shown in Fig. 5, f and g, SR-BIC323G transgenic mice did not exhibit a significant difference in plasma corticosterone levels during sepsis but were significantly more resistant to CLP-induced septic death when compared with nontransgenic SR-BI-null mice.
FIGURE 5.
Survival analysis of SR-BIC323G transgenic mice. a, transgenic construct. The construct contains enhancer-β-actin promoter-intron-SR-BIC323G cDNA. b, PCR genotyping of SR-BIC323G transgenic mice using P1/P2 primers. The transgenic mice were bred with SR-BI-null mice to generate transgenic mice in SR-BI-null background as described under “Experimental Procedures.” c, expression of SR-BIC323G in macrophages. Bone marrow cells from SR-BIC323G transgenic or nontransgenic littermates were cultured for 6 days. The expression of SR-BI was analyzed by Western blot (20 μg protein/lane). d and e, effect of SR-BIC323G on TNF-α and IL-6 generation in macrophages. The macrophages were treated with LPS for 20 h, and TNF-α and IL-6 levels were quantified (d and e, n = 4 per group with triplicate measurements). Mean ± S.D. *, p < 0.05 versus nontransgenic littermates. f and g, effect of SR-BIC323G on survival. The SR-BIC323G transgenic and nontransgenic littermates (n = 12 per group) were treated with CLP. Twenty h later, 10 μl serum was collected from tail vein for quantifying serum corticosterone (f, n = 6 per group). The survival was observed for 5 days (g). The data were expressed as the percentage of mice survived at indicated times. p = 0.01 versus nontransgenic littermates.
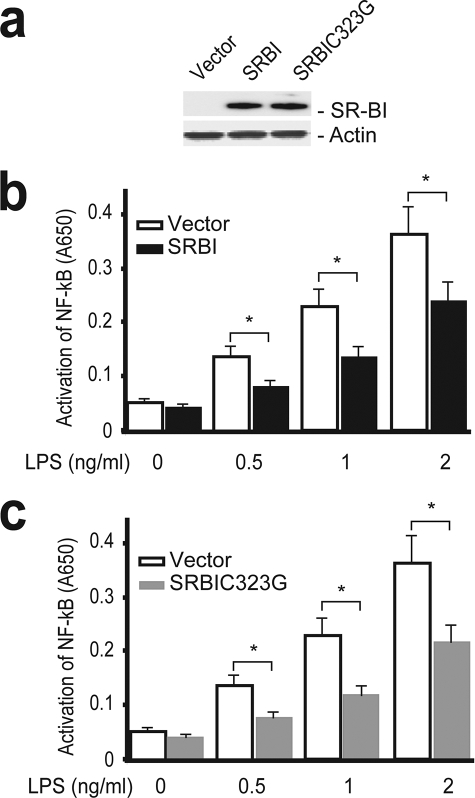
SR-BI Modulates TLR4-mediated NF-κB Activation
TLR4 is a key signaling molecule that mediates inflammatory response in macrophages during sepsis (38, 39). LPS released from Gram-negative bacteria activates TLR4 to initiate a downstream signaling cascade, leading to the activation of NF-κB, which then activates the transcription of inflammatory genes such as TNF-α, IL-6, and inducible nitric-oxide synthase (39, 40). We tested whether SR-BI modulates inflammatory response via TLR4 signaling using a HEK-Blue cell system. The HEK-Blue cell system stably expresses TLR4, CD14, MD2, and the NF-κB reporter, so it is a convenient system to elucidate TLR4 signaling. We generated HEK-Blue cells that stably express SR-BI or vector (Fig. 6a). As shown in Fig. 6b, LPS induced activation of NF-κB in an LPS dose-dependent manner. Importantly, the presence of SR-BI significantly suppressed LPS-stimulated NF-κB activation, indicating that SR-BI can modulate TLR4-mediated inflammatory signaling. We also generated HEK-Blue cells stably expressing SR-BIC323G (Fig. 6a). Similar to wild type SR-BI, the C323G mutant SR-BI effectively suppressed TLR4-mediated NF-κB activation (Fig. 6c).
FIGURE 6.
SR-BI suppresses TLR4-mediated NF-κB activation. a, Western blot analysis of HEK-Blue cells stably expressing SR-BI and C323G mutant SR-BI (5 μg protein/lane). b, effect of SR-BI on TLR4-mediated NF-κB activation. HEK-Blue vector (open bar) or HEK-Blue-SR-BI (solid bar) cells were treated with LPS at 0, 0.5, 1, and 2 ng/ml for 16 h, and the activation of NF-κB was quantified. c, effect of C323G mutant SR-BI on TLR4-mediated activation of NF-κB. HEK-Blue vector (open bar) or HEK-Blue SR-BIC323G (gray bar) cells were treated with LPS at 0, 0.5, 1, and 2 ng/ml for 16 h, and the activation of NF-κB was quantified. The data were from 4 independent experiments with triplicate measurements. mean ± S.D. *, p < 0.05 versus vector.
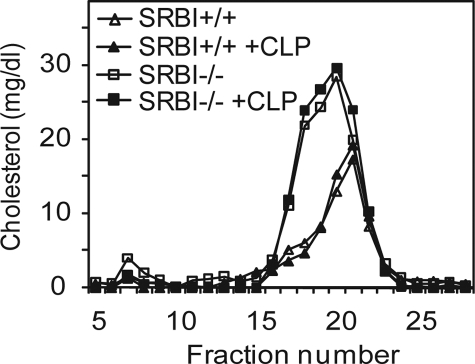
Effect of SR-BI on Lipoprotein Profiles during Sepsis
Lipoproteins, especially HDL, have been shown to bind LPS and neutralize LPS toxicity (41, 42). As an HDL receptor, SR-BI plays an important role in regulating plasma lipoprotein metabolism. Mice deficient in SR-BI have a 2-fold increase in plasma cholesterol levels and larger HDL particles as compared with wild type controls (10). This raises a possibility that changes in lipoprotein levels in SR-BI-null mice may contribute to susceptibility to sepsis. To determine the role of SR-BI in lipoprotein metabolism during sepsis, we measured serum cholesterol levels and analyzed lipoprotein profiles 20 h after CLP treatment. CLP treatment slightly increased plasma cholesterol levels in both SR-BI-null and wild type control mice, but the differences were not statistically significant: SR-BI−/− basal, 211.5 ± 16.1 mg/dl versus SR-BI−/− CLP, 232.3 ± 12.2 mg/dl; and SR-BI+/+ basal, 125.4 ± 6.5 mg/dl versus SR-BI+/+ CLP, 132.5 ± 8.2 mg/dl. The lipoprotein profiles were determined by gel filtration chromatography with fast protein liquid chromatography. As reported previously, SR-BI-null mice had larger HDL particles as compared with wild type controls. CLP treatment did not significantly alter lipoprotein profiles in either SR-BI-null or wild type control mice (Fig. 7). Using a Limulus amebocyte lysate assay, Cai et al. (26) showed that plasma from wild type and SR-BI-null mice had the same ability to bind and neutralize LPS, indicating that changes in lipoproteins in SR-BI-null mice did not affect the ability of lipoproteins to neutralize LPS.
FIGURE 7.
Effect of SR-BI on lipoprotein profiles during sepsis. SR-BI-null (solid square) and wild type littermates (solid triangle) were treated with CLP for 20 h. The serum was collected, and the lipoprotein profile was determined by gel filtration chromatography. Lipoprotein profiles of serum from SR-BI-null (open square) and wild type mice (open triangle) without CLP treatment were also determined (n = 3 per group). Representative data is shown.
Effect of SR-BI on Bacteremia during Sepsis
Previous in vitro studies showed that SR-BI facilitates the uptake of Gram-negative bacteria (27, 43), suggesting that SR-BI may play a role in bacteria clearance in sepsis. We collected blood and quantified the Gram-negative bacteria using blood agar culture. No significant difference in bacteria number in the blood was observed between SR-BI-null and wild type control mice 20 h after CLP challenge (SR-BI+/+, 267,050 ± 460,503/ml of blood versus SR-BI−/−, 234,972 ± 500,872/ml of blood (n = 12; p = 0.9)). Kupffer cells in the liver constitute the major pool of macrophages in the body. To determine whether SR-BI affects bacteria uptake by macrophages during sepsis, we isolated total DNA from the liver and quantified bacteria with qPCR using bacteria-specific primers. Wild type mice tended to have more bacteria in the liver compared with SR-BI-null mice 20 h after CLP challenge, but the difference is not statistically significant (SR-BI+/+, 183,333 ± 227,167/g of tissue versus SR-BI−/−, 39,166 ± 20,836/g of tissue (n = 6; p = 0.17)).
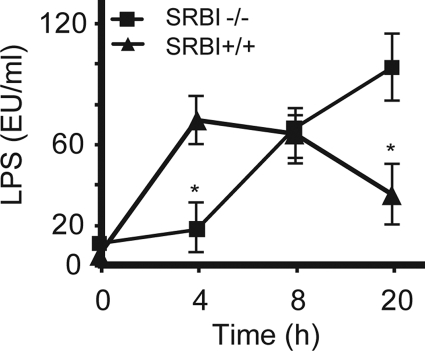
Effect of SR-BI on LPS Clearance during Sepsis
SR-BI has been shown to bind LPS and enhance LPS intracellular uptake in vitro(27, 43), which raises the possibility that SR-BI might facilitate LPS clearance during sepsis. We measured plasma LPS levels in CLP-challenged mice. SR-BI-null mice had a significantly higher plasma LPS levels than wild type controls 20 h following CLP treatment, indicating that SR-BI is required for LPS clearance during sepsis (Fig. 8). We also noticed that, compared with wild type controls, SR-BI-null mice had lower plasma LPS levels in the early stage of sepsis, indicating that SR-BI is required for LPS recruitment from inflammation site to circulation (Fig. 8).
FIGURE 8.
Effect of SR-BI on plasma LPS levels during sepsis. SR-BI-null mice and wild type littermates were treated with CLP for 4, 8, and 20 h, and the plasma LPS levels was quantified with a LPS kit (n = 6 per group with triplicate measurements). Mean ± S.D. *, p < 0.05 versus wild type.
DISCUSSION
This study demonstrates that SR-BI is a critical survival factor of sepsis in mice. In contrast to significant protection by SR-BI-mediated corticosterone production in LPS-induced endotoxic animal death, corticosterone did not provide protection against CLP-induced septic animal death. Further studies reveal that SR-BI protects against septic death through its role in modulating inflammatory responses in macrophages and facilitating LPS recruitment and clearance.
Role of SR-BI in Corticosterone Generation during Sepsis and Its Contribution to Septic Death
SR-BI is required for inducible corticosterone generation in stressful conditions such as endotoxemia, cold water swimming, and long term starvation (26, 44). Cai et al. (26) reported that supplementation of corticosterone to SR-BI-null mice effectively rescued mice from LPS-induced endotoxic death (decreasing mortality from 100% to 57%), indicating that SR-BI-mediated corticosteroid production plays a major protective role in endotoxemia. In this study, we determine the role of SR-BI in corticosterone generation during sepsis and its contribution to septic death. We demonstrate that SR-BI is required for inducible corticosterone production during sepsis. However, supplementation of corticosterone failed to rescue SR-BI-null mice from CLP-induced animal death, which is different from the efficient protection of corticosterone against LPS-induced endotoxic death. We speculate that the paradoxical effects of corticosterone on endotoxemia and sepsis are a result of the differential immune responses involved in endotoxemia and sepsis. For endotoxemia, an injection of LPS simply induces overproduction of inflammatory cytokines, leading to tissue damage and endotoxic death. Because corticosterone has anti-inflammatory activity, it can effectively reduce LPS-induced inflammatory response and therefore prevent endotoxic animal death (26). For sepsis, however, bacteria release LPS that stimulates the innate immune system to generate inflammatory cytokines, but the inflammatory cytokines now have both beneficial and deleterious effects. The inflammatory cytokines can potentially cause tissue damage, but they are also required for fighting against bacterial infections. If corticosterone supplementation suppresses inflammatory cytokine generation in sepsis, especially in its early stage, this would weaken the body's defense system, resulting in uncontrolled bacterial infections and ultimately septic death.
Role of SR-BI in Modulating Inflammatory Response in Macrophages and Its Contribution to Septic Death
We then looked for an alternative explanation for the protective role of SR-BI in sepsis. With the knowledge that SR-BI is expressed in macrophages and that the macrophage is one of the major players that mediate innate immune response, we conducted three groups of experiments to test our hypothesis that SR-BI protects against septic death through its role in modulating innate immune response of macrophages. First, we determined whether expression of SR-BI in macrophages modulates inflammatory response using bone marrow-derived macrophages. Macrophages deficient in SR-BI generated significantly higher levels of TNF-α and IL-6 by 20 h of LPS treatment when compared with wild type macrophages, indicating a prolonged and uncontrolled inflammatory response in the absence of SR-BI.
Next, we determined whether expression of SR-BI in macrophages contributes to protection against septic death. A number of approaches can be used to determine the role of SR-BI in macrophages in vivo (e.g. bone marrow transplantation has been used to determine the contribution of macrophage SR-BI to the development of atherosclerosis (23–25)). In this study, we employed transgenic mice expressing the C323G mutant SR-BI. We took advantage of several aspects of this animal model. 1) SR-BIC323G lacks cholesterol uptake activity so that we could exclude the contribution of SR-BI-mediated corticosteroid generation to sepsis. 2) As shown previously, SR-BI has multiple functions including induction of apoptosis and protection against NO-cytotoxicity (21, 22), which may contribute to its protection against septic death. SR-BIC323G has no apoptotic or anti-NO activity so that we could also exclude these effects. 3) We can avoid the sub-lethal radiation treatment used in bone marrow transplantation that may affect the outcomes of septic study (45). With this unique animal model, we confirmed that C323G-mutated SR-BI is capable of modulating inflammatory response in macrophages and, importantly, provides protection against CLP-induced septic death.
Then, we explored whether SR-BI suppresses inflammatory response by modulating TLR4 signaling using a HEK-Blue cell system. The HEK-Blue cell stably expresses TLR4 and its cofactors CD14 and MD2. The cell also expresses a NF-κB reporter, which makes it a convenient system to elucidate TLR4-mediated inflammatory signaling. We showed that LPS treatment stimulated TLR4 signaling resulting in NF-κB activation, and expression of SR-BI significantly suppressed NF-κB activation. Because TLR4 and NF-κB are upstream signaling molecules that control inflammatory cytokine production in macrophages, we conclude that SR-BI modulates the inflammatory response via TLR4 signaling.
Role of SR-BI in LPS Clearance during Sepsis
SR-BI enhances LPS uptake in vitro (43). By injection of radioisotope-labeled LPS, it has been shown that mice deficient in SR-BI have delayed LPS clearance from circulation (26). In this study, we measured plasma LPS levels to determine the effect of SR-BI on LPS metabolism during sepsis. As expected, SR-BI-null mice had significantly higher plasma LPS levels than the wild type controls 20 h following CLP treatment, indicating that SR-BI is required for LPS clearance during sepsis. We also noticed an unexpected phenomenon that SR-BI-null mice had lower plasma LPS levels in the early stage of sepsis compared with wild type controls, indicating that SR-BI is required for LPS recruitment from inflammation site to circulation. The defect in LPS recruitment may provide an explanation as to why SR-BI-null mice had delayed inflammatory responses during the early stage of sepsis.
It is worth noting that both the delay in cytokine production and subsequent enhancement in cytokine production by SR-BI-null mice treated with CLP are associated with decreased survival, but this relationship is not necessarily causal. More studies are required to determine the contribution of this delayed inflammatory response to septic death.
SR-BI may contribute to protection against other inflammatory diseases (e.g. atherosclerosis) through its role in modulating inflammatory response in macrophages. Using bone marrow transplantation, early studies have shown that expression of SR-BI in macrophages protects against the development of atherosclerosis (23–25). Interestingly, expression of SR-BI in macrophages does not alter plasma lipoprotein profiles or SR-BI-mediated cholesterol efflux in macrophages, suggesting that the macrophage SR-BI exerts its protective functions via a novel mechanism, independent of its role in regulating cholesterol metabolism. Although more detailed studies are required to determine the mechanisms underlying macrophage SR-BI protection against atherosclerosis, our findings that SR-BI modulates TLR4-mediated inflammatory response in macrophages provides a possible mechanism for the protection of SR-BI against atherosclerosis (46).
In summary, we demonstrate that SR-BI is a critical protective modulator in sepsis. We reveal that SR-BI protects against septic death likely through its role in modulating immune response via TLR4 signaling in macrophages and through its role in removal of LPS from circulation. Importantly, by using a transgenic mouse model, we showed that overexpression of the C323G mutant SR-BI provides protection against septic death. Our findings may provide a novel target for the intervention of sepsis.
Acknowledgments
We thank the members of the Kentucky Pediatric Research Institute for invaluable advice and assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants R01GM085231 (to X.-A. L.) and 2P0RR015592 (to T. Curry). This work was also supported by American Heart Association Grant 0530241N (to X.-A. L.) and the Children's Miracle Network (to X.-A. L.).
M. Chen, Q. Wu, L. Guo, G. Graf, A. Daugherty, and X.-A. Li, unpublished data.
- SR-BI
- scavenger receptor BI
- HDL
- high density lipoprotein
- IL
- interleukin
- TNF-α
- tumor necrosis factor-α
- CLP
- cecal ligation and puncture
- ELISA
- enzyme-linked immunosorbent assay
- LPS
- lipopolysaccharide.
REFERENCES
- 1.Bone R. C. (1991) Ann. Intern. Med. 115, 457–469 [DOI] [PubMed] [Google Scholar]
- 2.Angus D. C., Linde-Zwirble W. T., Lidicker J., Clermont G., Carcillo J., Pinsky M. R. (2001) Crit. Care Med. 29, 1303–1310 [DOI] [PubMed] [Google Scholar]
- 3.Martin G. S., Mannino D. M., Eaton S., Moss M. (2003) N. Engl. J. Med. 348, 1546–1554 [DOI] [PubMed] [Google Scholar]
- 4.Riedemann N. C., Guo R. F., Ward P. A. (2003) J. Clin. Investig. 112, 460–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sessler C. N., Perry J. C., Varney K. L. (2004) Curr. Opin. Crit. Care 10, 354–363 [DOI] [PubMed] [Google Scholar]
- 6.Krieger M. (2001) J. Clin. Investig. 108, 793–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chinetti G., Gbaguidi F. G., Griglio S., Mallat Z., Antonucci M., Poulain P., Chapman J., Fruchart J. C., Tedgui A., Najib-Fruchart J., Staels B. (2000) Circulation 101, 2411–2417 [DOI] [PubMed] [Google Scholar]
- 8.Acton S., Rigotti A., Landschulz K. T., Xu S., Hobbs H. H., Krieger M. (1996) Science 271, 518–520 [DOI] [PubMed] [Google Scholar]
- 9.Kozarsky K. F., Donahee M. H., Rigotti A., Iqbal S. N., Edelman E. R., Krieger M. (1997) Nature 387, 414–417 [DOI] [PubMed] [Google Scholar]
- 10.Rigotti A., Trigatti B. L., Penman M., Rayburn H., Herz J., Krieger M. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 12610–12615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kraemer F. B. (2007) Mol. Cell. Endocrinol. 265–266, 42–45 [DOI] [PubMed] [Google Scholar]
- 12.Arai T., Wang N., Bezouevski M., Welch C., Tall A. R. (1999) J. Biol. Chem. 274, 2366–2371 [DOI] [PubMed] [Google Scholar]
- 13.Braun A., Trigatti B. L., Post M. J., Sato K., Simons M., Edelberg J. M., Rosenberg R. D., Schrenzel M., Krieger M. (2002) Circ. Res. 90, 270–276 [DOI] [PubMed] [Google Scholar]
- 14.Kozarsky K. F., Donahee M. H., Glick J. M., Krieger M., Rader D. J. (2000) Arterioscler. Thromb. Vasc. Biol. 20, 721–727 [DOI] [PubMed] [Google Scholar]
- 15.Yu H., Zhang W., Yancey P. G., Koury M. J., Zhang Y., Fazio S., Linton M. F. (2006) Arterioscler. Thromb. Vasc. Biol. 26, 150–156 [DOI] [PubMed] [Google Scholar]
- 16.Van Eck M., Twisk J., Hoekstra M., Van Rij B. T., Van Der Lans C. A., Bos I. S., Kruijt J. K., Kuipers F., Van Berkel T. J. (2003) J. Biol. Chem. 278, 23699–23705 [DOI] [PubMed] [Google Scholar]
- 17.Li X. A., Titlow W. B., Jackson B. A., Giltiay N., Nikolova-Karakashian M., Uittenbogaard A., Smart E. J. (2002) J. Biol. Chem. 277, 11058–11063 [DOI] [PubMed] [Google Scholar]
- 18.Yuhanna I. S., Zhu Y., Cox B. E., Hahner L. D., Osborne-Lawrence S., Lu P., Marcel Y. L., Anderson R. G., Mendelsohn M. E., Hobbs H. H., Shaul P. W. (2001) Nat. Med. 7, 853–857 [DOI] [PubMed] [Google Scholar]
- 19.Mineo C., Yuhanna I. S., Quon M. J., Shaul P. W. (2003) J. Biol. Chem. 278, 9142–9149 [DOI] [PubMed] [Google Scholar]
- 20.Gong M., Wilson M., Kelly T., Su W., Dressman J., Kincer J., Matveev S. V., Guo L., Guerin T., Li X. A., Zhu W., Uittenbogaard A., Smart E. J. (2003) J. Clin. Investig. 111, 1579–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X. A., Guo L., Dressman J. L., Asmis R., Smart E. J. (2005) J. Biol. Chem. 280, 19087–19096 [DOI] [PubMed] [Google Scholar]
- 22.Li X. A., Guo L., Asmis R., Nikolova-Karakashian M., Smart E. J. (2006) Circ. Res. 98, e60–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Covey S. D., Krieger M., Wang W., Penman M., Trigatti B. L. (2003) Arterioscler. Thromb. Vasc. Biol. 23, 1589–1594 [DOI] [PubMed] [Google Scholar]
- 24.Zhang W., Yancey P. G., Su Y. R., Babaev V. R., Zhang Y., Fazio S., Linton M. F. (2003) Circulation 108, 2258–2263 [DOI] [PubMed] [Google Scholar]
- 25.Van Eck M., Bos I. S., Hildebrand R. B., Van Rij B. T., Van Berkel T. J. (2004) Am. J. Pathol. 165, 785–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai L., Ji A., de Beer F. C., Tannock L. R., van der Westhuyzen D. R. (2008) J. Clin. Investig. 118, 364–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vishnyakova T. G., Kurlander R., Bocharov A. V., Baranova I. N., Chen Z., Abu-Asab M. S., Tsokos M., Malide D., Basso F., Remaley A., Csako G., Eggerman T. L., Patterson A. P. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 16888–16893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eskandari M. K., Bolgos G., Miller C., Nguyen D. T., DeForge L. E., Remick D. G. (1992) J. Immunol. 148, 2724–2730 [PubMed] [Google Scholar]
- 29.Wesche-Soldato D. E., Chung C. S., Lomas-Neira J., Doughty L. A., Gregory S. H., Ayala A. (2005) Blood 106, 2295–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berwin B., Hart J. P., Rice S., Gass C., Pizzo S. V., Post S. R., Nicchitta C. V. (2003) EMBO J. 22, 6127–6136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinman C. R., Muralidhar B., Nuovo G. J., Rumore P. M., Yu D., Mukai M. (1997) Anal. Biochem. 244, 328–339 [DOI] [PubMed] [Google Scholar]
- 32.Cohen J. (2002) Nature 420, 885–891 [DOI] [PubMed] [Google Scholar]
- 33.Titheradge M. A. (1999) Biochim. Biophys. Acta 1411, 437–455 [DOI] [PubMed] [Google Scholar]
- 34.Beckman J. S., Koppenol W. H. (1996) Am. J. Physiol. 271, C1424–1437 [DOI] [PubMed] [Google Scholar]
- 35.Krieger M. (1999) Annu. Rev. Biochem. 68, 523–558 [DOI] [PubMed] [Google Scholar]
- 36.Su G. L. (2002) Am. J. Physiol. Gastrointest. Liver Physiol. 283, G256–265 [DOI] [PubMed] [Google Scholar]
- 37.Uesugi T., Froh M., Arteel G. E., Bradford B. U., Thurman R. G. (2001) Hepatology 34, 101–108 [DOI] [PubMed] [Google Scholar]
- 38.Poltorak A., He X., Smirnova I., Liu M. Y., Van Huffel C., Du X., Birdwell D., Alejos E., Silva M., Galanos C., Freudenberg M., Ricciardi-Castagnoli P., Layton B., Beutler B. (1998) Science 282, 2085–2088 [DOI] [PubMed] [Google Scholar]
- 39.Raetz C. R., Whitfield C. (2002) Annu. Rev. Biochem. 71, 635–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carmody R. J., Chen Y. H. (2007) Cell Mol. Immunol. 4, 31–41 [PubMed] [Google Scholar]
- 41.Ulevitch R. J., Johnston A. R., Weinstein D. B. (1979) J. Clin. Investig. 64, 1516–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ulevitch R. J., Johnston A. R., Weinstein D. B. (1981) J. Clin. Investig. 67, 827–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vishnyakova T. G., Bocharov A. V., Baranova I. N., Chen Z., Remaley A. T., Csako G., Eggerman T. L., Patterson A. P. (2003) J. Biol. Chem. 278, 22771–22780 [DOI] [PubMed] [Google Scholar]
- 44.Hoekstra M., Meurs I., Koenders M., Out R., Hildebrand R. B., Kruijt J. K., Van Eck M., Van Berkel T. J. (2008) J. Lipid Res. 49, 738–745 [DOI] [PubMed] [Google Scholar]
- 45.Létourneau I., Dorval M., Bélanger R., Légaré M., Fortier L., Leblanc M. (2002) Nephron 90, 408–412 [DOI] [PubMed] [Google Scholar]
- 46.Michelsen K. S., Wong M. H., Shah P. K., Zhang W., Yano J., Doherty T. M., Akira S., Rajavashisth T. B., Arditi M. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 10679–10684 [DOI] [PMC free article] [PubMed] [Google Scholar]