Abstract
Mitochondrial aldehyde dehydrogenase-2 (ALDH2) plays an essential role in nitroglycerin (GTN) bioactivation, resulting in formation of NO or a related activator of soluble guanylate cyclase. ALDH2 denitrates GTN to 1,2-glyceryl dinitrate and nitrite but also catalyzes reduction of GTN to NO. To elucidate the relationship between ALDH2-catalyzed GTN bioconversion and established ALDH2 activities (dehydrogenase, esterase), we compared the function of the wild type (WT) enzyme with mutants lacking either the reactive Cys-302 (C302S) or the general base Glu-268 (E268Q). Although the C302S mutation led to >90% loss of all enzyme activities, the E268Q mutant exhibited virtually unaffected rates of GTN denitration despite low dehydrogenase and esterase activities. The nucleotide co-factor NAD caused a pronounced increase in the rates of 1,2-glyceryl dinitrate formation by WT-ALDH2 but inhibited the reaction catalyzed by the E268Q mutant. GTN bioactivation measured as activation of purified soluble guanylate cyclase or release of NO in the presence of WT- or E268Q-ALDH2 was markedly potentiated by superoxide dismutase, suggesting that bioavailability of GTN-derived NO is limited by co-generation of superoxide. Formation of superoxide was confirmed by determination of hydroethidine oxidation that was inhibited by superoxide dismutase and the ALDH2 inhibitor chloral hydrate. E268Q-ALDH2 exhibited ∼50% lower rates of superoxide formation than the WT enzyme. Our results suggest that Glu-268 is involved in the structural organization of the NAD-binding pocket but is not required for GTN denitration. ALDH2-catalyzed superoxide formation may essentially contribute to oxidative stress in GTN-exposed blood vessels.
Aldehyde dehydrogenases (ALDH; EC 1.2.1.3)2 catalyze the oxidation of aliphatic and aromatic aldehyde substrates to the corresponding carboxylic acids with NAD(P) serving as electron accepting co-factor (1). The mitochondrial isoform (ALDH2), a homotetrameric protein with subunits of ∼54 kDa, appears to be essential for detoxification of ethanol-derived acetaldehyde, as indicated by significantly lowered alcohol tolerance of individuals expressing a low activity mutant of the protein (2, 3). Aldehyde oxidation by ALDH2 is thought to involve nucleophilic reaction of the substrate with a critical cysteine residue in the active site (Cys-302 in the human protein), resulting in formation of a thiohemiacetal intermediate, followed by hydride transfer to NAD, yielding a thioester intermediate that is hydrolyzed to the carboxylic acid product in a reaction that involves activation of H2O by an adjacent glutamate residue (Glu-268). In addition to aldehyde oxidation, ALDH2 catalyzes ester hydrolysis (4). The esterase activity is stimulated by NAD, but the co-factor is not essential for the reaction, which is initiated by nucleophilic attack of the substrate by Cys-302, resulting in formation of a thioester and release of the corresponding alcohol by hydrolysis of the intermediate through activation of water by Glu-268 (4).
The beneficial therapeutic effects of the antianginal drug GTN are thought to involve bioactivation of the organic nitrate in vascular smooth muscle to yield NO or a related species that activates sGC, resulting in cGMP-mediated vasorelaxation (5). In a seminal paper published in 2002, Stamler and co-workers (6) discovered that ALDH2 essentially contributes to vascular GTN bioactivation, and this has been confirmed in numerous later studies (for review see Ref. 7). Stamler and co-workers (6) proposed that GTN denitration involves the established esterase activity of ALDH2, i.e. nucleophilic attack of a nitro group of GTN by Cys-302, resulting in formation of a thionitrate intermediate and release of the corresponding alcohol, preferentially 1,2-glyceryl dinitrate (1,2-GDN). The thionitrate intermediate would then release nitrite either through nucleophilic attack of one of the adjacent cysteine residues (Cys-301 or Cys-303), resulting in formation of a disulfide in the active site, or through Glu-268-aided hydrolysis yielding a sulfenic acid derivative of Cys-302, which could undergo S-thiolation (8) to form a cysteinyl disulfide with one of the adjacent cysteine residues. This mechanism would be compatible both with the effect of NAD, which is not essential but increases reaction rates, and with GTN-triggered enzyme inactivation that is partially prevented by reduced thiols with two SH groups like DTT or dihydrolipoic acid. According to a brief statement in a paper on the structure of the East Asian (E487K) variant, mutation of Cys-302 and Glu-268 resulted in an almost complete loss of GTN reductase activity of ALDH2 (3), but so far the proposed role of these residues in GTN metabolism has not been thoroughly studied, and the mechanism underlying bioactivation of the nitrate is still unknown.
EXPERIMENTAL PROCEDURES
Materials
Bovine lung sGC was purified as previously described (9). Human ALDH2 was expressed in Escherichia coli BL21(DE3) and purified as described (10, 11). Sephacryl S-300 HR and [α-32P]GTP (400 Ci/mmol) were obtained from GE Healthcare. [2-14C]GTN (50–60 mCi/mmol) was from American Radiolabeled Compounds, purchased through Humos Diagnostica GmbH (Maria Enzersdorf, Austria). Nitropohl® ampoules (G. Pohl-Boskamp GmbH. & Co, Hohenlockstedt, Germany) containing 4.4 mm GTN in 250 mm glucose were obtained from a local pharmacy; dilutions were made in 50 mm TEA/HCl buffer. DEA/NO and SIN-1 were from Alexis Corporation (Lausen, Switzerland). All other chemicals, including hydralazine and CuZnSOD, were from Sigma-Aldrich.
Site-directed Mutagenesis
C302S and E268Q mutations were inserted using the QuikChange® II site-directed mutagenesis kit (Stratagene). The mutagenic primers 5′-GTTCTTCAACCAGGGCCAGTGCAGCTGTGCCGGATCCCGGACCTTCGTG-3′ (sense strand) and 5′-GAAGGTCCGGGATCCGGCACAGCTGCACTGGCCCTGGTTGAAGAAC-3′ (antisense strand) were used to introduce C302S mutation (bold type) and to add the BamHI restriction site (underlined) for a rapid screening of mutants but without changing the final amino acid sequence. The mutagenic primers 5′-GCAGCAACCTTAAGAGAGTGACCTTGCAGCTGGGGGGGAAG-3′ (sense) and 5′-CTTCCCCCCCAGCTGCAAGGTCACTCTCTTAAGGTTGCTGC-3′ (antisense) were used to introduce E268Q mutation (bold) and to add the AflII restriction site as a silent mutation (underlined) for screening of mutants. C302S- and E268Q-ALDH2 were expressed in E. coli BL21(DE3) and purified by p-hydroxyacetophenone affinity chromatography and size exclusion chromatography as described (11).
Determination of Dehydrogenase and Esterase Activities
Dehydrogenase activity of ALDH2 was measured as formation of NADH from NAD by monitoring the increase in absorbance at 340 nm (ϵ340 = 6.22 mm−1 cm−1) at 25 °C in the presence of 1.5 mm formaldehyde and 10 mm MgCl2 in 50 mm sodium pyrophosphate (pH 7.5 or 9.0) (12). Esterase activity was measured by monitoring formation of p-nitrophenolate from 0.1 mm p-nitrophenyl acetate (ϵ400 = 16 mm−1 cm−1) in 50 mm sodium pyrophosphate buffer (pH 7.5), containing 10 mm MgCl2 in the absence and presence of 1 mm NAD.
ALDH2 Inactivation by GTN
GTN-triggered inactivation of ALDH2 was determined in the absence and presence of 5,000 units/ml SOD as described (13). Briefly, the dehydrogenase activity of ALDH2 (33 μg/ml) was measured by monitoring the formation of NADH spectrophotometrically at 340 nm. The initial reaction mixture consisted of 0.4 mm acetaldehyde and 0.4 mm NAD in 50 mm phosphate buffer (pH 7.4) in the absence or presence of 5,000 units/ml SOD. After 1–2 min the reactions were started by the addition of ALDH2. Approximately 10 min after the start of the experiment, 50 μm GTN was added to inactivate the enzyme. After complete inactivation, 0.4 mm DTT was added to reactivate the enzyme. The linear absorbance increases after the addition of ALDH2 and DTT were used to calculate initial and restored activities, respectively. The gradual inactivation after GTN addition was fitted to a combination of a single exponential and a straight line, with the exponential component yielding the apparent inactivation rate constant and the linear part yielding the residual activity of the enzyme.
Determination of GTN Denitration
The rates of GTN metabolism yielding 1,2- and 1,3-GDN were determined according to a described protocol (14). Unless indicated otherwise, WT and mutated ALDH2 proteins (4 μg each) were incubated with 14C-labeled GTN (2 μm; ∼50,000 dpm) at 37 °C for 10 min in a final volume of 200 μl of 50 mm phosphate buffer (pH 7.4), containing 3 mm MgCl2 and 2 mm DTT in the absence and presence of 1 mm NAD. GTN concentrations ≥10 μm were adjusted by the addition of unlabeled GTN. Reaction products were extracted twice with 1 ml of diethyl ether, separated by thin layer chromatography, and quantified by liquid scintillation counting. Blank values were determined in the absence of protein under identical conditions and subtracted. The results are the mean values ± S.E. determined in three independent experiments. Because 1,2-/1,3-GDN ratios were not significantly affected by the two mutations described here, the rates of 1,3-GDN formation are not shown.
Determination of sGC Activity
Purified bovine lung sGC (50 ng) was incubated at 37 °C for 2 or 10 min as indicated in a final volume of 100 μl with the indicated concentrations of donor compounds (GTN and DEA/NO) in the presence of WT- or E268Q-ALDH2 as indicated in the text and figure legends. Assay mixtures contained 50 mm TEA/HCl (pH 7.4), 0.5 mm [α-32P]GTP (∼250,000 cpm), 3 mm MgCl2, 0.1 mm DTPA, and 1 mm cGMP. DTT, NAD, and SOD were present as indicated in the text and figure legends. To test for NO scavenging by ALDH2/GTN, sGC was incubated under otherwise identical conditions with WT- or E268Q-ALDH2 (25 μg each) in the presence of 1 μm DEA/NO. GTN (0.1 mm) and SOD (1,000 units/ml) were present as specified in the text. The reactions were terminated by the addition of 0.45 ml of zinc acetate (120 mm) and 0.45 ml of sodium bicarbonate (120 mm), followed by isolation and quantification of [32P]cGMP as previously described (15). The blank values were determined in the absence of sGC.
Determination of GTN-derived NO
NO formation was measured with a Clark-type electrode (World Precision Instruments, Berlin, Germany), calibrated daily with acidified nitrite as previously described (16). The electrode was equilibrated in 50 mm TEA/HCl (pH 7.4), containing SOD (1,000 units/ml unless indicated otherwise), 0.1 mm DTPA, 2 mm DTT, 3 mm MgCl2, and purified WT- or E268Q-ALDH2 (10- 100 μg/0.1 ml), followed by the addition of GTN (final concentration, 0.1 mm). The incubation volume was 0.5 ml. Output current was recorded in three separate experiments. The experiment shown in Fig. 2 was performed with 100 μg/0.1 ml of ALDH2 proteins. NO formation relative to 1,2-GDN formation (shown in Table 2) was calculated from the initial rates of product formation catalyzed by 10–100 μg/0.1 ml WT- and E268Q-ALDH2 in the absence and presence of NAD. The errors of the ratios were calculated according to Ref. 17.
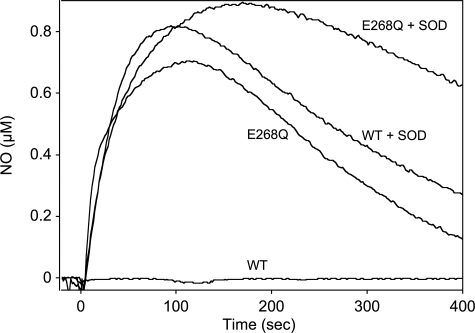
FIGURE 2.
Formation of GTN-derived NO by WT- and E268Q-ALDH2 in the absence and presence of SOD. WT- and E268Q-ALDH2 (0.5 mg each) were incubated in 0.5 ml of 50 mm TEA buffer (pH 7.4), containing 3 mm MgCl2, 0.1 mm DTPA, 2 mm DTT, and 0.1 mm GTN in the absence and presence of 1,000 units/ml SOD. NO formation was monitored with a Clark-type NO electrode. The traces shown are representative for three similar experiments.
TABLE 2.
Formation of GTN-derived NO relative to denitration catalyzed by WT- and E268Q-ALDH2
Initial rates of NO formation were divided by the initial rates of 1,2-GDN formation catalyzed by 10–100 μg/0.1 ml of WT- and E268Q-ALDH2. The data are the mean values ± S.E. of relative NO formation (expressed as percentages of 1,2-GDN formation) calculated from three independent experiments.
| ALDH2 |
vi(NO)/vi(1,2-GDN) × 100 |
|||
|---|---|---|---|---|
| WT |
E268Q |
|||
| −NAD | +NAD | −NAD | +NAD | |
| 10 μg/0.1 ml | 10.6 ± 0.55 | 13.4 ± 1.61 | 8.69 ± 2.43 | 31.7 ± 5.83a |
| 25 μg/0.1 ml | 6.39 ± 0.52 | 8.11 ± 0.84 | 8.12 ± 1.29 | 48.2 ± 6.59a |
| 50 μg/0.1 ml | 6.97 ± 0.34 | 6.34 ± 0.43 | 8.03 ± 0.48 | 37.4 ± 4.16a |
| 100 μg/0.1 ml | 6.06 ± 0.68 | 6.53 ± 1.29 | 9.12 ± 0.49 | 40.3 ± 1.85a |
ap < 0.001 vs. WT ± NAD and E268Q without NAD (analysis of variance, Holm-Sidak method).
Determination of GTN-derived Superoxide
Formation of superoxide/peroxynitrite was measured as hydroethidine oxidation. The reaction mixtures (final volume, 0.7 ml) contained 50 mm TEA buffer (pH 7.4), 3 mm MgCl2, 0.1 mm DTPA, 2 mm DTT, 2.5 μm hydroethidine, 0.45 mg WT- or E268Q-ALDH2 (corresponding to 65 μg/0.1 ml), and where indicated 0.1 mm GTN, 10 mm chloral hydrate, and 1000 units/ml SOD. The experiments were performed at 37 °C, and fluorescence was monitored at excitation and emission wavelengths of 490 nm (slit width, 5 nm) and 585 nm (slit width 20 nm), respectively. The data are the mean values ± S.E. of the increase in fluorescence read-outs (arbitrary units) monitored over 5 min in three to six independent experiments.
RESULTS
Basic Characterization of WT and Mutated ALDH2
Wild type, C302S-ALDH2, and E268Q-ALDH2 were assayed for oxidation of acetaldehyde (at pH 9.0 and 7.5), hydrolysis of p-nitrophenyl acetate (esterase activity), and denitration of GTN to 1,2-GDN. As shown in Table 1, mutation of the reactive cysteine residue Cys-302 resulted in virtual loss of dehydrogenase and esterase activities. Residual GTN reductase activity of the C302S mutant (∼10% of WT) may be due to reaction of GTN with one of the adjacent cysteine residues. Mutagenesis of Glu-268 caused virtually complete loss of dehydrogenase activity, but determination of the esterase activity in the absence and presence of NAD revealed an interesting, hitherto unrecognized effect of the E268Q mutation. Although NAD caused an approximately 6-fold increase of WT esterase activity, the activity of the E268Q mutant was decreased in the presence of the nicotinamide co-factor by ∼90%. The distinct effects of NAD on WT- and E268Q-ALDH2 were also apparent in assays of GTN denitration. Although both variants of the enzyme exhibited virtually identical rates of 1,2-GDN formation in the absence of NAD, the E268Q mutation resulted in an almost complete loss of GTN denitrating activity (∼3% of WT) in the presence of NAD. Thus, resembling ester hydrolysis, GTN denitration catalyzed by E268Q-ALDH2 was decreased ∼5-fold by the nicotinamide.
TABLE 1.
Dehydrogenase, esterase, and GTN reductase activities of WT-, C302S-, and E268Q-ALDH2
Enzyme activities were determined as described under “Experimental Procedures” and are expressed as nmol × min−1 × mg−1. The activities of the mutants are also given relative to WT (WT = 100%). The data are the mean values ± S.E. of three independent experiments.
| Enzyme activity | WT activity | C302S |
E268Q |
||
|---|---|---|---|---|---|
| Activity | Activity relative to WT | Activity | Activity relative to WT | ||
| % | % | ||||
| Dehydrogenase (pH 9.0) | 4,042 ± 57 | 11.9 ± 2.2b | 0.29 ± 0.06 | 56.1 ± 0.7b | 1.39 ± 0.01 |
| Dehydrogenase (pH 7.5) | 2,690 ± 26 | 8.7 ± 1.2b | 0.32 ± 0.05 | 23.5 ± 0.60b | 0.88 ± 0.02 |
| Esterase | |||||
| −NAD | 199.9 ± 0.5 | 0.71 ± 0.14b | 0.36 ± 0.07 | 5.33 ± 0.39b | 2.67 ± 0.20 |
| +NAD | 1,180 ± 31a | 0.40 ± 0.14b | 0.03 ± 0.01 | 0.64 ± 0.07b,a | 0.06 ± 0.01 |
| GTN reductase(1,2-GDN formation) | |||||
| −NAD | 1.21 ± 0.18 | 0.12 ± 0.03c | 9.37 ± 1.18 | 1.57 ± 0.13 | 133 ± 11 |
| +NAD | 8.73 ± 0.09a | 0.17 ± 0.06b | 1.97 ± 0.65 | 0.29 ± 0.18b,d | 3.36 ± 2.05 |
a p < 0.0001 vs. no NAD.
b p < 0.0001 vs. WT.
c p < 0.001 vs. WT.
d p < 0.005 vs. no NAD.
Effects of GTN and Protein Concentration on 1,2-GDN Formation
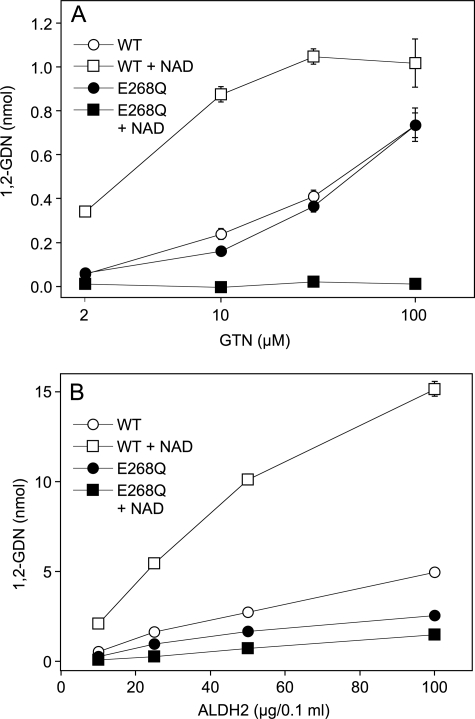
In the presence of NAD, GTN was denitrated by WT-ALDH2 with an EC50 of ∼3 μm (Fig. 1A). Maximal rates of 1,2-GDN formation, corresponding to a specific activity of ∼25 nmol × min−1 × mg−1 after 10 min were observed at ≥30 μm GTN. As reported previously (14), the reaction rates measured at low GTN concentrations were ∼5-fold lower in the absence of NAD, whereas the nicotinamide had a comparably moderate effect in the presence of high GTN (0.1 mm). In the absence of NAD the EC50 of GTN was increased to ≥30 μm. In contrast to the WT enzyme, E268Q-ALDH2 did not catalyze significant 1,2-GDN formation in the presence of NAD at any GTN concentration. In the absence of the nicotinamide, the mutant exhibited identical rates of GTN denitration as the WT enzyme over the whole GTN concentration range (Fig. 1A). A similar pattern was obtained when the initial rates of GTN denitration were measured as a function of the protein concentration after 1 min of incubation. As shown in Fig. 1B, 1,2-GDN formation increased in a roughly linear manner at ALDH2 concentrations up to 50 μg/0.1 ml. At the highest concentration of the WT enzyme tested (100 μg/0.1 ml), product formation declined, indicating rapid enzyme inactivation under high turnover conditions. Maximal activity of WT-ALDH2 measured with 10 μg of protein/0.1 ml in the presence of NAD after 1 min of incubation was 106 ± 8.1 nmol 1,2-GDN × min−1 × mg−1. This value is 3–4-fold higher than the activity measured after 10 min, confirming a previous study on turnover-dependent enzyme inactivation that is only partially prevented by DTT (13). The activity of the WT enzyme was markedly increased by NAD, whereas the activity of the mutant was decreased at all of the protein concentrations tested. However, higher concentrations of the mutant showed significant activity even in the presence of NAD, resulting in less pronounced inhibition of GTN denitration by the nicotinamide.
FIGURE 1.
Formation of 1,2-GDN from GTN by WT- and E268Q-ALDH2 in the absence and presence of NAD. A, effect of GTN concentration. Purified proteins (4 μg each) were incubated at 37 °C for 10 min in 0.2 ml phosphate buffer (pH 7.4) containing increasing concentrations of [14C]GTN, 3 mm MgCl2, and 2 mm DTT in the absence and presence of 1 mm NAD. B, effect of protein concentration. Purified proteins (20–200 μg each) were incubated at 37 °C for 1 min in 0.2 ml of phosphate buffer (pH 7.4) containing 0.1 mm [14C]GTN, 3 mm MgCl2, and 2 mm DTT in the absence and presence of 1 mm NAD. 1,2- and 1,3-GDN were extracted and quantified by radio thin layer chromatography as described previously (14). The data are the mean values ± S.E. of three independent experiments.
Formation of GTN-derived NO
As reported previously (11), the presence of SOD is essential for detection of GTN-derived NO formed by WT-ALDH2. However, as shown in Fig. 2, the E268Q mutant generated significant amounts of NO even in the absence of SOD, presumably because of reduced superoxide generation in the course of GTN turnover (see below). The time course of NO formation by E268Q-ALDH2 as compared with WT in the presence of SOD shows that the E268Q substitution led to significantly delayed NO decay, indicating slower rates of mechanism-based enzyme inactivation.
To see whether the partitioning between 1,2-GDN and NO formation was affected by the E268Q substitution and/or NAD, we measured the initial rates of NO formation catalyzed by 10–100 μg of WT- and E268Q-ALDH2/0.1 ml in the presence of 1,000 units/ml SOD with and without NAD and compared the data with the initial rates of 1,2-GDN formation (shown in Fig. 1B). As shown in Table 2, the rates of NO formation catalyzed by WT-ALDH2 were between 5 and 10% of the denitration rates, and product distribution was not significantly affected by NAD. However, the E268Q mutant showed an interesting difference. Although relative NO formation was similar to WT (8- 9%) in the absence of NAD, the decrease of GTN reductase activity was not paralleled by a comparable decrease in the rates of NO formation in the presence of the nucleotide, resulting in a consistent increase of relative NO formation to 30–50% of the denitration rates over the whole range of protein concentrations tested.
Activation of Purified sGC by GTN in the Presence of WT- and E268Q-ALDH2
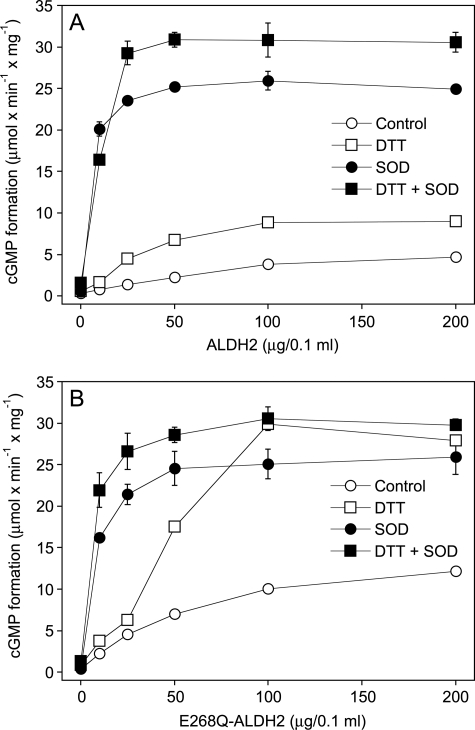
As shown in Fig. 3A, sGC was activated by GTN in the presence of increasing amounts of WT-ALDH2, but the effect reached a plateau of ∼15% of maximal sGC activity at 50–100 μg of ALDH2 (4.7 ± 0.34 μmol cGMP × min−1 × mg−1). In the presence of 2 mm DTT, the rates of cGMP formation were increased ∼2-fold, but again a plateau of submaximal sGC activation was reached with 50–100 μg of protein (9.0 ± 0.38 μmol cGMP × min−1 × mg−1). To test whether sGC activation is limited by co-generation of superoxide, which rapidly reacts with NO to yield peroxynitrite, we measured GTN-triggered cGMP accumulation in the presence of SOD. SOD (1,000 units/ml) markedly potentiated the effect of ALDH2, resulting in maximal sGC activation with 25 μg of protein, and DTT further increased the rates of cGMP formation by ∼20%. Fig. 3B shows that the rates of cGMP formation measured with E268Q-ALDH2 in the absence of DTT were ∼2-fold higher than with WT at all protein concentrations tested. In the presence of SOD, the effect of the mutant was virtually identical to that of WT-ALDH2. Intriguingly, a pronounced difference between the two ALDH2 variants became apparent when sGC activation was measured in the presence of DTT without SOD. Instead of reaching a plateau at ∼50 μg of protein/0.1 ml, sGC activity further increased with increasing E268Q-ALDH2 concentrations, and maximal sGC activation was observed with 100 μg/0.1 ml of the protein even in the absence of SOD. As shown in Fig. 2, this difference between the two ALDH2 variants was also apparent as formation of detectable GTN-derived NO by the E268Q mutant but not by the WT enzyme in the absence of SOD.
FIGURE 3.
Activation of sGC by GTN in the presence of WT-ALDH2 (A) and E268Q-ALDH2 (B). Purified sGC (50 ng) was incubated at 37 °C for 10 min with 0.1 mm GTN and the indicated concentrations of WT- or E268Q-ALDH2 in 0.1 ml of 50 mm TEA/HCl (pH 7.4), containing 0.5 mm [α-32P]GTP (∼250,000 cpm), 3 mm MgCl2, 0.1 mm DTPA, and 1 mm cGMP in the absence and presence of DTT (2 mm) and SOD (1,000 units/ml). The samples were analyzed for [32P]cGMP as described under “Experimental Procedures.” The data are the mean values ± S.E. of three independent experiments.
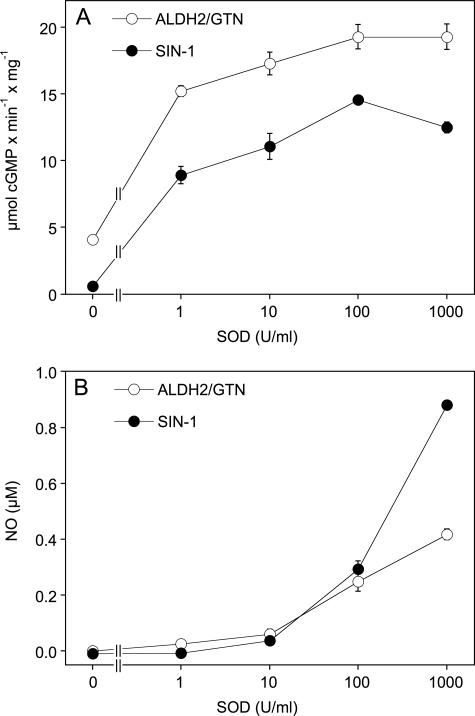
The SOD concentration-response curves shown in Fig. 4 revealed close similarities between ALDH2/GTN and the NO/superoxide donor SIN-1 in terms of sGC activation (Fig. 4A) and NO release (Fig. 4B), although SIN-1 released considerably more NO than ALDH/GTN at the highest SOD concentration tested (1,000 units/ml). The reason underlying this difference was not further investigated. The data also show that the sensitivity of the NO electrode is too low to detect the subnanomolar concentrations of NO that are sufficient for significant sGC activation.
FIGURE 4.
Effects of SOD concentration on sGC activation and NO formation from ALDH2/GTN and SIN-1. A, purified sGC was incubated for 10 min as described in the legend to Fig. 3 in the presence of 25 μg WT-ALDH2/100 μm GTN or 100 μm SIN-1 and the indicated concentrations of SOD. B, SIN-1 (100 μm) or 0.25 mg of WT-ALDH2/100 μm GTN was incubated in 0.5 ml of 50 mm TEA buffer (pH 7.4) in presence of the indicated concentrations of SOD as described in the legend to Fig. 2. NO formation was monitored with a Clark-type NO electrode. The data are the mean values ± S.E. of three experiments.
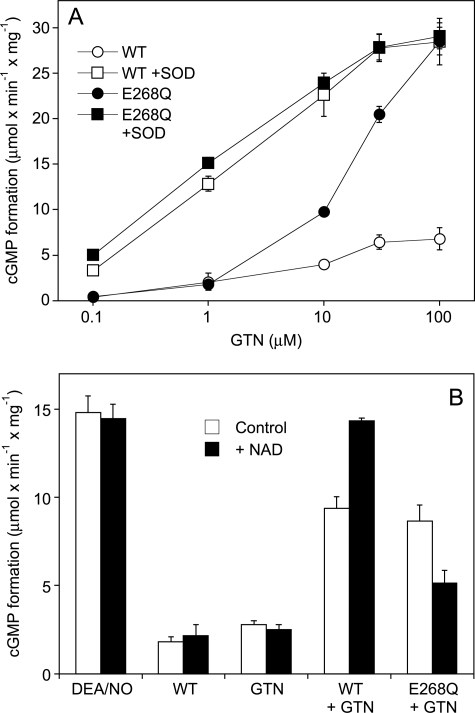
GTN Concentration Dependence and Effects of NAD
Fig. 5 A shows the effect of GTN concentration on sGC activation with WT- or E268Q-ALDH2 in the presence of 2 mm DTT. GTN moderately activated sGC in the presence of WT-ALDH2 with an EC50 of ∼5 μm. The E268Q mutant was identical to WT in the presence of up to 1 μm GTN but much more efficient at higher concentrations of the nitrate, causing maximal sGC activation in the presence of 100 μm GTN even in the absence of SOD. The EC50 of GTN was 20–30 μm, similar to denitration catalyzed by the E268Q mutant in the absence of NAD. SOD (1,000 units/ml) led to pronounced leftward shifts of the GTN concentration response curves (EC50 ∼ 1 μm) in the presence of both WT- and E268Q-ALDH2.
FIGURE 5.
Effects of GTN concentration and NAD on sGC activation. A, purified sGC was incubated for 10 min as described in the legend to Fig. 2 with increasing concentrations of GTN in the presence of 100 μg WT- or E268Q-ALDH2 with and without SOD (1,000 units/ml). B, purified sGC was incubated for 2 min as described in the legend to Fig. 2 with 0.1 mm GTN and WT- or E268Q-ALDH2 (2 μg each) in the presence of 1,000 units/ml SOD with and without 1 mm NAD. Control experiments were performed in the presence of 1 μm DEA/NO, WT-ALDH2 (2 μg) in the absence of GTN, and GTN (0.1 mm) in the absence of ALDH2. Samples were analyzed for [32P]cGMP as described under “Experimental Procedures”. Data are mean values ± S.E. of 3 independent experiments.
The effect of NAD on GTN-triggered sGC activation was measured in 2-min incubations in the presence of SOD to allow better comparison with the 1,2-GDN and NO data. As shown in Fig. 5B, NAD had no significant effect on cGMP formation in the presence of either WT-ALDH2 or GTN alone, and neither affected DEA/NO-stimulated sGC activity. Similarly to GTN denitration, the rates of cGMP formation measured in the presence of 2 μg/0.1 ml WT-ALDH2 were increased by NAD, whereas the nucleotide significantly inhibited sGC activation in the presence of the E268Q mutant.
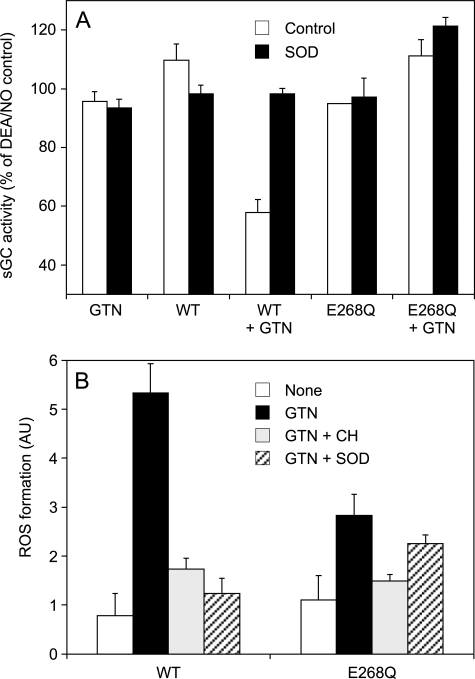
GTN Metabolism Resulting in Superoxide-mediated Scavenging of NO
The pronounced effect of SOD on GTN-triggered sGC activation suggested that ALDH2-catalyzed GTN metabolism is associated with superoxide formation. Therefore, we tested for interference of the ALDH2/GTN reaction with sGC activation by DEA/NO-derived NO. As shown in Fig. 6A, neither GTN nor WT- or E268Q-ALDH2 had any effect when given alone, but the combined presence of WT enzyme and GTN resulted in significant reduction of NO-stimulated sGC activity that was restored by SOD. Although NO scavenging was also prevented by the combined superoxide/peroxynitrite scavenger hydralazine (18), the peroxynitrite/NO2 scavengers methionine and urate (1 mm each) had no effect (data not shown). In contrast to WT-ALDH2, reaction of GTN with the E268Q mutant did not cause detectable NO scavenging. Fig. 6B shows that GTN turnover catalyzed by WT-ALDH2 resulted in significant oxidation of hydroethidine, reflecting formation of reactive oxygen species (superoxide and/or peroxynitrite). Hydroethidine oxidation was significantly inhibited by chloral hydrate and SOD and reduced by ∼50% when GTN was incubated with the E268Q mutant instead of the WT enzyme. E268Q-triggered hydroethidine oxidation was not significantly inhibited by SOD, suggesting the involvement of reactive species other than superoxide/peroxynitrite.
FIGURE 6.
Scavenging of NO and hydroethidine oxidation by ALDH2/GTN. A, purified sGC was incubated for 10 min as described in the legend to Fig. 2 with WT- or E268Q-ALDH2 (25 μg each) in the presence of 1 μm DEA/NO. GTN (0.1 mm) and SOD (1,000 units/ml) were present as indicated. The samples were analyzed for [32P]cGMP as described under “Experimental Procedures.” The data (mean values ± S.E.; n = 3) are expressed as percentages of maximally NO-stimulated sGC activity. B, purified proteins (0.45 mg each) were incubated at 37 °C in 0.7 ml of 50 mm TEA buffer (pH 7.4), containing 3 mm MgCl2, 0.1 mm DTPA, 2 mm DTT, 2.5 μm hydroethidine, and, where indicated, 0.1 mm GTN, 10 mm chloral hydrate (CH), and 1000 units/ml SOD. Fluorescence was monitored at excitation and emission wavelengths of 490 nm (slit width, 5 nm) and 585 nm (slit width, 20 nm), respectively. The data are the mean values ± S.E. of the increase in fluorescence read-outs (arbitrary units) monitored over 5 min in three to six independent experiments.
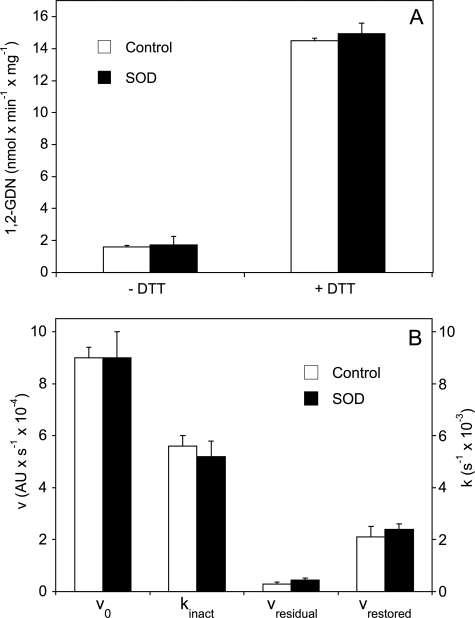
We considered that formation of superoxide and/or peroxynitrite in the course of GTN denitration could contribute to mechanism-based inactivation of ALDH2. However, as shown in Fig. 7, scavenging of superoxide by SOD had no effect on the rates of 1,2-GDN formation, and neither affected ALDH2 inactivation kinetics measured as acetaldehyde oxidation in the presence of GTN before and after the addition of DTT.
FIGURE 7.
Effects of SOD on GTN denitration (A) and ALDH2 inactivation (B). A, ALDH2 (50 μg/0.1 ml) was incubated for 10 min at 37 °C in 0.2 ml of phosphate buffer (pH 7.4) containing 100 μm [14C]GTN, 3 mm MgCl2 in the absence and presence of 2 mm DTT and 1,000 units/ml SOD. 1,2-GDN was extracted and quantified by radio thin layer chromatography as described under “Experimental Procedures.” The data shown are the mean values ± S.E. of three independent experiments. B, GTN-induced inactivation of the dehydrogenase activity of WT-ALDH2 in the absence and presence of SOD (5,000 units/ml). For experimental details see “Experimental Procedures” and Ref. 13. v0, rate of acetaldehyde oxidation; kinact, rate of GTN-triggered enzyme inactivation; vresidual, residual dehydrogenase activity of ALDH2 exposed to 50 μm GTN; vrestored, dehydrogenase activity restored by 0.4 mm DTT.
DISCUSSION
This study revealed several interesting and unexpected features of ALDH2-catalyzed bioactivation of GTN: (i) E268 is essential for aldehyde oxidation and ester hydrolysis but not for GTN denitration, (ii) E268Q-ALDH2-catalyzed GTN bioconversion is inhibited by NAD, and (iii) the ALDH2/GTN reaction results in mechanism-based superoxide formation that limits NO bioavailability and is significantly reduced upon mutation of Glu-268. In their original report Stamler and co-workers (6) proposed a reaction of GTN with the nucleophilic Cys-302 residue of ALDH2 as the initial step of GTN bioconversion. Our results with C302S-ALDH2 support this proposal, even though residual GTN reductase activity of the mutant (∼10% of WT) suggests minor contributions of other nucleophilic residues, e.g. Cys-301 or Cys-303, to the overall reaction. The loss of aldehyde dehydrogenase and esterase activities upon mutation of Glu-268 agrees well with a previous report showing that this residue is essentially involved in hydride transfer, activation of Cys-302, hydrolysis of thioester intermediates (19), and, of note, GTN denitration measured in the presence of NAD (3). Although the presence of NAD as hydride acceptor is obligatory for the dehydrogenase reaction, significant ester hydrolysis occurs also in the absence of NAD, indicating that the nucleotide is not an essential co-factor but accelerates reaction rates through a conformational change of the active site (see below). Surprisingly, NAD did not stimulate but inhibited the residual esterase activity of E268Q-ALDH2 by ∼90%. The divergent effects of NAD were also apparent in GTN denitration assays showing identical reaction rates of WT- and E268Q-ALDH2 in the absence of NAD, whereas the nicotinamide increased the activity of WT but decreased GTN denitration by the E268Q mutant. The effect of NAD on WT-ALDH2 was mainly because of an increase in apparent GTN affinity, resulting in diminished stimulation by the nucleotide with increasing GTN concentrations as observed previously (14). With the mutant, the inhibitory effect of NAD was not overcome by high GTN, but at increasing protein concentrations the E268Q variant exhibited significant activity even in the presence of NAD.
Structural Basis for the Divergent Effects of NAD
In the apo form of WT-ALDH2 two different side chain conformations of E268 have been observed (Protein Data Bank code 1o05) (20); in the first the carboxylate points toward Cys-302, whereas it is more remote (∼6.5 Å) in the second conformation. The latter state is supposed to be catalytically competent by accommodating and activating a water molecule positioned between the carboxylate and the thioester intermediate (19). Among the eight crystallographically independent active sites in the apo structure, five have the Glu-268 side chain in the “active” and three in the “inactive” conformation. The situation changes completely when NAD is bound (with the nicotinamide mononucleotide moiety in the hydrolysis conformation), because all Glu-268 side chains are in the active conformation (Protein Data Bank codes 1o00, 1o01, and 1o02) (19). This shift in the equilibrium in favor of the active conformation could explain the observed increase in esterase activity. Additional effects on the activity caused by interactions of the esterase substrate or the tetrahedral intermediate with the bound co-factor cannot be excluded. Similarly, alternate conformations have also been observed for the nicotinamide moiety in structures of ALDH2 with bound co-factor. In one conformation, termed “hydride transfer conformation,” the pyridine ring is close to Cys-302, whereas in the other, the “hydrolysis conformation,” it has moved away toward the entrance to the active site. It has been hypothesized that NAD can be bound in both states somewhat favoring the “hydride transfer” conformation. In the latter state, the nicotinamide ring is positioned between Glu-268 and Cys-302, which is very well compatible with hydride transfer (hence the name) but incompatible with hydrolysis of an acyl enzyme intermediate at Cys-302. The opposite applies to the other NAD conformation (19).
A possible explanation for the decrease of the esterolytic activity of the E268Q variant of ALDH2 upon NAD binding is, again, a shift in the equilibrium between the two co-factor conformations. If the hydride transfer conformation is relatively favored by the enzyme variant over the hydrolysis conformation, this would lead to the observed activity loss. In WT-ALDH2, Glu-268 forms a single hydrogen bond to the nicotinamide in the hydride transfer conformation. Thus, a shift toward this co-factor conformation (which inhibits ester hydrolysis) could be accomplished by additional interactions possible for a glutamine compared with a glutamate side chain (e.g. two hydrogen bonds formed with the carboxamide of the co-factor).
Formation of GTN-derived NO
Formation of 1,2-GDN correlated reasonably well with GTN bioactivation assayed as NO release or sGC activation in the presence of SOD, suggesting that 1,2-GDN and NO are derived from a common reaction intermediate, presumably a thionitrate involving Cys-302 or, to minor extent, one of the adjacent cysteine residues. The mechanism of NO formation is unknown, but our data indicate that the thionitrate intermediate is converted through two distinct pathways that both yield 1,2-GDN but either nitrite or NO as co-products. The three-electron reduction pathway yielding NO appears to account for 5–10% of total GTN turnover catalyzed by WT-ALDH2. However, in the reaction catalyzed by E268Q-ALDH2 in the presence of NAD, fractional NO formation increased to up to 50% of total turnover, suggesting that the two pathways are not necessarily linked. Although this pronounced increase in relative NO formation occurred under conditions of very low rates of GTN denitration, there was no obvious correlation between the rates of GTN turnover and the fraction of GTN converted to NO. At present we can only conclude that the partitioning between the two- and three-electron reduction pathways of GTN denitration appears to be determined by the active site structure involving Glu-268 of human ALDH2.
The present study showing significant cGMP accumulation in the presence of submicromolar concentrations of GTN strongly suggests that ALDH2-catalyzed three-electron reduction of GTN to yield NO mediates vascular GTN bioactivity in vivo. However, this may not be the complete story, because several laboratories failed to detect GTN-derived NO in blood vessels (21–23). There are several possibilities to explain this discrepancy. First, despite the impressive activation of sGC that we observed in the course of the ALDH2/GTN reaction, we cannot rule out the possibility that ALDH2-catalyzed NO formation is a peculiarity of the purified enzyme unrelated to vascular GTN bioactivation. Second, GTN-derived NO could be converted efficiently to a stable transport form that delivers NO to cytosolic sGC without significant release of free NO. Finally, taking into account that a considerable amount of vascular ALDH2 is found in soluble fractions of blood vessels (24), GTN bioactivation may occur in the cytosol rather than in mitochondria and involve local interaction of ALDH2 with sGC. Further work is needed to clarify this issue.
Mechanism-based Superoxide Formation
Our data provide strong evidence for co-generation of superoxide that limits the bioavailability of GTN-derived NO. First, SOD markedly potentiated sGC activation by GTN without having any effect on the rates of GTN denitration or turnover-dependent enzyme inactivation (Fig. 7). Second, ALDH2-catalyzed GTN turnover compromised NO stimulation of sGC, and this was prevented by the superoxide scavengers SOD and hydralazine. Finally, ALDH2-catalyzed GTN metabolism resulted in significant oxidation of hydroethidine that was inhibited by SOD and chloral hydrate. The source of the apparent superoxide generation is unknown. The two- and three-electron reduction pathways of GTN metabolism probably involve cysteine oxidation and could give rise to formation of reactive cysteine oxides, e.g. disulfide anion radical intermediates, that reduce O2 to superoxide (25, 26). Accordingly, lower reactivity of a critical cysteine residue in ALDH2 lacking the general base Glu-268 could explain reduced superoxide formation by the E268Q mutant and thus the observed increase in NO bioavailability as compared with the WT enzyme that was apparent as significant formation of detectable NO in the absence of SOD (Fig. 2).
Despite ample evidence that exposure of blood vessels to GTN causes superoxide formation that limits NO bioavailability in nitrate tolerance and beneficial effects of antioxidants documented by both animal and clinical studies, the actual source of superoxide in the vasculature is still a matter of debate (27). The proposed pathways of GTN-induced vascular superoxide formation include activation of NADPH oxidase (28), endothelial NO synthase uncoupling (29–31), and dysregulation of the mitochondrial respiratory chain (32–34). Considering the present findings in conjunction with the essential role of ALDH2 in vascular GTN bioactivation (35), it is tempting to speculate that superoxide-mediated nitrate tolerance is a direct consequence of the ALDH2/GTN reaction and that superoxide scavengers like ascorbate (36, 37) or Mn-SOD (33) exert their beneficial effects at least partially through scavenging of ALDH2/GTN-derived superoxide. Work is ongoing in our laboratory to study the potential implications of our results to the hemodynamic effects of GTN and development of nitrate tolerance in vivo.
Acknowledgment
We thank Margit Rehn for excellent technical assistance.
This work was funded by Fonds zur Förderung der Wissenschaftlichen Forschung in Austria Grants W901 (DK Molecular Enzymology) and P20669 and Deutsche Forsch ungs ge mein schaft Grant KO1157/4-1.
- ALDH
- mitochondrial aldehyde dehydrogenase (EC 1.2.1.3)
- cGMP
- 3′,5′-cyclic guanosine monophosphate
- DEA/NO
- 2,2-diethyl-1-nitroso-oxyhydrazine (DEA/NONOate)
- DTT
- dithiothreitol
- GDN
- glyceryl dinitrate
- GTN
- glyceryl trinitrate (nitroglycerin)
- NAD
- nicotinamide adenine dinucleotide
- sGC
- soluble guanylate cyclase
- SIN-1
- 3-morpholino sydnonimine
- TEA
- triethanolamine
- SOD
- superoxide dismutase
- WT
- wild type
- DTPA
- diethylene triamine pentaacetic acid.
REFERENCES
- 1.Racker E. (1949) J. Biol. Chem. 177, 883–892 [PubMed] [Google Scholar]
- 2.Crabb D. W., Edenberg H. J., Bosron W. F., Li T. K. (1989) J. Clin. Invest. 83, 314–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larson H. N., Zhou J., Chen Z., Stamler J. S., Weiner H., Hurley T. D. (2007) J. Biol. Chem. 282, 12940–12950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mann C. J., Weiner H. (1999) Prot. Sci. 8, 1922–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fung H. L. (2004) Annu. Rev. Pharmacol. Toxicol. 44, 67–85 [DOI] [PubMed] [Google Scholar]
- 6.Chen Z., Zhang J., Stamler J. S. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 8306–8311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayer B., Beretta M. (2008) Br. J. Pharmacol. 155, 170–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biswas S., Chida A. S., Rahman I. (2006) Biochem. Pharmacol. 71, 551–564 [DOI] [PubMed] [Google Scholar]
- 9.Russwurm M., Koesling D. (2005) Methods Enzymol. 396, 492–501 [DOI] [PubMed] [Google Scholar]
- 10.Zheng C. F., Wang T. T., Weiner H. (1993) Alcohol Clin. Exp. Res. 17, 828–831 [DOI] [PubMed] [Google Scholar]
- 11.Beretta M., Gruber K., Kollau A., Russwurm M., Koesling D., Goessler W., Keung W. M., Schmidt K., Mayer B. (2008) J. Biol. Chem. 283, 17873–17880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klyosov A. A., Rashkovetsky L. G., Tahir M. K., Keung W. M. (1996) Biochemistry 35, 4445–4456 [DOI] [PubMed] [Google Scholar]
- 13.Beretta M., Sottler A., Schmidt K., Mayer B., Gorren A. C. F. (2008) J. Biol. Chem. 283, 30735–30744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kollau A., Hofer A., Russwurm M., Koesling D., Keung W. M., Schmidt K., Brunner F., Mayer B. (2005) Biochem. J. 385, 769–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schultz G., Böhme E. (1984) in Methods of Enzymatic Analysis (Bergmeyer H. U., Bergmeyer J., Grassl M. eds) pp. 379–389, Verlag Chemie, Weinheim, Germany [Google Scholar]
- 16.Mayer B., Klatt P., Werner E. R., Schmidt K. (1995) J. Biol. Chem. 270, 655–659 [DOI] [PubMed] [Google Scholar]
- 17.Sachs L., Reynarowych Z. (1974) Applied Statistics: A Handbook of Techniques, 4th Ed., Springer-Verlag, New York [Google Scholar]
- 18.Daiber A., Oelze M., Coldewey M., Kaiser K., Huth C., Schildknecht S., Bachschmid M., Nazirisadeh Y., Ullrich V., Mülsch A., Münzel T., Tsilimingas N. (2005) Biochem. Biophys. Res. Commun. 338, 1865–1874 [DOI] [PubMed] [Google Scholar]
- 19.Perez-Miller S. J., Hurley T. D. (2003) Biochemistry 42, 7100–7109 [DOI] [PubMed] [Google Scholar]
- 20.Hurley T. D., Perez-Miller S., Breen H. (2001) Chem. Biol. Interact. 130–132, 3–14 [DOI] [PubMed] [Google Scholar]
- 21.Kleschyov A. L., Oelze M., Daiber A., Huang Y., Mollnau H., Schulz E., Sydow K., Fichtlscherer B., Mülsch A., Münzel T. (2003) Circ. Res. 93, e104–112 [DOI] [PubMed] [Google Scholar]
- 22.Núñez C., Víctor V. M., Tur R., Alvarez-Barrientos A., Moncada S., Esplugues J. V., D'Ocón P. (2005) Circ. Res. 97, 1063–1069 [DOI] [PubMed] [Google Scholar]
- 23.Miller M. R., Grant S., Wadsworth R. M. (2008) J. Vasc. Res. 45, 375–385 [DOI] [PubMed] [Google Scholar]
- 24.DiFabio J., Ji Y., Vasiliou V., Thatcher G. R., Bennett B. M. (2003) Mol. Pharmacol. 64, 1109–1116 [DOI] [PubMed] [Google Scholar]
- 25.Winterbourn C. C., Metodiewa D. (1994) Arch. Biochem. Biophys. 314, 284–290 [DOI] [PubMed] [Google Scholar]
- 26.Winterbourn C. C., Hampton M. B. (2008) Free Rad. Biol. Med. 45, 549–561 [DOI] [PubMed] [Google Scholar]
- 27.Münzel T., Daiber A., Mülsch A. (2005) Circ. Res. 97, 618–628 [DOI] [PubMed] [Google Scholar]
- 28.Münzel T., Kurz S., Rajagopalan S., Thoenes M., Berrington W. R., Thompson J. A., Freeman B. A., Harrison D. G. (1996) J. Clin. Invest. 98, 1465–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Münzel T., Li H., Mollnau H., Hink U., Matheis E., Hartmann M., Oelze M., Skatchkov M., Warnholtz A., Duncker L., Meinertz T., Förstermann U. (2000) Circ. Res. 86, E7–E12 [DOI] [PubMed] [Google Scholar]
- 30.Kaesemeyer W. H., Ogonowski A. A., Jin L., Caldwell R. B., Caldwell R. W. (2000) Br. J. Pharmacol. 131, 1019–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parker J. O., Parker J. D., Caldwell R. W., Farrell B., Kaesemeyer W. H. (2002) J. Am. Coll. Cardiol. 39, 1199–1203 [DOI] [PubMed] [Google Scholar]
- 32.Sydow K., Daiber A., Oelze M., Chen Z., August M., Wendt M., Ullrich V., Mülsch A., Schulz E., Keaney J. F., Jr., Stamler J. S., Münzel T. (2004) J. Clin. Invest. 113, 482–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daiber A., Oelze M., Sulyok S., Coldewey M., Schulz E., Treiber N., Hink U., Mülsch A., Scharffetter-Kochanek K., Münzel T. (2005) Mol. Pharmacol. 68, 579–588 [DOI] [PubMed] [Google Scholar]
- 34.Esplugues J. V., Rocha M., Nuñez C., Bosca I., Ibiza S., Herance J. R., Ortega A., Serrador J. M., D'Ocon P., Victor V. M. (2006) Circ. Res. 99, 1067–1075 [DOI] [PubMed] [Google Scholar]
- 35.Chen Z., Stamler J. S. (2006) Trends Cardiovasc. Med. 16, 259–265 [DOI] [PubMed] [Google Scholar]
- 36.Bassenge E., Fink N., Skatchkov M., Fink B. (1998) J. Clin. Invest. 102, 67–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watanabe H., Kakihana M., Ohtsuka S., Sugishita Y. (1998) J. Am. Coll. Cardiol. 31, 1323–1329 [DOI] [PubMed] [Google Scholar]