Abstract
We have previously reported that growth factor receptor-bound protein-7 (Grb7), an Src-homology 2 (SH2)-containing adaptor protein, enables interaction with focal adhesion kinase (FAK) to regulate cell migration in response to integrin activation. To further elucidate the signaling events mediated by FAK·Grb7 complexes in promoting cell migration and other cellular functions, we firstly examined the phos pho ryl a ted tyrosine site(s) of Grb7 by FAK using an in vivo mutagenesis. We found that FAK was capable of phos pho rylating at least 2 of 12 tyrosine residues within Grb7, Tyr-188 and Tyr-338. Moreover, mutations converting the identified Tyr to Phe inhibited integrin-dependent cell migration as well as impaired cell proliferation but not survival compared with the wild-type control. Interestingly, the above inhibitory effects caused by the tyrosine phos pho ryl a tion-deficient mutants are probably attributed to their down-regulation of phospho-Tyr-397 of FAK, thereby implying a mechanism by competing with wild-type Grb7 for binding to FAK. Consequently, these tyrosine phos pho ryl a tion-deficient mutants evidently altered the phospho-Tyr-118 of paxillin and phos pho ryl a tion of ERK1/2 but less on phospho-Ser-473 of AKT, implying their involvement in the FAK·Grb7-mediated cellular functions. Additionally, we also illustrated that the formation of FAK·Grb7 complexes and Grb7 phos pho ryl a tion by FAK in an integrin-dependent manner were essential for cell migration, proliferation and anchorage-independent growth in A431 epidermal carcinoma cells, indicating the importance of FAK·Grb7 complexes in tumorigenesis. Our data provide a better understanding on the signal transduction event for FAK·Grb7-mediated cellular functions as well as to shed light on a potential therapeutic in cancers.
Growth factor receptor bound protein-7 (Grb7)2 is initially identified as a SH2 domain-containing adaptor protein bound to the activated EGF receptor (1). Grb7 is composed of an N-terminal proline-rich region, following a putative RA (Ras-associating) domain and a central PH (pleckstrin homology) domain and a BPS motif (between PH and SH2 domains), and a C-terminal SH2 domain (2–6). Despite the lack of enzymatic activity, the presence of multiple protein-protein interaction domains allows Grb7 family adaptor proteins to participate in versatile signal transduction pathways and, therefore, to regulate many cellular functions (4–6). A number of signaling molecules has been reported to interact with these featured domains, although most of the identified Grb7 binding partners are mediated through its SH2 domain. For example, the SH2 domain of Grb7 has been demonstrated to be capable of binding to the phospho-tyrosine sites of EGF receptor (1), ErbB2 (7), ErbB3 and ErbB4 (8), Ret (9), platelet-derived growth factor receptor (10), insulin receptor (11), SHPTP2 (12), Tek/Tie2 (13), caveolin (14), c-Kit (15), EphB1 (16), G6f immunoreceptor protein (17), Rnd1 (18), Shc (7), FAK (19), and so on. The proceeding α-helix of the PH domain of Grb7 is the calmodulin-binding domain responsible for recruiting Grb7 to plasma membrane in a Ca2+-dependent manner (20), and the association between the PH domain of Grb7 and phosphoinositides is required for the phosphorylation by FAK (21). Two additional proteins, NIK (nuclear factor κB-inducing kinase) and FHL2 (four and half lim domains isoform 2), in association with the GM region (Grb and Mig homology region) of Grb7 are also reported, although the physiological functions for these interactions remain unknown (22, 23). Recently, other novel roles in translational controls and stress responses through the N terminus of Grb7 are implicated for the findings of Grb7 interacting with the 5′-untranslated region of capped targeted KOR (kappa opioid receptor) mRNA and the Hu antigen R of stress granules in an FAK-mediated phosphorylation manner (24, 25).
Unlike its member proteins Grb10 and Grb14, the role of Grb7 in cell migration is unambiguous and well documented. This is supported by a series of studies. Firstly, Grb7 family members share a significantly conserved molecular architecture with the Caenorhabditis elegans Mig-10 protein, which is involved in neuronal cell migration during embryonic development (4, 5, 26), suggesting that Grb7 may play a role in cell migration. Moreover, Grb7 is often co-amplified with Her2/ErbB2 in certain human cancers and tumor cell lines (7, 27, 28), and its overexpression resulted in invasive and metastatic consequences of various cancers and tumor cells (23, 29–33). On the contrary, knocking down Grb7 by RNA interference conferred to an inhibitory outcome of the breast cancer motility (34). Furthermore, interaction of Grb7 with autophosphorylated FAK at Tyr-397 could promote integrin-mediated cell migration in NIH 3T3 and CHO cells, whereas overexpression of its SH2 domain, an dominant negative mutant of Grb7, inhibited cell migration (19, 35). Recruitment and phosphorylation of Grb7 by EphB1 receptors enhanced cell migration in an ephrin-dependent manner (16). Recently, G7–18NATE, a selective Grb7-SH2 domain affinity cyclic peptide, was demonstrated to efficiently block cell migration of tumor cells (32, 36). In addition to cell migration, Grb7 has been shown to play a role in a variety of physiological and pathological events, for instance, kidney development (37), tumorigenesis (7, 14, 38–41), angiogenic activity (20), proliferation (34, 42, 43), anti-apoptosis (44), gene expression regulation (24), Silver-Russell syndrome (45), rheumatoid arthritis (46), atopic dermatitis (47), and T-cell activation (17, 48). Nevertheless, it remains largely unknown regarding the downstream signaling events of Grb7-mediated various functions. In particular, given the role of Grb7 as an adaptor molecule and its SH2 domain mainly interacting with upstream regulators, it will be interesting to identify potential downstream effectors through interacting with the functional GM region or N-terminal proline-rich region.
In this report, we identified two tyrosine phosphorylated sites of Grb7 by FAK and deciphered the signaling targets downstream through these phosphorylated tyrosine sites to regulate various cellular functions such as cell migration, proliferation, and survival. In addition, our study sheds light on tyrosine phosphorylation of Grb7 by FAK involved in tumorigenesis.
EXPERIMENTAL PROCEDURES
Reagents
Protein A-Sepharose 4B, human plasma fibronectin, poly-l-lysine, 5′-bromo-2-deoxyuridine (BrdUrd), puromycin, and mouse monoclonal α-vinculin and α-BrdUrd antibodies were purchased from Sigma-Aldrich (St. Louis, MO). Lipofectamine 2000TM, Dulbecco's modified Eagle's medium (DMEM), F-12 medium, CO2-independnet medium, and Opti-MEM were from Invitrogen. The mouse α-phosphotyrosine monoclonal antibody PY99, α-tubulin, and the rabbit polyclonal α-Grb7 (N-20), α-Grb10, α-Grb14, α-FAK (C-20), α-HA (Y-11), α-GFP, α-tubulin, and α-actin antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The rabbit polyclonal, α-pY397-FAK, α-pY861-FAK, α-pY925-FAK, and α-pY118-paxillin antibodies were from BD Transduction Laboratories. The rabbit polyclonal α-ERK1/2, α-pERK (pT202/pY204), α-38 MAPK, α-phospho-p38 MAPK (Thr-180/Tyr-182), and the monoclonal α-SAPK/JNK, α-phospho-SAPK/JNK (Thr-183/Tyr-185), α-AKT, and α-pS473-AKT antibodies were from Cell Signaling (Danvers, MA). The PD98059 was purchased from Calbiochem.
Cell Culture
The A431 epithelial carcinoma cells and 293T human kidney epithelial cells were maintained in DMEM supplemented with 10% fetal bovine serum (Invitrogen). Mouse fibroblast NIH 3T3 cells were cultured in DMEM containing 10% calf serum (Invitrogen), and the CHO cells were grown in F-12 medium (Invitrogen) containing 10% fetal bovine serum. The NIH 3T3 cells with stably expressing triple HA-tagged wild-type Grb7 and its point mutation mutants were generated by co-transfection of pKH3-Grb7 or its mutants along with the plasmid pPUR (containing a puromycin-resistant cassette) in a 10:1 ratio using Lipofectamine 2000TM transfection reagent (Invitrogen) according to the manufacturer's instructions, followed by selection and maintenance in the presence of 1.5 μg/ml puromycin culture medium. All cells were cultured under 5% CO2 at a 37 °C incubator.
Construction of Expression Vectors
The expression plasmids encoding Grb7, including pKH3-Grb7, pKH3-SH2, pKH3-Pro-GM, pKH3-Pro-GM-FAT, pDH-GST-Grb7, pHAN-Grb7, and pKH3-R239L, and plasmids pEGFP-FAK and pKH3-FAK have been described previously (19, 21, 35). Using pKH3-Grb7 as a template, individual point mutation, whose tyrosine residue is converted to phenylalanine (F), for each tyrosine residue within Grb7 was generated by an overlapping PCR mutagenesis method, as described previously (21), using the following primer sets (underlining represents the mutated amino acid sequences): 5′-AAGGTGTTCAGTGAGGATGGG-3′ (sense) and 5′-CCCATCCTCACTAACCACCTT-3′ (antisense) for Y107F; 5′-CTTCGCCAAGTTCGAACTGTTCAAGAGC-3′ (sense) and 5′-GCTCTTGAACAGTTCGAACTTGGCGAAG-3′ (antisense) for Y188F; 5′-CGATCTGGCCTCTTTTACTCCACCAAGG-3′ (sense) and 5′-CCTTGGTGGAGTAAAAGAGGCCAGATCG-3′ (antisense) for Y259F; 5′-CGATCTGGCCTCTATTTCTCCACCAAGG-3′ (sense) and 5′-CCTTGGTGGAGAAATAGAGGCCAGATCG-3′ (antisense) for Y260F; 5′-GGCACCTGCAGTTCGTGGCAGATG-3′ (sense) and 5′-CATCTGCCACGAACTGCAGGTGCC-3′ (antisense) for Y274F; 5′-AGTCCAACGTGTTCGTGGTGACGC-3′ (sense) and 5′-GCGTCACCACGAACACGTTGGACT-3′ (antisense) for Y284F; 5′-GCCGCAAGCTGTTCGGGATGCCC-3′ (sense) and 5′-GGGCATCCCGAACAGCTTGCGGC-3′ (antisense) for Y293F; 5′-CCTCTTCAACTTCGGGGTGCAGCTG-3′ (sense) and 5′-CAGCTGCACCCCGAAGTTGAAGAGG-3′ (antisense) for Y338F; 5′-GGGGTGCAGCTGTTCAAGAATTACC-3′ (sense) and 5′-GGTAATTCTTGAACAGCTGCACCCC-3′ (antisense) for Y343F; 5′-TACAAGAATTTCCAGCAGGC-3′ (sense) and 5′-GCCTGCTGGAAATTCTTGTA-3′ (antisense) for Y346F; 5′-AAAGTGAAGCATTTTCTCATCCTGC-3′ (sense) and 5′-GCAGGATGAGAAAATGCTTCACTTT-3′ (antisense) for Y480F; 5′-AGGGTCGCCTGTTCTTCAGCATGGATG-3′ (sense) and 5′-CATCCATGCTGAAGAACAGGCGACCCT-3′ (antisense) for Y492F; 5′-ACTTCGCCAAGGAAGAACTGTT-3′ (sense) and 5′-AACAGTTCTTCCTTGGCGAAGT-3′ (antisense) for Y188E; and 5′-TCTTCAAGGAAGGGGTGCAGCT-3′ (sense) and 5′-AGCTGCACCCCTTCCTTGAAGA-3′ (antisense) for Y338E. The final PCR products were digested with EcoRI and then inserted into pKH3 or pHAN vector at the corresponding site to generate pKH3-Y107F to pKH3-Y492F, respectively.
Immunoprecipitation and Western Blotting Analyses
Subconfluent cells were washed twice with ice-cold phosphate-buffered saline and then lysed with 1% Nonidet P-40 lysis buffer (20 mm Tris, pH 8.0, 137 mm NaCl, 1% Nonidet P-40, 10% glycerol, 1 mm Na3VO4, 1 mm phenylmethylsulfonyl fluoride, 10 mg/ml aprotinin, and 20 mg/ml leupeptin) on the ice at least 10 min. Next, lysates were collected and clarified by centrifugation for 20 min at 4 °C, and total protein concentration was determined using the Bio-Rad Protein Assay according to the manufacturer's instructions. Immunoprecipitations were carried out by incubating cell lysates with antibodies as indicated for 3 h at 4 °C, followed by incubation for 1 h with protein A-Sepharose 4B beads (Sigma-Aldrich). Washing four times with lysis buffer, immune complexes were resolved using SDS-PAGE. Western blotting was proceeded using horseradish peroxidase-conjugated IgG as a secondary antibody and the Western Lightning®–ECL system (PerkinElmer Inc.) for detection.
Cell Motility Assay
Cell motility assays for transiently transfected CHO cells were performed as described below. Briefly, CHO cells were co-transfected with a combination of the indicated expression vectors and pEGFP vector in a 5:1 ratio (3 μg of total plasmid DNA) using Lipofectamine 2000TM (Invitrogen) according to the manufacturer's instructions. Two days after transfection, the cells were trypsinized and replated onto a 60-mm tissue culture dish that had been coated with 5 μg/ml human plasma fibronectin. After 2-h incubation in a 5% CO2 incubator at 37 °C, the medium was replaced with CO2-independent medium (Invitrogen) supplemented with 0.1% fetal bovine serum, and the cells were transferred into a humidified 37 °C chamber at atmospheric CO2. A fluorescent image was captured to detect GFP. (i.e. positively transfected) cells. Time-lapse phase-contrast images were then captured at 15-min intervals using the Image-Pro Plus software program v3.0 and its specific cell motility macro called OMAware (Image Acquisition and Object Motility Analysis) v1.1 for Windows NT v4.0. These phase-contrast images were converted to black and white images using OMAware and saved as JPEG images. The OMAware program was then used to determine the velocity, distance traveled, and migration path for each cell based on its centroid as determined from the cell boundaries in the JPEG images. The velocity of each transfected cell (GFP+) was calculated relative to the mean velocity of the control (GFP−) cells. Data were collected using ∼30 positively transfected cells in three independent experiments for each expression vector.
Wound Healing Assay
Cells were cultured on the 60-mm culture plates until forming confluent monolayers followed by serum starvation for 12 h. Wounds were created in a line across the plates by scrapping in a 200-μl standard pipette tip. The wounded monolayers were then washed twice with serum-free media to remove cell debris and incubated with DMEM with 0.2% serum. Photographs were taken at 0 h and 20 or 24 h using a charge-coupled device camera (DP70, Olympus, Japan) connected to an inverted microscope (IX71, Olympus, Japan). Quantitative analysis of wound healing was determined by calculating the percentage of cells moved into the wounded space of each cell lines.
Cell Migration Assay
Cell migration assays were carried out using a Neuro Probe (Cabin John, MD) 48-well chemotaxis Boyden chamber as described previously (19). Approximately 1 × 105 cells were resuspended in DMEM and added in the upper wells of the Boyden chamber, and the cells were allowed to migrate toward fibronectin (10 μg/ml) in DMEM as the chemoattractant or DMED only as a control in the lower wells for 6 h in a 37 °C humidified incubator. At the end of experiments, cells on the upper side of the polycarbonate membrane were removed and the bottom-side cells were fixed with methanol for 8 min and stained with crystal violet (Sigma-Aldrich). The migrated cells were counted from five randomly selected fields of each well under light microscope at 20× magnification using Image-Pro Plus software, version 3.0 (Media Cybernetics, Silver Spring, MD).
BrdUrd Incorporation Assay
The subconfluent cells were firstly serum-starved for 24 h in DMEM with 0.5% fetal bovine serum or calf serum according to the cell lines used to arrest the cells in G0 phase. They were then washed twice with DMEM and incubated for 24 h in DMEM plus 10% serum plus 100 μm BrdUrd (Sigma-Aldrich). Next, cells were fixed in 4% paraformaldehyde for 15 min at room temperature, followed by trypsinization with 0.4% Triton X-100 for 8 min, and cellular DNA was digested with 0.5 unit/μl DNase I (New England Biolabs, Inc.) for 30 min at 37 °C. Cells were stained for 1 h with the affinity-purified mouse anti-BrdUrd antibody (1:200, Sigma-Aldrich), followed by 1-h exposure to streptavidin-conjugated Texas-Red (Amersham Biosciences). Cells were then counted in multiple fields and scored for BrdUrd-positive staining in each independent experiment.
Cell Survival Assay
NIH 3T3 cells were counted in a hemocytometer and seeded the same amount of cells at a confluence of 90% in the 6-well culture plates in DMEM plus 10% calf serum to allow growth overnight, followed by replaced with low serum DMEM containing 0.1% bovine serum albumin and 0.2% calf serum as described previously (49). At each indicated time point, the adherent cells were trypsinized and then collected by centrifugation at 1060 rpm for 5 min. Then, the numbers of trypan blue-positive dead cells and trypan blue-negative alive cells were counted on a hemocytometer under a microscope (IX71, Olympus, Japan).
Lentiviral Production and Infection
Lentiviruses encoding Grb7 small-hairpin RNAs (shRNA) or Luciferase small-hairpin RNA (shLuc) was obtained from the TRC lentiviral shRNA library in the National RNAi Core Facility of Academia Sinica, Taiwan. The targeting sequences of various shRNAs were as follows: #12 (clone ID: TRCN0000061386) 5′-GCCATCTGCATCCATCTTGTT-3′ and #13 (clone ID: TRCN0000061387) 5′-CCAGGGCTTTGTCCTCTCTTT-3′ are derived from Grb7. Production of lentiviruses was processed according to the guidelines of Broad Institute (available on the web). Briefly, 293T cells were co-transfected by individual pLKO.1-shRNA construct, the packaging plasmid pCMV-ΔR8.91 (containing gag, pol, and rev genes), and the envelope plasmid pMD.G (VSV-G expressing plasmid) in a 10:9:1 ratio. Two days after the transfection, a fraction of unconcentrated medium supernatant (containing shRNA lentiviruses) was added to cells followed by puromycin (2 μg/ml) selection for 3 days. The efficiency and specificity of knocking down Grb7 were examined by Western blotting using various antibodies as indicated.
Soft Agar Assay
Anchorage-independent growth was examined using soft agar assays. Briefly, the bottom layers of 6-well plates containing 0.5% agar in DMEM were prepared. Approximately 5 × 104 cells were seeded in a 1.5-ml volume of 0.35% agar containing DMEM/10% fetal bovine serum over the bottom agar layer and allowed to incubate for 2 weeks in a 37 °C, 5% CO2 humidified incubator and supplemented twice a week with complete medium. After the second week of treatment, viable colonies were assessed by the naked eye directly and by microscopy. Colony numbers were scored using a phase-contrast microscope at 20× magnification. Images were captured using Image-Pro Plus software under a light microscope (IX71, Olympus, Japan) and a 20× objective lens (Olympus) equipped with a chargecoupled device camera (DP70, Olympus, Japan).
RESULTS
Identification of Grb7 Tyrosine Phosphorylation Sites by FAK
We have previously shown that the formation of FAK·Grb7 complexes could promote cell migration, in which the tyrosine phosphorylation of Grb7 by FAK is essential (19, 21, 35). To further confirm that the tyrosine phosphorylation of Grb7 is orchestrated in an integrin-FAK·Grb7 signaling pathway, we examined the tyrosine phosphorylation of Grb7 by replating Grb7-tranfected CHO cells on fibronectin (in a serum-free condition) in a time-dependent manner. As expected, we observed that the occurrence of tyrosine phosphorylation of Grb7 was in concordance with the tyrosine phosphorylation of FAK in response to integrin activation (Fig. 1A), suggesting that the tyrosine phosphorylation of Grb7 takes place in integrin/FAK-mediated signaling pathways.
FIGURE 1.
Tyrosine phosphorylation of Grb7 by FAK. A, CHO cells that had been transfected with pKH3-Grb7 were plated on fibronectin-coated plates (10 μg/ml). Cell lysates were collected at varied time as indicated (0, 10, 20, 30, 60, 90, 120, 180, and 240 min) and immunoprecipitated (IP) by polyclonal anti-HA antibody (Y-11) or polyclonal anti-FAK antibody (C-20). The immune complexes were analyzed by Western blotting (IB) with anti-phosphotyrosine antibody (PY99), monoclonal anti-HA antibody (12CA5), or monoclonal anti-FAK antibody. The positions of phosphorylated Grb7 and FAK, total Grb7, and total FAK are marked on the left. B, CHO cells were transfected with pKH3 expression vectors encoding full-length Grb7, its SH2 domain or Pro-GM domain, or the chimeric molecule of Pro-GM fused FAT (focal adhesion targeting sequence) at its C terminus. Cell lysates were collected and immunoprecipitated by polyclonal anti-HA antibody (Y-11) followed by Western blotting with phosphotyrosine antibody (PY99, bottom panel) and monoclonal anti-HA antibody (12CA5, bottom panel). Single and double asterisks represent nonspecific blotted bands.
Because FAK serves as a tyrosine kinase for Grb7, we aim to determine the phosphorylation site(s) of Grb7 by FAK. We first attempted to delineate which part(s) or domain(s) of Grb7 were being potentially phosphorylated by FAK. To do so, CHO cells were co-transfected with GFP-FAK and various Grb7 truncated constructs. As shown in Fig. 1B, we found that the SH2 domain alone and the Pro-GM-FAT, whose carboxyl SH2 domain is replaced by the FAT sequence of FAK (by fusing the FAT, focal adhesion targeting, sequence to the Grb7 C terminus (50)), could be phosphorylated by FAK, indicating that both GM and SH2 domains could be phosphorylated by FAK. Interestingly, no phosphorylation occurred in the Pro-GM construct, suggesting the requirement of a physical interaction with FAK (via the SH2 domain binding of Grb7 to the phospho-Tyr-397 of FAK) or focal adhesion localization to facilitate Grb7 phosphorylated by FAK. Thus, the results could not provide a delimited region where tyrosine phosphorylation may occur. Under this circumstance, we next utilized a site-directed mutagenesis overlapping PCR method to generate point mutation constructs for each tyrosine residue within Grb7 by converting individual tyrosine residue into phenylalanine (Tyr → Phe). Herein, all of 12 resultant mutants (Tyr → Phe) are designated as Y107F, Y188F, Y259F, Y260F, Y274F, Y284F, Y293F, Y338F, Y343F, Y346F, Y480F, and Y492F, respectively (Fig. 2A). While co-transfection of these mutants (Tyr → Phe) with GFP-FAK into CHO cells and followed by Western blotting analyses as described above, two tyrosine-to-phenylalanine mutants, Y188F and Y338F, exhibited a significantly decreased tyrosine phosphorylation compared with that of wild-type Grb7 (Fig. 2B). Together, these results suggest that at least two tyrosine residues of Grb7 are capable of being phosphorylated by FAK upon formation of complexes with FAK and that the Tyr-338 is the truly phosphorylated site by FAK.
FIGURE 2.
Determination of Grb7 phosphorylated sites by FAK. A, schematic diagram of all the tyrosine residues of Grb7 subjected to generating Tyr to Phe point mutation mutants. B, CHO cells were co-transfected with pEGFP-FAK and wild-type Grb7 (WT) or its Tyr to Phe point mutation mutants in the pKH3 expression vector as indicated. They were lysed and immunoprecipitated (IP) by polyclonal anti-HA antibody Y-11. The immune complexes were analyzed by Western blotting (IB) with phospho-tyrosine antibody (PY99, upper panel) or monoclonal anti-HA antibody against Grb7 (12CA5, bottom panel). Two tyrosine mutants, Y188F and Y338F, exhibited reduction of their tyrosine-phosphorylated levels by FAK. C, CHO cells were co-transfected with GFP expression vector and pKH3-Grb7 or its tyrosine to phenylalanine mutants as indicated. They were then subjected to the cell motility assays as described under “Experimental Procedures.” The mean of relative migration rate (normalized to untransfected cells as 1.0) from three independent experiments are shown.
Phosphorylation of Grb7 at Tyr-188 and Tyr-338 Is Essential for Integrin-FAK·Grb7-dependent Cell Migration
To characterize the roles of tyrosine phosphorylation of Grb7, a computerized-based cell motility assay was firstly proceeded to examine the effects of its tyrosine-to-phenylalanine mutants on cell motility. Each mutant was assessed, and data were collected using ∼30 positively transfected cells in three independent experiments. We observed that both of Y188F and Y338F mutants were defective in cell motility compared with the wild type or other mutants except Y492F (Fig. 2C), which is consistent with reduced tyrosine phosphorylation on these two mutants. These results have therefore provided an insight on the requirement of Grb7 phosphorylation of Tyr-188 and Tyr-338 in integrin-FAK·Grb7-mediated cell migration. In addition, we performed other cell motility assays, such as wound healing assays and modified Boyden chamber assays, to ensure the migratory effect of these of tyrosine phosphorylation of Grb7-deficient mutants in NIH 3T3 cells that stably express the wild-type Grb7 or the point mutation mutants (Fig. 3B). Likewise, the tyrosine residues, Tyr-188 and Tyr-338, are phosphorylated targets of FAK in NIH 3T3 cells (Fig. 3A). As a control, the R239L mutant has been shown previously to be incapable of binding D3-phosphoinositides to its PH domain, thereby preventing Grb7 phosphorylation by FAK in an integrin-dependent manner (21). Consistently, compared with the mock cells, all of the tyrosine phosphorylation-deficient mutants, including Y188F, Y338F, and R239L, markedly decreased cell migration in the wound healing assay on fibronectin, because the cell growth rates did not show differences between the wild type and these mutants during the assay (Fig. 3B and data not shown). On the contrary, overexpression of the wild-type Grb7 could slightly increase cell migration compared with the mock control. Furthermore, employing the modified Boyden chamber assay, we again found that Grb7 phosphorylations of Tyr-188 and Tyr-338 were crucial for cells migrating toward fibronectin, because an approximate 40–80% reduction in cell migration in these tyrosine phosphorylation-deficient mutants was seen compared with the wild-type and mock controls (Fig. 3C). In addition, a double tyrosine-to-phenylalanine mutant, Y188F,Y338F (both Tyr-188 and Tyr-338 are converted into phenylalanine), exhibited a similar inhibitory effect. Conversely, the conversion of tyrosine to glutamic acid at Tyr-188 and Tyr-338, which mimicked the phosphorylated state of these tyrosine residues, apparently elevated cell migration to the extent of that of the wild type. To ensure the above effects indeed resulted from these tyrosine phosphorylation-deficient mutants, on the other hand, addition of lentiviruses encoding Grb7 shRNAs (for their knockdown efficiency and specificity, see also below in Fig. 7) significantly rendered a reversal migratory effect caused by these tyrosine phosphorylation-deficient mutants (Fig. 3D, upper panel) in correlation with the decrease or disappearance of their expressions in these NIH 3T3 stable cell lines (Fig. 3D, bottom panel). Notably, the motility for the mock control displayed equivalently in the absence and presence of Grb7 shRNAs, which might be explained by no change of endogenous proteins (NIH 3T3 cells do not contain detectable endogenous Grb7) by which the specificity of these shRNAs per se is indicated as well. Taken together, these results demonstrated that the phosphorylation of Grb7 at Tyr-188 and Tyr-338 by FAK plays an important role in mediating cell migration in an integrin-dependent manner.
FIGURE 3.
Tyrosine phosphorylation of Grb7 at Tyr-188 and Tyr-338 is required for cell migration. A, NIH 3T3 cells were co-transfected with pEGFP-FAK and wild-type Grb7 (WT) or its Tyr to Phe point mutation mutants in the pKH3 expression vector as indicated. They were lysed and immunoprecipitated (IP) by polyclonal anti-HA antibody Y-11. The immune complexes were analyzed by Western blotting (IB) with phospho-tyrosine antibody (PY99, upper panel) or monoclonal anti-HA antibody against Grb7 (12CA5, bottom panel). Two tyrosine mutants, Y188F and Y338F, exhibited reduction of their tyrosine phosphorylated levels by FAK. B, cell lysates from several NIH 3T3 clones with stably expressing Grb7 or its mutants, such as Y188F, Y274F, Y338F, and R239L, were analyzed by Western blotting with polyclonal anti-HA antibody (Y-11, upper panel) against the exogenous Grb7 expressions and monoclonal anti-vinculin antibody (bottom panel) as a loading control. In addition, one representative clone with stably expressing Grb7 or its mutants was subjected to wound healing assays as described under “Experimental Procedures.” Various Grb7 stably expressing NIH 3T3 cells were plated on fibronectin-coated plates (10 μg/ml), and a wound was made by scraping cell monolayer with a pipette tip (0 h) as indicated. The cells were then washed and incubated for 24 h to allow migration into the wounded area (24 h). Quantitation of cell migration was achieved by counting the number of cells that had migrated into the cell-free space. Four different areas were randomly chosen from each wounded area, and three independent experiments are shown. In comparison with cells stably expressing wild-type Grb7 (■) or mock cells (□), Y188F (▴), Y338F (X), and R239L (*) markedly reduced wound healing. C, one representative clone with stably expressing Grb7, as indicated, was subjected to cell migration assays toward fibronectin (10 μg/ml) using a modified Boyden chamber as described under “Experimental Procedures.” Mean cells counts from at lest nine fields of polycarbonate membrane (under the 20× objective microscopic lens, Olympus IX71) and three experiments are shown. Error bars represent ± S.D. D, one representative stably expressing Grb7 clone either infected with lentiviruses encoding short hairpin Grb7 (shGrb7) or luciferase (shLuc), as indicated, was subjected to cell migration assays toward fibronectin (10 μg/ml) using a modified Boyden chamber. Mean cells were counted, as above, and three experiments are shown. Error bars represent ± S.D. *, p ≦ 0.01.
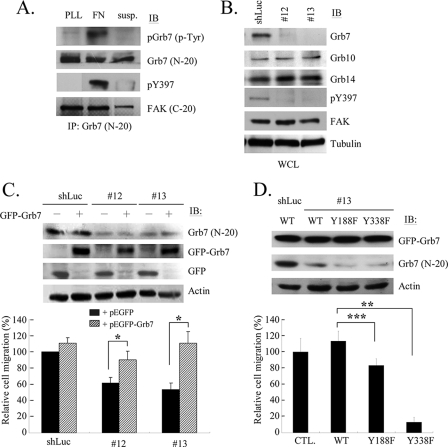
FIGURE 7.
Involvement of Grb7 and its tyrosine phosphorylation in A431 cell migration. A, A431 cells were detached by trypsin and replated on fibronectin (FN, 10 μg/ml) or poly-l-lysine (PLL, 10 μg/ml) or remained in suspension (Susp.), as indicated. Lysates were immunoprecipitated by anti-Grb7 antibody (N-19) followed by Western blotting (IB) with phosphotyrosine (PY99), anti-Grb7 (N-19), pY397, or anti-FAK (C-20). B, A431 cells were infected with lentiviruses harboring various short hairpin (sh) sequences targeting to Grb7 for knocking down its expression (#12 and #13) or to luciferase (shLuc) as a control. Cell lysates from the infected cells were subjected for Western blotting (IB) with various antibodies as indicated to confirm the efficiency and specificity of gene knockdown by Grb7 shRNAs. Interestingly, knockdown of Grb7 resulted in the diminishment of the phosphorylation of Tyr-397 and Tyr-861 but not Tyr-925 of FAK (data not shown for the latter two tyrosine sites.). C, cell migration of A431 cells with or without (including re-expressing exogenous Grb7) knocking down Grb7 was assessed with the modified Boyden chamber assay as previously described. Quantitation of cell migration by counting the number of cells that had migrated toward fibronectin (10 μg/ml) containing medium in the bottom wells. Mean cell counts from at lest nine fields of polycarbonate membrane (under the 20× objective microscopic lens, Olympus IX71) and three experiments are shown. Error bars represent ± S.D. D, re-introduction of wild-type Grb7 or the tyrosine phosphorylation-deficient mutants into Grb7 knockdown A431 cells (by #13 shRNA), and cell migration was assessed with the modified Boyden chamber assay. Results show the mean ± S.D. for at least three independent experiments by counting the relative percentage of BrdUrd-positive cells. *, p ≦ 0.02; **, p ≦ 0.06; and ***, p ≦ 0.01.
Involvement of Grb7 Phosphorylation at Tyr-188 and Tyr-338 in FAK-mediated Cell Proliferation but Modest in Survival
In line with the role of Grb7 acting as a mediator downstream of FAK-mediated signaling, we examined the effect of Grb7 in regard to the other cellular function regulated by FAK, such as cell proliferation. To test whether the tyrosine phosphorylation of Grb7 at Tyr-188 and Tyr-338 also participates in FAK-mediated cell proliferation, BrdUrd incorporation assays were performed. We found that cell proliferation was decreased in Y188F, Y338F, Y188–338F, and R239L mutants compared with the wild type and mock controls in NIH 3T3 cells (Fig. 4A). On the contrary, Y188E and Y338E showed no effect on cell proliferation. These data suggest that Grb7 may be involved in the regulation of cell proliferation in a tyrosine-phosphorylation manner.
FIGURE 4.
Role of Grb7's phosphorylation by FAK in cell proliferation and survival. A, NIH 3T3 cells stably expressing Grb7 or its mutants, as indicated, were subjected to BrdUrd incorporation assays as descried under “Experimental Procedures.” Results show the mean ± S.D. for at least three independent experiments by counting the relative percentage of BrdUrd-positive cells. *, p ≦ 0.01. B, NIH 3T3 cells stably expressing Grb7 or its mutants, as indicated, were subjected to cell survival assays as described under “Experimental Procedures.” Results show the mean ± S.D. for at least three independent experiments by counting the relative percentage of surviving cells at various time as indicated (0, 1, 2, 3, and 4 days) in a serum-free condition.
Remaining consistent with the enzyme-substrate relationship, in Fig. 2B, we have noticed that the tyrosine phosphorylation of Grb7 is more prominent in the presence of FAK overexpression. We further examined the effect of Grb7 phosphorylated by FAK in cell proliferation when FAK is activated by overexpression. Similarly, these tyrosine-deficient mutants showed an equivalently inhibitory effect on cell proliferation compared with the wild type or mock control, supporting the signaling role of the tyrosine phosphorylation of Grb7 in FAK-mediated cell proliferation (data not shown).
In addition, NIH 3T3 cells expressing the tyrosine phosphorylation of Grb7-deficient mutants with FAK caused a modest difference in cell survival in a time-dependent manner under a low serum condition compared with the wild-type Grb7 and mock controls, even in the presence of FAK overexpression (Fig. 4B and data not shown). Taken together, these results reveal that the phospho-Tyr-188 and phospho-Tyr-338 of Grb7 are also crucial for FAK-mediated cell proliferation but modest for cell survival.
Down-regulation of FAK Phosphorylation and Signaling by Tyrosine Phosphorylation-deficient Mutants of Grb7
Next, we sought to address how the tyrosine-deficient mutants led to the inhibitory effects observed above. First, to exclude a gross conformation change of these tyrosine phosphorylation-deficient mutants, which might cause the loss of their interaction with FAK, we examined the ability of the interaction between FAK and Y188F or Y338F. As expected, Fig. 5A shows that both mutants could remain in association with FAK to a comparable level as the wild-type Grb7 (we did not observe much difference in the binding affinity with FAK for the wild-type or Y188F/Y338F mutants). However, surprisingly, we found that either Y188F or Y338F mutant-associated FAK displayed lower phospho-Tyr-397 contents (upper panel, Fig. 5A), implying that the Grb7 tyrosine phosphorylation-deficient mutants may act as negative trap mutants for FAK activation and signaling. To further examine the effect of the Grb7 tyrosine phosphorylation-deficient mutants on FAK signaling, we found that phospho-Tyr861 but not phospho-Tyr925 is suppressed (Fig. 5B). The double tyrosine mutant, Y188F,Y338F, appeared to have a more profound effect than the individual tyrosine phosphorylation-deficient mutant, perhaps being due to nearly abrogation of tyrosine phosphorylation of this mutant by FAK. Interestingly, the phospho-Tyr-397 and phospho-Tyr861 of FAK were augmented during overexpression of wild-type Grb7 (Fig. 5B).
FIGURE 5.
Down-regulation of FAK phosphorylation by the Grb7 tyrosine phosphorylation-deficient mutants. A, cells were co-transfected with pEGFP-FAK and pKH3-Grb7, pKH3-Y188F, or pKH3-Y338F, as indicated. They were lysed and immunoprecipitated by polyclonal anti-HA antibody (Y-11) against HA-Grb7 and its mutants followed by Western blotting with phosphotyrosine antibody (PY99), monoclonal anti-HA antibody (12CA5), phospho-Tyr-397 antibody, or polyclonal anti-GFP antibody. The positions of phosphorylated Grb7, total Grb7, and associated FAK are marked. B, cells were transfected with pKH3-Grb7, pKH3-Y188F, pKH3-Y338F, pKH3-Y188,338F (a double mutant for both tyrosine 188 and 338 to phenylalanine) or pKH3 vector alone, as indicated. They were lysed and immunoprecipitated by polyclonal anti-GFP antibody against GFP-FAK followed by Western blotting analyses with phospho-Tyr-397, phospho-Tyr861, phospho-Tyr925 antibodies, polyclonal anti-GFP (for GFP-FAK) antibody, anti-HA (for HA-Grb7) antibody (Y-11), or anti-phospho-Grb7 (pY99) antibody. C, cells were co-transfected with pEGFP, pDH-GST-Grb7 (wild-type Grb7 tagged with GST at its N terminus), and pKH3-Y338F with various amounts as indicated. Cell lysates were collected and immunoprecipitated by polyclonal anti-FAK antibody (C-20) followed by Western blotting with anti-phospho-Tyr-397, total FAK (GFP-FAK), anti-GST against GST-Grb7 (wild-type), and anti-HA (12CA5) against HA-Y338F, respectively.
The inhibitory effects caused by these tyrosine phosphorylation-deficient mutants could presumably act by competing with wild-type Grb7 for binding to FAK thus reducing phosphorylation of the endogenous Grb7 by FAK. Although we were unable to detect any endogenous Grb7 in NIH 3T3 cells, it was evidently that the reduced exogenous wild-type Grb7 bound to FAK while increasing the expressions of Y338F tyrosine phosphorylation-deficient mutant in NIH3T3 cells in association with the decreased phospho-Tyr-397 of FAK (Fig. 5C).
Taken together, we hypothesize that these Grb7 tyrosine phosphorylation-deficient mutants bind to FAK in a phospho-Tyr-397-independent manner to suppress FAK phosphorylation, which may result in deficiency of FAK·Grb7-mediated signaling axis due to lack of phospho-Tyr-397. Thus, proper interaction and phosphorylation of Grb7 by FAK are essential for initiating the downstream signaling events.
Regulation of Phospho-Tyr-118 Paxillin, Erk1/2 Phosphorylation, and AKT Activity by FAK·Grb7 Complexes
To explore how FAK·Grb7-mediated signal pathways are involved in the regulation of the aforementioned cellular functions, a variety of known downstream signaling targets, such as phospho-paxillin, AKT activity, and MAPK cascades, of FAK were assayed. As shown in Fig. 6A (upper panel), the tyrosine phosphorylation-deficient mutants (i.e. Y188F, Y338F, and R239L), but not the wild-type or the non-phosphorylation-deficient mutant (i.e. Y274F), diminished the phospho-Tyr-118 of paxillin, which is essential for cell migration as described previously (51). Accordingly, this result can explain the observation of the tyrosine phosphorylation of Grb7-deficient mutants resulting in impaired cell migration (Figs. 2C and 3). The phosphorylation of Erk1/2 but not JNK (C-Jun N-terminal kinase) or p38 was also decreased in the tyrosine phosphorylation-deficient mutants compared with the wild-type or the Y274F mutant. Alternatively, to further evaluate the requirement of FAK activity for Grb7 in the regulation of Erk1/2 phosphorylation, NIH 3T3 cells co-expressed Grb7 and the kinase dead (KD) mutant of FAK evidently resulted in a decreased phosphorylation of Erk1/2 in concert with the reduction of tyrosine phosphorylation of Grb7, whereas the co-expression of Grb7 and wild-type FAK stimulated Erk1/2 phosphorylation compared with the mock control (Fig. 6B). With respect to the defects in cell proliferation mediated by the tyrosine phosphorylation of Grb7-deficient mutants (Fig. 4), the reduced phosphorylation of Erk1/2 was perhaps responsible for this negative outcome of cell proliferation but not cell migration. In accordance with this idea, we found that the effects of cell migration among the wild-type Grb7 or its tyrosine phosphorylation-deficient mutants were equivalent regardless of the presence or absence of PD98059, an inhibitor of MEK1 blocking the phosphorylation of Erk1/2 (data not shown), thereby suggesting that the phosphorylation of Erk1/2 is probably mainly responsible for cell proliferation in FAK·Grb7-mediated signaling. Finally, AKT has long been thought to act as an important regulator in cell survival. Here, we showed that the activity of AKT was modestly inhibited by these Grb7 tyrosine phosphorylation-deficient mutants (Fig. 6A), consistent with the less important role for Grb7 in FAK-mediated cell survival.
FIGURE 6.
FAK·Grb7-mediated downstream signaling. A, cell lysates of NIH 3T3 cells with stably expressing Grb7 or its mutant together with pEGFP-FAK expression plasmid were analyzed by Western blotting with various antibodies as indicated. Results show reduction of pY118-paxillin, pS473-AKT, and pErk1/2 in the presence of the Grb7 tyrosine phosphorylation-deficient mutants, Y188F, Y338F, and R239L. B, NIH 3T3 cells with stably expressing Grb7 with pEGFP-FAK or its kinase-dead mutant were lysed and a fraction of which was analyzed by Western blotting (IB) using pErk1/2, total Erk1/2 antibodies, and polyclonal anti-FAK antibody (C-20). The remaining lysates were immunoprecipitated by polyclonal anti-HA antibody (Y-11) followed by Western blotting with phosphotyrosine antibody (PY99) and monoclonal anti-HA antibody (12CA5).
FAK·Grb7 Complexes Regulate Cell Proliferation, Migration, and Anchorage-independent Growth in Cancer Cells
Previous studies showed that Grb7 is often co-amplified with Her2/ErbB2 in numerous cancers (29–32), highlighting its potential role in tumor progress. Thus, we further examined whether the formation and signaling of FAK·Grb7 complexes contribute to tumorigenesis and/or tumor progression. The A431 cell line, an epithelial carcinoma cell line, was used to test this hypothesis due to the abundant expressions of Grb7 as well as FAK (data not shown). Consistently, we found that, in A431 cells, the elevated tyrosine phosphorylation of endogenous Grb7 was robust in response to cell adherent onto fibronectin, which is in concordance with FAK activation as seen in the presence of phospho-Tyr-397, suggesting that the formation of FAK·Grb7 complexes could serve as a prominent signal transducer in integrin-mediated signaling in tumor cells (Fig. 7A). Interestingly, we also noticed that the formation of endogenous FAK·Grb7 complexes was detected in a phospho-Tyr-397-independent manner, although Grb7 still remained little tyrosine phosphorylated while A431 cells adhered to poly-l-lysine (Fig. 7A, lane 1). Likewise, the formation of FAK·Grb7 complexes, although to a less extent, was also observed during A431 cell suspension (Fig. 7A, lane 3). These results are consistent with the above observations in regard to the preferential binding of the tyrosine phosphorylation-deficient mutants to FAK in a Tyr-397-independent manner in NIH 3T3 cells (Fig. 5).
Next, we took advantage of a lentivirus-based gene-knockdown approach that allowed us to knock down Grb7 expression up to 90–100% in A431 cells but not influence the expressions of FAK or two other Grb7 family members, Grb10 and Grb14 (Fig. 7B). Interestingly, we have seen that the phospho-Tyr-397 of FAK was dramatically decreased when Grb7 was knocked down, implying an intimate signaling link between FAK and Grb7 in A431 cells. Hence, these lentivirus-transducing cells were subjected to being used for analyzing the effect on cell migration by conducting wound healing assays. As shown in Fig. 7C, the knockdown of Grb7 exhibited a delayed migration into wounded space compared with the lentivirus control, shLuc. A reversal of the inhibitory effect caused by the knockdown of Grb7 was seen while re-expressing exogenous Grb7 in the lentivirus-transducing A431 cells (Fig. 7C), which demonstrating that Grb7 indeed influences the cell migration of A431 cells. Furthermore, while re-expressing Grb7 tyrosine phosphorylation-deficient mutants, Y188F and Y338F, in the lentivirus-transducing A431 cells, the inhibitory effect of cell migration was less pronounced compared with the re-expression of wild-type Grb7 or control cells (Fig. 7D). This implicated a pivotal role of phospho-Tyr-188 and phospho-Tyr-338 of Grb7 for cell motility in A431 cells, although we have found a modest effect of phospho-Tyr-188 compared with phospho-tyr338 in cell migration in A431 cells.
Because Grb7 is often co-amplified with Her2/ErbB2 in certain human cancers and tumor cell lines (7, 27, 28), it implies the involvement of Grb7 in tumorigenesis. To furthermore evaluate whether Grb7 does play a role in mediating tumorigenesis in A431 carcinoma cells, we first asked whether Grb7 is required for A431 cell proliferation. Utilizing the BrdUrd incorporation assay as described above, we found that the knockdown of Grb7 resulted in an evident inhibition of cell proliferation in A431 cells compared with the lentivirus control (Fig. 8A).
FIGURE 8.
FAK·Grb7 complex-mediated tumorigenesis in A431 cells. A, proliferative levels of A431 cells with or without knockdown Grb7 were also determined by BrdUrd incorporation assays. Results show the mean ± S.D. for at least three independent experiments by counting the relative percentage of BrdUrd-positive cells. B, A431 cells with (#12 and #13) or without (shLuc) knockdown Grb7 were subjected to soft agar assays to determine the role of Grb7 in tumorigenesis. Data are the means ± S.D. (error bars) of three independent experiments performed in duplicate. *, p ≦ 0.01; **, p ≦ 0.01.
Then, an in vitro soft agar assay was performed to evaluate the requirement for Grb7 in tumorigenesis. Our data demonstrated that the loss of Grb7 expression in A431 cells could lead to the reduction of colony formation on soft agar, whereas both control lentivirus-transducing cells and the parental cells retained comparable capabilities of anchorage-independent growth on soft agar (Fig. 8B). Thus, our data imply a potential role for Grb7 as a promoter during oncogenesis of tumor cells. Collectively, these data suggest that Grb7 serves as a pivotal mediator downstream of integrin signaling in the regulation of cell migration and proliferation in cancer cells.
DISCUSSION
Grb7 is the stereotype member of the emerging Grb7 adaptor protein family with potential roles in cell migration and tumor progression. However, the molecular mechanisms and signal transduction pathways of Grb7-mediated cellular functions are yet to be elucidated. In this study, we identified that at least two tyrosine residues on Grb7, namely Tyr-188 and Tyr-338, were capable of being phosphorylated by FAK. Upon integrin activation, FAK could form a complex with Grb7 and that the phospho-Tyr-118 of paxillin and Erk1/2 phosphorylation, but less AKT activity, were affected through these two tyrosine sites of Grb7 phosphorylated by FAK, thereby regulating cell migration and proliferation. In addition, our data also demonstrated that FAK·Grb7-mediated signaling importantly took part in tumorigenesis in A431 cells. These findings provide further evidence to indicate how Grb7 responds to integrin activation mediated by its subsequent exploitation of tyrosine phosphorylation by FAK to affect a variety of downstream signaling targets in the regulation of cell migration, proliferation, and even tumorigenesis.
A number of protein kinase molecules enable interacting with Grb7 in response to their corresponding upstream stimuli and/or activations, fitting the molecular characteristic of Grb7 as an adaptor protein (see review in Ref. 5). To further transmit the signals from these kinases into inside of a cell, one possible way for Grb7 is to become phosphorylated by these kinases directly. In fact, upon integrin activation, the recruitment of Grb7 by FAK could further lead to the tyrosine phosphorylation of Grb7 by FAK activity in a phospho-Tyr-397-dependent manner and in the prerequisite of phosphoinositides bound to the PH domain of Grb7 (21, 35). However, the consequence and following downstream signaling events for Grb7 phosphorylated by FAK are largely unknown at present. By generating a serial Grb7 tyrosine to phenylalanine mutants (total of 12 tyrosine sites on Grb7, thereby 12 Tyr → Phe mutants were created), they allowed us to identify the potential phosphorylation site(s) in vivo by FAK in this study. Two of twelve tyrosine residues, corresponding to Tyr-188 and Tyr-338, were revealed as the phosphorylated tyrosine residues by FAK due to the diminished tyrosine phosphorylation levels compared with that of the wild-type Grb7 (Fig. 2). Nevertheless, we could not rule out possible false-negative results by just measuring the phosphorylation levels of the point mutation mutants for each Grb7 tyrosine residue. It is very likely that the decreased phosphorylation levels of certain tyrosine mutant(s) might have been neglected because of the existence of multiple phosphorylation sites. Indeed, we could not consistently detect the tyrosine-phosphorylated residue(s) within the SH2 domain of Grb7, although the SH2 domain has been shown capable of being phosphorylated by FAK as shown in Fig. 1B and the recent studies by Tsai et al. (24, 25). Beside the SH2 domain, Siamakpour-Reihani et al. (23) have reported several phosphorylated tyrosine residues within the PH domains of Grb7 required for its interaction with four and half lim domains isoform 2, a transcriptional regulator linked with oncogenesis, although there is no clue which of the one or more kinases are responsible for the tyrosine phosphorylation of Grb7 in this event and in which none of the identified phosphotyrosine residues in their study matched with ours. To ensure the indentified Tyr-188 and Tyr-338 truly as being phosphorylated targets by FAK, an in vitro mass spectrometric analysis for phosphotyrosine mapping should be employed for validation. In our preliminary experiments for the in vitro mass spectrometric analysis, we retain an inconclusive result due to technical difficulty on erasing the high background of phospho-serine/threonine of the endogenous/exogenous Grb7. Nonetheless, our data still provide a strong support for tyrosine phosphorylation of Grb7 at Tyr-188 and Tyr-338 by FAK.
The crucial role of Grb7 phosphorylation at Tyr-188 and Tyr-338 in the integrin/FAK·Grb7 signaling axis is strongly substantiated by the dominant-negative effects of the tyrosine phosphorylation-deficient mutation (Tyr → Phe) as well as the reversal effect of the phosphorylated mimicry mutation (Tyr → Glu) of these tyrosine residues. Although there are slightly different effects by these two tyrosine phosphorylation sites on downstream signaling events (Fig. 6) and affecting differential levels of cell migration and proliferation (Figs. 3 and 4), at present, we do not know how these two phosphorylation sites act together. Nevertheless, phosphorylation at both tyrosine residues is necessary for mediating FAK downstream events, and it is likely that a conformational change and/or interactions with downstream effector protein(s) require(s) the phosphorylation of both tyrosine sites (both sites are located within the GM domain, which has been shown to be essential for downstream signaling). Thus, structural analyses and comparison among these mutants and wild-type Grb7 ought to provide a clear insight. Interestingly, we have observed that these tyrosine phosphorylation-deficient mutants remain strong binding affinity with FAK in a phospho-Tyr-397-independent manner. Although previous studies suggested the requirement of phospho-Tyr-397 for interacting with Grb7, this phospho-Tyr-397-independent interaction between Grb7 and FAK is further warranted by the co-immunoprecipitation between less-/unphosphorylated Grb7 and unphosphorylated Tyr-397 FAK while A431 cells were suspended and replated on poly-l-lysine (Fig. 7A). The importance of this phospho-Tyr-397-independent interaction/complex between Grb7 and FAK remains to be clarified. However, the importance of Grb7 in FAK-mediated signaling in A431 is obvious, because knockdown of Grb7 affected the FAK activation (phosphorylation of Tyr-397, Fig. 7B) and signaling (phosphorylation at Tyr-861 but not Tyr-925; data not shown). Hence, it again supports the potential role of Grb7 in tumorigenesis (Fig. 8).
Grb7 is initially identified as a bound protein partner of the cytoplasmic domain of epidermal growth factor receptor (1). Since then, more than a dozen upstream receptors or kinases are reported to associate with Grb7 in response to corresponding cognate ligand binding and/or activation, including Her2/ErbB2, platelet-derived growth factor receptor, insulin receptor, Met, EphB1, and FAK (see review in Refs 2, 4, 5). Conceivably, Grb7 ought to link with diverse downstream signal transduction pathways to transmit these upstream signals into the inside of a cell. In Fig. 6, we have demonstrated that the tyrosine phosphorylation of paxillin at Tyr-118, Erk1/2 phosphorylation, and AKT activity, but less are altered in the tyrosine phosphorylation-deficient mutants compared with the wild-type Grb7, suggesting that these signaling molecules could act as downstream signaling targets in an integrin-FAK·Grb7 signaling axis. Indeed, the functional relevance modulated by these signaling targets is in concordance with Grb7-regulated cellular functions, such as cell migration, proliferation, and survival, which is also consistently demonstrated by several reports. For example, in breast cancer cells overexpressing both of HER-2/Neu and Grb7, it has been reported that Grb7 is capable of modulating AKT phosphorylation at both Thr-308 and Ser-473 to facilitate tumor growth (40), correlating to the well characterized cell survival role for AKT. Moreover, the RA domain of Grb7 possessing the Ras-binding region similar to that in c-Raf (52) presents a high affinity in association with K-Ras and N-Ras (53). Since Ras proteins are well known small GTPases in mediating MAPK cascades, including Erk1/2 activation, it is possible for them to serve as candidates in directing the activation of Erk1/2 activation downstream of FAK·Grb7 complexes. Alternatively, the phospho-tyrosine residues on Grb7 could provide an additional binding site for other SH2 domain-containing signaling molecules or might change the affinity for interacting with its binding partners. The latter has been implicated in a recent study by Tsai et al. (24, 25). The interaction of Grb7 with Hu protein, a component of stress granules, is modulated by the phosphorylation states of Grb7, of which the hypophosphorylated Grb7 bound to Hu protein but the hyperphosphorylated Grb7 by FAK lost the affinity with it. However, these links remain to be verified in future studies.
Constitution of FAK·Grb7 complexes is thought to be mediated by the interaction of the SH2 domain of Grb7 with the phospho-Tyr-397 of FAK in response to integrin activation (19, 35). Surprisingly, we have noticed that Grb7 might bind to FAK in a phospho-Tyr-397-independent manner by the co-immunoprecipitation assays (Fig. 7A). In particular, this phospho-Tyr-397-independent interaction predominantly appeared during cell suspension or adhesion onto poly-l-lysine, presumably, under the circumstance of integrin inactivation, suggesting that a pre-complex of FAK and Grb7 might exist prior to FAK activation. In the light of integrin activation, Grb7, promptly and eventually, binds to the phospho-Tyr-397 of FAK at focal adhesion sites to form a proper signaling complex. Noteworthy, this scenario likely requires the tyrosine phosphorylation of Grb7 by FAK to accomplish the formation of an effective FAK·Grb7 signaling complex, because the tyrosine phosphorylation-deficient mutants could remain bound to non-phospho-Tyr-397 FAK (Fig. 5). In this regard, the tyrosine phosphorylation of Grb7 by FAK not only accounts for its importance in transmitting signals as discussed above but also may enable release of the autoinhibitory mechanism of Grb7 proposed by the Lyons group (23). Nevertheless, more studies are needed to establish and elucidate these possibilities in detail. To be noted, the tyrosine phosphorylation-deficient mutants used in this study, Y188F and Y338F, and the double mutation of both tyrosine residues (Y188F,Y338F), were found to act primarily as dominant negative mutants for FAK, because overexpression of these mutants blocked autophosphorylation of FAK at Tyr-397 and certain downstream signaling events, thereby providing a mechanism by which these mutants enable competition with wild-type Grb7 from binding to FAK due to lack of the proper binding site (phospho-Tyr-397) for Grb7 (Fig. 5). Paradoxically, how do these mutants bind and retain their negative trapping effects on FAK while lacking the phospho-Tyr-397? Indeed, a similar question is raised during cell suspension or replating on poly-l-lysine as seen in Fig. 7A. As discussed above, these tyrosine-deficient mutants may exert a Tyr-397-independent preferential binding to an unidentified region of FAK as well as result in and maintained inactivation of FAK.
A number of reports have documented the involvement of Grb7 in tumor progression (7, 14, 38–41), however, it remains unclear how Grb7 takes part in tumorigenesis. In this study, we demonstrated that endogenous Grb7 plays a pivotal role in regulating cell migration, proliferation, and tumorigenesis in A431 carcinoma cell line (Figs. 7 and 8). Although overexpression of EGF receptor is thought as a main cause resulting in the tumorigenic phenomenon, knocking down Grb7 could lead to a reversal, thereby suggesting a role of Grb7 in tumorigenesis in A431 carcinoma as well (Fig. 7A and data not shown). Indeed, the fact has been observed on the close association between EGF receptor and Grb7 (7). Beside, FAK enables being activated in response to EGF signaling as well (54).Together, it is rationalized to speculate that the involvement of Grb7 in coherent with FAK activation in tumorigenesis. In agreement with the above speculation, our data have shown that Grb7 is required for tumorigenesis in the A431 carcinoma, thereby highlighting the importance of the FAK·Grb7 signaling complexes in tumorigenesis.
In conclusion, we have identified two phosphorylation tyrosine sites at Tyr-188 and Tyr-338 of Grb7 by using FAK, by which phospho-Tyr-118 of paxillin and phospho-Erk1/2 might be involved in the regulation of FAK-dependent cell migration and proliferation. In addition, a reciprocal regulation of FAK by Grb7 is evidently revealed to further illustrate a novel mechanism of FAK activation.
Acknowledgments
We are grateful to Dr. Ming-Chuan Huang of National Taiwan University for discussion and assistance in some of the experiments. We also thank Drs. Chun-Che Chang, Shan-Li Wang, Jun-Yang Liou, and Irene Cheng for critical reading of the manuscript and helpful comments.
This work was supported by National Science Council Grant NSC-96-2311-B-002-023-MY3 and Frontier Research Grant of National Taiwan University 97R0343 (to T.-L. S.).
- Grb7
- growth factor receptor-bound protein-7
- PH
- pleckstrin homology
- EGF
- epidermal growth factor
- GM
- Grb and Mig homology
- SH2
- Src homology-2
- CHO
- Chinese hamster ovary
- GFP
- green fluorescent protein
- HA
- hemagglutinin
- shRNA
- short-hairpin RNA
- MAPK
- mitogen-activated protein kinase
- ERK
- extracellular signal-regulated kinase
- SAPK
- stress-activated protein kinase
- DMEM
- Dulbecco's modified Eagle's medium
- BrdUrd
- bromodeoxyuridine
- JNK
- c-Jun N-terminal kinase
- FAT
- focal adhesion targeting
- FAK
- focal adhesion kinase.
REFERENCES
- 1.Margolis B., Silvennoinen O., Comoglio F., Roonprapunt C., Skolnik E., Ullrich A., Schlessinger J. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 8894–8898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daly R. J. (1998) Cell. Signal. 10, 613–618 [DOI] [PubMed] [Google Scholar]
- 3.Morrione A. (2000) Int. J. Mol. Med. 5, 151–154 [DOI] [PubMed] [Google Scholar]
- 4.Han D. C., Shen T. L., Guan J. L. (2001) Oncogene 20, 6315–6321 [DOI] [PubMed] [Google Scholar]
- 5.Shen T. L., Guan J. L. (2004) Front. Biosci. 9, 192–200 [DOI] [PubMed] [Google Scholar]
- 6.Holt L. J., Siddle K. (2005) Biochem. J. 388, 393–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stein D., Wu J., Fuqua S. A., Roonprapunt C., Yajnik V., D'Eustachio P., Moskow J. J., Buchberg A. M., Osborne C. K., Margolis B. (1994) EMBO J. 13, 1331–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiddes R. J., Campbell D. H., Janes P. W., Sivertsen S. P., Sasaki H., Wallasch C., Daly R. J. (1998) J. Biol. Chem. 273, 7717–7724 [DOI] [PubMed] [Google Scholar]
- 9.Pandey A., Liu X., Dixon J. E., Di Fiore P. P., Dixit V. M. (1996) J. Biol. Chem. 271, 10607–10610 [DOI] [PubMed] [Google Scholar]
- 10.Yokote K., Margolis B., Heldin C. H., Claesson-Welsh L. (1996) J. Biol. Chem. 271, 30942–30949 [DOI] [PubMed] [Google Scholar]
- 11.Kasus-Jacobi A., Béréziat V., Perdereau D., Girard J., Burnol A. F. (2000) Oncogene 19, 2052–2059 [DOI] [PubMed] [Google Scholar]
- 12.Keegan K., Cooper J. A. (1996) Oncogene 12, 1537–1544 [PubMed] [Google Scholar]
- 13.Jones N., Master Z., Jones J., Bouchard D., Gunji Y., Sasaki H., Daly R., Alitalo K., Dumont D. J. (1999) J. Biol. Chem. 274, 30896–30905 [DOI] [PubMed] [Google Scholar]
- 14.Lee H., Volonte D., Galbiati F., Iyengar P., Lublin D. M., Bregman D. B., Wilson M. T., Campos-Gonzalez R., Bouzahzah B., Pestell R. G., Scherer P. E., Lisanti M. P. (2000) Mol. Endocrinol. 14, 1750–1775 [DOI] [PubMed] [Google Scholar]
- 15.Thömmes K., Lennartsson J., Carlberg M., Rönnstrand L. (1999) Biochem. J. 341, 211–216 [PMC free article] [PubMed] [Google Scholar]
- 16.Han D. C., Shen T. L., Miao H., Wang B., Guan J. L. (2002) J. Biol. Chem. 277, 45655–45661 [DOI] [PubMed] [Google Scholar]
- 17.De Vet E. C., Aguado B., Campbell R. D. (2003) Biochem. J. 375, 207–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vayssière B., Zalcman G., Mahé Y., Mirey G., Ligensa T., Weidner K. M., Chardin P., Camonis J. (2000) FEBS Lett. 467, 91–96 [DOI] [PubMed] [Google Scholar]
- 19.Han D. C., Guan J. L. (1999) J. Biol. Chem. 274, 24425–24430 [DOI] [PubMed] [Google Scholar]
- 20.Li H., Sánchez-Torres J., del Carpio A. F., Nogales-González A., Molina-Ortiz P., Moreno M. J., Török K., Villalobo A. (2005) Oncogene 24, 4206–4219 [DOI] [PubMed] [Google Scholar]
- 21.Shen T. L., Han D. C., Guan J. L. (2002) J. Biol. Chem. 277, 29069–29077 [DOI] [PubMed] [Google Scholar]
- 22.Chen D., Xu L. G., Chen L., Li L., Zhai Z., Shu H. B. (2003) Oncogene 22, 4348–4355 [DOI] [PubMed] [Google Scholar]
- 23.Siamakpour-Reihani S., Argiros H. J., Wilmeth L. J., Haas L. L., Peterson T. A., Johnson D. L., Shuster C. B., Lyons B. A. (2009) J. Mol. Recognit. 22, 9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsai N. P., Bi J., Wei L. N. (2007) EMBO J. 26, 1522–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai N. P., Ho P. C., Wei L. N. (2008) EMBO J. 27, 715–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manser J., Roonprapunt C., Margolis B. (1997) Dev. Biol. 184, 150–164 [DOI] [PubMed] [Google Scholar]
- 27.Akiyama N., Sasaki H., Ishizuka T., Kishi T., Sakamoto H., Onda M., Hirai H., Yazaki Y., Sugimura T., Terada M. (1997) Cancer Res. 57, 3548–3553 [PubMed] [Google Scholar]
- 28.Kishi T., Sasaki H., Akiyama N., Ishizuka T., Sakamoto H., Aizawa S., Sugimura T., Terada M. (1997) Biochem. Biophys. Res. Commun. 232, 5–9 [DOI] [PubMed] [Google Scholar]
- 29.Dahlberg P. S., Jacobson B. A., Dahal G., Fink J. M., Kratzke R. A., Maddaus M. A., Ferrin L. J. (2004) Ann. Thorac. Surg. 78, 1790–1800 [DOI] [PubMed] [Google Scholar]
- 30.Haran M., Chebatco S., Flaishon L., Lantner F., Harpaz N., Valinsky L., Berrebi A., Shachar I. (2004) Leukemia 18, 1948–1950 [DOI] [PubMed] [Google Scholar]
- 31.Walch A., Specht K., Braselmann H., Stein H., Siewert J. R., Höpt U., Hofler H., Werner M. (2004) Int. J. Cancer 112, 747–753 [DOI] [PubMed] [Google Scholar]
- 32.Tanaka S., Pero S. C., Taguchi K., Shimada M., Mori M., Krag D. N., Arii S. (2006) J. Natl. Cancer Inst. 98, 491–498 [DOI] [PubMed] [Google Scholar]
- 33.Itoh S., Taketomi A., Tanaka S., Harimoto N., Yamashita Y., Aishima S., Maeda T., Shirabe K., Shimada M., Maehara Y. (2007) Mol. Cancer Res. 5, 667–673 [DOI] [PubMed] [Google Scholar]
- 34.Kao J., Pollack J. R. (2006) Genes Chromosomes Cancer 45, 761–769 [DOI] [PubMed] [Google Scholar]
- 35.Han D. C., Shen T. L., Guan J. L. (2000) J. Biol. Chem. 275, 28911–28917 [DOI] [PubMed] [Google Scholar]
- 36.Pero S. C., Oligino L., Daly R. J., Soden A. L., Liu C., Roller P. P., Li P., Krag D. N. (2002) J. Biol. Chem. 277, 11918–11926 [DOI] [PubMed] [Google Scholar]
- 37.Leavey S. F., Arend L. J., Dare H., Dressler G. R., Briggs J. P., Margolis B. L. (1998) Am. J. Physiol. 275, F770–F776 [DOI] [PubMed] [Google Scholar]
- 38.Skotheim R. I., Monni O., Mousses S., Fosså S. D., Kallioniemi O. P., Lothe R. A., Kallioniemi A. (2002) Cancer Res. 62, 2359–2364 [PubMed] [Google Scholar]
- 39.Hodgson J. G., Malek T., Bornstein S., Hariono S., Ginzinger D. G., Muller W. J., Gray J. W. (2005) Cancer Res. 65, 9695–9704 [DOI] [PubMed] [Google Scholar]
- 40.Bai T., Luoh S. W. (2008) Carcinogenesis 29, 473–479 [DOI] [PubMed] [Google Scholar]
- 41.van Agthoven T., Veldscholte J., Smid M., van Agthoven T. L., Vreede L., Broertjes M., de Vries I., de Jong D., Sarwari R., Dorssers L. C. (2009) Breast Cancer Res. Treat. 114, 23–30 [DOI] [PubMed] [Google Scholar]
- 42.Reiske H. R., Zhao J., Han D. C., Cooper L. A., Guan J. L. (2000) FEBS Lett. 486, 275–280 [DOI] [PubMed] [Google Scholar]
- 43.Pero S. C., Shukla G. S., Cookson M. M., Flemer S., Jr., Krag D. N. (2007) Br. J. Cancer 96, 1520–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoon S. W., Kim T. Y., Sung M. H., Kim C. J., Poo H. (2005) Proteomics 5, 1987–1995 [DOI] [PubMed] [Google Scholar]
- 45.Hitchins M. P., Abu-Amero S., Apostolidou S., Monk D., Stanier P., Preece M. A., Moore G. E. (2002) J. Med. Genet 39, E13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim C. W., Cho E. H., Lee Y. J., Kim Y. H., Hah Y. S., Kim D. R. (2006) J. Korean. Med. Sci. 21, 478–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.An J. J., Kim S. Y., Lee S. H., Kim D. W., Ryu H. J., Yeo S. I., Jang S. H., Kwon H. J., Kim T. Y., Lee S. C., Poo H., Cho S. W., Lee K. S., Park J., Eum W. S., Choi S. Y. (2007) J. Biochem. Mol. Biol. 40, 189–195 [DOI] [PubMed] [Google Scholar]
- 48.Chu P., Pardo J., Zhao H., Li C. C., Pali E., Shen M. M., Qu K., Yu S. X., Huang B. C., Yu P., Masuda E. S., Molineaux S. M., Kolbinger F., Aversa G., de Vries J., Payan D. G., Liao X. C. (2003) J. Biol. 2, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen T. L., Park A. Y., Alcaraz A., Peng X., Jang I., Koni P., Flavell R. A., Gu H., Guan J. L. (2005) J. Cell Biol. 169, 941–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen T. L., Guan J. L. (2001) FEBS Lett. 499, 176–181 [DOI] [PubMed] [Google Scholar]
- 51.Petit V., Boyer B., Lentz D., Turner C. E., Thiery J. P., Vallés A. M. (2000) J. Cell Biol. 148, 957–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wojcik J., Girault J. A., Labesse G., Chomilier J., Mornon J. P., Callebaut I. (1999) Biochem. Biophys. Res. Commun. 259, 113–120 [DOI] [PubMed] [Google Scholar]
- 53.Rodriguez-Viciana P., Sabatier C., McCormick F. (2004) Mol. Cell. Biol. 24, 4943–4954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sieg D. J., Hauck C. R., Ilic D., Klingbeil C. K., Schaefer E., Damsky C. H., Schlaepfer D. D. (2000) Nat. Cell Biol. 2, 249–256 [DOI] [PubMed] [Google Scholar]