Abstract
We have studied the role of carbonic anhydrase 9 (CA9), a cancer-associated extracellular isoform of the enzyme carbonic anhydrase in multicellular spheroid growths (radius of ∼300 μm) of human colon carcinoma HCT116 cells. Spheroids were transfected with CA9 (or empty vector) and imaged confocally (using fluorescent dyes) for both intracellular pH (pHi) and pH in the restricted extracellular spaces (pHe). With no CA9 expression, spheroids developed very low pHi (∼6.3) and reduced pHe (∼6.9) at their core, associated with a diminishing gradient of acidity extending out to the periphery. With CA9 expression, core intracellular acidity was less prominent (pHi = ∼6.6), whereas extracellular acidity was enhanced (pHe = ∼6.6), so that radial pHi gradients were smaller and radial pHe gradients were larger. These effects were reversed by eliminating CA9 activity with membrane-impermeant CA inhibitors. The observation that CA9 activity reversibly reduces pHe indicates the enzyme is facilitating CO2 excretion from cells (by converting vented CO2 to extracellular H+), rather than facilitating membrane H+ transport (such as H+ associated with metabolically generated lactic acid). This latter process requires titration of exported H+ ions with extracellular HCO3−, which would reduce rather than increase extracellular acidity. In a multicellular structure, the net effect of CA9 on pHe will depend on the cellular CO2/lactic acid emission ratio (set by local oxygenation and membrane HCO3− uptake). Our results suggest that CO2-producing tumors may express CA9 to facilitate CO2 excretion, thus raising pHi and reducing pHe, which promotes tumor proliferation and survival. The results suggest a possible basis for attenuating tumor development through inhibiting CA9 activity.
The carbonic anhydrases (CAs)3 are a family of enzymes that reversibly catalyze CO2 hydration to H+ and HCO3− (1, 2). Recent studies have identified several CA isoforms, such as CA4, CA9, CA12, and CA14, with extracellular-facing catalytic sites (2). Many cells express extracellular CA (CAe) isoforms, but their physiological role remains unclear. In particular, the strong link between cancer and CA9 expression (1–5) has provoked great interest in the role of CAe in tumor biology.
Based on their topology, CAe isoforms are likely to regulate the concentration of extracellular H+, CO2, and HCO3−. Cell metabolism drives transmembrane fluxes of H+ ions, CO2 and HCO3−, and can provide substrate for the CAe-assisted reaction. For example, CO2 is released from aerobically respiring cells. By consuming or producing H+ ions, the CAe-catalyzed reaction will affect extracellular pH (pHe). Many membrane proteins are modulated by pHe (6–8). Some of these are acid/base transporters that regulate intracellular pH (pHi) (9). Such modulation allows pHe to cross-talk with pHi (10, 11), thus helping to shape the plethora of effects that pHi has on cellular physiology (3, 9, 12, 13). Extracellular pH can also affect tissue structure through the release or modulation of proteolytic enzymes that act on the extracellular matrix (14, 15). In addition, the pHe-pHi difference is important in determining the distribution of membrane-permeant weak acids/bases, which include many drugs used clinically (e.g. doxorubicin).
A complete understanding of pH regulation at tissue level requires characterization of events occurring within cells, at their surface membrane, and in the surrounding extracellular space. To date, many pH studies have treated the extracellular space as an infinite, well-stirred, and equilibrated compartment of constant pH. This condition is compatible with experimentally superfused, isolated cells, but it may not apply to all cells in situ. Blood plasma is a major component of extracellular fluid. In health, plasma pH is regulated to ∼7.4 by the lungs and kidneys, acting in concert to remove excess acid/base that has been added to blood from dietary or cellular sources. Tissue fluid occupies the gap between plasma and cells (with the exception of blood-borne cells). Under conditions of ideal diffusive coupling between cells and capillaries, pHe in tissue fluid would be held close to plasma pH. However, pHe close to the cell surface can diverge from 7.4, particularly when the cell-capillary distance is increased (e.g. as a result of poor blood perfusion), when the excreted acid/base load is elevated, or when the local buffering capacity is compromised.
Regulation of pHe is particularly important in tumors because these are characterized by a high metabolic rate (16, 17) and abnormal blood perfusion (18, 19). Studies have shown that tumors develop low pHe (∼6.9) in response to the mismatch between metabolic demand and the capacity to remove metabolic waste products (14, 18, 20). Tumors can survive in considerably more acidic interstitium than their non-neoplastic counterparts, partly because of their ability to maintain a favorably alkaline pHi for growth and development (21). It has been argued that tumors can survive selectively by maintaining a level of pHe that is lethal to normal cells but not sufficiently acidic to kill the tumor itself (2, 14, 22).
A major fraction of cell-derived acid is excreted in the form of CO2, generated directly from the Krebs cycle or from titration of intracellular H+ with HCO3−. To maintain a steep outward gradient for CO2 excretion, extracellular CO2 must not accumulate. This can be achieved by venting CO2 to the nearest capillary or by reacting CO2 locally to produce H+ and HCO3−. The balance between these two fluxes is set by the diffusion distance and CO2 hydration kinetics, respectively. Diffusion is anecdotally considered to be fast. However, over long distances, CO2 diffusion may be slower than its local reactive flux. Assuming a CO2 diffusion coefficient, DCO2, of 2500 μm2/s and a spontaneous CO2 hydration rate, kf, of 0.14 s−1 (23), local CO2 consumption by reaction will be faster than CO2 diffusion over distances >190 μm (√(2 × DCO2/kf)). The reactive flux can be augmented enzymatically by CAe, to increase further the importance of reactive versus diffusive consumption of CO2. If, for instance, hydration is catalyzed 10-fold, reactive CO2 removal would exceed diffusive CO2 removal over distances of >60 μm.
The remainder of transmembrane acid efflux takes the form of lactic acid, generated from anaerobic respiration or aerobic glycolysis (Warburg effect) (16). Lactic acid efflux can be accelerated if its extracellular concentration is kept low by diffusive dissipation or by CAe-catalyzed extracellular titration of H+ with HCO3−. It is important to note that CAe-catalyzed hydration of extracellular CO2 will reduce pHe, whereas titration of extracellular lactic acid by HCO3− (to form CO2, a weaker acid) will raise pHe. Therefore, the capacity of CAe to regulate pHe will depend on the chemistry of the excreted acid. In most healthy tissues at rest, the majority of cellular acid is emitted as CO2. Recent work on tumors also suggests a dominance of CO2 over lactic acid (22, 24).
The role for CAe in facilitating CO2 removal has been demonstrated for CA4 in skeletal muscle (25) and proposed for CA9 in tumors (2, 26). Furthermore, CA9 expression is strongly up-regulated in hypoxia (5), providing a mechanism by which CA9 levels are linked to diffusion distance. A consequence of facilitated CO2 removal is the attainment of a more uniformly alkaline pHi across the tissue. We demonstrated this recently in three-dimensional in vitro tissue models imaged for pHi (23). One prediction from that study is that CA9, although reducing pHi nonuniformity, will give rise to local extracellular acidity, particularly at the core of multicellular growths.
If pHe is indeed acidified by CA9, the enzyme expression may be doubly beneficial for CO2-excreting tumors: it will help to attain (i) a favorable alkaline pHi for growth and (ii) an acidic pHe to facilitate invasiveness. Clinically, CA9 may serve as a target for drugs. In the present work, we image pHe using a novel, membrane-impermeant fluorescent pH dye in multicellular spheroid growths (∼35,000 cells) derived from the colon carcinoma cell line HCT116. We demonstrate a key role for CA9 in regulating both pHi and pHe. Furthermore, we show that, even in the hypoxic core of spheroids, the principal substrate for CA9 is cell-excreted CO2 and that the precise effect of CA9 on pHe depends on the relative efflux from cells of lactic acid versus CO2.
MATERIALS AND METHODS
Solutions
The culture medium was Dulbecco's modified Eagle's medium containing 11 mm d-glucose or 2-deoxy-d-glucose (DOG) or d-galactose, buffered by 20 mm Hepes or 5% CO2, 22 mm HCO3−. The superfusate was normal Tyrode solutions containing 4.5 mm KCl, 1 mm CaCl2, 1 mm MgCl2, 11 mm glucose (or DOG or galactose), buffered by either 5% CO2 with 22 mm HCO3− (pH 7.4, 37 °C), 5% CO2 with 14 mm HCO3− (pH 7.2, 37 °C), or 1% CO2 with 2.2 mm HCO3− (pH 7.2, 37 °C) or a mixture of 2 mm + 2 mm, 10 mm + 10 mm, or 20 mm + 20 mm Hepes + Mes (pH 7.4, 37 °C). NaCl was added for a final osmolarity of 300 mOsm/kg. All of the superfusates were delivered at 2 ml/min and at 37 °C maintained by a feedback heater. Superfusates were changed rapidly (<5 s) using a switcher, supplied by two solutions in parallel.
pH Reporter Dyes
To measure pHi, carboxy-SNARF-1 (23) was loaded into cells as the membrane-permeant acetoxymethyl ester (10 μm) for 10 min (single cells) or 45 min (spheroids). Excitation at 514 nm was provided by an argon laser. Fluorescence was measured at 580 and 640 nm, ratioed, and converted to pHi using nigericin calibration (23). Although carboxy-SNARF-1 can also, in theory, be used to measure pHe, it is prohibitively expensive for use in superfusion experiments. For this reason, we have implemented novel fluorescein-based, dual excitation pH dyes for imaging pHe: fluorescein-5-(and-6-)-sulfonic acid (FS) and N-(fluorescein-5-thiocarbamoyl)-1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine (DF). The dyes were obtained from Invitrogen. FS was used in aqueous solutions at 30 μm. DF is lipid-soluble and was loaded into cell membranes by exposing cells to 10 μm dye for 10min (27). The dyes were excited alternately at two wavelengths: near (450–456 nm) and far (488–490 nm) from the absorption isosbestic point (∼460 nm). Fluorescence was collected >515 nm, ratioed, and calibrated with superfusates at different pH, buffered by 10 mm Hepes + 10 mm Mes.
Inhibitors
The CAe inhibitor 1-([4-sulfamoylphenyl)ethyl]-2,4,6-trimethyl pyridinium perchlorate (AP105) was synthesized in house and validated previously for inhibition potency (1). Acetazolamide (ATZ), a membrane-permeant CA inhibitor, was obtained from Sigma.
Cells
HCT116 cells (radius 8.6 μm) were transfected with plasmid vector only (referred to as “empty vector” (or EV)) or plasmid vector with the cDNA for human CA9 (referred to as “CA9 expressor”) and grown in McCoy's 5A medium (23). CA9 cDNA was a gift from Dr. J. Pastorek (Bratislava, Slovakia).
Tests for Carbonic Anhydrase Expression
Ice-cold cell suspension was homogenized 30–50 times and centrifuged at 3000 rpm for 10 min. Supernatant was microcentrifuged (Ti70 rotor; 8000 rpm, 45 min) to deposit the membrane fraction, which tested positive for Na+/K+ pump protein. Western blots tested for CA9 protein using mouse monoclonal M75 primary antibodies (a gift from Dr. J. Pastorek) (23). The clone with the highest CA9 expression was selected for growing CA9-positive spheroids.
Spheroid Growth
CA9 expressor and EV HCT116 cells were cultured in McCoy's 5A medium. Aggregation into spheroids (28) was initiated by plating 4 × 106 cells in 250-ml spinner flasks (Techne MCS, UK) spun at 40 rpm for 2–9 days. Unless stated otherwise, the media were buffered by 5% CO2, 22 mm HCO3− (pH 7.4 at 37 °C).
Epifluorescence Measurement of pHe
Single cells were superfused in a chamber mounted on a Nikon Diaphot inverted microscope with a ×40 oil immersion objective. Excitation light alternating every 250 ms between 450 and 490 nm was provided by a xenon lamp monochromator. Fluorescence was collected by a photomultiplier tube at >515 nm.
Confocal Imaging of pHe and pHi
Confocal imaging offers high spatial resolution. Single cells or spheroids were imaged in a superfusion chamber mounted on a Leica IRBE microscope with a ×10 dry objective. The system was coupled to an normal Tyrode TCS confocal system with argon visible (514-, 458-, or 488-nm) and argon UV (351-nm) lasers. For single cell experiments, the pinhole was set to 1 Airy unit, and each cell was defined as a region of interest (ROI). For spheroid experiments, the Airy diameter in μm was set to 15% of spheroid diameter, a compromise between adequate confocality and good photon capture. The fluorescence images were used to identify the equatorial plane of the spheroid and to produce a spheroid outline. This outline was used to generate 10 concentric, nonoverlapping layered ROIs of width equal to a tenth of spheroid radius. ROI1 was defined as the ROI at the core, and ROI10 was defined as the peripheral ROI.
Statistics
The data are presented as the means ± S.E. and tested for statistical differences with t test (significant differences for p < 0.05).
RESULTS
Characterization of Fluorescein-based Dyes
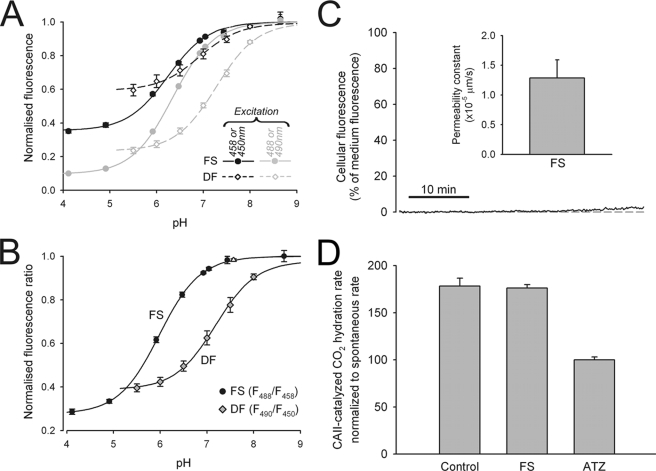
Fluorescein derivatives FS and DF were characterized for their fluorescent properties. Cell-free, 30 μm solutions of FS at different pH were studied in the confocal system under argon laser excitation, alternating between 458 and 488 nm every 4 s. To characterize DF, the cells were preloaded with dye at 10 μm for 10 min, and then dye-tagged cells were superfused with dye-free solutions at different pH under xenon lamp excitation, alternating between 450 and 490 nm every 0.25 s. Fig. 1A shows FS and DF emission at different pH. Fluorescence excited at the lower wavelength was less pH-sensitive, as it is nearer to the isosbestic point. The fluorescence excited at the two wavelengths was ratioed and plotted as a function of pHe in Fig. 1B. The pK values for FS and DF were estimated to be 6.4 and 7.6 (corrected for the ratio of maximum-to-minimum fluorescence at the lower wavelength, which offsets empirically estimated affinity (29)).
FIGURE 1.
Characterizing fluorescein-derived dyes. A, fluorescence from 30 μm FS (circles) or from cell membranes loaded with 10 μm DF (diamonds), plotted versus superfusate pH. FS was excited at 458 nm (black) or 488 nm (gray) with an argon laser; DF was excited at 450 nm (black) or 490 nm (gray) with a xenon lamp. Data (n = 6–12/bin) were fitted to sigmoid curves. B, FS and DF fluorescence ratio versus pH. C, intracellular fluorescence during superfusion of EV cells with 30 μm FS, relative to superfusate fluorescence. Cellular dye uptake is negligible, indicative of low FS membrane permeability. D, rate of CO2 hydration at 4 °C in the presence of 10 nm bovine red cell CA2. The rates were also measured in the presence of 100 μm ATZ or FS (n = 5/bin). The data were normalized to the ATZ (spontaneous) rate. FS does not inhibit CA2 activity.
For a dye to report only pHe, it must not permeate cell membranes. The membrane permeability of FS was measured in EV cells, superfused with 30 μm dye, and imaged confocally. 45 min of exposure to FS increased cellular fluorescence by <3%, suggesting low membrane permeability (Fig. 1C). To determine whether DF inserts at the inner or outer leaflet of the membrane, DF-loaded cells were superfused with solutions that altered pHe alone (changing superfusate pHe) or pHi alone (superfusing with ammonium or acetate salts at constant pHe). DF fluorescence was only responsive to the former maneuver, indicating outer leaflet insertion.
We also tested whether the dyes inhibit CA activity. A 10 nm solution of bovine red cell CA2, buffered by 20 mm Hepes, was tested for CA activity (23). 0.5 ml of 100% CO2-saturated water was injected rapidly to 1.5 ml of enzyme suspension in a stirred chamber at 4 °C. Medium pH time courses were fitted with kinetic equations for CO2 hydration. The protocol was repeated in the presence of 30 μm FS and 100 μm ATZ. Catalysis by CA was inhibited by ATZ but unaffected by FS (Fig. 1D) or DF (not shown).
Measuring CA9 Activity in Single Cells
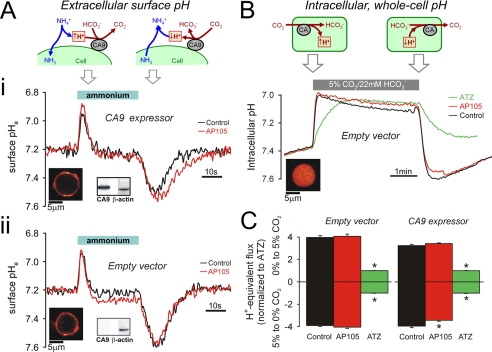
Single HCT116 cells, transfected with vector alone, or vector with the CA9 gene, were assayed for CAe activity using the epifluorescence set-up. The cells were loaded with DF to report surface membrane pHe (Fig. 2, A and B) (27) and superfused with solutions buffered by 1% CO2, 2.2 mm HCO3− (pH 7.2). Superfusates of low buffering capacity optimized the amplitude of surface pHe changes. The superfusate was switched rapidly to one containing 30 mm ammonium (pH 7.2) for a period of 30 s. Exposure to an equilibrated solution of NH4+/NH3 drives rapid entry of NH3 into cells. Surface pHe is reduced as extracellular NH4+ deprotonates to replenish NH3 that has entered the cell. After ∼20 s, surface pHe re-equilibrates to 7.2. On subsequent removal of the extracellular weak base, NH3 is driven rapidly out of the cell, protonates at the outer cell surface, and transiently raises surface pHe. Because CO2/HCO3− buffer contributes to the release and consumption of H+ ions at the cell surface, the time courses of recorded pHe transients depend on CO2/HCO3− buffer kinetics, i.e. CAe activity.
FIGURE 2.
Measuring CA activity in intact HCT116 cells. A, panel i, CA9-expressing cells loaded with membrane-inserting dye DF (see fluorescence map). Membrane fraction tested positive for CA9 protein by Western blotting (β-actin as control). Intact cells were superfused with 1% CO2, 2.2 mm HCO3− buffer (pH 7.2). Superfusion was switched rapidly for 30s to buffer containing 30 mm NH4Cl (pH 7.2). Transmembrane NH3 flux produces transient changes to surface membrane pHe (see cartoon). Surface pHe transients were smaller and briefer in the absence of CA9 inhibitor, 500 nm AP105 (red). Panel ii, protocol repeated on EV cells. Surface pHe transients are not different in the presence or absence of AP105, indicating a lack of CAe activity. B, EV cells AM-loaded with carboxy-SNARF-1 to measure pHi (see fluorescence map). The cells were superfused with CO2-free solution buffered by 20 mm Hepes (pH 7.4) and exposed for 4min to superfusate buffered by 5% CO2, 22 mm HCO3− (pH 7.4). Transmembrane CO2 flux produces pHi changes, at a rate determined by CA catalysis (see cartoon). Protocol was repeated in the presence of AP105 (500 nm) or ATZ (100 μm). ATZ blocks all CA activity. In EV cells, AP105 has no effect on catalysis, indicating that the drug does not enter cells. C, initial pHi slope on the addition and removal of CO2 for EV (n = 30) and CA9 expressor cells (n = 30). AP105 had a small but significant effect only in CA9-expressing cells, in response to extracellular CO2 removal.
Fig. 2A (panel i) shows surface pHe transients measured in CA9-expressing cells in the absence and presence of the CAe inhibitor, AP105 (500 nm). Expression of CA9 protein in the membrane fraction was confirmed by Western blotting. The pHe transients were smaller and briefer in the absence of AP105. This was more evident in the alkaline direction; the area under the pHe transient was 57% larger in the presence of inhibitor. The smaller size of pHe transients recorded in the absence of drug is indicative of CAe activity. The same experimental protocol was performed on EV cells that lack CA9 protein in the membrane. In this case, the pHe transients (Fig. 2A, panel ii) were not affected by AP105, indicating the absence of CAe activity.
To confirm the membrane impermeability of AP105 (and hence selectivity for CAe isoforms), we tested the inhibitory potency of AP105 (500 nm) on intracellular CA activity in EV cells and compared this with the potency of ATZ (100 μm), a membrane-permeant CAe inhibitor. EV cells were loaded with the pHi reporter dye carboxy-SNARF-1 (Fig. 2B) and superfused with dye-free solution. The superfusate was switched from one buffered by 20 mm Hepes (nominally CO2-free) to one buffered by 5% CO2, 22 mm HCO3−. Rapid solution change from 0 to 5% CO2 drives CO2 influx, intracellular hydration, and pHi acidification. The reverse solution change produces the opposite response (23). Fig. 2B shows the pHi time course associated with these maneuvers. ATZ slowed the rate of pHi change, in both directions, but AP105 had no significant effect. The difference in response to the two drugs confirms that AP105 does not penetrate the cell membrane.
The pHi time courses are in agreement with high membrane CO2 permeability (estimated to be ≥10 μm/s). The rate of pHi change upon the addition or removal of CO2 provides a measure of intracellular CO2/HCO3− buffering kinetics. Fig. 2C plots the initial rates of these pHi changes, normalized to the rate measured in ATZ. Catalysis by intracellular CA (CAi) isoforms alone (CAe blocked with AP105) is 4.0- and 3.4-fold in EV and CA9-expressing cells, respectively, i.e. slightly greater in EV cells. This finding is consistent with the inverse relationship between CA9 and CAi expression observed previously in RT112 cells (23). Additional CAe activity in drug-free CA9-expressing cells augmented (by 15%) the kinetics of pHi change on removal of extracellular CO2, but not on its addition. CA9 has an asymmetric effect on CO2 hydration and HCO3− dehydration, because the spontaneous rate of the former is slower, making it more responsive to CA9 catalysis. The pHi time courses can also be used to derive intrinsic (non-CO2) buffering capacity (βi), which is given by the change in [HCO3−]i (which is equal to [HCO3−]o × 10pHi−pHo divided by the pHi change (23)). βi was 27.3 ± 0.2 and 23.5 ± 0.2 mm/pH (n = 30) in CA9-expressing and EV cells, respectively (Fig. 2B).
In summary, cells transfected with CA9 were positive for CA9 protein and displayed functional CAe activity. EV cells did not express CA9 protein and showed no CAe activity but a modestly higher CAi activity than CA9-expressing cells.
Optical Measurements in Spheroids
The geometry of spheroids was characterized by confocal imaging with the membrane-permeant dye, 7-amino-4-methylcoumarin (AMC) (supplemental Fig. S1). Superfusion of spheroids with 10 μm AMC increased fluorescence throughout the spheroid, with an effective AMC mobility of 212 ± 12 μm2/s, ∼5-fold lower than that predicted from its molecular weight. This reduction suggests that the fraction of spheroid volume occupied by cells is 80%. The restricted extracellular space is suitable for driving CO2/HCO3− buffer out-of-equilibrium, a condition that allows CAe isoforms to perform net catalysis. Steady state AMC fluorescence throughout the spheroid indicated a high degree of spherical symmetry (supplemental Fig. S1).
CA9 Enzymatic Activity Reduces Intracellular pH Gradients but Increases Extracellular pH Gradients
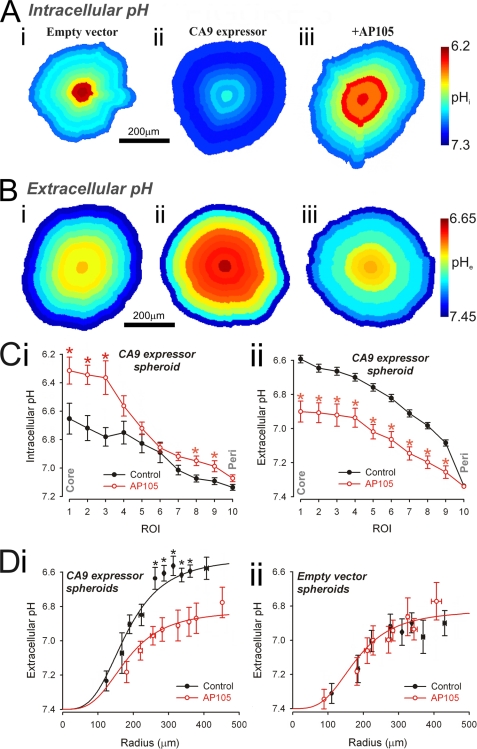
An earlier work (23) has shown that CA9 catalytic activity raises pHi and reduces its spatial heterogeneity in RT112 spheroids. This observation was tested in HCT116 spheroids (radius of 150–200 μm), loaded intracellularly with carboxy-SNARF-1. The experiment could not be performed on larger spheroids because of poor dye penetrability. Steady state fluorescence was converted to pHi maps (Fig. 3A) for an EV spheroid (panel i), a CA9-expressing spheroid under control conditions (panel ii), and one pretreated with 500 nm AP105 (panel iii). With CA9 activity, pHi was more alkaline and spatially nearly uniform. In the absence of CA9 activity, large spatial pHi gradients were evident, with significant acidity in core regions. Radial pHi data are summarized in Fig. 3C (panel i).
FIGURE 3.
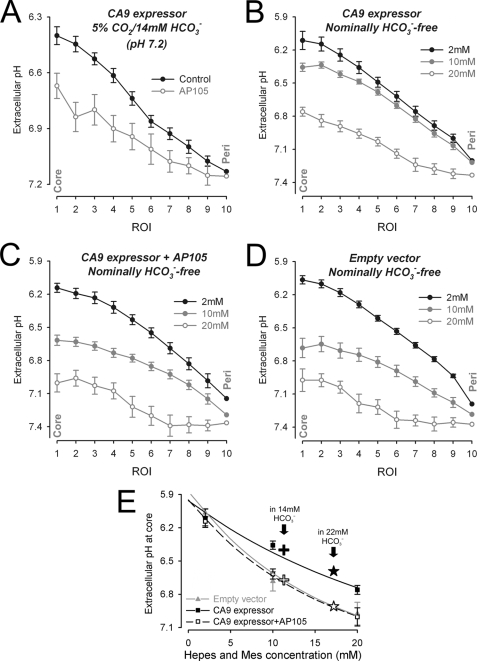
Extracellular CA activity acidifies spheroid extracellular space and alkalinizes spheroid intracellular space. A and B, maps of pHi (A) (measured with carboxy-SNARF-1) and pHe (B) (measured with fluorescein-5-(and-6-)-sulfonic acid) in EV spheroid (panel i), CA9-expressing spheroid (panel ii), and CA9-expressing spheroid preincubated with 500 nm AP105 (panel iii). C, radial profile of pHi (panel i) and pHe (panel ii) recorded in CA9-expressing spheroids (black; n = 20; spheroid radius of 222.0 ± 10.6 μm (panel i) or of 299.0 ± 2.7 μm) (panel ii) and in CA9-expressing spheroids treated with 500 nm AP105 (red; n = 20; spheroid radius of 229.2 ± 13.7 μm (panel i) or of 307.0 ± 6.3 μm) (panel ii). *, significant difference between control and AP105. CA9 activity decreases pHe and increases pHi. D, core pHe measured in the presence (red; n = 6–25/bin) or absence (black; n = 7–30/bin) of AP105 in CA9-expressing spheroids (panel i) and EV spheroids (panel ii). As spheroid size increases, core pHe decreases. The difference between CA9-expressing and EV spheroids is abolished in the presence of AP105 (best fit with three-parameter sigmoid curve).
In separate experiments, we imaged pHe to test the hypothesis that the effect of CA9 activity on pHe is opposite to that on pHi (23). Spheroids (radius of 250–350 μm) were superfused with 30 μm FS solution buffered by 5% CO2, 22 mm HCO3− and imaged confocally. The dye penetrates the extracellular space of the spheroid within 2 min (supplemental Fig. S2). Fig. 3B shows pHe maps at steady state for an EV spheroid (panel i), a CA9-expressing spheroid (panel ii), and one pretreated with 500 nm AP105 (panel iii). Core pHe was ∼6.9 in the absence of CA9 (Fig. 3B, panel i) and considerably more acidic (∼6.6) in its presence (Fig. 3B, panel ii). Preincubation with 500 nm AP105 for 2 h raised core pHe to ∼6.9. Radial pHe data are summarized in Fig. 3C (panel ii). The lower pHe measured in CA9-expressing spheroids was not due to a higher cellular metabolic rate, because both EV and CA9-expressing single cells acidified culture medium to a similar extent (supplemental Fig. S3).
In summary, spheroids expressing CA9 developed intracellular and extracellular acidosis that was greatest at the core. Inhibition of CA9 with AP105 reduced the radial pHe gradient (Fig. 3B) but increased the radial pHi gradient (Fig. 3C). The data suggest that CA9 catalytic activity accelerates the efflux of cell-derived acid to the extracellular space and helps to maintain a more uniform pHi at the cost of a more acidic and heterogeneous pHe.
Larger CA9-expressing Spheroids Deposit Greater Extracellular Acid Load
Acid generated by respiring cells is deposited into the extracellular space, whence it diffuses to the superfusate. The diffusion distance will determine the pace at which acid can be vented. Spheroids made from CA9 expressor or EV cells were grown to radii between 100 and 500 μm, and pHe in the core ROI was measured in the presence and absence of 500 nm AP105 (Fig. 3D). In both types of spheroid, core pHe acidified in a saturating manner as spheroid size increased. Core pHe was 54% more acidic in CA9 expressors. Preincubation with AP105 for 2 h alkalinized core pHe in CA9-expressing spheroids but had no effect on EV spheroids (Fig. 3D). These data suggest that AP105-sensitive extracellular acidity is due to CAe activity, present in CA9-expressing spheroids but absent in EV spheroids.
Extracellular, Not Intracellular, CA Isoforms Play a Dominant Role in Acidifying pHe
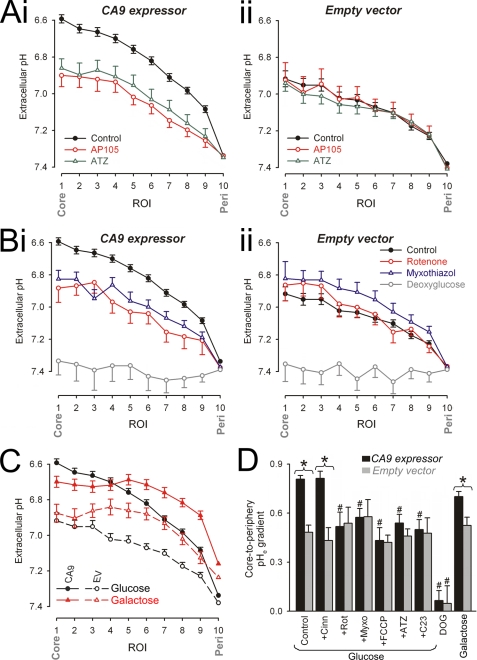
We have investigated whether inhibition of intracellular CA isoforms has a supplementary effect on pHe gradients. This was examined by comparing pHe data from spheroids (radius, 250–350 μm) preincubated with membrane-impermeant AP105 (500 nm) and membrane-permeant ATZ (100 μm). AP105 and ATZ reduced pHe gradients by similar amounts in CA9-expressing spheroids (Fig. 4A, panel i) but had no significant effect on EV spheroids (Fig. 4A, panel ii). This indicates that, in the absence of CAe activity, CAi isoforms do not affect spatial pHe gradients.
FIGURE 4.
Pharmacological studies of extracellular pH gradients. A–C, imaging for pHe with dye FS in spheroids superfused with 5% CO2, 22 mm HCO3− buffer. The data are presented as radial pHe profiles. *, significant difference between control and drug. A, data for CA9-expressing (panel i) and EV spheroids (panel ii). Black filled circles, drug-free conditions (spheroid radius of 299.0 ± 2.7 (panel i) and 297.6 ± 2.67 μm (panel ii)). Red open circles, with 500 nm AP105 (spheroid radius of 307.0 ± 6.3 (panel i) and 303.0 ± 5.7 μm (panel ii)); Green open triangles, with 100 μm ATZ (spheroid radius of 308.6 ± 3.6 (panel i) and 296.6 ± 6.4 μm (panel ii)). B, data for CA9-expressing (panel i) and EV spheroids (panel ii). Black filled circles, drug-free conditions. Red open circles, with 1 μm rotenone (spheroid radius of 310.0 ± 5.9 (panel i) and 314.7 ± 6.2 μm (panel ii)). Blue open triangles, with 5 μm myxothiazole (spheroid radius of 325.1 ± 7.2 (panel i) and 319.5 ± 4.2 μm (panel ii)). Gray open squares, d-glucose replaced with DOG (spheroid radius of 278.7 ± 3.6 (panel i) and 305.0 ± 6.6 μm (panel ii)). C, pHe gradients in CA9-expressing spheroids (filled symbols) or EV spheroids (open symbols) respiring glucose (black symbols) or galactose (red symbols; spheroid radius of 299.4 ± 6.1 (panel i) and 29.5 ± 4.3 μm (panel ii)). D, core-to-periphery pHe gradient under different conditions. *, significant difference between CA9-expressing and EV spheroids. #, significant difference between data from the same spheroid type (EV or CA9 expressor). Cinn, α-cyanohydroxycinnamate.
Principal Source of Substrate for Extracellular Acidification Is CO2 Production
In experiments described thus far, glucose was the sole superfused metabolic substrate. It is thus likely to underpin the appearance of extracellular acid. To test this, spheroids (radius of 250–350 μm) were equilibrated in medium containing DOG in place of glucose to block glycolysis (30). Spheroids were subsequently superfused in DOG-containing, 5% CO2, 22 mm HCO3−-buffered medium and imaged for pHe. The spheroids did not acidify pHe (Fig. 4B), confirming that substrate metabolism is ultimately the source of extracellular acid.
Cells can generate acid metabolically in the form of CO2 or lactic acid (2, 16, 17). One major source of CO2 is decarboxylation of Krebs cycle intermediates under aerobic conditions. To investigate whether the Krebs cycle contributes to extracellular acidification, spheroids (radius 250–350 μm) were incubated for 2 h in glucose-containing medium with 1 μm rotenone or 5 μm myxothiazol, inhibitors of mitochondrial complexes I and III, respectively (31) (Fig. 4B). CA9-expressing spheroids, when forced to respire without Krebs cycle activity, still generated acidic pHe gradients, but these gradients were up to 40% smaller, and were no longer significantly different from those measured in EV spheroids, i.e. CAe activity under these conditions had no apparent effect on pHe. Uncoupling of mitochondria with FCCP (25 μm) (31) had a similar effect to rotenone and myxothiazol (Fig. 4D). The applied doses of mitochondrial drugs were higher than typically used in isolated cells, to ensure adequate inhibition throughout the large, restricted spheroid volume. Mitochondrial inhibitors did not target in vitro CA9 activity (supplemental Fig. S4). In isolated cells, rotenone and myxothiazol did not have a significant effect on resting pHi, but FCCP produced a small, transient acidification (supplemental Fig. S4).
In a different set of experiments, spheroids were grown in medium containing galactose instead of glucose. Oxidation of galactose to pyruvate yields no ATP and forces cells to commit to the Krebs cycle (32). Radial pHe profiles were measured in CA9-expressing and EV spheroids (Fig. 4C). When tracking in from the oxygenated periphery, galactose-respiring spheroids displayed a steeper fall of pHe, in agreement with a higher Krebs cycle turnover under these metabolic conditions (32). The degree of extracellular acidosis saturated toward the core, suggesting a fall in metabolic activity in the deeper, more hypoxic regions. CA9 activity thus acidified pHe, as expected from a CO2-generating process.
Our data indicate that the Krebs cycle normally operates in spheroids as large as 300 μm in radius, and its output underlies much of the difference in pHe gradients observed in CA9-expressing spheroids and EV spheroids. Fig. 4D summarizes the effects of different incubation conditions on the amplitude of radial pHe gradients.
Majority of Cell Acid Is Excreted across Membranes as CO2 Not Lactic Acid
Spheroids larger than 150 μm in radius are known to develop hypoxic cores (33, 34). Low O2 levels activate hypoxia-inducible factor and up-regulate the anaerobic respiration of glucose to lactic acid (35). Lactic acid production is also raised when mitochondria are blocked pharmacologically. In the absence of the Krebs cycle, lactic acid production would appear to underlie much of the acidic pHe in spheroids (Fig. 4B). Lactic acid production in hypoxic regions may explain why pHe continues to fall with increasing depth in glucose-respiring spheroids but levels off in galactose-respiring spheroids (Fig. 4C).
Cells can remove lactic acid in two ways. The first is by intracellular titration with HCO3− (sequestered from the extracellular space). The titration reaction generates CO2, which diffuses to the extracellular space, where it is subject to catalysis by CA9, if active. The second way is by extruding lactic acid directly via the monocarboxylic acid transporter (MCT) (36). Alternatively, the H+ ion component of lactic acid can be extruded by transporters such as Na+-H+ exchange (9). In HCT116 cells, however, H+ extrusion by Na+-H+ exchange is low and ∼4-fold smaller than acid-neutralizing HCO3− uptake transport (supplemental Fig. S4). A similar observation has been made in RT112 cells (23). Functional MCT activity in HCT116 cells was demonstrated by measuring pHi in isolated cells during transient exposure to 5 mm sodium lactate to activate lactic acid influx via MCT (supplemental Fig. S4). The MCT blocker α-cyanohydroxycinnamate (2.5 mm) (36) inhibited a large fraction of the acidification induced by this procedure, indicating that direct lactic acid transport is mainly MCT-mediated (supplemental Fig. S4). The contribution of MCT-mediated lactic acid excretion to pHe nonuniformity was studied in glucose-respiring spheroids, preincubated with 2.5 mm α-cyanohydroxycinnamate for 2 h. This compound did not significantly affect pHe gradients in either CA9 expressor or EV spheroids (Fig. 4D). Transmembrane lactic acid efflux therefore does not contribute greatly toward acidic pHe under control conditions. It is therefore likely that the majority of anaerobically produced acid exits cells as CO2, formed by titration with intracellular HCO3−.
Extracellular Acidification Can Be Altered by Extracellular [HCO3−] and Buffering Capacity
The extracellular buffering regime has a 2-fold effect on pHe gradients. First, the magnitude of pHe changes in response to acid depends inversely on extracellular buffering capacity. Second, extracellular CO2/HCO3− buffer provides substrate (HCO3−) for cellular uptake and titration with intracellular acid. To investigate the effect of extracellular buffering capacity and composition, the spheroids were preincubated for 6 h under one of two conditions. In one set of experiments, incubation and subsequent superfusion was performed in lower extracellular [HCO3−] (5% CO2, 14 mm HCO3−, pH 7.2). Radial pHe profiles were measured in CA9-expressing spheroids. Compared with experiments in 22 mm HCO3− media, acidic pHe gradients were greater in low HCO3− medium, in agreement with its lower buffering capacity. Inhibition of CA9 with AP105 had a similar effect on pHe gradients under both buffering conditions (Fig. 5A).
FIGURE 5.
Extracellular pH gradients in low or nominally nil HCO3−superfusates. Spheroids were equilibrated in media buffered by 5% CO2/14 mm HCO3− (pH 7.2) (A) or an equimolar mixture (2 + 2, 10 + 10, 20 + 20 mm) of Hepes + Mes buffer (pH 7.4) instead of CO2/HCO3− (B–D). Radial pHe profiles (n = 10–22/data set) in CA9-expressing spheroids with and without 500 nm AP105 (A; radius of 377.6 ± 6.2 and 362.6 ± 6.6 μm), CA9-expressing spheroids (B; radius of 274.3 ± 5.7, 285.3 ± 13.8, and 285.0 ± 7.2 μm), CA9-expressing spheroids preincubated with 500 nm AP105 (C; radius of 271.3 ± 7.3, 272.6 ± 4.5, and 277.6 ± 6.9 μm), and EV spheroids (D; radius of 280.9 ± 7.0, 261.9 ± 4.1, and 263.3 ± 6.2 μm). E, core-to-periphery pHe gradient versus Hepes + Mes concentration. Stars and crosses, superimposition of pHe gradient measured in 5% CO2, 22 mm HCO3− buffer and 5%CO2, 14 mm HCO3− buffer with (black filled symbols) or without (open symbols) CA9 activity.
In a second set of experiments, spheroids were preincubated and then superfused with Hepes and Mes buffer (2 + 2 mm, 10 + 10 mm, or 20 + 20 mm) in place of CO2/HCO3−. An equimolar mixture of Hepes + Mes ensures that buffering capacity is near constant over the pHe range 6–7.5. Overall, increasing the extracellular buffering capacity in CA9-expressing or EV spheroids decreased pHe gradients (Fig. 5, B–D). Spheroids expressing CA9 activity produced larger pHe gradients than EV spheroids (Fig. 5, B and D). Preincubation with 500 nm AP105 reduced pHe gradients in CA9-expressing spheroids to levels observed in EV spheroids (Fig. 5C). The relationship between pHe gradient size and Hepes + Mes buffering (Fig. 5E) indicates that extracellular buffering by 5% CO2, 22 mm HCO3− corresponds to an equivalent Hepes + Mes concentration of ∼17 mm.
In summary, our data show that CA9 can acidify pHe even in the absence of superfusate CO2/HCO3− buffer. This confirms that the principal source of extracellular acid is cell-derived CO2, much of this being generated from the Krebs cycle.
DISCUSSION
Carbonic Anhydrase 9 as a Regulator of Both Intracellular and Extracellular pH
The extracellular topology of the CA9 active site has hinted at a role in acidifying pHe. Expression of the enzyme in a population of isolated cells reduces medium pH (26). In single cells, expression of CA9 has been shown to raise steady state pHi (3), and our previous work (23) has shown that CA9 activity raises pHi and reduces its heterogeneity in multicellular spheroid growths. All of these results suggest that CA9, in effect, increases the transmembrane acid efflux.
In the present work, we have tested this hypothesis by in vitro imaging of both pHi and pHe in spheroids grown from cells of the HCT116 human colon carcinoma line. This cell line has a high metabolic rate (supplemental Fig. S3) and can grow into multicellular spheroids that are accessible to confocal imaging (Fig. 3 and supplemental Figs. S1 and S2). Spheroids are a good model for poorly perfused, developing tumors (23, 28, 33) because they have a restricted extracellular space, with a considerable core-to-surface diffusion distance for solutes such as CO2 and H+ ions. Spheroids may also offer a useful experimental model for simulating pH control during interrupted vascular perfusion of normal tissue, such as brain and myocardium. We have characterized a novel dye, fluorescein-5-(and-6)-sulfonic acid, and we find that it provides excellent spatial measurements of pHe in spheroids. Under control conditions, spheroids develop radial gradients of pHi and pHe, with the lowest levels reached at the core (Fig. 3C). Expression of CA9 decreases core pHe and increases core pHi (Fig. 3, A–C), supporting a role for CA9 in facilitating transmembrane acid efflux.
Sources of Extracellular Acidosis and the Role of CA9
We studied the factors, including CA9 activity, that affect pHe. The degree of extracellular acidity increases with spheroid radius (Fig. 3D), as expected from the increasing diffusion distance. The ultimate source of acid in the extracellular space is cellular respiration, because removal of metabolizable substrate causes spheroid pHe to equilibrate with the superfusate (Fig. 4B). There are three possible ways for cells to generate extracellular acid (Fig. 6A): (i) Jmito, mitochondrial CO2 production and its efflux; (ii) Jextr, lactic acid production and H+-lactate extrusion by MCT or H+ ion extrusion by e.g. Na+-H+ exchange, and (iii) Jtitr, lactic acid production, its titration by intracellular HCO3− and subsequent efflux in the form of CO2. In general, these acid fluxes will occur in all tissues, but the distribution among the three pathways will depend on factors such as oxygenation, mitochondrial status, membrane transporter activity, and the chemistry of the respiratory substrate. For example, removing extracellular HCO3−, by eliminating HCO3− reuptake into cells, will reduce Jtitr to 0 but increase Jextr, to ensure sustained removal of lactic acid. Another example is that substituting glucose with galactose forces cells to rely entirely on oxidative phosphorylation rather than anaerobic respiration (32). Under these conditions, Jtitr and Jextr will fall to 0, whereas Jmito and O2 consumption will rise.
FIGURE 6.
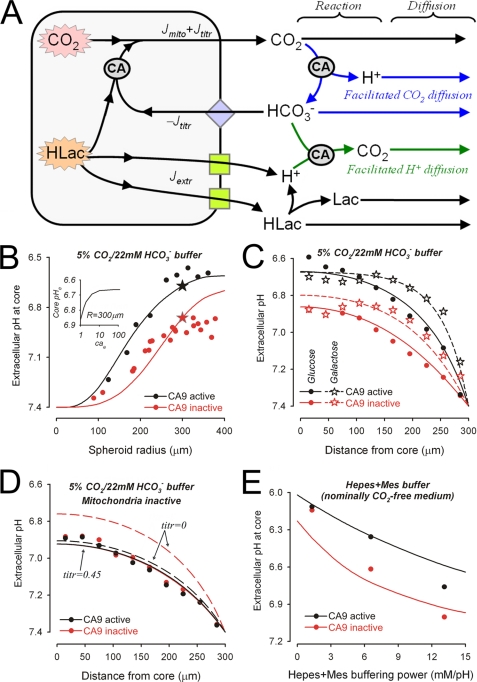
Model of spheroid pHe regulation. A, proposed scheme of acid efflux pathways. Cell metabolism generates acid which takes the form of CO2 or lactic acid (HLac). CO2 generated directly by mitochondria (Jmito) can exit across the membrane. The acidity caused by lactic acid can be extruded across the membrane as H+ ions (Jextr) by H+-lactate co-transport or H+ extrusion (green squares). Alternatively lactic acid can be titrated with intracellular HCO3−, imported on transmembrane HCO3− carriers (blue diamond), to produce CO2 (Jtitr). Extracellular pH depends on acid output and the extracellular diffusion and reaction fluxes. Carbonic anhydrases (circled CA) catalyze the reaction CO2 + H2O ↔ H+ + HCO3−. B, experimental data (from Fig. 3D) with model simulation for the relationship between core pHe and spheroid radius in 5% CO2, 22 mm HCO3− buffer. To obtain the sigmoidal relationship, Fsubs μm/s) is 180−(0.3 × radius). Red, CA inactive (CAe = 1); black, CA active (CAe = 10); stars, core pHe for radius = 300 μm. Inset, relationship between core pHe and CA activity (CAe). C, experimental data (from Figs. 4, A and C) with model simulation for radial pHe gradients in glucose-respiring (circles) or galactose-respiring (stars) spheroids in 5% CO2, 22 mm HCO3− buffer. D, experimental data (from Fig. 4B) with model simulation (continuous line) for radial pHe gradients in 5% CO2, 22 mm HCO3− buffer in the presence of mitochondrial inhibitors. The fraction of intracellular lactic acid titrated was 0.45. Dashed line, model simulation assuming nil intracellular lactic acid titration. E, experimental data (from Fig. 5) with model simulation for core pHe at increasing Hepes + Mes buffer capacity.
Intracellular and extracellular CA isoforms could potentially play a role in all three acid efflux pathways (Fig. 6A). Our data, however, show that inhibition of CAi isoforms has no supplementary effect on pHe gradients when CA9 activity is absent, indicating that the major influence of CA on pHe is exerted through CAe (Fig. 4A). CA9 activity accelerates both extracellular CO2 hydration and the reverse dehydration reaction. If cellular acid is excreted as CO2, CA9 will increase its extracellular hydration, yielding more H+ (thus lowering pHe), thereby facilitating further transmembrane CO2 efflux. If cellular acid is excreted as lactic acid, CA9 will facilitate its extracellular titration with HCO3−. This will augment transmembrane lactic acid efflux but will increase pHe. This is because CO2 is a weaker and more diffusible acid than lactic acid. Therefore, CA9 will have opposing effects on pHe, depending on whether CO2 or lactic acid are principally extruded. Either way, CA9 will facilitate the efflux of acid (CO2 or lactic acid). The fact that, in control spheroids CA9 acidifies pHe indicates that its principal effect is to facilitate the efflux of cellular CO2. In support of this model, radial pHe gradients were not significantly affected by blocking MCT (Fig. 4D). Furthermore, the activity of other H+ ion extruding transporters (e.g. Na+-H+ exchange) in HCT116 cells is low (supplemental Fig. S4), and so their contribution to H+ efflux will be minimal. This is in agreement with data from in situ colon carcinomas, showing that ∼70% of acid output is as CO2 (22, 24).
In well oxygenated regions of spheroids, most CO2 will originate from the Krebs cycle (Jmito). Indeed, in galactose-respiring spheroids, this is the only source of cellular acid. It is therefore notable that radial pHe profiles in these spheroids were steep near the periphery but leveled off sharply with depth, in contrast to a smoother and more gradual pHe acidification toward the core of glucose-respiring spheroids (Fig. 4C). The steepness of the radial pHe gradient at the periphery of galactose-respiring spheroids can be explained by a high respiration rate in these regions, as observed previously in cultured cells (32). The flatter pHe gradient at depths >170 μm indicates a sharp drop in acid production, correlating with the predicted fall in O2 tension. In contrast, pHe continues to fall with depth in glucose-respiring spheroids, suggesting a sustained acid production that persists under hypoxia.
As O2 tension falls, lactic acid production will increase. Because CA9 has an acidifying effect on pHe, even at the hypoxic core of spheroids (Fig. 3C, panel ii), it is likely that cell-generated lactic acid in these regions is titrated (Jtitr) with HCO3− to produce CO2, which then vents from cells to act as a substrate for CAe. For this process to be maintained, HCO3− must be continuously transported back into the cell from the extracellular space. The role of HCO3− influx transporters in determining Jtitr could be studied with pharmacological inhibitors. However, many inhibitors of acid/base transport, such as DIDS, also inhibit CA activity directly (37); therefore it was not feasible to use these drugs in our studies of CA9. However, the role that Jtitr plays in providing substrate (CO2) for CA9 can be appreciated by removing extracellular HCO3−, thus blocking HCO3− re-uptake (Fig. 5). Over a comparable pHe range, the acidifying effect of CA9 on pHe was 15% smaller in spheroids superfused in Hepes/Mes buffer than in 5% CO2, 22 mm HCO3− buffer (Fig. 5E).
In the presence of mitochondrial inhibitors, spheroids continued to produce an acidic pHe, but there was now no observable influence of CA9 activity (Fig. 4B). Under these conditions, Jmito is abolished, and all acid efflux is carried by Jextr + Jtitr. The lack of effect of CA9 under these conditions argues for a balance between Jextr and Jtitr, at which there is no net CA9 catalysis, i.e. catalyzed CO2 hydration (driven by Jtitr) and its catalyzed reverse reaction (driven by Jextr) cancel out.
Mathematical Model of the Role of CA9
To understand the complex acid/base events occurring in spheroids, it was necessary to construct a quantitative diffusion reaction model (see supplemental material for details). By fitting experimental data to the model, we were able to estimate Jmito, Jextr, and Jtitr, as well as extracellular buffering capacity (βe), extracellular H+ mobility (De), and CAe activity (CAe). These parameters could not be derived directly from any one set of data, because they require a unifying mathematical framework to fit several data sets simultaneously. The very good fits to experimental data (Fig. 6, B–E) further support the conclusions made above.
Total acid production depends on the rate of metabolic substrate respiration (Fsubs). The model predicts Fsubs = 90 μm/s in glucose-respiring spheroids (radius, 300 μm). This rate is in agreement with measurements of 30–190 μm/s, previously made in spheroids (34). But glucose consumption rates in tumors have been reported to be an order of magnitude lower than this (2–15 μm/s) (18). These latter data, however, may have been underestimated Fsubs values, because they were normalized to total tumor mass, including its blood supply, stroma, and dead cells, which do not contribute to tumor cell Fsubs.
In control spheroids, the local O2 tension determines whether metabolic substrate is respired aerobically to CO2 (Jmito) or anaerobically to lactic acid (Jtitr + Jextr). Our model predicts that the rate of aerobic respiration decays exponentially toward the spheroid core, with a space constant of 250 μm. This is in agreement with radial O2 measurements in spheroids (33, 34) that fit to an exponential decay, with depth (x), exp(−x/λO2), giving a space constant (λO2) of 150–650 μm. For galactose-respiring spheroids, the model predicts that Fsubs is raised 4-fold, and λO2 is reduced to 45 μm, in agreement with the higher oxidative phosphorylation rate measured in cultured cells (32).
Lactic acid production shows an opposite depth dependence, 1-exp(-x/λO2), i.e. it increases with depth, unless the metabolic substrate is galactose, which is not respired anaerobically. A certain fraction of lactic acid is titrated by intracellular HCO3− (Jtitr), and the remainder is extruded by H+ transporters (Jextr). The relationship between Jtitr and Jextr can be estimated by best fitting the model to experimental data. Under control conditions (Fig. 6, B and C), a large fraction of lactic acid is predicted to undergo intracellular titration. In nominally HCO3−-free superfusate (Fig. 6E), all lactic acid is predicted to be extruded directly.
Under control conditions, the lowering of pHe by CA9 activity is consistent with a 10-fold catalysis of CO2 hydration (CAe = 10; Fig. 6B). However, in spheroids with drug-inhibited mitochondria (Jmito = 0), CA9 activity had no apparent effect on pHe gradients. This is expected to occur at a certain balance between Jtitr and Jextr, because these acid fluxes drive the CA9-assisted reaction in opposite directions. Using the model it is possible to calculate that this balance is attained when 45% of lactic acid is titrated intracellularly (Fig. 6D).
Our data cannot provide independent estimates for βe and De, but best fitting their product, De × βe, yields a value of 1.6 × 104 mm·μm2/s. This implies that as βe increases, De must decrease, in agreement with the finding that H+ buffers lower the effective H+ ion mobility (38).
Conditions for CA9 Catalysis
Using our experimental data and computational modeling, we can now define the conditions under which CA9 catalysis exerts a significant effect on pHe. Condition 1: Cells must engage in metabolic acid production (Fig. 4B). Our results suggest that the ability of CA9 to decrease pHe increases with respiration rate (Fsubs). In the presence of galactose, the elevated Fsubs increases the steepness of radial pHe profiles within the aerobic zone (Fig. 6C). Condition 2: The effect of CA9 on pHe will depend on the type of acid released across the cell membrane. Our model predicts that the greatest acidifying effect of CA9 on pHe is observed if all substrate is respired aerobically to CO2 (Fig. 6C). The greatest alkalinizing effect of CA9 on pHe will be observed if all glucose is respired to lactic acid, without any titration by HCO3− (dashed curves in Fig. 6D). The observation that, in vivo, pHe is low in most cancers (14, 18, 20) suggests that CO2 excretion is important in CA9-expressing tumors, such as colon cancer (2, 22, 24), and that efflux of lactic acid may be dominant in tumors lacking CAe activity, such as gastric cancer (39). This may define different phenotypes of cancer pHe, with implications for the selection of therapies that target their metabolism. Condition 3: Cellular uptake of HCO3− can drive extracellular CO2/HCO3− buffer out-of-equilibrium and allow CA9 to exert a net catalytic effect, particularly when HCO3− influx is combined with CO2 efflux (i.e. counter flux). This arrangement between CA and an acid/base transporter has been called a HCO3− transport metabolon (40, 41). Condition 4: The buffering properties of the extracellular space will affect the equilibrium between H+, CO2, and HCO3−. If, in our model, De and/or βe are increased, then the predicted pHe gradients become smaller. When [H+]e is “buffered” by raised De × βe, CO2/HCO3− buffer is more prone to be driven out-of-equilibrium by transmembrane CO2 or HCO3− flux. Therefore, the effect of CA9 on pHe gradients will be greater at higher De × βe (Fig. 6E). Condition 5: The level of CA9 catalysis can vary because of spatial differences in expression. Such variations in CA9 activity may therefore affect local pHe. CA9 expression levels in stably transfected spheroids are nearly uniform (23), but as CA9 expression is hypoxia-induced (5), the enzyme may not be uniformly distributed in poorly perfused tissues or tumors. Our computational model, however, shows that the effect of CA9 on pHe is saturable (Fig. 6B, inset); therefore spatial variations in CA9 catalysis above ∼20-fold will not affect pHe greatly in regions where overall expression is high. In addition, the need for CA9 catalysis to maintain CO2/HCO3− equilibrium is less at shorter distances from the blood supply. Therefore, CA9 activity in well oxygenated peripheral areas will be redundant, and expression levels there will not affect pHe. This redundancy may explain the correlation between CA9 expression and hypoxia in tumors (42).
Conclusions
Carbonic anhydrases are found in virtually all cells. CAe isoforms have generated considerable attention because of their postulated role in facilitating acid removal from cells. CA9 is of particular interest because of its association with cancer and its hypothesized role in regulating metabolism. To elucidate the physiological role for CAe isoforms, extracellular CO2/HCO3− buffer must be driven out-of-equilibrium. This will happen in the restricted extracellular environment of respiring spheroids and in poorly perfused but metabolically active tissue. CA9 activity facilitates acid removal by accelerating the conversion of excreted product (CO2 or lactic acid) to its conjugate pair (HCO3− or lactate). The CA9-dependent reaction flux then becomes significant, compared with diffusive removal of waste products. Inhibition of CA9 activity impedes acid efflux and increases intracellular acidity (Fig. 3C, panel i). In well perfused tissue (including the peripheral regions of spheroids), diffusion distances are small enough to ensure rapid waste product removal, making CA9 catalysis redundant. In deeper regions, simple diffusion of acid waste products cannot be accelerated, but expression of CAe isoforms offers a tool by which cells can engage in facilitated diffusion. A consequence of CAe activity is extracellular acidification (when CO2 is hydrated) or alkalinization (when HCO3− is dehydrated). Therefore, expression of CAe isoforms is a means by which cells can modulate pHe. Because the source of acid/base disturbance affecting pHe is the cell, changes in pHe will be mirrored by opposite changes in pHi. Extracellular CA9 (and presumably other CAe isoforms, such as CA4, CA12, and CA14) therefore acts as a “catalytic converter” for the acid exhaust system of respiring cells. Inhibition of CA9 activity in CO2-excreting tumors may thus provide a strategy for suppressing their development, by acidifying pHi (to attenuate growth (2, 21)) and alkalinizing pHe (to eliminate the selective advantage of neoplastic cells (8, 22)).
Supplementary Material
Acknowledgments
We acknowledge I. Ledaki, A. Hulikova, and P. Cobden for excellent assistance in culturing spheroids.
This work was supported by funds from the Royal Society and Medical Research Council (to P. S.), the British Heart Foundation and Wellcome Trust (to R. D. V.-J.), EUROXY, and Cancer Research UK (to A. L. H.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4 and details of the mathematical model.
- CA
- carbonic anhydrase
- DOG
- 2-deoxy-d-glucose
- FS
- fluorescein 5(6)-sulfonic acid
- DF
- N-(fluorescein 5-thiocarbamoyl)-1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine
- AP105
- 1-([4-sulfamoylphenyl)ethyl]-2,4,6-trimethyl pyridinium perchlorate
- ATZ
- acetazolamide
- EV
- empty vector
- ROI
- region of interest
- AMC
- 7-amino-4-methylcoumarin
- FCCP
- carbonyl cyanide p-trifluoromethoxyphenylhydrazone
- MCT
- monocarboxylic acid transporter
- Mes
- 4-morpholineethanesulfonic acid
- DIDS
- 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid.
REFERENCES
- 1.Supuran C. T. (2008) Nat. Rev. Drug Discov. 7, 1–14 [DOI] [PubMed] [Google Scholar]
- 2.Swietach P., Vaughan-Jones R. D., Harris A. L. (2007) Cancer Metastasis Rev. 26, 299–310 [DOI] [PubMed] [Google Scholar]
- 3.Chiche J., Ilc K., Laferrière J., Trottier E., Dayan F., Mazure N. M., Brahimi-Horn M. C., Pouysségur J. (2009) Cancer Res. 69, 358–368 [DOI] [PubMed] [Google Scholar]
- 4.Pastorek J., Pastoreková S., Callebaut I., Mornon J. P., Zelník V., Opavský R., Zat'ovicová M., Liao S., Portetelle D., Stanbridge E. J., Závada J., Burny A., Kettmann R. (1994) Oncogene 9, 2877–2888 [PubMed] [Google Scholar]
- 5.Wykoff C. C., Beasley N. J., Watson P. H., Turner K. J., Pastorek J., Sibtain A., Wilson G. D., Turley H., Talks K. L., Maxwell P. H., Pugh C. W., Ratcliffe P. J., Harris A. L. (2000) Cancer Res. 60, 7075–7083 [PubMed] [Google Scholar]
- 6.Ludwig M. G., Vanek M., Guerini D., Gasser J. A., Jones C. E., Junker U., Hofstetter H., Wolf R. M., Seuwen K. (2003) Nature 425, 93–98 [DOI] [PubMed] [Google Scholar]
- 7.Waldmann R., Champigny G., Lingueglia E., De Weille J. R., Heurteaux C., Lazdunski M. (1999) Ann. N.Y. Acad. Sci. 868, 67–76 [DOI] [PubMed] [Google Scholar]
- 8.Huang W. C., Swietach P., Vaughan-Jones R. D., Ansorge O., Glitsch M. D. (2008) Curr. Biol. 18, 781–785 [DOI] [PubMed] [Google Scholar]
- 9.Boron W. F. (2004) Adv. Physiol. Educ. 28, 160–179 [DOI] [PubMed] [Google Scholar]
- 10.Jean T., Frelin C., Vigne P., Barbry P., Lazdunski M. (1985) J. Biol. Chem. 260, 9678–9684 [PubMed] [Google Scholar]
- 11.Stewart A. K., Kurschat C. K., Vaughan-Jones R. D., Alper S. L. (2009) J. Biol. Chem. 284, 6126–6139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chambard J. C., Pouyssegur J. (1986) Exp. Cell Res. 164, 282–294 [DOI] [PubMed] [Google Scholar]
- 13.Pouysségur J., Sardet C., Franchi A., L'Allemain G., Paris S. (1984) Proc. Natl. Acad. Sci. U.S.A. 81, 4833–4837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gatenby R. A., Gawlinski E. T., Gmitro A. F., Kaylor B., Gillies R. J. (2006) Cancer Res. 66, 5216–5223 [DOI] [PubMed] [Google Scholar]
- 15.Kato Y., Nakayama Y., Umeda M., Miyazaki K. (1992) J. Biol. Chem. 267, 11424–11430 [PubMed] [Google Scholar]
- 16.Gatenby R. A., Gillies R. J. (2004) Nat. Rev. Cancer 4, 891–899 [DOI] [PubMed] [Google Scholar]
- 17.Gillies R. J., Robey I., Gatenby R. A. (2008) J. Nucl. Med. 49, (Suppl. 2) 24S–42S [DOI] [PubMed] [Google Scholar]
- 18.Vaupel P., Kallinowski F., Okunieff P. (1989) Cancer Res. 49, 6449–6465 [PubMed] [Google Scholar]
- 19.Stubbs M., McSheehy P. M., Griffiths J. R., Bashford C. L. (2000) Mol. Med. Today 6, 15–19 [DOI] [PubMed] [Google Scholar]
- 20.Gillies R. J., Raghunand N., Karczmar G. S., Bhujwalla Z. M. (2002) J. Magn. Reson. Imaging 16, 430–450 [DOI] [PubMed] [Google Scholar]
- 21.Griffiths J. R., Stevens A. N., Iles R. A., Gordon R. E., Shaw D. (1981) Biosci. Rep. 1, 319–325 [DOI] [PubMed] [Google Scholar]
- 22.Griffiths J. R., McIntyre D. J., Howe F. A., Stubbs M. (2001) Novartis Found. Symp. 240, 46–62 [DOI] [PubMed] [Google Scholar]
- 23.Swietach P., Wigfield S., Cobden P., Supuran C. T., Harris A. L., Vaughan-Jones R. D. (2008) J. Biol. Chem. 283, 20473–20483 [DOI] [PubMed] [Google Scholar]
- 24.Holm E., Hagmüller E., Staedt U., Schlickeiser G., Günther H. J., Leweling H., Tokus M., Kollmar H. B. (1995) Cancer Res. 55, 1373–1378 [PubMed] [Google Scholar]
- 25.Geers C., Gros G. (2000) Physiol. Rev. 80, 681–715 [DOI] [PubMed] [Google Scholar]
- 26.Svastová E., Hulíková A., Rafajová M., Zat'ovicová M., Gibadulinová A., Casini A., Cecchi A., Scozzafava A., Supuran C. T., Pastorek J., Pastoreková S. (2004) FEBS Lett. 577, 439–445 [DOI] [PubMed] [Google Scholar]
- 27.Stock C., Mueller M., Kraehling H., Mally S., Noël J., Eder C., Schwab A. (2007) Cell Physiol. Biochem. 20, 679–686 [DOI] [PubMed] [Google Scholar]
- 28.Sutherland R. M. (1988) Science 240, 177–184 [DOI] [PubMed] [Google Scholar]
- 29.Grynkiewicz G., Poenie M., Tsien R. Y. (1985) J. Biol. Chem. 260, 3440–3450 [PubMed] [Google Scholar]
- 30.Pelicano H., Martin D. S., Xu R. H., Huang P. (2006) Oncogene 25, 4633–4646 [DOI] [PubMed] [Google Scholar]
- 31.Wallace K. B., Starkov A. A. (2000) Ann. Rev. Pharmacol. Toxicol. 40, 353–388 [DOI] [PubMed] [Google Scholar]
- 32.Marroquin L. D., Hynes J., Dykens J. A., Jamieson J. D., Will Y. (2007) Toxicol. Sci. 97, 539–547 [DOI] [PubMed] [Google Scholar]
- 33.Carlsson J., Acker H. (1988) Int. J. Cancer 42, 715–720 [DOI] [PubMed] [Google Scholar]
- 34.Kunz-Schughart L. A., Doetsch J., Mueller-Klieser W., Groebe K. (2000) Am. J. Physiol. Cell Physiol. 278, C765–C780 [DOI] [PubMed] [Google Scholar]
- 35.Semenza G. L. (2003) Nat. Rev. Cancer 3, 721–732 [DOI] [PubMed] [Google Scholar]
- 36.Halestrap A. P., Price N. T. (1999) Biochem. J. 343, 281–299 [PMC free article] [PubMed] [Google Scholar]
- 37.Villafuerte F. C., Swietach P., Vaughan-Jones R. D. (2007) FASEB J. 21, A1284 [Google Scholar]
- 38.Swietach P., Vaughan-Jones R. D. (2005) J. Physiol. 566, 793–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pastoreková S., Parkkila S., Parkkila A. K., Opavský R., Zelník V., Saarnio J., Pastorek J. (1997) Gastroenterology 112, 398–408 [DOI] [PubMed] [Google Scholar]
- 40.Sterling D., Reithmeier R. A., Casey J. R. (2001) J. Biol. Chem. 276, 47886–47894 [DOI] [PubMed] [Google Scholar]
- 41.Morgan P. E., Pastoreková S., Stuart-Tilley A. K., Alper S. L., Casey J. R. (2007) Am. J. Physiol. Cell Physiol. 293, C738–C748 [DOI] [PubMed] [Google Scholar]
- 42.Airley R. E., Loncaster J., Raleigh J. A., Harris A. L., Davidson S. E., Hunter R. D., West C. M., Stratford I. J. (2003) Int. J. Cancer 104, 85–91 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.