Abstract
Although there is a consensus that mitochondrial function is somehow linked to the aging process, the exact role played by mitochondria in this process remains unresolved. The discovery that reduced activity of the mitochondrial enzyme CLK-1/MCLK1 (also known as COQ7) extends lifespan in both Caenorhabditis elegans and mice has provided a genetic model to test mitochondrial theories of aging. We have recently shown that the mitochondria of young, long-lived, Mclk1+/− mice are dysfunctional, exhibiting reduced energy metabolism and a substantial increase in oxidative stress. Here we demonstrate that this altered mitochondrial condition in young animals paradoxically results in an almost complete protection from the age-de pend ent loss of mitochondrial function as well as in a significant attenuation of the rate of development of oxidative biomarkers of aging. Moreover, we show that reduction in MCLK1 levels can also gradually prevent the deterioration of mitochondrial function and associated increase of global oxidative stress that is normally observed in Sod2+/− mutants. We hypothesize that the mitochondrial dysfunction observed in young Mclk1+/− mutants induces a physiological state that ultimately allows for their slow rate of aging. Thus, our study provides for a unique vertebrate model in which an initial alteration in a specific mitochondrial function is linked to long term beneficial effects on biomarkers of aging and, furthermore, provides for new evidence which indicates that mitochondrial oxidative stress is not causal to aging.
Because it is well known that the aging process is characterized by declines in basal metabolic rate and in the general performance of energy-dependent processes, many aging studies have focused on mitochondria because of their central role in producing chemical energy (ATP) by oxidative phosphorylation (1). Among the various theories of aging that have been proposed, the mitochondrial oxidative stress theory of aging is the most widely acknowledged and studied (2–4). It is based on the observation that mitochondrial energy metabolism produces reactive oxygen species (ROS),2 that mitochondrial components are damaged by ROS, that mitochondrial function is progressively lost during aging, and that the progressive accumulation of global oxidative damage is strongly correlated with the aged phenotype. However, the crucial question of whether these facts mean that mitochondrial dysfunction and the related ROS production cause aging remains unproven (5–7). Furthermore, recent observations made in various species, including mammals, have begun to directly challenge this hypothesis, notably by relating oxidative stress to long (8) or increased (9) lifespans, by demonstrating that overexpression of the main antioxidant enzymes does not extend lifespan (10) as well as by showing that mitochondrial dysfunction could protect against age-related diseases (11).
A direct and powerful approach to attempt to clarify this major question and to test the theory is to characterize the mitochondrial function of long-lived mutants (12). CLK-1/MCLK1 is an evolutionary conserved protein (13) and has been found to be located in the mitochondria of yeast (14), worms (15), and mice (16). The inactivation of the Caenorhabditis elegans gene clk-1 substantially increases lifespan (17). Moreover, the elimination of one functional allele of its murine orthologue also resulted in an extended longevity for Mclk1+/− mice in three distinct genetic backgrounds (18). These findings have provided for an evolutionarily conserved pathways of animal aging that is affected by the function of a mitochondrial protein (19, 20). In mitochondria CLK1/MCLK1 acts as an hydroxylase and is implicated in the biosynthesis of ubiquinone (coenzyme Q or UQ), a lipid-like molecule primarily known as an electron carrier in the mitochondrial respiratory chain and as a membrane antioxidant but which is also associated with an increasing number of different aspects of cellular metabolism (20, 21). Taken together, these observations indicate that the long-lived Mclk1+/− mouse is a model of choice for the understanding of the links between mitochondrial energy metabolism, oxidative stress, and the aging process in mammals.
Previous analysis of Mclk1+/− mice, which show the expected reduction of MCLK1 protein levels (22), have revealed that their tissues as well as their mitochondria contain normal levels of UQ at 3 months of age (23). Yet the same study also revealed a host of phenotypes induced by Mclk1 heterozygosity (see below). Thus, it appears that MCLK1 has an additional function that is unrelated to UQ biosynthesis but responsible for the phenotypes observed in young Mclk1+/− mutants. This is consistent with several results from nematodes which also strongly suggest that CLK-1 has other functions (24, 25).
In depth characterization of the phenotype of young Mclk1+/− mutants has revealed that the reduction of MCLK1 levels in these animals profoundly alters their mitochondrial function despite the fact that UQ production is unaffected (23). In fact, we have shown that Mclk1 heterozygosity induces a severe impairment of mitochondrial energy metabolism as revealed by a reduction in the rates of mitochondrial electron transport and oxygen consumption as well as in ATP synthesis and ATP levels in both the mitochondria and the whole cell. ATP levels in several organs were surprisingly strongly affected with, for example, a 50% reduction of overall cellular ATP levels in the livers of Mclk1+/− mutants (23). Moreover, we have found that the Mclk1+/− mice sustain high mitochondrial oxidative stress by a variety of measurements, including aconitase activity, protein carbonylation, and ROS production (23). Additionally, we have shown that this early mitochondrial dysfunction is associated with a reduction in some aspects of cytosolic oxidative damage and global oxidative stress that can be measured via recognized plasma biomarkers such as 8-isoprostanes and 8-hydroxy-2-deoxyguanosine (8-OHdG). Considering that the accumulation of global oxidative damage is known to be tightly linked to the aging process (26), this latter result suggests that the anti-aging effect triggered by low MCLK1 levels might already act at a young age.
To further investigate the clk-1/Mclk1-dependent mechanism of aging as well as to try to elucidate the still unclear relation between mitochondrial dysfunction, oxidative stress, and aging, we have now carefully analyzed the evolution of the phenotype of Mclk1+/− mutants over time. We have also studied the effects of reduced MCLK1 levels on the phenotype of mice heterozygous for the mitochondrial superoxide dismutase (Sod2), which represent a well known model of mitochondrial oxidative stress (27). In addition of confirming the long lifespan phenotype of the Mclk1+/− mutants in a mixed background (129S6 x BALB/c), we also report here a study of mutants and controls on a completely isogenic background where we find that the condition of Mclk1+/− mutants unexpectedly results in protection against the age-dependent loss of mitochondrial function. Moreover, we found that the mutants are characterized by a significant attenuation of the age-associated increase in global oxidative stress normally observed in mammals. We also show that the Mclk1+/− condition can gradually reverse the deterioration of mitochondrial function and the associated increase of global oxidative stress that is normally observed in Sod2+/− mutants. Thus, this study provides for a unique vertebrate model in which reduced levels of a specific mitochondrial protein causes early mitochondrial dysfunction but has long term beneficial effects that slow down the rate of aging, as established with appropriate biomarkers, and can ultimately prolong lifespan in mice. Furthermore, in line with recent studies that have raised doubts about the validity of the mitochondrial oxidative stress theory of aging (4, 8, 10), our results, which relate to a recognized long-lived mice model, represent a novel and crucial indication that mitochondrial oxidative stress might not by itself be causal to aging.
EXPERIMENTAL PROCEDURES
Animals
All of the mice were housed in a pathogen-free animal facility at McGill University and were given a standard rodent diet and water ad libitum. At the time of analysis animals were anesthetized, sacrificed by cervical dislocation, and perfused with phosphate buffer. Tissues were then rapidly removed, rinsed, and placed in ice-cold mitochondrial isolation buffer or immediately frozen in liquid nitrogen. All procedures were approved by McGill's Animal Care and Ethics committees. The mice were separated from their mother at 21 days of age and housed 3–5 per cage, with both genotypes present in each cage. Lifespan was determined by recording the age of spontaneous death or when one of the following criteria was met: unresponsiveness to touch, slow respiration, coldness to touch, a hunched up position with matted fur, sudden weight loss, or the presence of a tumor large enough to inhibit the animal's normal behavior.
All of the mice used in the experiments comparing animals of different ages (3, 12, and 23 months) were F1 hybrid progeny generated by crossing mice of two different pure inbred strains. Indeed, these animals were produced by mating Mclk1+/− males from the original knock-out background (129S6) to females on a pure BALB/c background. These animals were, therefore, all genetically identical (isogenic) except at the Mclk1 locus. In contrast, all mice used in the survival curves analysis were from a mixed background derived at an earlier time (18) from the same two backgrounds that were used to produce the isogenic F1 mice. Note that the original knock-out background was previous described as 129/SvJ (22). However recent changes in nomenclature mean that it should now more correctly be called 129S6/SvEvTac, which can be abbreviated to 129S6. The F1 animals of the mutant and wild-type groups were siblings and were co-housed immediately after weaning.
Because homozygous Sod2−/− mice have the longest survival in the DBA/2J/B6 F1 background (29), we generated the Sod2+/− Mclk1+/− double mutant mice used in the present study by mating Sod2+/− Mclk1+/− mice in the C57BL/6J background to DBA/2J wild-type animals to create a mixed background.
Identification of Quinones
The extraction of quinones as well as their quantification by high performance liquid chromatography (HPLC) were performed as described previously (18). The total amount of quinone was normalized to the amount of protein.
Isolation of Mitochondria and Determination of Mitochondrial Oxygen Consumption
On the day of the experiment fresh livers were homogenized in 10 volumes (w/v) of an homogenization buffer consisting of 0.25 m sucrose, 10 mm Hepes buffer, pH 7.4, 1 mm EDTA. Liver mitochondria were then isolated by standard differential centrifugation according to detailed procedures described elsewhere (30). The purity of the mitochondrial as well as cytosolic preparations obtained was then checked with antisera against porin and α-tubulin as reported previously (23). Oxygen consumption of isolated mitochondria was measured polarographically with a Clark-type oxygen electrode connected to a suitable recorder (Yellow Springs Instrument Co.) by following published procedures (23). Mitochondrial oxygen consumption in the presence of substrates and ADP (0.8 mm) is reported as state 3, whereas the state corresponding to the period after all added ADP has been converted into ATP or in the presence of 1.25 mg/ml oligomycin is defined as state 4 respiration (Table 1). The respiratory control ratio (state 3/state 4) and the adenosine diphosphate-to-oxygen ratio (ADP/O), which are indicators of the intactness of the inner mitochondrial membrane and of the level of coupling of mitochondrial respiration, have been measured for all the animals used in the aging study (Table 1). Also, the quality of mitochondria preparation was confirmed by at least a 3-fold increase in respiration rate in the presence of the uncoupler.
TABLE 1.
Mitochondrial oxygen consumption parameters
RCR, respiratory control ratio; ADP/O, adenosine diphosphate-to-oxygen ratio.
| 3 months |
12 months |
23 months |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Males |
Females |
Males |
Females |
Males |
Females |
|||||||
| Mclk1+/+ | Mclk1+/− | Mclk1+/+ | Mclk1+/− | Mclk1+/+ | Mclk1+/− | Mclk1+/+ | Mclk1+/− | Mclk1+/+ | Mclk1+/− | Mclk1+/+ | Mclk1+/− | |
| O2 consumption state 4 (glutamate) (nmol/mg/min) | 31.8 ± 3.1a | 27.8 ± 1.7 | 28.9 ± 2.1 | 27.5 ± 0.5 | 34 ± 2.7 | 32 ± 1.4 | 31 ± 2.4 | 34 ± 1.9 | 29.2 ± 1.5 | 31.1 ± 1.8 | 28.4 ± 0.6 | 27.8 ± 2.9 |
| O2 consumption state 4 (succinate) (nmol/mg/min) | 102 ± 7.8 | 101.1 ± 6 | 93.5 ± 3.4 | 97.9 ± 3.4 | 104 ± 3.7 | 99.6 ± 4.9 | 99.8 ± 3.8 | 95.6 ± 4 | 88.5 ± 3.3 | 90.8 ± 4.7 | 96.7 ± 4.3 | 103.3 ± 5 |
| RCR (glutamate) | 7.3 ± 0.7 | 6.9 ± 0.5 | 7.5 ± 0.4 | 6.1 ± 0.4b | 6 ± 0.4 | 5.6 ± 0.3b | 6.3 ± 0.6 | 5.5 ± 0.4 | 6.1 ± 0.5 | 6.4 ± 0.5 | 5.1 ± 0.3 | 5.9 ± 0.6 |
| RCR (succinate) | 3.8 ± 0.2 | 3.5 ± 0.1 | 4.1 ± 0.2 | 3.2 ± 0.2b | 3.1 ± 0.2 | 2.7 ± 0.2 | 3.8 ± 0.2 | 3.4 ± 0.2 | 3.1 ± 0.2 | 3.6 ± 0.2 | 3.2 ± 0.2 | 3.3 ± 0.2 |
| ADP/O (glutamate) | 3 ± 0.1 | 3 ± 0.1 | 2.6 ± 0.2 | 2.8 ± 0.2 | 3 ± 0.08 | 2.9 ± 0.06 | 2.8 ± 0.07 | 2.9 ± 0.2 | 3.1 ± 0.06 | 3.1 ± 0.05 | 2.7 ± 0.1 | 2.9 ± 0.1 |
| ADP/O (succinate) | 2 ± 0.1 | 2.1 ± 0.1 | 2 ± 0.2 | 2 ± 0.1 | 1.9 ± 0.08 | 2 ± 0.07 | 2 ± 0.1 | 1.9 ± 0.07 | 1.8 ± 0.05 | 1.7 ± 0.06 | 1.7 ± 0.1 | 1.8 ± 0.07 |
a Data are the means ± S.E. of 8–12 samples.
b Significantly different from the controls (Mclk1+/+) at p < 0.05.
Determination of Oxidative Damage
Lipid peroxidation was determined in cytosolic and mitochondrial fractions of liver by the indirect measurement of free malondialdehyde (MDA) using a standard published method with some modifications (31). Briefly, one volume of cytosolic or mitochondrial fraction (0.1 ml of sample and 0.4 ml of 50 mm Tris-HCl, pH 7.3) was mixed with a 0.5 volume of trichloroacetic acid (150 mg/ml) and centrifuged at 2000 rpm for 10 min. The resulting supernatant was mixed with 0.5 ml of thiobarbituric acid (0.7 mg/ml) and boiled for 15 min. After cooling, absorbance was measured at 535 nm on a Beckman DU 640 spectrophotometer. MDA concentration was calculated using an extinction coefficient of 1.56 × 105 mol/liter·cm. Results were expressed in nmol of MDA/mg of protein (determined using Bio-Rad Bradford kit). The level of protein carbonyl contents in liver tissues was determined with the Protein Carbonyl Assay kit (Cayman Chemical) according to the manufacturer's instructions. Plasma free and esterified 8-isoprostanes were quantified with an 8-isoprostanes enzyme immunoassay kit (Cayman Chemical) according to the provided protocol. The plasma levels of 8-OHdG, a biomarker for oxidative damage to DNA, were determined using an enzyme-linked immunosorbent assay kit according to the manufacturer's protocol (Stressgen).
Enzymatic Antioxidant Assays
Aconitase activity in liver mitochondrial and cytosolic extracts was determined as described elsewhere (32). SOD2 activity was assessed in presence of 1 mm KCN using a commercially available kit according to the manufacturer's instructions (Cayman Chemical). All activities were normalized to the quantity of proteins used in the assays.
Statistical Analysis
Comparisons between Mclk1+/+ and Mclk1+/− as well as between Sod2+/− and Sod2+/− Mclk1+/− animals were performed using an unpaired two-tailed Student's t test, and differences between the two genotypes were considered to be significant when p was <0.05. Statistical comparisons between control Sod2+/+ Mclk1+/+ mice and each of the three other genotypes individually have been performed by using a one-way analysis of variance followed by the Dunnett's post-hoc test, and the differences were considered to be significant at p < 0.05. For evaluating survival data, the log-rank (Mandel-Cox) test was performed using GraphPad Prism Version 5.00 for Windows, GraphPad Software, San Diego. The Gompertz equation, Rm = R0eat, where Rm is the mortality rate as a function of time or age (t), R0 is the nonexponential factor in mortality, and a is the exponential (Gompertz) mortality rate coefficient, used to model the aging process. The linear regression was obtained from ln(Rm) = ln(R0) + αt and the mortality rate doubling time, a measure of the rate of aging, was estimated from the slope as described (33).
RESULTS
Increased Survival of Mclk1+/− Mutants
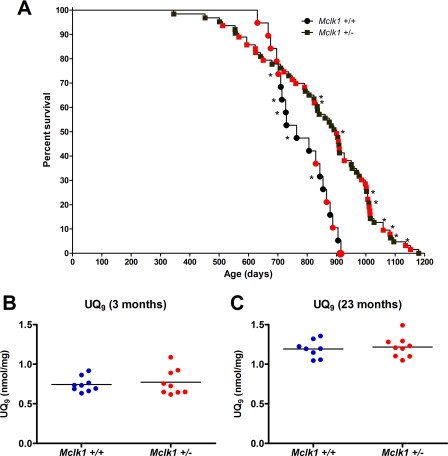
In a previous study (18) in which the effect of Mclk1 heterozygosity on three genetic backgrounds was examined, we found that Mclk1+/− mutants from a mixed background (129S6 x BALB/c) showed the greatest increase in lifespan (31% longer on average). However, because the sample sizes examined were relatively small and contained both sexes in unequal ratios, we have carried out a new study with larger cohorts to verify that Mclk1 heterozygosity could indeed increase the average and maximum lifespan, as the earlier results suggested. We scored the lifespan of 14 Mclk1+/+ mice, of which 8 were males, and of 54 Mclk1+/− mutants, of which 24 were males. The two survival curves (not shown) were different by the log-rank (Mantel-Cox) test (p = 0.0231). There was no significant difference of survival between the sexes within each genotype. As there was also no significant difference for each genotype between the previous data and the new data, we pooled the data from the two experiments for the best possible visualization of the difference of survival between the genotypes in this background. In Fig. 1A, males are identified with red symbols, and the symbols corresponding to animals from the previous dataset are marked with an asterisk. The median survival for the pooled samples was 764 days with a maximum of 915 days for Mclk1+/+ animals and 900 days with a maximum of 1180 for the Mclk1+/− mutants. The survival curves were different by the log-rank (Mantel-Cox) test (p = 0.0008). Visually, the two curves are strikingly different, with the wild-type animals all dying within a relatively short period, but the mutants showing a much greater diversity of survival, including animals that died substantially before any wild-type animal and animals that lived considerably longer than any wild-type animal. Furthermore, we used the data from the survival curve in the Gompertz model, which is commonly used to evaluate the intrinsic rate of mortality in different populations (34) (see “Experimental Procedures”). From the Gompertz analysis we obtained the mortality rate doubling time, a recognized measure of the rate of aging (33), and we found that it was significantly different between the cohorts (0.23 year for the Mclk1+/+ animals and 0.41 year for the Mclk1+/− mutants). This suggests that the Mclk1+/− mutants have indeed a slower rate of aging.
FIGURE 1.
Increased lifespan of Mclk1+/− mice is confirmed in a mixed (129S6 x BALB/c) background and is independent of MCLK1 function in ubiquinone biosynthesis. A, Kaplan-Meier survival curves of Mclk1+/+ (n = 19) and Mclk1+/− (n = 63) mice are shown. Males are identified with red symbols, and the symbols corresponding to animals from the previous dataset are marked with an asterisk (18). Ubiquinone levels are normal at all ages in Mclk1+/− mice. Quantification of UQ9 levels in whole liver homogenates from 3-month-old (B) and 23-month-old (C) Mclk1+/+ and Mclk1+/− isogenic F1 males from a 129S6 x BALB/c cross was performed by HPLC analysis. UQ10 is barely detectable in liver (not shown). Each dot in the graphs represents an individual mouse.
UQ Biosynthesis Is Unaffected in Young and Old Mclk1+/− Mutants
We have shown previously that various tissues from 3-month-old Mclk1+/− mice in several genetic backgrounds exhibit the expected decrease in Mclk1 mRNA and MCLK1 protein levels but that whole tissue homogenates as well as mitochondrial extracts from the same organs display no reduction in either UQ9 or UQ10 levels (22, 23). The very same mice, however, displayed numerous mitochondrial phenotypes (23). This has revealed that MCLK1 has an additional activity unrelated to UQ biosynthesis but necessary for normal mitochondrial function. Here, we have used HPLC to determine the levels of quinones in liver mitochondria of young isogenic animals from an additional genetic background, 129S6 x BALB/c, in both 3- and 23-month-old F1 animals to determine whether quinone content changes with age. As in the previously investigated backgrounds, the UQ9 levels were unaffected by Mclk1 heterozygosity in 3-month-old mice (Fig. 1B). In addition, we also found no differences in UQ9 levels between Mclk1+/− and Mclk1+/+ mice at 23 months of age (Fig. 1C).
Reduction in Age-dependent Loss of Mitochondrial Function
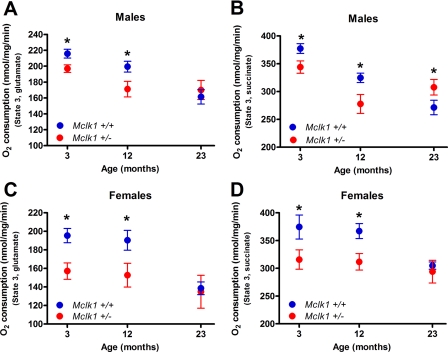
We have analyzed mitochondrial function in young (3 months), middle-aged (12 months), and old (23 months) F1 males and females from the 129S6 x BALB/c cross. Mitochondria isolated from both genotypes are well coupled as revealed by standard respiratory control ratio and ADP/O ratio values, and no significant differences between the genotypes was observed for state 4 respiration (Table 1). In wild-type Mclk1+/+ controls from both sexes, oxygen consumption of isolated mitochondria measured in the presence of ADP was found to decrease with increasing age (Fig. 2), as has been found in other studies (1, 35). We have recently reported that the rate of oxygen consumption from intact isolated liver mitochondria was reduced with both complex I substrates (glutamate in combination with malate) and a complex II substrate (succinate) in 3-month-old Mclk1+/− males from the BALB/c and the C57BL/6J backgrounds and that these defects in oxidative phosphorylation are accompanied by reduced ATP production (23). We show here that this phenotype of low oxygen consumption with both types of substrates is also observe in young males and females originating from the 129S6 x BALB/c cross (Fig. 2). Furthermore, we found that this difference between both genotypes persist in 12-month animals as the mitochondria isolated from Mclk1+/− mutants also consumed less oxygen than the controls with all studied substrates. However, after 23 months, the age-dependent decrease in oxygen consumption in Mclk1+/− mutants is much less pronounced than in Mclk1+/+ control siblings. Thus, at 23 months oxygen consumption is now similar in both genotypes in the case of females (Fig. 2, C and D) and even significantly higher than controls in Mclk1+/− mutant males when tested with succinate (Fig. 2B).
FIGURE 2.
Protection against age-dependent decrease in mitochondrial function in Mclk1+/− animals. Oxygen consumption (state 3) levels of isolated liver mitochondria from young (3 months), middle-aged (12 months), and old (23 months) male (A and B) and female (C and D) isogenic F1 mice of both genotypes from a 129S6 x BALB/c cross. Oxygen consumption levels were measured with glutamate/malate (A and C) and succinate (B and D) as respiratory substrates. Mitochondrial function in young Mclk1+/− mice is altered in both sexes and with both substrates. The Mclk1+/+ mitochondria show the known age-dependent decrease in oxygen consumption, whereas the Mclk1+/− mitochondria are only very mildly affected by the aging process. Each point in the graphs represents the mean ± S.E. of 10–12 animals. The asterisk denotes statistical significance of the difference between Mclk1+/+ and Mclk1+/− animals for a specific age; p < 0.05.
Age-related Decrease in Mitochondrial Oxidative Damage
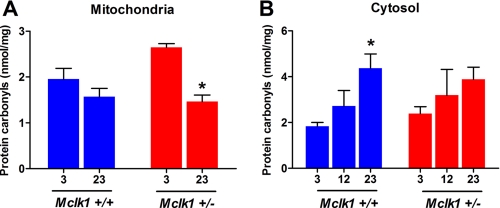
By studying recognized oxidative stress biomarkers, we have previously reported a substantial increase in the level of intramitochondrial oxidative damage in mitochondria from young Mclk1+/− animals (23). Among the variety of biomarkers that were tested, the levels of protein carbonyls was one of the most significant increases in mitochondrial extracts from Mclk1+/− mutants. Thus, we have now quantified the levels of protein carbonyls, known to be a reasonable index of the oxidative status (36), in mitochondrial extracts at different ages. In mitochondria, oxidative damage, including protein carbonylation, is known to result principally from ROS produced at the electron transport chain level (37). Therefore, the level of mitochondrial protein carbonyls could decrease with age as electron transport slows down, as was previously shown in rodents (38). However, this is likely compensated at least in part by age-dependent accumulation of damage to the mitochondria resulting in inefficient function and increased ROS production (39). In fact, we observe a mild, non-significant decrease in carbonyl content between the 3-month and the 23-month cohorts of Mclk1+/+ controls (Fig. 3A) (the 12-month cohort was not examined). However, the decrease of mitochondrial carbonyl production is much more pronounced in Mclk1+/− mutants, as at 23 months it is now similar to that of controls (Fig. 3A). Consequently, at 23 months Mclk1+/− mutants and controls have similar electron transport and similar carbonyl levels, suggesting a similar but not a lower level of mitochondrial oxidative stress.
FIGURE 3.
Age-related changes in the levels of mitochondrial and cytosolic oxidative damage. Levels of protein carbonyls were quantified in mitochondrial (A) and cytosolic (B) extracts from young (3 months), middle-aged (12 months, cytosolic only), and old (23 months) Mclk1+/+ and Mclk1+/− isogenic F1 males from a 129S6 X BALB/c cross. Data are the means ± S.E. of 8–10 animals. The asterisk denotes statistical significance of the differences between 3- and 23-month-old Mclk1+/+ as well as between 3- and 23-month-old Mclk1+/− animals; p < 0.05.
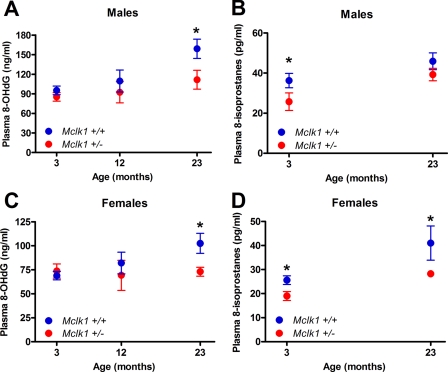
Attenuation of the Age-associated Increases in Cytosolic and Global Oxidative Stress
We also tested cytoplasmic oxidative stress by measuring the level of cytoplasmic protein carbonyls for both genotypes at 3, 12, and 23 months. We observed that the levels of cytoplasmic carbonyls gradually increase with age in the Mclk1+/+ cohort, whereas the change in Mclk1+/− animals was much reduced (Fig. 3B). Furthermore, we have measured plasma levels of 8-isoprostanes, which result from oxidative damage to membrane lipids, which are secreted into the plasma from multiple tissues and which represent a measure of global oxidative stress (40). The 8-isoprostanes levels were reduced in Mclk1 heterozygotes compared with Mclk1+/+ in young (3 months) and old (23 months) males and females mice (Fig. 4, B and D) (the 12-month cohort was not examined). We have also measured oxidative damage to DNA, which can be measured by measuring the plasma levels of 8-OHdG, a modified nucleoside base that is present in the plasma and excreted in the urine (41), another circulating marker of age-dependent oxidative damage to tissues (42, 43). We found no significant differences between genotypes in 8-OHdG levels in 3- and 12-month-old animals, but a significant difference in 23-month-old animals was observed with both males and females (Fig. 4, A and C). Indeed, our analysis revealed that in contrast to the expected gradual increase in plasma levels of 8-OHdG that we observed in old Mclk1+/+ controls, 8-OHdG levels increased minimally with age in the plasma of Mclk1+/− animals (Fig. 4, A and C). Given the tight association of global oxidative stress with physiological age, our results are consistent with the finding that the Mclk1+/− mutants are long-lived and with the notion that their increased lifespan is the result of a lower rate of aging.
FIGURE 4.
Reduction in age-dependent systemic oxidative stress. Biomarker of oxidative damage to DNA, 8-OHdG, and to membrane lipids, 8-isoprostanes, were quantified in plasma samples of young (3 months), middle-aged (12 months, 8-OHdG only), and old (23 months) male (A and B) and female (C and D) isogenic F1 mice of both genotypes from a 129S6 x BALB/c cross. Each point in the graphs represents the mean ± S.E. of 10–12 animals. The asterisk denotes statistical significance of the difference between Mclk1+/+and Mclk1+/− animals; p < 0.05.
Suppression of the Loss of Mitochondrial Oxygen Consumption and the Increased Mitochondrial Oxidative Stress of Sod2+/− Mutants
To further investigate the link between the conditions in young and old mutants and to better understand the long term protective effect against oxidative damage induced by low MCLK1 levels, we took advantage of a genetic model of increased mitochondrial oxidative stress. Sod2 encodes the mitochondrial superoxide dismutase. Loss of both copies of the gene is lethal, but loss of one copy leads to increased mitochondrial oxidative stress, a gradual increase in oxidative damage, and a concomitant loss in mitochondrial oxygen consumption (27, 44). We have created a double-heterozygous strain of Mclk1+/− Sod2+/− mutants in a mixed background (see “Experimental Procedures”) and, using liver homogenate, have analyzed mitochondrial and cytoplasmic oxidative stress as well as mitochondrial function of all four viable genetic classes of female siblings that result from crosses of double-heterozygous animals.
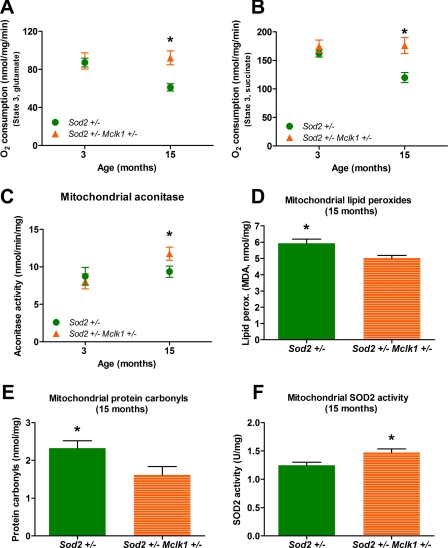
At 3 months there is already a reduction in the level of oxygen consumption of liver mitochondria with both complex I and complex II substrates in Sod2+/−, Mclk1+/−, and Mclk1+/− Sod2+/− mutants (Table 2). At 15 months the reduction remains mild in Mclk1+/− mutants but is now more severe in Sod2+/− mutants. However, the reduction in mitochondrial respiration with complex I and complex II substrates induced by the decreased superoxide detoxification is completely suppressed in the double mutants (Fig. 5, A and B). These results, which were also confirmed in kidney mitochondria (data not shown), reveal that when the rate of loss of mitochondrial activity is accelerated as in Sod2+/− mutants, Mclk1 heterozygosity can already have an important protective impact in the first 15 months of life.
TABLE 2.
Mitochondrial electron transport chain and oxidative stress-related measurements
ND, note determined.
| Sod2+/+Mclk1+/+ | Mclk1+/− | Sod2+/− | Sod2+/−Mclk1+/− | |||||
|---|---|---|---|---|---|---|---|---|
| Age (months) | 3 | 15 | 3 | 15 | 3 | 15 | 3 | 15 |
| Oxygen consumption (nmol/mg/min) | ||||||||
| Glutamate | 100 ± 5a | 89 ± 9 | 83 ± 7 | 72 ± 5 | 87 ± 5 | 61 ± 4b | 88 ± 8 | 92 ± 7 |
| Succinate | 177 ± 7 | 173 ± 11 | 159 ± 9 | 146 ± 8 | 162 ± 6 | 119 ± 9b | 175 ± 11 | 176 ± 14 |
| Mitochondrial ROS damage | ||||||||
| Aconitase (nmol/mg/ml) | 11.7 ± 1 | 16.5 ± 1.1 | 9.4 ± 0.9 | 11.6 ± 1.1b | 8.7 ± 1.2c | 9.3 ± 0.8b | 8.1 ± 1c | 11 ± 0.8b |
| Carbonyl (nmol/mg) | ND | 1.54 ± 0.25 | ND | 1.83 ± 0.27 | ND | 2.34 ± 0.2b | ND | 1.61 ± 0.22 |
| MDA (nmol/mg) | ND | 5.2 ± 0.24 | ND | 5.2 ± 0.13 | ND | 6 ± 0.26b | ND | 5 ± 0.16 |
| SOD2 activity (units/mg) | ND | 1.67 ± 0.14 | ND | 1.56 ± 0.06 | ND | 1.25 ± 0.05b | ND | 1.47 ± 0.06 |
| Cytosolic ROS damage | ||||||||
| Carbonyl (nmol/mg) | ND | 1.65 ± 0.23 | ND | 1.53 ± 0.17 | ND | 2.03 ± 0.12b | ND | 1.46 ± 0.08 |
| Aconitase (nmol/mg/ml) | 19.2 ± 0.9 | 22.1 ± 1.8 | 18.7 ± 0.9 | 22 ± 2.6 | 19 ± 0.7 | 22 ± 1.6 | 18.4 ± 0.8 | 23 ± 1 |
| MDA (nmol/mg) | ND | 1.17 ± 0.05 | ND | 1.16 ± 0.05 | ND | 1.3 ± 0.08 | ND | 1.07 ± 0.03 |
| Global ROS damage | ||||||||
| 8-OHdG (ng/ml) | 76.2 ± 5.2 | 80.2 ± 6.5 | 78.5 ± 6.5 | 69.1 ± 6.2 | 83.2 ± 6.2 | 85.2 ± 6.5 | 72.4 ± 8.4 | 57.9 ± 7.5b |
aData are the means ± S.E. of 8–12 samples.
b Significantly different from the 15-month controls (Sod2+/+ Mclk1+/+) at p < 0.05, Dunnett's test.
c Significantly different from the 3-month controls (Sod2+/+ Mclk1+/+) at p < 0.05, Dunnett's test.
FIGURE 5.
Mclk1 heterozygosity suppresses the increased mitochondrial oxidative stress and the loss of mitochondrial oxygen consumption of Sod2+/− mutants. The partial loss of Mclk1 in Sod2+/− Mclk1+/− mutants suppresses the defective mitochondrial oxygen consumption (state 3) phenotype of Sod2+/− mice with both complex I (A) and complex II (B) substrates and positively affects the Sod2+/− phenotype by reducing the mitochondrial oxidative stress as shown by increased mitochondrial aconitase activity (C), decreased lipid peroxidation (D), and reduced levels of protein carbonyls (E). Mclk1 heterozygosity also increases the level of SOD2 enzymatic activity in Sod2+/− Mclk1+/− mutants (F). Each point or bar in the graphs represents the mean ± S.E. of 8–10 animals. The statistical significance of the differences between Sod2+/− and Sod2+/− Mclk1+/− animals (*) is shown on the graphs; p < 0.05.
In 3-month-old animals we observed the expected decrease in mitochondrial aconitase in Mclk1+/− and Sod2+/− mutants as well as in Mclk1+/− Sod2+/− double mutants, where the effect is most severe, likely because of additivity of the effect of the two mutations (Table 2). At 15 months the effect on mitochondrial aconitase of each mutation is more severe than at 3 months in single-mutant animals, particularly in Sod2+/− mutants (Table 2). However, in the double mutants the level of mitochondrial aconitase activity is significantly higher than in Sod2+/− mutants, indicating that Mclk1 heterozygosity has had a positive impact on mitochondrial oxidative stress by that time (Fig. 5C).
Aconitase activity is but one measure of mitochondrial oxidative stress, and we have also measured mitochondrial lipid peroxidation and carbonyls levels at 15 months to confirm the protective effect of Mclk1 heterozygosity. Both types of damage were not increased in Mclk1+/− mutants but were significantly increased in Sod2+/− mutants (Table 2). Furthermore, we observed that Mclk1 heterozygosity could again completely suppress the increased damage produced by the partial loss of Sod2 (Fig. 5, D and E). Additionally, we have confirmed that SOD2 activity is decreased in isolated liver mitochondria from Sod2+/− mutants (Table 2). This decrease of SOD2 activity, which is highly significant (p < 0.01), did not reach the expected 50%, but it is well documented that the level of SOD2 activity in various tissues of the Sod2+/− mice is highly variable when compared with controls and can be higher or lower than the expected 50% (45, 46). Surprisingly, we found that this decrease was suppressed in Mclk1+/− Sod2+/− double mutants (Table 2 and Fig. 5F). The observation is consistent with our previous finding that SOD2 activity is up-regulated in mitochondrial extracts from 3-month-old Mclk1+/− BALB/c mutants (23).
Reduction in the Levels of Non-mitochondrial Oxidative Damage Observed in Sod2+/− Mice
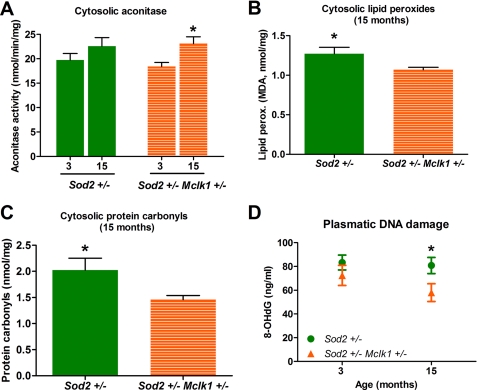
To better characterize the beneficial effects of Mclk1 heterozygosity on the Sod2+/− phenotype, we have measured the levels of aconitase activity in the cytosolic compartment. Although cytoplasmic aconitase activity increases slightly from 3 to 15 months in Sod2+/− animals, this effect is only statistically significant in double mutants (Fig. 6A). However, aconitase activity at 15 months is not different between the Sod2+/− animals and the double heterozygotes. We also quantified the levels of cytosolic lipid peroxidation and protein carbonylation, two markers of oxidative stress that are not affected in Mclk1+/− mutants but are increased in 15-month Sod2+/− mutants (Table 2). We found that at 15 months, cytoplasmic lipid peroxidation and protein carbonylation were significantly reduced in double mutants compared with Sod2+/− animals (Fig. 6, B and C). Overall, our analyses indicate that reduction of MCLK1 expression induces a decrease in non-mitochondrial oxidative stress in the double mutants at 15 months.
FIGURE 6.
2-Fold reduction of Mclk1 expression significantly reduces the levels of systemic oxidative damage observed in Sod2+/− mice. Mclk1 heterozygosity reduced the levels of systemic oxidative stress as revealed by an increased mitochondrial aconitase activity (A) as well as by a reduction in the amounts of cytosolic lipid peroxidation (B), cytosolic carbonyls (C), and plasmatic 8-OHdG (D) observed in 15-month-old Sod2+/− Mclk1+/− mutants. Each point or bar in the graphs represents the mean ± S.E. of 8–10 animals. The statistical significance of the differences between 3- and 15-month-old Sod2+/− Mclk1+/− animals (A) or between Sod2+/− and Sod2+/− Mclk1+/− animals (B–D) were shown on the respective graphs; p < 0.05.
Partial Loss of Sod2 Enhances the Protective Effects of Mclk1 Heterozygosity
The data in Table 2 indicate that oxygen consumption in the double mutants at 15 months is not only better than in Sod2+/− mutant but, rather, returns to the control level and are, thus, even better than in Mclk1+/− mutants at the same age. This suggests that Sod2 heterozygosity enhances the beneficial effect of Mclk1 heterozygosity. To gain further insight into this phenomenon, we have measured 8-OHdG levels, which are lowered by Mclk1 heterozygosity in aging males and females Mclk1+/− mice at 23 months (Fig. 4, A and C). Again, we find that although each single mutation has no, or only a very slight, effect at 15 months, 8-OHdG levels are significantly reduced in double mutants (Fig. 6D). Strikingly, the levels of DNA damage in Sod2+/− Mclk1+/− animals are even lower than in the controls at 15 months (Table 2).
DISCUSSION
The Effects of the Reduction of MCLK1 Levels on Biomarkers of Aging and Lifespan Are Independent of the Role of the Protein in UQ Biosynthesis
We have found previously that UQ levels were unchanged in whole tissue homogenates as well as in mitochondria of young Mclk1+/− mutants (18, 22, 23). As young Mclk1+/− mice display a variety of mitochondrial phenotypes, we concluded that MCLK1 carries out another, yet unidentified mitochondrial function in addition to its hydroxylase function in UQ biosynthesis. Here we have shown that no UQ phenotype could be evidenced in Mclk1+/− mutants compared with isogenic controls at 23 months of age; that is, at a time at which we observe a substantial reduction in the appearance of biomarkers of aging. Thus, it is unlikely that the loss of heterozygosity in liver cells and MCLK1 function in UQ biosynthesis are crucial to the longevity of the Mclk1+/− mutants.
Mclk1+/− Mutants Display a Slow Rate of Aging
To confirm previously published data (18) revealing an extended lifespan for Mclk1+/− mice, we have obtained survival data from larger cohorts (Fig. 1A) which are sufficiently extensive to be used in the Gompertz model and calculate the mortality rate doubling time, a recognized measure of the rate of aging that has been found to be significantly affected in several long-lived mice mutants (33). The calculated mortality rate doubling times were statistically different between the Mclk1+/+ and Mclk1+/− genotypes, indicating that the Mclk1+/− mutants survive longer on average than Mclk1+/+ siblings, and they do this at least in part thanks to a lower rate of increase of their age-specific mortality. Surprisingly, in addition to a slower increase in mortality, we observed an earlier onset of death in the mutants. This might be because of the greater sample size of the mutant cohort combined with the potential presence of genetic variation in this mixed background (see “Experimental Procedures”). However, this possibility is made less likely by the fact that the wild-type controls, which should not be any less genetically variable than the mutants, display a much more uniform age of death than the mutants. Thus, a variable age of death might be an intrinsic property of Mclk1+/− mutants, possibly because the mutant phenotype is the result of a reduction in MCLK1 levels and not of a complete elimination of the protein, which might entail that animal-to-animal variation in the severity of the reduction produces a range of phenotypes. Such variation would not be observed in the wild type as the wild-type level of MCLK1 is likely somewhat in excess of what is necessary to produce a fully wild-type function. However, this is hypothetical as we have no physiological or biochemical information on the animals in the survival cohort.
In addition to the slower rate of aging suggested by the survival curves, we have shown that Mclk1+/− mutants display a slower rate of development of classical biomarkers of aging, such as the levels of mitochondrial oxygen consumption (Fig. 2) and mitochondrial oxidative stress (Fig. 3) as well as non-mitochondrial oxidative stress, in particular as measured by plasma metabolites resulting from stress to membranes (8-isoprostanes) and DNA (8-OHdG) (Fig. 4). Indeed, we found that the levels of 8-isoprostanes, one of the most reliable markers to assess global oxidative stress (40, 47), are lower at all ages in mutants. Interestingly, the levels of plasmatic 8-isoprostanes are also reduced in other long-lived mice (48, 49). Similarly, 8-OHdG is known to be a sensitive indicator of global oxidative stress (43), to increase with physiological age, and to be lowered by caloric restriction (50). It is of note that measurement of 8-OHdG from plasma greatly provides more accurate results than from tissue samples where there is a risk of auto-oxidation of deoxyguanosine (dG) during the preparation of samples as well as during the work-up procedure (51). Given the likely importance of mitochondrial function in resistance to disease and of oxidative stress in the etiology of age-dependent diseases, it is likely, but unproven at this time, that the reduction in the rate of physiological aging of the mutants is at the heart of their increased longevity.
Are the Phenotypes Observed in Mclk1+/− Mutants at a Young Age Related to Their Slow Rate of Aging and Their Increased Survival?
The phenotypes of young and old Mclk1+/− mutants are substantially different. As pointed out in the previous paragraph, it is the phenotype of the old animals that is consistent with a slow rate of aging and an increased longevity. Thus, it is legitimate to wonder whether the phenotype observed in the younger animals is causal to that of the old animals. We have approached this question by studying the effect of Mclk1 heterozygosity on the first part of the lifespan of Sod2+/− mutants (up to 15 months), whose phenotype is an early increase in mitochondrial oxidative stress and a partial loss of mitochondrial respiration as well as a gradual increase in non-mitochondrial oxidative stress (27, 52). These previously described phenotypes were all observed in the Sod2+/− animals on the DBA/2J/B6 mixed background that we used. We have found that all these phenotypes can be suppressed by loss of one copy of Mclk1. This suggests that partial loss of Mclk1 can indeed confer a gradual protection against oxidative and age-related damage and have early beneficial effects that are very similar to those we observe in the old (23 months) animals. This protection could in part be the consequence of an up-regulation of SOD2 activity in the double heterozygotes, a phenotype which is also observed in the sibling Mclk1+/− mutants and which was also previously reported in a pure isogenic background (BALB/c) (23).
The animals in this double-mutant experiment were from a mixed background. Thus, it is possible that genetic differences between individuals could have affected their phenotype. However, the reduction of mitochondrial function and the increase in mitochondrial oxidative stress as well as the changes in non-mitochondrial oxidative stress that have previously been observed in pure backgrounds for both the Sod2+/− and Mclk1+/− mutants were also observed in this mixed background for these genotypes, indicating that genetic variation within these groups or within the wild-type group was insufficient to mask the effect from the mutant loci. Although this makes it unlikely that the same hypothetical genetic variation was sufficient to significantly affect the phenotype of the double mutant sibling group, additional experiments with Sod2+/− Mclk1+/− double mutants in a pure genetic background will be the only way to completely exclude this possibility.
The Increased Mitochondrial Oxidative Stress of Mclk1+/− Mutants Appears to Be Involved in the Mechanism That Attenuates the Development of Biomarkers of Aging
Several features of the phenotype of Sod2+/− Mclk1+/− double mutants at 15 months are closer to the wild-type phenotype than to the phenotype of Mclk1+/− mutants at that age, including mitochondrial oxygen consumption and mitochondrial carbonyl levels. Furthermore, the level of cytosolic lipid peroxidation and of plasma 8-OHdG in the double mutants is even lower than in the wild-type controls. These observations suggest that the mitochondrial oxidative stress produced by partial loss of Sod2 reinforces the effect of partial loss of Mclk1, which by itself induces increased oxidative stress as well. Although the effect of the double-mutant genotype on mouse lifespan remains to be established, a positive interaction similar to what we describe here is observed in long-lived C. elegans clk-1 mutants whose longevity is strongly enhanced by RNA interference against sod-2, the worm homologue of Sod2 (53, 54). However, the effects of partial loss of Mclk1 must be because of additional changes in mitochondrial function than just an increase in oxidative stress, as it produces the beneficial gradual reversal in phenotype that we have observed.
A Model for the Mechanism of Increased Longevity of Mclk1+/− Mutants
We have previously proposed that the primary defect in Mclk1+/− mutants is a different equilibrium between electron transport, ATP synthesis, NAD(H) synthesis, and ROS detoxification (23). A slow rate of electron transport leads to low ATP production and NAD(H) synthesis, which leads in turn to a deficit in those mechanisms of ROS detoxification that require regeneration, such as those that use glutathione. In fact, we have observed that Mclk1+/− mitochondria sustain high oxidative stress despite a significant up-regulation of the enzymatic activity of the mitochondrial SOD and the glutathione peroxidases. Thus, the deficit in detoxification leads to higher oxidative stress, which in turn, together with the low levels of NAD(H), impairs electron transport further. We have also proposed that the low levels of ATP and NAD(H) found in Mclk1+/− mutants might lead to a depression of cytoplasmic ROS-generating processes to explain the mild decrease in non-mitochondrial oxidative damage that we observe in young Mclk1+/− animals (23). It is important to remember that the majority of the oxidative metabolites that find their way into the plasma do not originate in the mitochondria but in the nucleus and in cellular membranes. The longevity of these animals might, therefore, spring from a lower rate of accumulation of irreversible non-mitochondrial damage. Our finding here fully supports this hypothesis, as we find a slowing down of overall damage accumulation with age without a reduction of mitochondrial oxidative stress below that of the wild type. In addition, we find that mitochondrial function also is lost more slowly in the mutants than in the wild type. We interpret this to mean that the overall sparing because of decreased cytoplasmic ROS production also spares mitochondrial function. This is not surprising as a majority of the protein components of the mitochondria are manufactured in the cytoplasm. An overall lower level of damaged proteins will benefit mitochondrial function as much as it will benefit other cellular processes. Our findings support the venerable rate-of-living theory of aging, which postulates that the rate of energy metabolism determines the rate of the aging process (55). Note, however, that this theory is often formulated to embody secondary statements about the relation between the maximum lifespan of species and the variation in their mass-specific rate of metabolism that are controversial and that are not addressed by our study (55, 56).
The Detrimental Effect of Mitochondrial Oxidative Stress Is Not the Link between Mitochondrial Function and Aging
The mitochondrial oxidative stress theory of aging postulates that ROS produced during mitochondrial respiration are the primary cause of aging by damaging mitochondria and leading to loss of mitochondrial function and reduced energy production, which would ultimately lead to widespread cellular dysfunction and death (2). However, recently studies conducted in different organisms including mammals have begun to seriously challenge the theory (9, 10, 53, 54). Although our study confirms the well known association between the accumulation of oxidative damage to cellular constituents and the aging process (28), virtually all our findings with Mclk1 appear difficult to reconcile with the major statement of the mitochondrial oxidative stress theory of aging. We have found that young Mclk1+/− mutant mitochondria sustain high oxidative stress, yet their altered function ultimately results in slow aging by a mechanism that remains to be fully clarified. Also, despite their high oxidative stress, the function of Mclk1+/− mitochondria declines less rapidly than that of the wild type, indicating that even age-dependent damage to mitochondria is not principally caused by mitochondrial oxidative stress. Moreover, the beneficial effects we observe are not the result of low mitochondrial oxidative stress in the aged animal. Indeed, at 23 months of age the mitochondrial oxidative stress of Mclk1+/− is the same as that of the controls, not lower. Finally, the partial loss of mitochondrial superoxide detoxification in Sod2+/− mutants enhances the protective effects of Mclk1 heterozygosity, suggesting that the oxidative stress observed in Mclk1+/− mutants is an integral part of the mechanism that allows for their slow rate of aging.
Acknowledgments
We thank XingXing Liu for help in setting up the aging cohorts and organ harvesting. We also thank Aurélie Masurel for help with animal colony maintenance and genotyping.
This work was funded in part by grants from the National Science and Engineering Research Council of Canada and the Canadian Institutes of Health Research (to S. H.).
- ROS
- reactive oxygen species
- UQ
- coenzyme Q
- 8-OHdG
- 8-hydroxy-2-deoxyguanosine
- HPLC
- high performance liquid chromatography
- MDA
- malondialdehyde.
REFERENCES
- 1.Navarro A., Boveris A. (2007) Am. J. Physiol. Cell Physiol 292, C670–686 [DOI] [PubMed] [Google Scholar]
- 2.Harman D. (1972) J. Am. Geriatr. Soc. 20, 145–147 [DOI] [PubMed] [Google Scholar]
- 3.Balaban R. S., Nemoto S., Finkel T. (2005) Cell 120, 483–495 [DOI] [PubMed] [Google Scholar]
- 4.Muller F. L., Lustgarten M. S., Jang Y., Richardson A., Van Remmen H. (2007) Free Radic. Biol. Med. 43, 477–503 [DOI] [PubMed] [Google Scholar]
- 5.Austad S. (2008) Aging Cell 7, 119–124 [DOI] [PubMed] [Google Scholar]
- 6.Lambert A. J., Brand M. D. (2007) Aging Cell 6, 417–420 [DOI] [PubMed] [Google Scholar]
- 7.Sohal R. S., Mockett R. J., Orr W. C. (2002) Free Radic. Biol. Med. 33, 575–586 [DOI] [PubMed] [Google Scholar]
- 8.Andziak B., O'Connor T. P., Qi W., DeWaal E. M., Pierce A., Chaudhuri A. R., Van Remmen H., Buffenstein R. (2006) Aging Cell 5, 463–471 [DOI] [PubMed] [Google Scholar]
- 9.Schulz T. J., Zarse K., Voigt A., Urban N., Birringer M., Ristow M. (2007) Cell Metab. 6, 280–293 [DOI] [PubMed] [Google Scholar]
- 10.Pérez V. I., Van Remmen H., Bokov A., Epstein C. J., Vijg J., Richardson A. (2009) Aging Cell 8, 73–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pospisilik J. A., Knauf C., Joza N., Benit P., Orthofer M., Cani P. D., Ebersberger I., Nakashima T., Sarao R., Neely G., Esterbauer H., Kozlov A., Kahn C. R., Kroemer G., Rustin P., Burcelin R., Penninger J. M. (2007) Cell 131, 476–491 [DOI] [PubMed] [Google Scholar]
- 12.Hekimi S. (2006) Nat. Genet. 38, 985–991 [DOI] [PubMed] [Google Scholar]
- 13.Ewbank J. J., Barnes T. M., Lakowski B., Lussier M., Bussey H., Hekimi S. (1997) Science 275, 980–983 [DOI] [PubMed] [Google Scholar]
- 14.Jonassen T., Larsen P. L., Clarke C. F. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 421–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felkai S., Ewbank J. J., Lemieux J., Labbé J. C., Brown G. G., Hekimi S. (1999) EMBO J. 18, 1783–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang N., Levavasseur F., McCright B., Shoubridge E. A., Hekimi S. (2001) J. Biol. Chem. 276, 29218–29225 [DOI] [PubMed] [Google Scholar]
- 17.Lakowski B., Hekimi S. (1996) Science 272, 1010–1013 [DOI] [PubMed] [Google Scholar]
- 18.Liu X., Jiang N., Hughes B., Bigras E., Shoubridge E., Hekimi S. (2005) Genes Dev. 19, 2424–2434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hekimi S., Guarente L. (2003) Science 299, 1351–1354 [DOI] [PubMed] [Google Scholar]
- 20.Stepanyan Z., Hughes B., Cliche D. O., Camp D., Hekimi S. (2006) Exp. Gerontol. 41, 940–951 [DOI] [PubMed] [Google Scholar]
- 21.Turunen M., Olsson J., Dallner G. (2004) Biochim. Biophys. Acta 1660, 171–199 [DOI] [PubMed] [Google Scholar]
- 22.Levavasseur F., Miyadera H., Sirois J., Tremblay M. L., Kita K., Shoubridge E., Hekimi S. (2001) J. Biol. Chem. 276, 46160–46164 [DOI] [PubMed] [Google Scholar]
- 23.Lapointe J., Hekimi S. (2008) J. Biol. Chem. 283, 26217–26227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Branicky R., Nguyen P. A., Hekimi S. (2006) Mol. Cell. Biol. 26, 3976–3985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hihi A. K., Kebir H., Hekimi S. (2003) J. Biol. Chem. 278, 41013–41018 [DOI] [PubMed] [Google Scholar]
- 26.Sohal R. S., Weindruch R. (1996) Science 273, 59–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams M. D., Van Remmen H., Conrad C. C., Huang T. T., Epstein C. J., Richardson A. (1998) J. Biol. Chem. 273, 28510–28515 [DOI] [PubMed] [Google Scholar]
- 28.Sohal R. S. (2002) Free Radic. Biol. Med. 33, 573–574 [DOI] [PubMed] [Google Scholar]
- 29.Huang T. T., Johnson M. S., Figueroa-Colon R., Dwyer J. H., Goran M. I. (2001) Obes. Res. 9, 283–289 [DOI] [PubMed] [Google Scholar]
- 30.Sohal R. S. (1993) Free Radic. Biol. Med. 14, 583–588 [DOI] [PubMed] [Google Scholar]
- 31.Gonzales S., Polizio A. H., Erario M. A., Tomaro M. L. (2005) World J. Gastroenterol. 11, 3533–3538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nulton-Persson A. C., Szweda L. I. (2001) J. Biol. Chem. 276, 23357–23361 [DOI] [PubMed] [Google Scholar]
- 33.de Magalhães J. P., Cabral J. A., Magalhães D. (2005) Genetics 169, 265–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riggs J. E., Millecchia R. J. (1992) Mech. Ageing Dev. 62, 191–199 [DOI] [PubMed] [Google Scholar]
- 35.Shigenaga M. K., Hagen T. M., Ames B. N. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 10771–10778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Argüelles S., García S., Maldonado M., Machado A., Ayala A. (2004) Biochim. Biophys. Acta 1674, 251–259 [DOI] [PubMed] [Google Scholar]
- 37.Cadenas E., Boveris A., Ragan C. I., Stoppani A. O. (1977) Arch. Biochem. Biophys. 180, 248–257 [DOI] [PubMed] [Google Scholar]
- 38.Davies S. M., Poljak A., Duncan M. W., Smythe G. A., Murphy M. P. (2001) Free Radic. Biol. Med. 31, 181–190 [DOI] [PubMed] [Google Scholar]
- 39.Sohal R. S., Sohal B. H. (1991) Mech. Ageing Dev. 57, 187–202 [DOI] [PubMed] [Google Scholar]
- 40.Morrow J. D. (2000) Drug Metab. Rev. 32, 377–385 [DOI] [PubMed] [Google Scholar]
- 41.Chiou C. C., Chang P. Y., Chan E. C., Wu T. L., Tsao K. C., Wu J. T. (2003) Clin. Chim. Acta 334, 87–94 [DOI] [PubMed] [Google Scholar]
- 42.Hamilton M. L., Van Remmen H., Drake J. A., Yang H., Guo Z. M., Kewitt K., Walter C. A., Richardson A. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 10469–10474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park K. S., Kim J. H., Kim M. S., Kim J. M., Kim S. K., Choi J. Y., Chung M. H., Han B., Kim S. Y., Lee H. K. (2001) Diabetes 50, 2837–2841 [DOI] [PubMed] [Google Scholar]
- 44.Gardner P. R., Raineri I., Epstein L. B., White C. W. (1995) J. Biol. Chem. 270, 13399–13405 [DOI] [PubMed] [Google Scholar]
- 45.Asimakis G. K., Lick S., Patterson C. (2002) Circulation 105, 981–986 [DOI] [PubMed] [Google Scholar]
- 46.Van Remmen H., Salvador C., Yang H., Huang T. T., Epstein C. J., Richardson A. (1999) Arch. Biochem. Biophys. 363, 91–97 [DOI] [PubMed] [Google Scholar]
- 47.Montuschi P., Barnes P. J., Roberts L. J., 2nd (2004) FASEB J. 18, 1791–1800 [DOI] [PubMed] [Google Scholar]
- 48.Cai W., He J. C., Zhu L., Chen X., Wallenstein S., Striker G. E., Vlassara H. (2007) Am. J. Pathol. 170, 1893–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choksi K. B., Roberts L. J., 2nd, DeFord J. H., Rabek J. P., Papaconstantinou J. (2007) Biochem. Biophys. Res. Commun. 364, 761–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sohal R. S., Agarwal S., Candas M., Forster M. J., Lal H. (1994) Mech. Ageing Dev. 76, 215–224 [DOI] [PubMed] [Google Scholar]
- 51.Shigenaga M. K., Aboujaoude E. N., Chen Q., Ames B. N. (1994) Methods Enzymol. 234, 16–33 [DOI] [PubMed] [Google Scholar]
- 52.Melov S., Coskun P., Patel M., Tuinstra R., Cottrell B., Jun A. S., Zastawny T. H., Dizdaroglu M., Goodman S. I., Huang T. T., Miziorko H., Epstein C. J., Wallace D. C. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 846–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang W., Li J., Hekimi S. (2007) Genetics 177, 2063–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van Raamsdonk J. M., Hekimi S. (2009) PLoS Genetics 5, e1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pearl R. (1922) The Biology of Death, J. B. Lippincott, Philadelphia [Google Scholar]
- 56.Speakman J. R. (2005) J. Exp. Biol. 208, 1717–1730 [DOI] [PubMed] [Google Scholar]