Abstract
β1-Adrenergic receptor (β1AR) stimulation confers cardioprotection via β-arrestin-de pend ent transactivation of epidermal growth factor receptors (EGFRs), however, the precise mechanism for this salutary process is unknown. We tested the hypothesis that the β1AR and EGFR form a complex that differentially directs intracellular signaling pathways. β1AR stimulation and EGF ligand can each induce equivalent EGFR phos pho ryl a tion, internalization, and downstream activation of ERK1/2, but only EGF ligand causes translocation of activated ERK to the nucleus, whereas β1AR-stimulated/EGFR-transactivated ERK is restricted to the cytoplasm. β1AR and EGFR are shown to interact as a receptor complex both in cell culture and endogenously in human heart, an interaction that is selective and undergoes dynamic regulation by ligand stimulation. Although catecholamine stimulation mediates the retention of β1AR-EGFR interaction throughout receptor internalization, direct EGF ligand stimulation initiates the internalization of EGFR alone. Continued interaction of β1AR with EGFR following activation is dependent upon C-terminal tail GRK phos pho ryl a tion sites of the β1AR and recruitment of β-arrestin. These data reveal a new signaling paradigm in which β-arrestin is required for the maintenance of a β1AR-EGFR interaction that can direct cytosolic targeting of ERK in response to catecholamine stimulation.
β1-Adrenergic receptor (β1AR)3 stimulation regulates a number of signaling pathways and has been recently shown to transactivate epidermal growth factor receptor (EGFR) and increase ERK activation in both in vitro cell culture systems and in vivo mouse models (1). The key signaling components required for EGFR transactivation following β1AR stimulation include: 1) C-terminal phosphorylation of activated β1ARs by GRK5/6, and 2) recruitment of both β-arrestins 1 and 2 to phosphorylated β1ARs. Recruitment of β-arrestins to activated β1ARs allow subsequent activation of c-Src and matrix metalloproteinases, cleavage of HB-EGF and activation of EGFR, processes that contribute to transactivation pathways defined for other 7TMRs (2–7). Trafficking of EGFR has been shown to be critical in defining downstream signaling pathways regulated by ligand-induced activation (8). Although direct EGFR stimulation via its ligand EGF leads to internalization of the receptor as well as ERK1/2 translocation to the nucleus and activation of Elk-1-mediated transcription (9, 10), the precise mechanism by which ERK signaling pathways are regulated following β1AR-mediated EGFR stimulation is poorly understood.
Recruitment of β-arrestin1/2 to activated 7TMRs allows these multifunctional scaffold proteins to target other signaling proteins that are involved in receptor internalization, desensitization, and intracellular signaling complexes. Members of the mitogen-activated protein kinase family such as ERK are among the proteins recruited to receptors by β-arrestins (11). Several recent studies have shown that β-arrestin1/2 recruitment to activated 7TMRs may direct both cytosolic ERK1/2 signaling, via formation of internalized β-arrestin signalosomes containing the receptor and activated ERK (12, 13), and nuclear targeting of ERK (14, 15), depending upon the 7TMR, cell-type, and culture conditions tested. Although β-arrestin recruitment is essential for β1AR-mediated transactivation of EGFR, it remains unclear if β-arrestins play a role in regulating and targeting the downstream ERK response.
In this study we sought to determine whether the mode of EGFR activation, via β1AR-mediated transactivation versus direct ligand activation, induces differential effects on ERK1/2 signaling and if so, to elucidate the mechanism by which this process occurs. Here, we show that β1AR and EGFR form a receptor complex at the plasma membrane that is dynamically regulated by ligand stimulation, leading to differential ERK1/2 targeting and intracellular effects. Moreover, the recruitment of β-arrestin to the agonist-occupied β1AR is required to maintain prolonged β1AR-EGFR interaction during simultaneous receptor internalization and to retain ERK1/2 activation in the cytosol. These findings illustrate the importance of β-arrestin in mediating receptor-receptor interaction and the targeting of downstream signaling pathways.
EXPERIMENTAL PROCEDURES
Plasmids
WT-β1AR and GRK−β1AR plasmids have been described (16). FLAG-EGFR, EGFR-GFP, ERK2-RFP, MyrPalm-mYFP, and βarr2-mYFP plasmids and HA-AT1AR cells were provided by Dr. Robert J. Lefkowitz, Duke University. βarr2-RRK/Q-GFP was a gift of Dr. Christopher D. Nelson, Duke University. Mouse β1AR and human EGFR were ligated into mCFP or mYFP vectors with XhoI and HindIII using T4 ligase (Promega). pFA2-ELK1 and pFA2-CREB vectors were purchased from Stratagene, and pGL4-firefly luciferase and pRL-TK Renilla luciferase from Promega.
Cell Culture
WT-β1AR, GRK−β1AR, and β1AR-mCFP cells (each ∼1 pmol/mg protein), HA-AT1AR cells (∼2 pmol/mg protein), or native cells were grown in minimal essential medium, 10% fetal bovine serum and 1% penicillin-streptomycin at 37 °C and transfected using FuGENE 6 (Roche Diagnostics). Cells were incubated overnight in serum-free medium and pretreated with ICI-118,551 (ICI, β2AR antagonist, 0.1 μm, 20 min, Sigma) ± AG 1478 (EGFR inhibitor, 1 μm, 5 min, Calbiochem) or H89 (PKA inhibitor, 1 μm, 5 min, Sigma) followed by agonists.
Confocal Laser Microscopy
After treatment, cells were phosphate-buffered saline-rinsed, fixed in 4% paraformaldehyde for 20 min, permeabolized with ice-cold 0.2% Triton X-100 for 5 min, rinsed, and blocked with 1% bovine serum albumin for 1 h. Incubation with anti-phosphorylated ERK1/2 rabbit antibody (1:250 for 16 h, Cell Signaling) or anti-FLAG mouse antibody (1:10000 for 20 min, Sigma) in 1% bovine serum albumin was followed by either fluorescein-conjugated goat anti-rabbit IgG or Texas Red-conjugated goat anti-mouse IgG (1:500 each for 1 h; Molecular Probes). Samples were visualized as previously described (1). Each condition was performed independently 4–6 times with 50 cells/dish %EGFR-GFP internalization calculation was (cellular EGFR-GFP intensity excluding plasma membrane/cellular EGFR-GFP intensity including plasma membrane) × 100.
Luciferase Assay
WT-β1AR cells transfected with luciferase constructs were stimulated in 96-well plates as indicated for 5 h, followed by lysis with the Dual Luciferase® Reporter Assay System (Promega). Relative light units were detected using a Veritas Microplate luminometer (Turner Biosystems/Promega). Luciferase activity is reported relative to the non-stimulated group.
Cell Fractionation
After treatment, cells were rinsed, collected in 1 ml of phosphate-buffered saline (4 °C) and spun for 10 s at 6,000 × g. Pellets were resuspended in ∼10 times pellet volume in hypotonic lysis buffer (10 mm HEPES-KOH, pH 7.5, 10 mm KCl, 3 mm MgCl2, 0.05% Nonidet P-40, 1 mm EDTA, pH 8) and incubated on ice for 10 min. Lysates were spun at 3,000 × g for 5 min, after which the supernatants were spun at 13,200 × g for 30 min (cytosolic fraction). Pellets from the 3,000 × g spin were washed 5 times in 500 μl of hypotonic lysis buffer and resuspended in ∼10 times the original pellet volume with a second lysis buffer (50 mm HEPES-KOH, pH 7.9, 250 mm KCl, 0.1% Nonidet P-40, 0.1 mm EDTA, pH 8, 0.1 mm EGTA, pH 8, 10% glycerol) and incubated on ice for 30 min. Samples were spun at 13,200 × g for 10 min (nuclear fraction). Protein estimation, immunoblotting, and analysis are previously described (1).
HB-EGF Enzyme-linked Immunosorbent Assay
The media of WT-β1AR cells stimulated with Dob for 5 min was collected, concentrated with Amicon Ultra-15 centrifugal filters (10,000 NMWL, Millipore), and HB-EGF levels assayed via enzyme-linked immunosorbent assay following the manufacturer's protocol (R&D Systems, Inc.).
Chemical Cross-linking and Immunoprecipitation
Cross-linking has been described (17). Immunoprecipitation (IP) of samples was performed with 500 to 1000 μg of protein and overnight incubation with 30 μl of either anti-FLAG M2 or anti-HA affinity gel (Sigma).
Membrane Preparation and βAR Radioligand Binding
Membrane preparation and receptor binding were performed using [125I]cyanopindolol as described previously (1, 18, 19). Membrane pellets were lysed for 1 h at 4 °C with lysis buffer containing 1% dodecyl maltoside to maintain native oligomeric protein configurations (20, 21) and underwent IP with rabbit anti-EGFR antibody or IgG control antibody (Upstate).
Fluorescence Resonance Energy Transfer (FRET)
Imaging buffer (125 mm NaCl, 5 mm KCl, 1.5 mm MgCl2, 1.5 mm CaCl2, 10 mm glucose, 0.2% bovine serum albumin, 10 mm HEPES, pH 7.4) was added to β1AR-mCFP cells expressing EGFR-mYFP in 35-mm dishes on a 37 °C heated stage. Images were acquired on a Zeiss Axiovert 200M microscope (Carl Zeiss MicroImaging, Inc.) with a Roper Micromax cooled charge-coupled device camera (Photometrics) and SlideBook 4.0 (Intelligent Imaging Innovations). Agonist-induced increases or decreases in FRET was monitored using cells emitting ∼10% basal FRET. All calculations used have been described (22).
β-Arrestin1/2 siRNA Experiments
Control (CTL) and β-arr1 and β-arr2 siRNA sequences and protocol have been used by us previously and described (1, 17, 23). For microscopy experiments, 35-mm dishes of WT-β1AR cells or β1AR-mCFP cells were transfected with CTL or a combination of β-arr1 and β-arr2 siRNA (7 μg) and EGFR-GFP or EGFR-mYFP (0.3 μg). After FRET, cells were lysed and immunoblotted to confirm β-arr1/2 knockdown.
Statistical Analysis
Statistical tests were performed using either two-tailed unpaired t test or one-way analysis of variance with the Newman-Keuls multiple comparison post hoc test using Prism 5.0 software. p value (*, p < 0.05; †, p < 0.01, ‡, p < 0.001) of <0.05 was considered significant.
RESULTS
β1AR-mediated Transactivation of EGFR Induces Cytosolic Retention of ERK
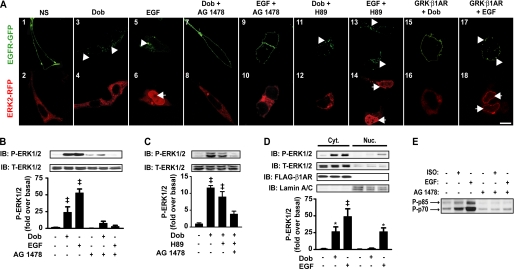
We tested whether the mechanism of EGFR activation, β1AR-mediated transactivation by catecholamine (Dob or ISO) versus direct ligand stimulation (EGF), differentially effects intracellular ERK targeting. HEK 293 cells stably expressing FLAG-tagged WT-β1AR (WT-β1AR cells) and transiently expressing green fluorescent protein-tagged EGFR (EGFR-GFP) and red fluorescent protein-tagged ERK2 (ERK2-RFP) were stimulated with agonists and assessed via confocal microscopy. In a non-stimulated state, EGFR-GFP was localized to the cell membrane, whereas ERK2-RFP was cytosolic (Fig. 1A, panels 1 and 2). Simulation for 20 min with Dob (β1AR agonist) or EGF (direct EGFR ligand) each induced internalization of EGFR-GFP as indicated by puncta formation (Fig. 1A, panels 3 and 5, arrowheads), which was prevented by pretreatment with AG 1478 (EGFR inhibitor, Fig. 1A, panels 7 and 9). Conversely, ERK2-RFP underwent differential targeting in response to agonist stimulation; EGF caused nuclear translocation of ERK2-RFP (Fig. 1A, panel 6, arrow), which was sensitive to AG 1478 (Fig. 1A, panel 10), whereas ERK remained in the cytosol following Dob stimulation (Fig. 1A, panel 4). AG 1478 did not alter ERK2-RFP targeting in response to Dob (Fig. 1A, panel 8). H89 had no effect on agonist-mediated EGFR-GFP internalization (Fig. 1A, panels 11 and 13, arrowheads) or ERK2-RFP targeting (Fig. 1A, panels 12 and 14, arrow), indicating that these effects are independent of PKA activity downstream of G protein signaling. In addition, we tested whether stimulation of β1ARs that lack all GRK phosphorylation sites in the C-terminal tail (GRK−β1AR) induce a different outcome on ERK translocation. We have previously shown GRK−β1ARs are significantly impaired in their ability to induce EGFR transactivation and ERK1/2 phosphorylation (1, 17). Consistent with these previous observations, Dob stimulation of GRK−β1AR cells did not induce EGFR-GFP internalization (Fig. 1A, panel 15). ERK2-RFP localization was not altered in response to Dob stimulation in these cells (Fig. 1A, panel 16), however, EGF stimulation produced both EGFR-GFP internalization (Fig. 1A, panel 17, arrowheads) and nuclear translocation of ERK2-RFP (Fig. 1A, panel 18, arrows). Similar data were obtained in U2S cells containing endogenous β1AR (supplemental Fig. 1A) and using a different β1AR agonist, ISO (supplemental Fig. 1B).
FIGURE 1.
β1AR-mediated transactivation of EGFR and direct ligand stimulation of EGFR differentially target ERK. A, WT-β1AR cells expressing EGFR-GFP and ERK2-RFP were stimulated for 20 min with Dob (1 μm) or EGF (1 nm) ± AG 1478 (1 μm) or H89 (1 μm). EGFR-GFP underwent internalization in response to Dob and EGF (panels 3 and 5, arrowheads), which was blocked by AG1478 (panels 7 and 9) but not H89 (panels 11 and 12, arrowheads). Only EGF induced nuclear translocation of ERK2-RFP (panel 6, arrow), which was prevented by AG 1478 (panel 12) but not H89 (panel 14, arrow). Dob stimulation of GRK−β1AR cells did not induce EGFR-GFP internalization (panel 15) nor altered ERK2-RFP localization (panel 16), whereas EGF stimulation did induce both EGFR-GFP internalization (panel 17, arrowheads) and nuclear translocation of EKR2-RFP (panel 18, arrows). Scale bar = 10 μm. Images shown are representative of at least four independent experiments. B, Dob (1 μm) or EGF (1 nm) stimulation for 5 min increased P-ERK1/2 in WT-β1AR cells transfected with FLAG-EGFR, whereas AG 1478 (1 μm) pretreatment attenuated P-ERK1/2 as shown in the histogram. Data represent the mean ± S.E. from at least five independent experiments. ‡, p < 0.001. C, Dob (1 μm) stimulation for 5 min increased P-ERK1/2, which was not significantly attenuated by H89 (1 μm), whereas pretreatment with H89 and AG 1478 (1 μm) significantly attenuated phosphorylation as summarized in the histogram. Data represent the mean ± S.E. from four independent experiments. ‡, p < 0.001. D, WT-β1AR cells stimulated with Dob (1 μm) or EGF (1 nm) for 5 min underwent cellular fractionation (confirmed by FLAG-β1AR and Lamin A/C enrichment). Dob induced cytosolic P-ERK1/2 only, whereas EGF increased both cytosolic and nuclear P-ERK1/2, summarized in the histogram. Data represent the mean ± S.E. from six independent experiments. *, p < 0.05; ‡, p < 0.001. E, WT-β1AR cells transiently expressing FLAG-EGFR were stimulated with ISO (1 μm) or EGF (1 nm) for 5 min in the presence or absence of AG 1478 (1 μm) and phospho (P)-p75/85 S6K was measured via immunoblotting. Results are representative of three independent experiments. IB, immunoblot.
To determine whether cellular localization of ERK2-RFP in response to Dob and EGF stimulation correlated with ERK activation, we performed immunoblotting with an anti-phosphorylated ERK1/2 (P-ERK1/2) antibody. Dob and EGF each increased P-ERK1/2, effects that were significantly attenuated by EGFR inhibition (Fig. 1B). Although AG 1478 decreased Dob-induced P-ERK1/2 by ∼70%, we tested whether β1AR-mediated PKA signaling accounts for the residual Dob-induced P-ERK1/2 (Fig. 1C). In the presence of the PKA inhibitor H89, Dob-induced P-ERK1/2 levels were reduced by ∼25% indicating that PKA-dependent signaling is responsible for only a small portion of P-ERK1/2 with the majority accounted for by β1AR-mediated EGFR transactivation. Additionally, cell fractionation experiments confirmed that Dob significantly induced only cytosolic ERK1/2 phosphorylation, whereas EGF significantly increased both cytosolic and nuclear ERK1/2 phosphorylation (Fig. 1D). Indeed, p75/p85 S6 kinase (S6K), a downstream target of cytosolic P-ERK, is phosphorylated in response to both catecholamine and EGF stimulation in an AG 1478-sensitive (sp) manner (Fig. 1E).
Consistent with the above data, Dob stimulation resulted in P-ERK2-RFP being targeted only to the cytosol (Fig. 2A, panel 3), whereas EGF stimulation resulted in the targeting of P-ERK2-RFP to both cytosol and nucleus (Fig. 2A, panel 5, arrow). Each of these responses was abrogated with the inclusion of AG 1478 (Fig. 2A, panels 7 and 9). We then tested whether nuclear translocation of ERK1/2 would induce gene transcription as measured by Elk-1-Gal4 luciferase reporter activity. EGF stimulation resulted in a significant increase in the amount of Elk-1-Gal4-driven luciferase activity, indicative of increased ERK1/2 activity in the nucleus (Fig. 2B), which was blocked by AG 1478 pretreatment. Conversely, activation of Elk-1-Gal4 luciferase was not observed with Dob (Fig. 2B) or ISO (Fig. 2D). To ensure catecholamine stimulation produced a β1AR-mediated signal in these assays, CREB-Gal4 luciferase activity was assessed, indicative of cAMP signaling downstream of β1AR activation. Using this system, both Dob (Fig. 2C) and ISO (Fig. 2D) caused significant induction of CREB-Gal4-mediated luciferase activity. Therefore, whereas β1AR-mediated transactivation and direct EGF ligand stimulation each induce EGFR internalization and downstream phosphorylation of ERK, these stimuli result in differential intracellular targeting and function of ERK1/2.
FIGURE 2.
Impact of differential activation and targeting of ERK in response to β1AR-mediated transactivation versus direct ligand stimulation of EGFR. A, WT-β1AR cells transfected with ERK2-RFP were stained with an ERK1/2-specific phosphoantibody following 5 min agonist treatment. Dob (10 μm) increased P-ERK2-RFP in the cytosol only (panel 3), whereas EGF (1 nm) increased P-ERK2-RFP in both the cytosol and nucleus (panel 5, arrow). AG 1478 (1 μm) prevented both Dob- and EGF-induced P-ERK2-RFP (panels 7 and 9). Scale bar = 10 μm. Images shown are representative of at least four independent experiments. B, WT-β1AR cells expressing Elk-1-Gal4, Firefly, and Renilla luciferase were stimulated with Dob (1 μm) or EGF (1 nm) for 5 h. Luciferase activity increased with EGF, but not Dob stimulation, which was ablated by AG 1478 (1 μm). Data represent the mean ± S.E. from at least seven independent experiments. ‡, p < 0.001. C, WT-β1AR cells expressing CREB-Gal4, Firefly, and Renilla luciferase were stimulated with Dob (1 μm) or EGF (1 nm) for 5 h. Luciferase activity increased in response to Dob, but not EGF stimulation. Data represent the mean ± S.E. from at least five independent experiments. ‡, p < 0.001. D, WT-β1AR cells transiently expressing either Elk-1-Gal4 or CREB-Gal4 with Firefly luciferase and Renilla luciferase, were stimulated with ISO (1 μm) for 5 h and luciferase activity measured. Only CREB-controlled luciferase activity increased in response to ISO. Data represent the mean ± S.E. from four independent experiments. ‡, p < 0.001.
Ligand Concentrations Matched for EGFR and ERK1/2 Activation Maintain Differential ERK Targeting
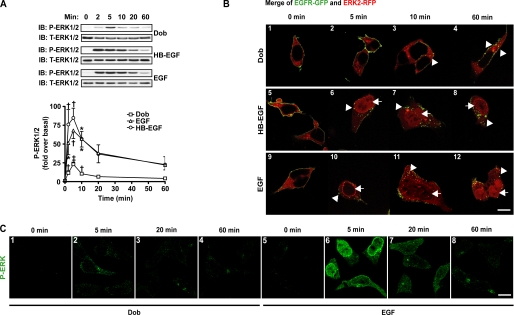
HB-EGF is an endogenously expressed membrane protein known to be cleaved by matrix metalloproteinases in response to βAR stimulation (1, 4, 17). To test the effect of bypassing β1AR-mediated EGFR transactivation with exogenously added HB-EGF and EGF, we measured ERK1/2 activation and cellular targeting at various time points following HB-EGF, EGF, and Dob stimulation. In WT-β1AR cells transiently transfected with FLAG-EGFR, Dob induced an early and significant increase in P-ERK1/2, peaking at 5 min and approaching basal levels by 60 min (Fig. 3A). Direct EGFR stimulation with HB-EGF or EGF induced rapid and significant P-ERK1/2 that peaked at 5 min and returned to ∼⅓ of maximum by 60 min (Fig. 3A). Confocal microscopy of Dob-stimulated WT-β1AR cells transfected with EGFR-GFP and ERK2-RFP showed marked EGFR internalization after 10 min (Fig. 3B). Importantly, at no time point did Dob stimulation result in ERK2-RFP translocation to the nucleus, even with continued EGFR internalization (Fig. 3B). In contrast, both EGF and HB-EGF began to initiate EGFR internalization and ERK2-RFP translocation to the nucleus after 5 min of stimulation that persisted for 60 min (Fig. 3B). Consistent with our immunoblotting data, cell staining at various time points revealed peak ERK phosphorylation at 5 min in response to either Dob or EGF (Fig. 3C) and returning close to basal levels by 60 min.
FIGURE 3.
Time-dependent activation of ERK1/2 by β1AR-mediated transactivation and direct ligand stimulation of EGFR. A, WT-β1AR cells transfected with FLAG-EGFR were stimulated with Dob (10 μm), HB-EGF (1 nm), or EGF (1 nm) for 0–60 min and cell lysates immunoblotted (IB) for P-ERK1/2 and T-ERK1/2. The histogram depicts the time course of P-ERK1/2 response for each ligand. Data represent the mean ± S.E. from at least four independent experiments. *, p < 0.05; †, p < 0.01; ‡, p < 0.001. B, WT-β1AR cells were transiently transfected with EGFR-GFP and ERK2-RFP and stimulated with Dob (1 μm), HB-EGF (1 nm), or EGF (1 nm) for 0–60 min. Dob (panels 3 and 4), HB-EGF (panels 6-8), and EGF (panels 10-12) each induced EGFR-GFP internalization (arrowheads), whereas only HB-EGF (panels 6-8) and EGF (panels 10-12) caused translocation of ERK2-RFP to the nucleus (arrows). Scale bars = 10 μm. Images shown are representative of at least four independent experiments. C, WT-β1AR cells were transiently transfected with ERK2-RFP and stained with anti-P-ERK1/2 antibody following Dob (1 μm) or EGF (1 nm) stimulation for increasing periods of time. Peak ERK2-RFP phosphorylation was achieved within 5 min for both Dob (panel 4) and EGF (panel 6). EGF stimulation induced prolonged P-ERK2-RFP in both the cytosol and the nucleus. Scale bar = 10 μm. Composite images shown are representative of at least four independent experiments.
We next compared the effects of increasing Dob, EGF, and HB-EGF concentrations on ERK1/2 phosphorylation (Fig. 4A). Analysis of the concentration-response curves revealed that stimulation with 1 μm Dob, 0.1 nm EGF, and 0.01 nm HB-EGF resulted in a similar level of P-ERK1/2 (Fig. 4A). Although Dob did not induce nuclear ERK2-RFP trafficking even up to concentrations of 100 μm, both 0.1 nm EGF and 0.01 nm HB-EGF resulted in robust nuclear accumulation of ERK2-RFP (Fig. 4B). To assess the effects of increasing concentrations of Dob, EGF, and HB-EGF on EGFR-GFP internalization, we calculated the loss of EGFR-GFP from the plasma membrane in response to ligand stimulation (Fig. 4C). The concentrations of 0.1 nm EGF and 0.01 nm HB-EGF induced equivalent EGFR-GFP internalization as Dob at concentrations ≥1 μm. Consistent with the internalization data, the level of P-EGFR in response to 1 μm Dob was equivalent to that of 0.1 nm EGF indicating similar EGFR activation (Fig. 4D). To determine whether the concentrations of exogenous HB-EGF added in these experiments were within the range of endogenous HB-EGF shed in response to β1AR activation, we collected the media of WT-β1AR cells stimulated with Dob for 5 min and measured the amount of HB-EGF released (Fig. 4E). Under non-stimulated conditions, cells released a basal level of 0.94 ± 0.15 pm HB-EGF into the media. In contrast, the media of Dob-stimulated cells contained 3.54 ± 0.13 pm HB-EGF. Thus, whereas Dob causes release of HB-EGF into the media that induces a similar level of EGFR internalization and P-ERK1/2 as 0.01 nm HB-EGF, differential mechanisms must be involved to account for the subsequent cytosolic retention of ERK.
FIGURE 4.
Ligand concentrations matched for ERK1/2 phosphorylation, EGFR phosphorylation, and EGFR internalization responses produce differential ERK targeting. A, WT-β1AR cells were transiently transfected with FLAG-EGFR and stimulated for 5 min with increasing concentrations of Dob (1 nm to 100 μm), HB-EGF and EGF (100 fmol to 10 nm) following which cell lysates were immunoblotted (IB) for P-ERK1/2 and T-ERK1/2. The histogram depicts the concentration-response curves for each ligand. Data represent the mean ± S.E. from at least four independent experiments. B, WT-β1AR cells were transiently transfected with EGFR-GFP and ERK2-RFP (shown as merged images) and were stimulated for 20 min with increasing concentrations of Dob (1–100 μm) and HB-EGF and EGF (0.01–1 nm). Dob (panels 2-4), HB-EGF (panels 6-8), and EGF (panels 11 and 12) each induced EGFR-GFP internalization (arrowheads), whereas only HB-EGF (panels 6-8) and EGF (panels 11 and 12) caused nuclear translocation of ERK2-RFP (arrows). Scale bar = 10 μm. Images shown are representative of at least seven independent experiments. C, EGFR-GFP internalization was assessed in response to ligand concentrations described above. Data represent the mean ± S.E. from at least seven independent experiments. NS, non-stimulated. †, p < 0.01; ‡, p < 0.001. D, WT-β1AR cells were transiently transfected with FLAG-EGFR, stimulated for 5 min with either Dob (1 μm) or EGF (0.1 nm), and immunoblotted for P-EGFR and T-EGFR, as summarized in the histogram. Data represent the mean ± S.E. from three independent experiments. *, p < 0.05. E, WT-β1AR cells were stimulated with Dob (1 μm) for 5 min, the media collected, concentrated, and the concentration of HB-EGF shed into the media assessed via sandwich enzyme-linked immunosorbent assay. Data represent the mean ± S.E. from four independent experiments. ‡, p < 0.001.
β1AR and EGFR Associate with Specificity and Their Association Is Differentially Regulated by Ligand Stimulation
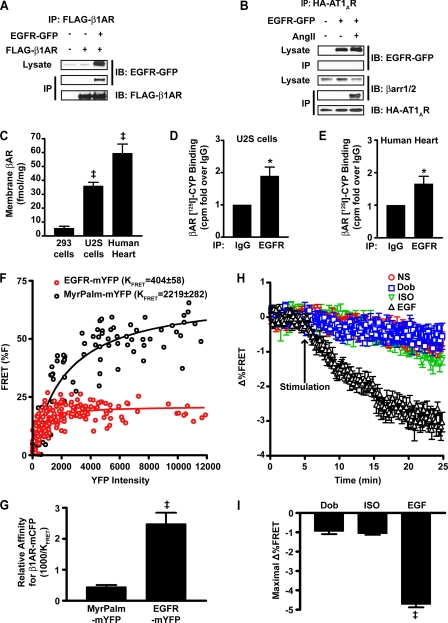
To address the mechanism by which differential targeting of ERK in response to ligand may occur, we tested the possibility that β1AR and EGFR may interact and direct the cellular localization of ERK. Recently, a number of studies have reported an interaction between other 7TMRs and EGFR (5, 24–28). In FLAG-β1AR cells transfected with EGFR-GFP, IP of FLAG-β1AR resulted in co-IP of EGFR (Fig. 5A). In contrast, EGFR-GFP overexpression in HEK 293 cells stably expressing the hemagglutinin-tagged angiotensin type 1A receptor (HA-AT1AR) did not result in the co-IP of EGFR with AT1AR, even following angiotensin II stimulation (Fig. 5B). Angiotensin II stimulation did induce association between AT1AR and β-arrestin1/2, confirming receptor activation and ability to interact with predicted proteins. Thus, simple overexpression of another 7TMR (AT1AR), which is known to transactivate EGFR (27, 29, 30), is insufficient to induce its interaction with EGFR in this system.
FIGURE 5.
Interaction of β1AR and EGFR is regulated by ligand stimulation. A, HEK 293 cells were transiently transfected with FLAG-WT β1AR ± EGFR-GFP and underwent IP with FLAG-M2-agarose gel. EGFR-GFP associated with WT-β1AR. Data shown are representative of three independent experiments. B, HA-AT1AR cells were transiently transfected with EGFR-GFP and underwent IP with HA-agarose gel following stimulation with angiotensin II (1 μm, 5 min). HA-AT1AR did not co-IP with EGFR in stimulated or non-stimulated states, but did associate with β-arr1/2 following angiotensin II treatment. Data shown are representative of three independent experiments. C, comparison of membrane βAR density in native HEK 293 cells, U2S cells, and human heart as assessed via [125I]cyanopindolol (CYP) binding analysis. Data represent the mean ± S.E. from at least four independent experiments. ‡, p < 0.001. D, βAR measured in EGFR IP versus IgG IP, shown as fold-over IgG, as determined by [125I]cyanopindolol binding analysis in U2S cell membranes. Data represent the mean ± S.E. from five independent experiments. *, p < 0.05. E, βAR measured in EGFR IP versus IgG IP, shown as fold-over IgG, as determined by [125I]cyanopindolol binding analysis in human heart membranes. Data represent the mean ± S.E. from five independent experiments. *, p < 0.05. F, HEK 293 cells stably expressing β1AR-mCFP were transiently transfected with EGFR-mYFP or MyrPalm-mYFP. The %FRET between β1AR-mCFP and either mYFP-tagged protein was measured over increasing YFP intensities. Nonlinear regression analysis was performed to fit the data to curves. G, the relative affinities of EGFR-mYFP and MyrPalm-mYFP for β1AR-mCFP. Data represent the mean ± S.E. from six independent experiments with 123 (MyrPalm-mYFP) and 195 (EGFR-mYFP) total cells. ‡, p < 0.001. H, β1AR-mCFP stable cells were transiently transfected with EGFR-mYFP and underwent stimulation with agonist after 5 min of baseline FRET was obtained. EGF (1 nm) caused a reduction in %FRET, whereas neither Dob (1 μm) nor ISO (1 μm) stimulation altered %FRET from that of non-stimulated cells (NS). Data represent the mean ± S.E. from eight independent experiments. I, nonlinear regression analysis of the %FRET signals in response to stimulation determined EGF significantly reduced the calculated %FRET (−4.72 ± 0.16%) compared with Dob (−0.95 ± 0.15%) and ISO (−1.06 ± 0.07%). Data represent the mean ± S.E. from at least six independent experiments. ‡, p < 0.001.
To determine whether β1AR and EGFR interact at endogenous levels and in vivo, we performed co-IP experiments in U2S cells and human heart tissue, which basally express both the EGFR and ∼35–60 fmol/mg protein of βARs (Fig. 5C). Membrane preparations from both U2S cells and heart tissue were used to IP endogenous EGFR followed by radioligand binding with the highly specific βAR ligand [125I]iodocyanopindolol. Nonspecific binding was determined by the separate addition of IgG followed by IP. Within the EGFR immunoprecipitates we detected a significant level of endogenous βAR compared with IgG control immunoprecipitates in both U2S cells (0.16 ± 0.04 fmol of receptor, Fig. 5D) and human heart tissue (0.13 ± 0.06 fmol of receptor, Fig. 5E). These experiments support our data that under basal conditions there is an interaction between β1ARs and EGFRs.
To determine the specificity of β1AR-EGFR interaction, we performed FRET experiments in HEK 293 cells stably expressing monomeric cyan fluorescent protein-tagged β1AR (β1AR-mCFP cells) and transiently expressing either EGFR-mYFP or myristoylated-palmitoylated mYFP (MyrPalm-mYFP). MyrPalm-mYFP is targeted to the plasma membrane (31), thereby providing a nonspecific membrane-bound FRET partner for β1AR-mCFP for comparison with EGFR-mYFP to determine specificity of interaction. As levels of MyrPalm-mYFP and EGFR-mYFP increased, the amount of detectable FRET also increased, achieving maximal %FRET of ∼50 and 20%, respectively (Fig. 5F). Because the maximal FRET efficiency between β1AR-mCFP and either mYFP-tagged protein is influenced by both proximity and orientation of mCFP and mYFP, and dependent upon mYFP concentration, it does not necessarily represent the true amount of specific interaction between the partners. Therefore, we compared the relative affinities of EGFR-mYFP and MyrPalm-mYFP for β1AR-mCFP calculated via saturation binding analysis. A 5-fold greater affinity of EGFR for β1AR than that of MyrPalm-mYFP for β1AR was attained (Fig. 5G), indicating that the interaction of β1AR with EGFR has significantly higher specificity than with a general membrane-bound protein.
We next used FRET analysis to explore the possibility that agonist stimulation can regulate β1AR-mCFP-EGFR-mYFP association. Catecholamine stimulation (either Dob or ISO) induced a small reduction in FRET over time that was indistinguishable from non-stimulated cells (Fig. 5H). In contrast, EGF stimulation caused an immediate decrease in FRET signal that persisted. Nonlinear regression analysis of the data revealed approximately a 5-fold greater loss in FRET (%Fmax) with EGF stimulation compared with Dob or ISO (Fig. 5I). Thus, EGF stimulation significantly disrupts β1AR-EGFR interaction, whereas catecholamine stimulation maintains receptor interaction.
β1AR and EGFR Interaction Is Regulated by C-terminal GRK Phosphorylation Sites and β-Arrestin-mediated Internalization
GRK-mediated phosphorylation of the β1AR is required for transactivation of EGFR by inducing β-arrestin recruitment (1). We have previously shown that GRK−β1ARs are significantly impaired in their ability to induce EGFR transactivation and ERK1/2 phosphorylation (1, 17). Therefore, we tested whether β1AR GRK phosphorylation sites are required for β1AR-EGFR interaction. In non-stimulated WT-β1AR cells transfected with EGFR-GFP, β1AR and EGFR were expressed on the plasma membrane (Fig. 6A, panels 1 and 2), and co-localize in puncta upon stimulation with ISO (Fig. 6A, panels 3 and 4, arrowheads). In contrast, EGF stimulation caused EGFR-GFP internalization alone, whereas β1ARs remained on the cell surface (Fig. 6A, panels 5 and 6, arrowhead). Because GRK−β1ARs can undergo catecholamine-stimulated internalization by a clathrin/β-arrestin-independent mechanism (16), we tested whether they would co-localize with EGFR. ISO stimulation induced internalization of GRK− β1AR, leaving EGFR-GFP at the cell surface (Fig. 6A, panels 9 and 10, arrowheads), whereas EGF stimulation again produced internalization of EGFR-GFP without inducing GRK−β1AR internalization (Fig. 6A, panels 11 and 12, arrowheads). Thus, GRK phosphorylation sites in the β1AR are essential for β1AR-EGFR interaction following catecholamine stimulation. Additionally, we assessed the requirement of β1AR GRK phosphorylation sites for the basal association of β1AR and EGFR. HEK 293 cells stably expressing either FLAG-tagged WT-β1AR or GRK−β1AR and transfected with EGFR-GFP underwent IP with FLAG M2-agarose and the amount of EGFR-GFP associated with each β1AR was assessed. In comparison to WT-β1AR, GRK−β1AR had a significantly decreased ability to associate with EGFR-GFP (Fig. 6B). Densitometric analysis revealed a decrease of ∼90% in β1AR-EGFR association when β1AR GRK phosphorylation sites were absent, suggesting the C-terminal conformation of β1AR may be an important factor in determining its interaction with EGFR.
FIGURE 6.
β1AR and EGFR interaction is regulated by C-terminal GRK phosphorylation sites and β-arrestin-mediated internalization. A, WT-β1AR or GRK−β1AR cells were transiently transfected with EGFR-GFP, stimulated with ISO (1 μm) or EGF (1 nm) for 20 min, and assayed via confocal microscopy (shown as merged images of EGFR-GFP and Texas Red-labeled β1AR). ISO stimulation caused co-internalization of WT-β1AR and EGFR-GFP into puncta (panels 3 and 4, arrowheads), whereas GRK−β1AR underwent internalization alone without EGFR-GFP (panels 9 and 10, arrowheads). EGF induced internalization of only EGFR-GFP in both WT-β1AR and GRK−β1AR cells (panels 5 and 6, 11 and 12, respectively, arrowheads). Scale bars = 10 μm, upper panels; 1 μm, lower panels. Images shown are representative of at least four independent experiments. NS, non-stimulated. B, HEK 293 cells expressing WT-β1AR or GRK−β1AR with or without EGFR-GFP underwent IP with FLAG-M2-agarose gel. EGFR-GFP association with GRK−β1AR was significantly reduced in comparison to WT-β1AR. Data are summarized in histogram, and represent the mean ± S.E. from at least four independent experiments. †, p < 0.01. C, HEK 293 cells expressing WT-β1AR or GRK−β1AR and stimulated with ISO (1 μm) or EGF (1 nm) at different time points underwent IP with FLAG-M2-agarose gel. β-arr1/2 associated with ISO-stimulated WT-β1AR at 5 and 30 min, whereas EGF stimulation produced no β-arr1/2 accumulation in the IP. β-arr1/2 did not IP with GRK−β1AR in response to ISO. Data are summarized in the histogram, and represent the mean ± S.E. from at least four independent experiments. ‡, p < 0.001. D, WT-β1AR cells were transiently transfected with either WT-βarr2-mYFP or RRK/Q-βarr2-GFP, stimulated with ISO (1 μm, 10 min), and assayed via confocal microscopy. ISO caused recruitment of WT-βarr2-mYFP to the plasma membrane (panel 5, arrow) and internalization of WT-β1AR (panel 4, arrowhead), whereas RRK/Q-βarr2-GFP was recruited to the plasma membrane (panel 11, arrow) but WT-β1AR internalization was abrogated, although punctae still formed on the cell surface (panel 10, arrowhead). Scale bar = 10 μm. Images shown are representative of at least four independent experiments. E, WT-β1AR cells transiently transfected with EGFR-GFP (1st image), WT-βarr2-mYFP or RRK/Q-βarr2-GFP (3rd image) and stimulated with ISO (1 μm, 5 min) underwent IP with FLAG-M2 agarose gel. Both WT-βarr2-mYFP and RRK/Q-βarr2-GFP were similarly recruited to β1AR upon ISO stimulation (4th image, normalized to total WT-βarr2-mYFP and RRK/Q-βarr2-GFP expression, summarized in the bottom left histogram). In the presence of WT-βarr2-mYFP, EGFR-GFP associated with WT-β1AR before and after ISO stimulation (2nd image). RRK/Q-βarr2-GFP overexpression caused a reduction in the amount of EGFR-GFP associated with WT-β1AR following ISO stimulation (2nd image, normalized to total EGFR-GFP expression, summarized in bottom right histogram). Data represent the mean ± S.E. from five independent experiments. *, p < 0.05.
To explore the possible requirement of β-arrestin in mediating β1AR-EGFR trafficking after catecholamine stimulation, we assessed the ability of WT-β1AR and GRK−β1AR cells to recruit β-arrestin. Stimulation with catecholamine induced a significant rapid increase in β-arrestin recruitment to the WT-β1AR, peaking at 5 min and remaining elevated up to 30 min post-stimulation (Fig. 6C). Importantly, stimulation with EGF in WT-β1AR cells or with ISO in GRK−β1AR cells did not result in β-arrestin association suggesting that β-arrestin recruitment to the receptor complex is required for their continued association in the presence of catecholamine.
We next tested the possibility that β1AR-EGFR co-localization following catecholamine stimulation depends on β-arrestin-mediated internalization of the receptor complex. We used a β-arr2 mutant with altered C-terminal amino acid residues (RRK/Q) that render it unable to induce receptor internalization despite retaining its ability to be recruited to activated βAR (32, 33). To ensure this β-arr2 mutant could be recruited to agonist-stimulated β1ARs in our system, we treated WT-β1AR cells transfected with either WT-βarr-2-mYFP or RRK/Q-β-arr2-GFP with ISO and assessed β-arr2 recruitment via confocal microscopy (Fig. 6D). ISO stimulation induced the recruitment of both WT- and RRK/Q-β-arr2 to β1ARs. Subsequently, performing FLAG-β1AR IP, we compared the effects of WT- and RRK/Q-β-arr2 on β1AR-EGFR association in WT-β1AR cells transfected with EGFR-GFP and either WT-β-arr2-mYFP or RRK/Q-β-arr2-GFP (Fig. 6E). In non-stimulated cells EGFR associated with β1AR in the presence of either WT- or RRK/Q-β-arr2. Following β1AR stimulation, recruitment of both WT- and RRK/Q-β-arr2 increased similarly. Association of β1AR and EGFR did not change in the presence of WT-βarr2, however, in the presence of RRK/Q-β-arr2, ISO stimulation caused a significant decrease in β1AR-EGFR interaction (78.6 ± 6.4%).
Knockdown of β-Arrestin1/2 Prevents β1AR-EGFR Interaction following Catecholamine Stimulation
Because overexpression of the internalization-deficient β-arr2 RRK/Q led to impairment of catecholamine-induced β1AR-EGFR association, we sought to determine whether knockdown of endogenous β-arr1/2 would perturb the trafficking of the β1AR-EGFR complex following catecholamine stimulation. FRET analysis was performed in β1AR-mCFP cells transfected with EGFR-mYFP and CTL siRNA or a combination of two siRNAs targeting either β-arr1 or β-arr2 (β-arr1/2 siRNA). Dob stimulation of cells that received CTL siRNA produced minimal loss of FRET over time (Fig. 7A), comparable with the response to Dob shown in Fig. 5H. In contrast, Dob stimulation of cells transfected with βarr1/2 siRNA, causing ≥90% ablation of β-arr1/2 expression, reduced calculated %Fmax by 2.55 ± 0.11%, as determined by nonlinear regression analysis.
FIGURE 7.
β-Arrestin-mediated retention of β1AR-EGFR interaction following catecholamine stimulation. A, β1AR-mCFP cells transiently transfected with EGFR-mYFP and either CTL or βarr1/2 siRNA underwent stimulation with Dob (1 μm) after 5 min of baseline FRET was obtained. Addition of Dob did not significantly reduce %FRET in the presence of CTL siRNA, but did cause a marked reduction in %FRET in cells transfected with βarr1/2 siRNA (>90% βarr1/2 knockdown as determined by immunoblotting (IB)). Data represent the mean ± S.E. from 10 independent experiments. B, WT-β1AR cells were transfected with either CTL or βarr1/2 siRNA and EGFR-GFP (shown as merged images of EGFR-GFP and Texas Red-labeled β1AR). β1AR and EGFR-GFP co-localized at the plasma membrane in nonstimulated cells (panels 1, 2, 5, and 6). Treatment with ISO (1 μm, 20 min) caused formation of puncta containing both β1AR and EGFR in CTL siRNA-treated cells (panels 3 and 4, arrowheads), but only β1AR in βarr1/2 siRNA-treated cells (panels 7 and 8, arrowheads). Scale bars = 10 μm, upper panels; 1 μm, lower panels. Images shown are representative of at least four independent experiments. C, WT-β1AR cells transiently transfected with EGFR-GFP and either CTL or βarr1/2 siRNA underwent IP with FLAG-M2-agarose gel. In CTL-treated cells, EGFR-GFP associated with WT-β1AR in the presence or absence of ISO (1 μm, 5 min), whereas β-arr1/2 and P-ERK1/2 were detected in the IP only following ISO stimulation. In βarr1/2 siRNA-treated cells (∼90% knockdown), both EGFR-GFP and P-ERK1/2 association with β1AR was reduced following agonist stimulation. As summarized in the histograms, ISO-induced β1AR-EGFR association and β1AR-P-ERK1/2 interaction were significantly reduced in the presence of βarr1/2 siRNA versus CTL siRNA. Data represent the mean ± S.E. from five independent experiments. *, p < 0.05; †, p < 0.01. D, proposed mechanism of regulation of β1AR-EGFR interaction via transactivation. β1AR and EGFR associate at the plasma membrane. Catecholamine stimulation induces GRK-mediated phosphorylation of the cytoplasmic tail of β1AR and recruitment of βarr1/2 bound to ERK. Transactivation of EGFR, phosphorylation of ERK, and internalization of the β1AR-EGFR complex occurs in a β-arr-dependent manner. β-arr1/2 dissociates from the receptor complex and P-ERK is restricted to the cytosol (green arrow) and does not translocate to the nucleus (capped red arrow). E, direct ligand stimulation of EGFR with EGF does not initiate GRK or β-arr1/2 signaling, but induces dissociation of EGFR from β1AR, recruitment and phosphorylation of non-β-arr1/2-bound ERK, and internalization of EGFR away from the plasma membrane. P-ERK is targeted throughout the cytosol and the nucleus (green arrows). Yellow circles indicate phosphorylation.
Furthermore, we investigated the co-localization of β1AR and EGFR in the presence of CTL versus βarr1/2 siRNA via confocal microscopy. Cells transfected with either siRNA maintained β1AR and EGFR co-localization at the cell surface in a nonstimulated state (Fig. 7B, panels 1, 2, 5, and 6). Treatment with ISO caused the formation of internalized puncta containing both β1AR and EGFR in CTL siRNA-treated cells (Fig. 7B, panels 3 and 4, arrowheads), but only β1AR in βarr1/2 siRNA-treated cells (Fig. 7B, panels 7 and 8, arrowhead). EGF stimulation induced internalization of only EGFR-GFP in both CTL and βarr1/2 siRNA-treated cells (not shown). These data demonstrate that whereas the basal association of receptors at the cell surface does not require β-arr1/2, following catecholamine stimulation endogenous β-arr1/2 recruitment is necessary to maintain β1AR-EGFR interaction and co-internalization.
To determine whether β-arr1/2 interaction with the β1AR-EGFR complex directs ERK signaling, IP of FLAG-β1AR with or without catecholamine stimulation was performed in the presence of CTL or βarr1/2 siRNA and associating proteins assessed. In WT-β1AR cells transfected with EGFR-GFP and CTL siRNA, EGFR associated with β1AR before and after ISO stimulation (Fig. 7C), corroborating our FRET and confocal microscopy data. β-arr1/2 and P-ERK1/2 were observed to interact with β1AR only following catecholamine stimulation. In accordance with our previous studies (1, 17), β-arr1/2 siRNA abolished the P-ERK1/2 response to catecholamine stimulation in the cell lysate. Overall, βarr1/2 siRNA mediated the loss of β1AR association with both EGFR (81.5 ± 8.9%) and P-ERK1/2 (72.8 ± 5.4%) following β1AR stimulation (Fig. 7C). Thus, catecholamine-induced β-arr1/2 recruitment to β1AR-EGFR is required not only to direct ERK1/2 activation and targeting, but to maintain the association of β1AR and EGFR in a complex.
DISCUSSION
Although 7TMR-mediated transactivation of EGFR has been shown to induce phosphorylation of ERK1/2, the mechanisms responsible for this effect vary as widely as the 7TMRs that transactivate EGFR (17, 24, 28, 34, 35). Previously, we have shown a role for β-arrestin in the regulation of β1AR-mediated transactivation of EGFR (1, 17) and in this study we show that β1AR and EGFR interact as a complex whose continued association following agonist stimulation is dependent upon GRK phosphorylation sites in the C-terminal tail of β1AR, and on the recruitment of β-arrestin. We identify that β1AR-mediated EGFR transactivation leads to differential intracellular trafficking of ERK1/2 compared with direct ligand stimulation of EGFR. Each stimulus induces EGFR activation and internalization, but despite this common feature, the biological consequences of these distinct stimuli are divergent. Others have reported that β-arrestin functions as a scaffold for ERK and their upstream kinases, thus providing a pool of β-arrestin-bound ERK1/2 in the cytosol (36–38). Thus, we propose that upon catecholamine stimulation, GRK-mediated phosphorylation of the cytoplasmic tail of β1AR favors recruitment of βarr1/2-bound ERK1/2 over free cytosolic ERK1/2. β-Arrestin induces transactivation of EGFR, which allows for phosphorylation of the β-arrestin-recruited ERK1/2. Following β-arrestin-mediated internalization of the β1AR-EGFR complex, β-arrestin dissociates from the receptor complex and restricts bound P-ERK1/2 to the cytosol (Fig. 7D). Whether ERK1/2 phosphorylation is dependent on β1AR-EGFR internalization, or occurs simultaneously but independent of receptor internalization, is not known. Direct ligand stimulation of EGFR with EGF, however, does not initiate GRK or β-arrestin signaling, but induces dissociation of EGFR from β1AR, recruitment and phosphorylation of non-β-arr1/2-bound ERK1/2, and internalization of EGFR away from the plasma membrane. This pool of P-ERK1/2 is targeted throughout both the cytosol and nucleus (Fig. 7E). These findings provide new insight into the mechanism of β-arrestin-mediated signaling following catecholamine stimulation, which not only induces EGFR transactivation, but also induces internalization of a β1AR-EGFR complex and directs intracellular ERK1/2 targeting.
The dynamics of ERK1/2 phosphorylation in response to 7TMR stimulation, including AT1AR, β2AR, and parathyroid hormone receptor, have been studied in detail by others (12, 39, 40). Both β-arrestin-dependent and -independent signaling pathways contribute to phosphorylation of ERK1/2, however, the extent to which each pathway induces this effect differs for various 7TMRs. In our study we show that, in response to β1AR stimulation, a majority of the resulting ERK1/2 phosphorylation is dependent on β-arrestin-mediated EGFR transactivation. Approximately 70–75% of catecholamine-induced P-ERK1/2 was abolished via inhibition of EGFR with AG 1478 (Fig. 1B) or siRNA-directed ablation of β-arrestin1/2 expression (Fig. 7C). Consistent with these data, blocking PKA signaling with H89 reduced the P-ERK1/2 response by ∼25% following β1AR stimulation (Fig. 1C). In terms of β1AR-mediated EGFR transactivation, inhibition of PKA signaling with H89 had no effect on EGFR internalization, whereas blockade of EGFR with AG 1478 or activation of GRK−β1AR (Fig. 1A) or knockdown of β-arr1/2 completely prevented this process (Fig. 7B). Thus, whereas β-arrestin-independent signaling plays a small role in ERK1/2 phosphorylation following β1AR stimulation, the majority of ERK1/2 activation is achieved via β-arrestin-dependent EGFR transactivation.
EGFR is known to undergo rapid internalization and targeting for recycling or degradation following stimulation with EGF in a concentration-dependent manner (8, 41, 42). Catecholamine stimulation of βARs induces a β-arrestin-associated internalization that leads to receptor recycling (11). Our data demonstrate co-localization of β1AR, EGFR, and β-arrestin following adrenergic stimulation that maintains cytosolic ERK1/2 signaling. Interestingly, even low concentrations of EGF or HB-EGF, which produced similar P-ERK1/2 levels as β1AR stimulation, did not lead to cytosolic-restricted ERK1/2. Therefore, β-arrestin recruitment to the receptor complex is necessary to induce EGFR transactivation, as we previously reported (1, 17), and to subsequently promote the maintenance of the receptor complex and cytosolic retention of ERK1/2. Indeed, our data demonstrate that an internalization-deficient β-arrestin mutant (RRK/Q), or the absence of β-arrestins altogether (siRNA knockdown), decreases β1AR-EGFR association by ∼80% following catecholamine stimulation and significantly reduces interaction with phosphorylated ERK1/2 by ∼70%. Thus, β-arrestin recruitment is essential not only for the induction of β1AR-mediated EGFR transactivation but also for directing β1AR-EGFR internalization and P-ERK1/2 trafficking in the cell. Conversely, direct ligand stimulation of EGFR does not recruit β-arrestins, leading to rapid dissociation of the receptor complex, internalization of EGFR alone, and simultaneous cytosolic and nuclear targeting of P-ERK1/2. Proximal to β-arrestin signaling, we also show a role for GRK phosphorylation sites in the regulation of β1AR-EGFR interaction, suggesting the C-terminal conformation of β1AR is important in allowing its interaction with EGFR.
ERK1 and −2 lack both NLS and NES motifs, but do typically traffic into the nucleus following mitogenic stimulation via regulatory proteins (43). Indeed, several studies have shown that β-arrestins act as scaffolds for the components of ERK1/2 signaling following stimulation of various 7TMRs, including the AT1AR and β2AR, and that stronger receptor-β-arrestin interactions usually lead to cytosolic retention of ERK1/2 (11, 12). In this study we show the cytosolic retention of ERK1/2 in response to β1AR stimulation is β-arrestin-dependent despite βARs classically having a lower affinity for β-arrestin (11). This may be reflective of either a small pool of β-arrestin-bound ERK1/2 available for phosphorylation via β1AR-mediated EGFR transactivation or the potential association of a protein phosphatase in the β1AR-β-arrestin1/2-EGFR-ERK1/2 complex, because β-arrestins have been shown to interact with several phosphatases (44).
The spectrum of 7TMRs that induce EGFR transactivation is an ever-expanding cohort that highlights the importance of EGFR as an alternative 7TMR signaling pathway beyond the classical G protein-dependent paradigm (1–7, 24–30, 35, 45–50). Although a number of 7TMRs have been shown to induce transactivation of EGFR, only a handful have been demonstrated to interact with EGFR, although the mechanism(s) of these associations have not been elucidated (5, 24–28). In our study we demonstrate a ligand-dependent, dynamic interaction between β1AR and EGFR using overexpression systems and show that this interaction occurs at endogenous receptor levels in cells and human heart tissue. Moreover, the recruitment of endogenous β-arr1/2 directs internalization of the β1AR-EGFR complex and is essential for their continued association upon catecholamine stimulation, because direct EGFR stimulation does not recruit β-arrestin and leads to the disruption of the receptor complex. Thus, our study provides new mechanistic insights into the regulation of this receptor complex by β-arrestin and the impact of this regulation on intracellular ERK1/2 targeting and activity. We propose that this dynamic β-arrestin-regulated receptor-receptor interaction may be a mechanism by which the β1AR can regulate EGFR signaling to exert differential cellular effects. Furthermore, we believe our findings may provide an attractive explanation for the cardioprotective effects of β1AR-mediated EGFR transactivation we have previously observed (1).
Supplementary Material
Acknowledgments
We thank Weili Zou and Rhonda L. Carter for outstanding technical assistance, Dr. Robert J. Lefkowitz for thoughtful discussion, and Dr. Dawn E. Bowles for use of luciferase assay equipment.
This work was supported, in whole or in part, by National Institutes of Health Grant HL-56687 (to H. A. R.). This work was also supported by a Heart and Stroke Foundation of Canada Fellowship Award (to D. G. T.), an American Heart Association Fellowship Award 0825499E (to I. M. K.), and a Howard Hughes Medical Institute Medical Student Research Training Fellowship (to P. A. P.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- β1AR
- β1 adrenergic receptor
- EGFR
- epidermal growth factor receptor
- ERK
- extracellular regulated kinase
- GRK
- G protein-coupled receptor kinase
- 7TMR
- 7 transmembrane receptor
- Dob
- dobutamine
- ISO
- isoproterenol
- HB-EGF
- heparin-binding EGF
- ICI
- ICI 118,551
- CREB
- cAMP response element-binding protein
- U2S
- U2 sarcoma cells
- WT
- wild type
- RFP
- red fluorescent protein
- GFP
- green fluorescent protein
- YFP
- yellow fluorescent protein
- CFP
- cyan fluorescent protein
- IP
- immunoprecipitate
- FRET
- fluorescence resonance energy transfer
- siRNA
- small interfering RNA
- CTL
- control
- PKA
- protein kinase A
- HA
- hemagglutinin
- MyrPalm
- myristoylated palmitoylated
- HEK
- human embryonic kidney.
REFERENCES
- 1.Noma T., Lemaire A., Naga Prasad S. V., Barki-Harrington L., Tilley D. G., Chen J., Le Corvoisier P., Violin J. D., Wei H., Lefkowitz R. J., Rockman H. A. (2007) J. Clin. Invest. 117, 2445–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buvinic S., Bravo-Zehnder M., Boyer J. L., Huidobro-Toro J. P., González A. (2007) J. Cell Sci. 120, 4289–4301 [DOI] [PubMed] [Google Scholar]
- 3.Drube S., Stirnweiss J., Valkova C., Liebmann C. (2006) Cell. Signal. 18, 1633–1646 [DOI] [PubMed] [Google Scholar]
- 4.Kim J., Eckhart A. D., Eguchi S., Koch W. J. (2002) J. Biol. Chem. 277, 32116–32123 [DOI] [PubMed] [Google Scholar]
- 5.Maudsley S., Pierce K. L., Zamah A. M., Miller W. E., Ahn S., Daaka Y., Lefkowitz R. J., Luttrell L. M. (2000) J. Biol. Chem. 275, 9572–9580 [DOI] [PubMed] [Google Scholar]
- 6.Mendez M., LaPointe M. C. (2005) Am. J. Physiol. Heart Circ. Physiol 288, H2111–2117 [DOI] [PubMed] [Google Scholar]
- 7.Yano S., Macleod R. J., Chattopadhyay N., Tfelt-Hansen J., Kifor O., Butters R. R., Brown E. M. (2004) Bone 35, 664–672 [DOI] [PubMed] [Google Scholar]
- 8.Vieira A. V., Lamaze C., Schmid S. L. (1996) Science 274, 2086–2089 [DOI] [PubMed] [Google Scholar]
- 9.Baass P. C., Di Guglielmo G. M., Authier F., Posner B. I., Bergeron J. J. (1995) Trends Cell Biol. 5, 465–470 [DOI] [PubMed] [Google Scholar]
- 10.Jorissen R. N., Walker F., Pouliot N., Garrett T. P., Ward C. W., Burgess A. W. (2003) Exp. Cell Res. 284, 31–53 [DOI] [PubMed] [Google Scholar]
- 11.DeWire S. M., Ahn S., Lefkowitz R. J., Shenoy S. K. (2007) Annu. Rev. Physiol. 69, 483–510 [DOI] [PubMed] [Google Scholar]
- 12.Ahn S., Shenoy S. K., Wei H., Lefkowitz R. J. (2004) J. Biol. Chem. 279, 35518–35525 [DOI] [PubMed] [Google Scholar]
- 13.Shenoy S. K., Barak L. S., Xiao K., Ahn S., Berthouze M., Shukla A. K., Luttrell L. M., Lefkowitz R. J. (2007) J. Biol. Chem. 282, 29549–29562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi H., Narita Y., Nishida M., Kurose H. (2005) Cell. Signal. 17, 1248–1253 [DOI] [PubMed] [Google Scholar]
- 15.Zheng H., Loh H. H., Law P. Y. (2008) Mol. Pharmacol. 73, 178–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rapacciuolo A., Suvarna S., Barki-Harrington L., Luttrell L. M., Cong M., Lefkowitz R. J., Rockman H. A. (2003) J. Biol. Chem. 278, 35403–35411 [DOI] [PubMed] [Google Scholar]
- 17.Kim I. M., Tilley D. G., Chen J., Salazar N. C., Whalen E. J., Violin J. D., Rockman H. A. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 14555–14560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perrino C., Naga Prasad S. V., Mao L., Noma T., Yan Z., Kim H. S., Smithies O., Rockman H. A. (2006) J. Clin. Invest. 116, 1547–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naga Prasad S. V., Barak L. S., Rapacciuolo A., Caron M. G., Rockman H. A. (2001) J. Biol. Chem. 276, 18953–18959 [DOI] [PubMed] [Google Scholar]
- 20.Christenn M., Kindler S., Schulz S., Buck F., Richter D., Kreienkamp H. J. (2007) FEBS Lett. 581, 5173–5177 [DOI] [PubMed] [Google Scholar]
- 21.Davidson A. L., Nikaido H. (1991) J. Biol. Chem. 266, 8946–8951 [PubMed] [Google Scholar]
- 22.Violin J. D., Ren X. R., Lefkowitz R. J. (2006) J. Biol. Chem. 281, 20577–20588 [DOI] [PubMed] [Google Scholar]
- 23.Ahn S., Wei H., Garrison T. R., Lefkowitz R. J. (2004) J. Biol. Chem. 279, 7807–7811 [DOI] [PubMed] [Google Scholar]
- 24.Basu S., Pathak S. K., Chatterjee G., Pathak S., Basu J., Kundu M. (2008) J. Biol. Chem. 283, 32369–32376 [DOI] [PubMed] [Google Scholar]
- 25.Han C., Michalopoulos G. K., Wu T. (2006) J. Cell. Physiol. 207, 261–270 [DOI] [PubMed] [Google Scholar]
- 26.Guerrero J., Santibañez J. F., González A., Martínez J. (2004) Exp. Cell Res. 292, 201–208 [DOI] [PubMed] [Google Scholar]
- 27.Seta K., Sadoshima J. (2003) J. Biol. Chem. 278, 9019–9026 [DOI] [PubMed] [Google Scholar]
- 28.Fuentes L. Q., Reyes C. E., Sarmiento J. M., Villanueva C. I., Figueroa C. D., Navarro J., González C. B. (2008) Cell. Signal. 20, 1642–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhai P., Galeotti J., Liu J., Holle E., Yu X., Wagner T., Sadoshima J. (2006) Circ. Res. 99, 528–536 [DOI] [PubMed] [Google Scholar]
- 30.Mifune M., Ohtsu H., Suzuki H., Nakashima H., Brailoiu E., Dun N. J., Frank G. D., Inagami T., Higashiyama S., Thomas W. G., Eckhart A. D., Dempsey P. J., Eguchi S. (2005) J. Biol. Chem. 280, 26592–26599 [DOI] [PubMed] [Google Scholar]
- 31.Zacharias D. A., Violin J. D., Newton A. C., Tsien R. Y. (2002) Science 296, 913–916 [DOI] [PubMed] [Google Scholar]
- 32.Nelson C. D., Kovacs J. J., Nobles K. N., Whalen E. J., Lefkowitz R. J. (2008) J. Biol. Chem. 283, 21093–21101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaidarov I., Krupnick J. G., Falck J. R., Benovic J. L., Keen J. H. (1999) EMBO J. 18, 871–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rozengurt E. (2007) J. Cell. Physiol. 213, 589–602 [DOI] [PubMed] [Google Scholar]
- 35.Arora P., Cuevas B. D., Russo A., Johnson G. L., Trejo J. (2008) Oncogene 27, 4434–4445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luttrell L. M., Roudabush F. L., Choy E. W., Miller W. E., Field M. E., Pierce K. L., Lefkowitz R. J. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 2449–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meng D., Lynch M. J., Huston E., Beyermann M., Eichhorst J., Adams D. R., Klusmann E., Houslay M. D., Baillie G. S. (2009) J. Biol. Chem. 284, 11425–11435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song X., Coffa S., Fu H., Gurevich V. V. (2009) J. Biol. Chem. 284, 685–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shenoy S. K., Drake M. T., Nelson C. D., Houtz D. A., Xiao K., Madabushi S., Reiter E., Premont R. T., Lichtarge O., Lefkowitz R. J. (2006) J. Biol. Chem. 281, 1261–1273 [DOI] [PubMed] [Google Scholar]
- 40.Gesty-Palmer D., Chen M., Reiter E., Ahn S., Nelson C. D., Wang S., Eckhardt A. E., Cowan C. L., Spurney R. F., Luttrell L. M., Lefkowitz R. J. (2006) J. Biol. Chem. 281, 10856–10864 [DOI] [PubMed] [Google Scholar]
- 41.Haugh J. M. (2002) Mol. Interv. 2, 292–307 [DOI] [PubMed] [Google Scholar]
- 42.Sigismund S., Argenzio E., Tosoni D., Cavallaro E., Polo S., Di Fiore P. P. (2008) Dev. Cell 15, 209–219 [DOI] [PubMed] [Google Scholar]
- 43.Caunt C. J., Rivers C. A., Conway-Campbell B. L., Norman M. R., McArdle C. A. (2008) J. Biol. Chem. 283, 6241–6252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao K., McClatchy D. B., Shukla A. K., Zhao Y., Chen M., Shenoy S. K., Yates J. R., 3rd, Lefkowitz R. J. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 12011–12016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Voisin L., Foisy S., Giasson E., Lambert C., Moreau P., Meloche S. (2002) Am. J. Physiol. Cell Physiol. 283, C446–455 [DOI] [PubMed] [Google Scholar]
- 46.Ancha H. R., Kurella R. R., Stewart C. A., Damera G., Ceresa B. P., Harty R. F. (2007) Int. J. Biochem. Cell Biol. 39, 2143–2152 [DOI] [PubMed] [Google Scholar]
- 47.Kodama H., Fukuda K., Takahashi T., Sano M., Kato T., Tahara S., Hakuno D., Sato T., Manabe T., Konishi F., Ogawa S. (2002) J. Mol. Cell Cardiol. 34, 139–150 [DOI] [PubMed] [Google Scholar]
- 48.McCole D. F., Truong A., Bunz M., Barrett K. E. (2007) J. Biol. Chem. 282, 13303–13315 [DOI] [PubMed] [Google Scholar]
- 49.Kodali R., Hajjou M., Berman A. B., Bansal M. B., Zhang S., Pan J. J., Schecter A. D. (2006) Cardiovasc. Res. 69, 706–715 [DOI] [PubMed] [Google Scholar]
- 50.Belcheva M. M., Tan Y., Heaton V. M., Clark A. L., Coscia C. J. (2003) Mol. Pharmacol. 64, 1391–1401 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.