Abstract
Nuclear import of proteins with nuclear localization signals (NLSs) is mediated by shuttling carriers, the importins. Some cargoes display more than a single NLS, and among these are homeodomain proteins such as Arx, which is critical for development of multiple tissues. Arx has two functional NLSs. The present studies show that several pathways can import Arx via its NLS2, which is within its DNA binding homeodomain. Using an in vitro nuclear import assay, we show that import of Arx via NLS2 can be mediated by importin β1, importin 9, or importin 13, with binding being strongest to importin β1. All binding is sensitive to RanGTP. Experiments based on precise domain deletions indicate that NLS2 binds impβ1, imp9, and imp13 and includes both an importin binding subdomain and a regulatory subdomain with arginine residues being important for function. Moreover, Arx can be co-precipitated with these importins when NLS2 is present. Although nuclear import of Arx can be mediated by these three importin βs, importin β1 seems to play the major role judging from in vivo small interfering RNA ablations and the in vitro import assay. This is the first evidence to show the role of importin β1 in nuclear import of paired-type homeodomain proteins. We propose a novel and possibly quite general mechanism for nuclear import of paired-type homeodomain proteins which is critical for development.
Precise nucleocytoplasmic distribution of the homeodomain superfamily of transcription factors is critical for development. Several studies have demonstrated that NLS activity resides within the 60-amino acid DNA binding homeodomain itself, which is composed of three helices (1, 2). The “paired type” subgroup of the superfamily is characterized by a set of highly conserved residues. There are 26 members of this subgroup in man. The aristaless-related homeobox (ARX)4 protein, a paired-type homeodomain containing protein, is mutated in multiple human conditions (3–5). ARX is expressed most strongly in the brain (6) and is important for development of the forebrain, pancreas, and testis (7). Arx proteins are highly conserved (Fig. 1) and contain 562 amino acids (564 amino acids in mouse) with four poly(A) (alanine) tracts of variable length, a paired-type homeodomain, and a conserved “aristaless” domain (8). Endogenous Arx was found in the nucleus of some types of nerve cells (9, 10). Because Arx has a molecular mass larger than 60 kDa, its nuclear import is likely to be signal-dependent. Three putative basic NLSs in Arx have been proposed (8, 11): NLS1 from aa 82 to 89, BC1 from aa 327 to 334, and BC2 from aa 381 to 388 (Fig. 1). It is not clear whether or how these putative NLSs function in the nuclear localization of Arx.
FIGURE 1.
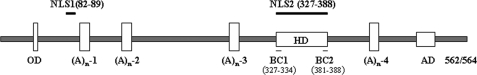
Structure of Arx protein. Arx contains four poly(A) tracts ((A)n), an N-terminal octapeptide domain (OD), a C-terminal aristaless domain (AD), and a homeobox domain (HD) (aa 331–388). Two NLSs are indicated: NLS1, aa 82–89; NLS2, aa 327–388. Two basic clusters of amino acids are shown as BC1 (aa 327–334) and BC2 (aa 381–388).
As for other trinucleotide-repeat-containing genes (12, 13), the first poly(A) tract can expand in Arx. Expansion of this tract or loss of the 3′ aristaless domain in Arx are both associated with infantile spasms syndrome and mental retardation (14, 15). Moreover, expansion of the first poly(A) tract of Arx results in formation of nuclear inclusions and an increase in cell death (16). X-linked mental retardation and dystonia are related to expansion in the second poly(A) tract of Arx (8, 17). Like Arx, the paired-type homeodomain protein Pax6 is important in neural development (18). Ploski et al. (19) found that Pax6 has only one NLS which overlaps with its homeodomain. It is recognized by importin 13, which is required for its import and is a member of importin β superfamily (19). These authors suggested that most paired-type homeodomain transcription factors including Arx are imported by importin 13 (19). Interestingly, imp13 can interact with Arx. However, whether imp13 is the only receptor responsible for nuclear import of Arx (11) is an open question.
Nuclear localization of transcription factors is essential in eukaryotes, and members of the importin β superfamily (importin βs) play key roles in signal-dependent nuclear import and export (20–31). Cargo binding and release by importin βs are regulated by the asymmetric distribution of RanGTP, which is more concentrated in the nucleus than in the cytoplasm (32). Proteins containing classic nuclear localization sequences (cNLS) are imported by the importin α/β heterodimer (26, 33), which interacts with short stretches of positively charged amino acids. cNLSs can be monopartite with five basic amino acids or bipartite, with two short basic clusters separated by a spacer (25, 34). In addition to the cNLS-mediated pathway, importin βs can function in the absence of importin α. In this situation, cargoes use a nonclassical NLS (23). Known nonclassical NLS lack conserved sequences or structures, and different nonclassical NLS are generally recognized by different importin βs.
Many proteins have at least two NLSs. Among these is the glucocorticoid receptor (GR) (35). For the GR, NLS1 is a cNLS which localizes to the C-terminal region of the DNA binding domain (36). The second GR NLS (NLS2), a nonclassical NLS, is in the ligand binding domain (35). NLS2-mediated import of the GR is slower than import via NLS1 and is hormone-dependent (37). It, therefore, is likely to require a structural change that affects NLS1. Recent observations suggest that nuclear import of the GR is accomplished by multiple transport proteins, providing the opportunity for context-specific regulation (38, 39); however, the exact contribution of import receptor interactions with NLS2 in the context of full-length GR has not yet been elucidated.
Importin β1 can transport many cargoes directly as well as cargoes with basic NLSs via importin α and import uridine-rich small nuclear ribonucleoproteins via snurportin. Importin 9 imports histones and ribosomal proteins (23). Importin 13 (imp13, also known as lgl2, IPO13, or Kap13) is one of the few importins that can transport cargoes bi-directionally (40). Its expression and function are developmentally regulated (41, 42), and the expression level of imp13 is higher in the brain than in other tissues (41–43). Several cargoes of imp13 have been identified: Rbm8, Ubc9 (40), Pax6 (19), the NF-YB/NF-YC dimer (44), the glucocorticoid receptor (39), and myopodin (45). imp13 exports eIF-1A (40). imp13 plays a role in meiotic differentiation of mouse germ cells by regulating the nuclear import of ubiquitin-conjugating enzyme 9 (46).
There is a so-called redundancy in terms of multiple importin βs being able to mediate nuclear import of certain proteins. A good example is that of the importin βs binding NLS of ribosomal protein rpL23a, which can be recognized by importin β1, importin β2, importin 5, and importin 7 (47). Moreover, c-Jun can be transported into the nucleus by importin β1, importin β2, importin 7, and importin 9 (48). In the present study two functional NLS domains of Arx have been characterized. Nuclear import of Arx via NLS2, which largely overlaps with the homeobox domain, is mediated by multiple pathways including imporin β1, importin 9, and importin 13, with importin β1 playing the major role. Moreover, nuclear import of Arx via importin β1 is not enhanced by the addition of either importin 9 or importin 13. Yet-unidentified factors, therefore, may influence the choice of nuclear import pathway of Arx.
EXPERIMENTAL PROCEDURES
Construction of ARX Plasmids
All expression vectors were constructed by integrating specific PCR products into different expression vectors. All PCR reactions for ARX mutation were done with the Takara LA Taq with GC buffer (#DRR20AG, Takara, Japan), and restriction enzymes and T4 ligase were purchased from Takara Co., Japan. The sequences of primers and the description of plasmids are summarized as supplemental materials. All plasmids were sequence-verified. Schematic diagrams of the ARX mutants are shown in Figs. 2A and 7A.
FIGURE 2.
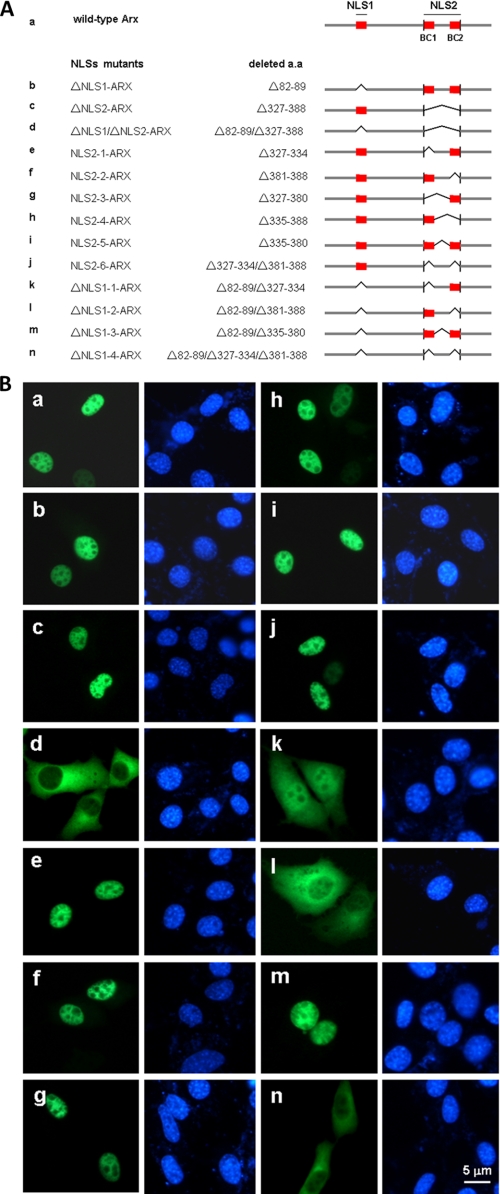
Arx is a nuclear protein and has two functional NLSs. EGFP-tagged murine Arx and its mutants were transiently expressed in NIH3T3 cells. In panel A, schematics represent deletion mutants of NLSs of Arx. Bridges indicate deleted segments. BC represents basic clusters in the homeodomain. Domains of NLS1, BC1, and BC2 are colored in red. Subcellular localization of Arx and its mutants were shown in panel B. Wild-type Arx (a) is primarily in the nucleus. ΔNLS1-Arx lacking either putative NLS1 (aa 82–89) (b) or ΔNLS2-Arx lacking putative NLS2 (aa 327–388) (c) still localizes to the nucleus. Deletion of both putative NLS1 and NLS2 blocks nuclear localization of Arx (d). When NLS1 is present, deletion of each basic cluster of the homeodomain in NLS2 or both does not result in mislocation of Arx (e, f, g, h, and j). Deletion of the intervening fragment in the homeodomain also does not change the subcellular location of Arx (i). Note that both basic clusters of the homeodomain are necessary for nuclear import of Arx when NLS1 is absent (k and l). Deletion of the intervening fragment of the homeodomain did not change the nuclear location of Arx even when NLS1 is absent (m). When NLS1 is absent, deletion of both BC domains relocates Arx into the cytoplasm (n).
FIGURE 7.
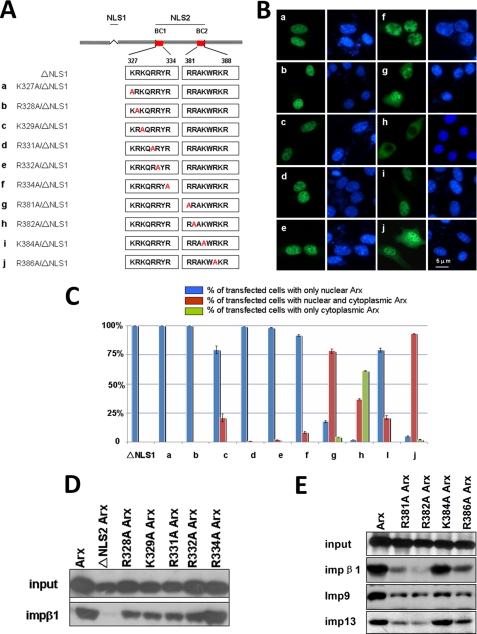
The R382A mutation in NLS2 abolishes its function in Arx by blocking its interaction with impβ1. Most positively charged amino acids in the homeobox were substituted by alanine residues as shown in panel A. These mutants tagged with EGFP were transiently expressed in NIH3T3 cells. As shown in panel B, mutations K327A, R328A, K329A, R331A, R332A, and R334A in the BC1 domain (a–f) did not mislocalize ΔNLS1-Arx. These mutants are still in the nuclei. By contrast, mutations R381A, R382A, and R386A in the BC2 domain of (g, h, and j) mislocalized ΔNLS1-Arx into the cytoplasm of most cells. Mutant K384A still shows a dominant nuclear location of Arx (i). Statistical data of these experiments is summarized in panel C. Among these mutants, mutant R382A showed that about 60% of transfected cells had only cytoplasmic signal, and more than 30% of R382A mutant-expressing cells had both cytoplasmic and nuclear Arx. By contrast, cells expressing ΔNLS1-Arx show only nuclear signal, suggesting that arginine 382 is an important residue for NLS2 function. In panel D, most substitution mutants of positively charged amino acids in the homeobox were expressed with an EGFP tag, and lysates were used to test their interactions with GST-tagged impβ1. Bound Arx and its mutants were detected by an anti-EGFP antibody. As shown, mutants R328A, K329A, R331A, R332A, and R334A in the BC1 domain still show significant bindings to impβ1, in comparison with the binding of ΔNLS2-Arx to impβ1. In panel E, each of R381A, R382A, K384A, and R386A in the BC2 domain in the homeobox was expressed, and lysates were used to test their interactions with GST-tagged impβ1, imp9, and imp13. As shown, mutants of R381A, R382A, or R386A show modest binding to impβ1 and show reduced interactions with imp9 and imp13. Note that mutant K384A shows significant interactions with impβ1, imp9, and imp13. Error bars show S.D.
Site-directed Mutagenesis
Site-directed mutagenesis in plasmids pARX or pΔNLS1-ARX was done by PCR. A pair of primers for the PCR reaction was designed to overlap with each other to create a substitution of arginine or lysine with an alanine residue at specific sites (supplemental materials). The total volume for PCR reaction was 20 μl. The extension time is 8 min, and 14 cycles were set for each mutagenesis reaction. After PCR, 0.5 μl of DpnI (New England Biolabs) and 2.2 μl of its buffer were added to the reaction mixture, and then these mixtures were incubated at 37 °C for 3 h. The mixtures were then transfected into DH5α competent cells and screened for correct mutants by sequencing.
Preparation of Cell Lysates
Twenty-four hours after transfection, cells grown on 100-mm plates were washed with cold phosphate-buffered saline. Cells were scraped into 1 ml of lysis buffer (50 mm HEPES-NaOH (pH 7.5), 100 mm NaCl, 0.5% Nonidet P-40, 2.5 mm EDTA, 10% glycerol, 1 mm dithiothreitol, 1 mm phenylmethylsulfonyl fluoride, and 1% proteinase inhibitor mixture (#P8340, Sigma)) and lysed on ice for 30 min with gentle shaking. The mixture was spun at 15,000 × g for 30 min at 4 °C, and the supernatant was collected for GST pulldown assays or Western blotting.
GST Pulldown Assay
Both GST-tagged impβ1, imp4, imp9, and imp13 and Arx-His6 were overexpressed by isopropyl 1-thio-β-d-galactopyranoside induction in bacteria. Cells expressing GST-tagged proteins were sonicated in 1× phosphate-buffered saline, and the supernatants were incubated with glutathione-Sepharose 4B beads. The purified Arx-His6 and lysates of cells expressing EGFP-tagged Arx and its mutants were incubated with glutathione-Sepharose 4B beads saturated with either impβ1, imp4, imp9, and imp13-GST or GST for 3 h at 4 °C. Washed beads were mixed with protein loading buffer and boiled for SDS-PAGE-Western blotting. About 3% of each input and 50% of each bound sample were loaded for each Western blotting analysis.
Co-immunoprecipitation
Immunoprecipitation was performed as described previously (49). In brief, extracts from transfected cells were prepared with Nonidet P-40-containing transport buffer (20 mm Hepes, 110 mm potassium acetate, 2 mm magnesium acetate, 5 mm sodium acetate, 0.5 mm EGTA, 1 μg/ml protease inhibitor mixture of aprotinin, leupeptin, and pepstatin, and 0.2% Nonidet P-40). About 5 × 106 cells were suspended in 200 μl of buffer. Samples were precleared by incubation with protein G-plus-agarose beads (#sc2002, Santa Cruz) at 4 °C for 1 h and then incubated with 1 μg of a monoclonal antibody against GFP (#ab1218, Abcam Co.) with 20 μl of protein G-plus-agarose at 4 °C for 4 h. Agarose beads were washed with transport buffer and suspended in SDS-PAGE sample buffer and analyzed subsequently by Western blotting. Endogenous impβ1, imp9, and imp13 in the precipitated complex were detected by an anti-impβ1 antibody (#ab2811, Abcam), anti-imp9 antibody (#ab52605, Abcam), and anti-imp13 antibody (39), respectively. About 10% of each input and 25% of each bound sample were loaded for each Western blotting analysis.
RanGTP Binding Assay
Wild-type Ran-GST and RanQ69L-GST were expressed in DH5α cells using isopropyl 1-thio-β-d-galactopyranoside induction. Bacterial cell lysates were incubated with glutathione-Sepharose 4B beads, which were then subjected to cleavage with thrombin in 200 μl of binding buffer, followed by the addition of 1 mm phenylmethylsulfonyl fluoride. 2 mm GDP and 2 mm GTP were incubated with purified Ran and RanQ69L, respectively, in the presence of 50 mm Hepes-NaOH (pH 7.3), 200 mm NaCl, 5 mm MgCl2, 5 mm β-mercaptoethanol for 2 h at room temperature. impβ1-GST, imp4-GST, imp9-GST, and imp13-GST fusion proteins were bound to glutathione-Sepharose 4B beads in a binding buffer containing 50 mm Tris-HCl (pH 7.5), 200 mm NaCl, 5 mm MgCl2, and 5 mm β-mercaptoethanol overnight at 4 °C. Beads were washed with the binding buffer and then incubated with bacterial lysates containing Arx-His6 in the binding buffer at 4 °C for 4 h. Supernatants were removed, and beads were washed with the binding buffer. Then beads were separated into three equal parts, which were incubated with RanGDP, RanQ69LGTP, or the control buffer only at room temperature for 30 min. Samples were separated on 10% SDS-PAGE and analyzed by the Western blot with an anti-His6 antibody. About 10% of each input and 50% of each bound sample were loaded for each Western blotting analysis.
In Vitro Import Assay
Briefly, HeLa cells were grown on 10-mm glass coverslips to 40–80% confluence. Permeabilization was done with 40 μg/ml digitonin (#D5628, Sigma) for 5 min on ice. The permeabilized cells were incubated for 30 min at 30 or 4 °C with 25 μl of a transport reaction mix consisting of cargo (1 μm) and recombinant importin βs (1 μm) in transport buffer (20 mm Hepes-KOH (pH 7.3), 110 mm potassium acetate, 5 mm magnesium acetate, 1 mm EGTA, 2 mm dithiothreitol, and 250 mm sucrose) supplemented with an energy regenerating system (0.5 mm ATP, 0.5 mm GTP, 10 mm creatine phosphate, and 50 μg/ml creatine kinase and 3 μm Ran (GDP)). The import reaction was stopped by adding 500 μl of transport buffer. Cells were fixed by using 4% paraformaldehyde for 15 min on ice. For negative controls, the assay was done in the absence of recombinant transport factors. Cells were visualized by a Leica confocal microscope (model SPII AOBS). The coding regions for the His6-tag fusion proteins were cloned as follows; murine Arx and R382A/ΔNLS1-Arx were inserted at BamHI/HindIII sites of pET28a vector, and then an EGFP fragment was inserted at HindIII/XhoI sites. A DNA sequence encoding the fragment of vitamin D receptor (aa 4–232) (VDR) was inserted into the vector pGEX4T-2-EGFP to express a truncated VDR tagged with both GST and EGFP. This purified protein was used as a positive control for the activity of imp4 (50). In all experiments, a minimum of 25 cells was analyzed for each determination in each of three experiments.
Kinetic Assay
The kinetic assay was followed as previously described (41, 51). In brief, purified ΔNLS1-Arx-His6-EGFP (1 μm) was added to digitonin-permeabilized cells as the transport cargo along with purified impβ1 (1 μm), imp9 (1 μm), imp13 (1 μm), or mixtures of these importins. The influx of the cargo into nuclei was recorded in real time by a Leica confocal microscope (model SPII AOBS). A series of images was then acquired (5-s intervals) to visualize nuclear fluorescence (excluding the signal at the perimeter of the nucleus). Nuclear fluorescence was quantitated using Metamorph software (Universal Imaging Corp.). Background values from outside the cell were subtracted. Values of nuclear fluorescence were calculated by averaging fluorescence values from three random areas in each nucleus. In all assays at least five nuclei were analyzed for each determination in each of three experiments. The value of nuclear fluorescence at the point of 250 s after the addition of cargoes was used as 100%. Half-times for nuclear accumulation of cargoes were determined when nuclear fluorescence reached to 50% that of the 250-s point of nuclear import. Import was performed at 20 °C in the transport buffer.
Cell Culture and Transfection
Cells of N2a (a mouse neuroblastoma cell line, kindly provided by Dr. Huaxi Xu from the Burnham Institute), NIH3T3, HeLa, and 293T were grown in Dulbecco's modified Eagle's medium (Invitrogen) plus 10% de-complemented fetal bovine serum and incubated at 37 °C in a 5% CO2, air incubator. For microscopic examination, cells on glass coverslips (12 mm) were usually seeded at 5.0 × 104 cells/coverslip the day before transfection. Plasmid DNA (0.5 μg/coverslip) was transfected into cells using the Lipofectamine 2000 kit (#11668-019′Invitrogen). Subcellular distributions of Arx and its mutants were documented by either a Nikon Eclipse 80i fluorescence microscope or a Leica confocal microscope (model SPII AOBS). In all experiments a minimum of 25 cells was analyzed for each determination in each of three experiments. For preparation of cell lysates, cells were usually seeded at 1 × 106 cells/100-mm plate the day before transfection. 5–10 μg of plasmid DNA/plate were transfected into cells. Transfected cells were lysed for further analysis after 24 h.
Indirect Immunofluorescence Staining
Coverslips of adherent cells were fixed with 3.7% formaldehyde for 30 min on ice and then quenched and permeabilized with 0.1 m glycine (pH 7.0), 0.1% Triton X-100 in phosphate buffer saline (52). These coverslips were stained with an anti-impβ1 or anti-imp13 antibody for 1 h at room temperature and subsequently labeled with a rhodamine-conjugated secondary antibody for another hour at room temperature. In all experiments a minimum of 25 cells was analyzed for each determination in each of three experiments.
Small Interfering RNA (siRNA) Silencing
To silence imp13, a pGCsilencer U6/Neo/GFP/imp13 plasmid expressing a mouse imp13 siRNA (5′-ccgaccaguaugaaagcuuaa-3′) (45, 46) (GENECHEM, Shanghai, China) or a pSilencer1.0-U6/GFP/imp13 plasmid expressing a human imp13 siRNA (5′-ggugccugagauccaguactt-3′) (Ambion) (39) was transfected in NIH3T3, 293T, and HeLa cells. To silence impβ1, plasmids pGENEsil-1/U6/EGFP expressing a mouse impβ1 siRNA (5′-gaguugcagcuggucuacaaauuaa-3′) (53) or a human impβ1 siRNA (5′-aagggagcacuacaguaucug-3′) (Genesil Co., Wuhan, China) was transfected in NIH3T3, 293T, and HeLa cells. A plasmid to express an RNA without homology to human or mouse sequences was used as a control in silencing experiments (39). After 24 h, cells were transfected with DsRed-tagged ΔNLS1-Arx. After 48–72 h, cells were fixed or lysed, and protein levels of endogenous impβ1 and imp13 were evaluated by indirect immunofluorescence or Western blotting. Subcellular localization of DsRed-tagged ΔNLS1-Arx was documented by either a Nikon Eclipse 80i fluorescence microscope or a Leica confocal microscope (model SPII AOBS). In all experiments a minimum of 25 cells was analyzed for each determination in each of three experiments.
Statistical Analysis
All data are presented as the mean ± S.E. Statistical significance was determined by two-way analysis of variance. Pair-wise group comparisons were then assessed using Student-Neuman-Keuls test.
RESULTS
Arx Is a Nuclear Protein and Has Two Functional NLSs
Because Arx is important for brain development, we have studied Arx in N2a cells, which are of neuronal origin, as well as in NIH3T3 cells. Like endogenous Arx in the nucleus of GABAergic neurons (9, 10), EGFP-tagged Arx localizes primarily to the nucleus of NIH3T3 (Fig. 2B) and N2a cells (data not shown). By scanning the sequences of human and mouse Arx, we and others (8) predicted that there are three putative cNLSs at amino acids 82–89, 327–334, and 381–388 of mouse Arx (Fig. 1). To test whether these domains are functional, we deleted them from EGFP-tagged mouse Arx (Fig. 2, A and B). Plasmids (Fig. 2A) expressing Arx and its mutants were transiently expressed in NIH3T3, N2a, and 293T cells. As shown in Fig. 2, B, b, e, and f, deletion of each single putative NLS separately did not change the nuclear location of Arx. However, when all three domains were removed, Arx no longer localizes to the nucleus (Fig. 2Bn), suggesting that the putative NLSs have overlapping import function and that there is no other NLS in Arx. Each of the putative cNLSs could function in nuclear import of Arx. The subcellular distributions of Arx and its mutants in N2a and 293T cells are identical to those in NIH3T3 cells (data not shown).
To learn whether each of these domains functions in nuclear import of Arx, we first removed putative NLS1 (Δ82–89) and then deleted either putative BC1 (Δ327–334) or BC2 (Δ381–388) from NLS1-deleted Arx. Although NLS1-deleted Arx localizes to the nucleus (Fig. 2Bb), further deletion of either BC1 (Fig. 2Bk) or BC2 (Fig. 2Bl) mislocalized Arx to the cytoplasm. These data suggest that neither BC1 nor BC2 can function independently in nuclear import of Arx. Because the double deletion of residues 82–89 and 327–388 mislocalized Arx (Fig. 2Bd) but the double-deletion of residues 82–89 and 335–380 (Fig. 2Bm) did not, we conclude that the two basic clusters (327–334 and 381–388) work together as a functional NLS. We, therefore, call the fragment from aa 82–89 NLS1 and the fragment from aa 327–388 NLS2.
Arx Interacts with Multiple Importin βs via NLS2 and Has the Greatest Affinity for Importin β1
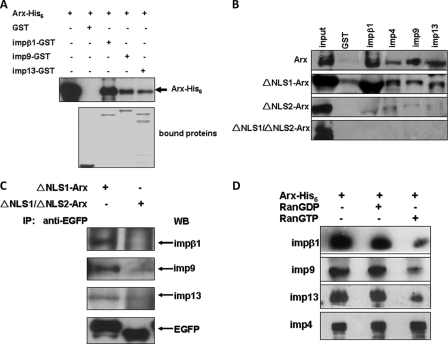
Importin 13 was proposed to be the transportor of nuclear Arx (11, 19), and the interaction of imp13 with Arx was also detected in our yeast two-hybrid screening with a C-terminal fragment of imp13 lacking the Ran binding domain as bait (data not shown). To test whether other importin βs are able to function in nuclear import of Arx, several importin βs available in this laboratory were tested. Beside imp13, importin β1 (impβ1) and importin 9 (imp9) interact with Arx (Fig. 3A) in GST pulldown assays when Arx-His6 and GST-tagged importin βs were expressed in Escherichia coli, respectively, suggesting that these interactions are direct. Interestingly, although the amount of immobilized impβ1-GST was less than for imp9 or imp13 (Fig. 3A, bottom panel), the amount of bound Arx was much greater for impβ1 than for imp9 or imp13. Therefore, Arx has a higher affinity for impβ1 than for imp9 or imp13. EGFP-tagged Arx expressed in 293T cells also binds impβ1, imp9, imp13, and also importin 4 (imp4) as judged by incubating lysates of the EGFP-Arx-expressing cells with the GST-tagged importin βs (Fig. 3B, top panel).
FIGURE 3.
Arx interacts with impβ1, imp4, imp9, and imp13 via its NLS2, and bindings of Arx to impβ1, imp9, and imp13 are sensitive to RanGTP. In panel A, His6-tagged full-length Arx and GST-tagged impβ1, imp9, and imp13 were expressed in E. coli and purified. In GST pulldown experiments, bound Arx was detected by an anti-His6 antibody. As shown, Arx interacted with impβ1, imp9, and imp13. Bound GST alone was used as a negative control. The amounts of bound GST and GST-tagged importin βs were indicated by Coomassie Blue stain. Judging from the intensity of the anti-His6 signal, the amount of Arx bound to impβ1 is much stronger than to the other importin βs. In panel B, lysates of 293T cells expressing EGFP-tagged wild-type Arx, ΔNLS1-Arx, ΔNLS2-Arx, and ΔNLS1/NLS2-Arx were incubated with GST-tagged impβ1, imp4, imp9, or imp13. Bound Arx was detected by an anti-EGFP antibody. As shown, both wild-type Arx and ΔNLS1-Arx bind impβ1, imp4, imp9, and imp13 (top two panels). ΔNLS2-Arx and ΔNLS1/ΔNLS2-Arx did not bind these importin βs (bottom two panels), suggesting that interactions of Arx with these importin βs are mediated by NLS2. In panel C, EGFP-tagged ΔNLS1-Arx and ΔNLS1/ΔNLS2-Arx were expressed in 3T3 cells. An antibody against GFP was incubated with cell lysates containing either EGFP-tagged ΔNLS1-Arx or ΔNLS1/ΔNLS2-Arx. Precipitated complexes (IP) were analyzed by SDS-PAGE, and endogenous impβ1, imp9, and imp13 were detected using the corresponding antibodies. As shown, all of these importin βs bind ΔNLS1-Arx, but none of them forms complexes with ΔNLS1/ΔNLS2-Arx, suggesting that the binding of Arx to these importin βs is NLS2-dependent. WB, Western blot. In panel D, Arx-His6 and GST-tagged impβ1, imp4, imp9, and imp13 were expressed and purified form E. coli. Immobilized impβ1, imp4, imp9, and imp13 on glutathione-Sepharose beads were incubated with Arx-His6. After washing, mixtures of recombinant impβ1-GST, imp4-GST, imp9-GST, or imp13-GST and Arx-His6 were incubated with either GDP-loaded Ran or GTP-loaded RanQ69L. After washing, the bound proteins were eluted with SDS-PAGE sample buffer and subjected to SDS-PAGE followed by Western blot analysis. Note that in the presence of GTP-loaded RanQ69L, the amount of Arx bound to impβ1, imp9, or imp13 was significantly reduced by comparison to bound Arx in the presence of GDP-loaded Ran. Note that RanGTP did not disassociate the imp4·Arx complex.
Importin βs directly or indirectly bind NLSs of transport cargoes. Given that Arx has two NLSs, we sought to determine which one is recognized by these importins. Based on our yeast two-hybrid screening, the C-terminal fragment (aa 288–562) of human Arx, which includes NLS2 (Fig. 1) can bind imp13 (data not shown). Because we cannot exclude the possibility of interaction of NLS1 with these importin βs, we expressed wild-type Arx and several Arx mutants (lacking NLS1, NLS2, or both NLS1 and NLS2). In GST pulldown experiments (Fig. 3B, second panel), as for wild-type Arx, deletion of NLS1 did not affect the binding of impβ1, imp4, imp9, or imp13 to Arx; however, deletion of NLS2 (Fig. 3B, third panel) or double-deletion of NLS1 and NLS2 (Fig. 3B, bottom panel) eliminated binding of these importin βs to Arx. To further confirm that Arx binds these importins via NLS2 in vivo, we expressed EGFP-tagged ΔNLS1-Arx and ΔNLS1/ΔNLS2-Arx in NIH3T3 cells. The lysates were incubated with an anti-EGFP antibody. As shown in Fig. 3C, endogenous impβ1, imp9, and imp13 were found to form the complexes with ΔNLS1-Arx and show modest binding to ΔNLS1/ΔNLS2-Arx. Thus, binding of Arx to these importin βs is mediated by NLS2.
As shown in Fig. 3, B and C, Arx lacking NLS2 did not bind impβ1, imp4, imp9, or imp13. We also tested possible interactions between Arx fragments (aa 1–90 or 1–326) containing NLS1 with these importin βs. None of them showed binding (data not shown), suggesting that other pathways transport Arx via NLS1.
Nuclear Import of Arx via NLS2 Is Mediated by Multiple Pathways
Because impβ1, imp4, imp9, and imp13 bind Arx via NLS2, it is important to test whether they can transport Arx into the nucleus. Upon import, binding of RanGTP to import complexes releases cargoes from importin βs (25). Thus, if Arx can be released from these importin βs by RanGTP, we can consider that Arx is an import cargo. To this end, GDP-loaded Ran and GTP-loaded RanQ69L, a non-hydrolyzable form of Ran (40, 41), were incubated with the complex of Arx-His6·impβ1-GST, Arx-His6·imp4-GST, Arx-His6·imp9-GST, or Arx-His6·imp13-GST. After incubation, bound Arx-His6 was analyzed by Western blotting with an anti-His6 antibody. As shown in Fig. 3D, the amounts of Arx bound to either impβ1, imp9, or imp13 was greatly reduced in the presence of RanGTP when compared with RanGDP controls, suggesting that Arx is indeed an import cargo of impβ1, imp9, and imp13. Binding to imp4 was not affected by RanGTP.
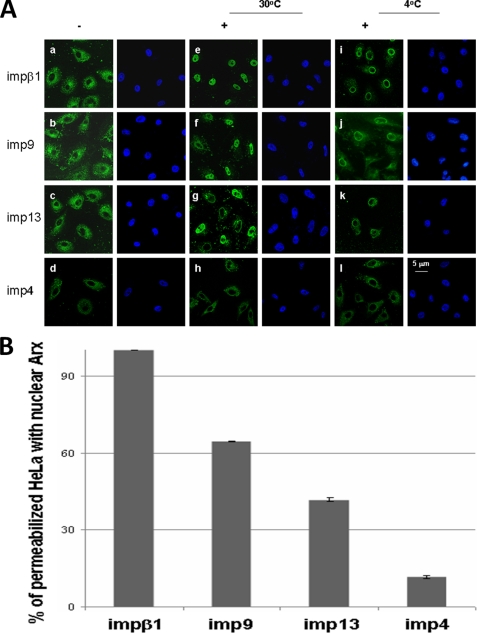
To test whether these importin β family members can transport Arx, we have used the digitonin-permeabilized HeLa cell in in vitro import assay (55). Both EGFP-tagged Arx-His6 and GST-tagged importin βs were overexpressed and purified from E. coli. The GST tag was then removed by thrombin. As shown, impβ1 (Fig. 4Ae), imp9 (Fig. 4Af), and imp13 (Fig. 4Ag) but not imp4 (Fig. 4Ah) can transport Arx into the nucleus at 30 °C. Nuclear Arx-EGFP can be found in more than 90% of permeabilized HeLa cells in the presence of impβ1 (Fig. 4B). More than 60% of permeabilized cells had nuclear Arx-EGFP in the presence of imp9. Less than 50% of permeabilized cells had nuclear Arx-EGFP in the presence of imp13. The VDR is a cargo of imp4 (50), and VDR was imported in the nucleus of permeabilized cells (supplemental figure). By contrast less than 15% of permeabilized cells had nuclear Arx-EGFP in the presence of imp4. Arx was not found in the nucleus with any of these importin βs after incubation at 4 °C (i, j, k, and l in Fig. 4A). These results suggest that Arx can be transported into the nucleus by impβ1, imp9, and imp13. impβ1 imports Arx more efficiently than imp9 or imp13, and imp4 plays modest a lesser role (if any) in import of Arx.
FIGURE 4.
impβ1, imp9, and imp13 mediate the import of Arx into nuclei of digitonin-permeabilized cells. HeLa cells were permeabilized with digitonin, incubated for 30 min with the import mixtures at 30 °C, washed, and fixed. In panel A, EGFP-Arx-His6 is imported into the nuclei in the presence of impβ1 (e), imp9 (f), or imp13 (g) but not imp4 (h). EGFP-Arx-His6 was not found in nuclei in the absence of impβ1 (a), imp9 (b), or imp13 (c). At 4 °C, none of these importin βs could transport the cargo into the nuclei (i, j, and k). A rim signal that indicates binding to the nuclear pore complexes is visible in all cases (i, j, and k). Interestingly, imp4 does not import Arx (d, h, and l). –, without importins; +, with importins. Note in panel B that nuclear Arx-EGFP can be found in more than 90% of permeabilized HeLa cells in the presence of impβ1. More than 60% of permeabilized cells had nuclear Arx-EGFP in the presence of imp9, but less than 50% of permeabilized cells had nuclear Arx signal in the presence of imp13. imp4 had little effect on importing Arx (less than 15% of permeabilized cells). Error bars show S.D.
Importin β1 Plays a Major Role in Nuclear Import of Arx via NLS2
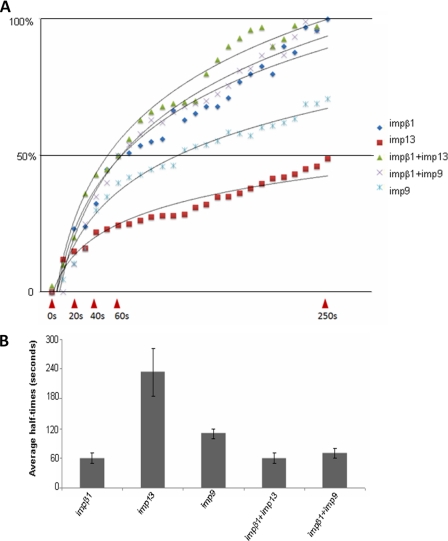
Although in vitro experiments show that multiple importin βs (impβ1, imp9, and imp13) bind and transport Arx into the nucleus, it is of interest to ask whether nuclear import of Arx via NLS2 could be enhanced by the combinations of these importin βs. To test this hypothesis, we used the permeabilized cell assay to measure the kinetics of import of ΔNLS1-Arx in the presence of either impβ1 alone, imp9 alone, imp13 alone, impβ1 with imp9, or impβ1 with imp13. As shown in Fig. 5, the half-times for nuclear accumulation of Arx mediated by impβ1, imp9, and imp13 are 60 ± 10, 110 ± 10, and 230 ± 50 s, respectively. The addition of imp13 to impβ1 did not shorten the half-time (60 ± 10 s) for nuclear accumulation of the cargo, although we did see a slight increase of nuclear Arx. Similar results were observed when imp9 was added to impβ1 in this assay. These observations suggest that impβ1 plays a major role in nuclear import of Arx via NLS2 in vitro.
FIGURE 5.
Quantitation of Arx influx into nuclei with different importin βs in vitro. In in vitro nuclear import assays the rates of nuclear accumulation of EGFP-tagged ΔNLS1-Arx with different importin βs were measured in real-time by confocal microscopy. In panel A, the fluorescence of intranuclear regions of interest of digitonin-permeabilized cells incubated with cargo EGFP-tagged ΔNLS1-Arx and different importin βs (see Fig. 4) was quantitated using Metamorph software. Five representative influx curves of EGFP-tagged ΔNLS1-Arx with impβ1, imp9, imp13, impβ1/imp9, and impβ1/imp13 are shown. Curves are normalized to facilitate comparisons. In panel B, half-times for nuclear accumulation of cargo EGFP-tagged ΔNLS1-Arx imported by different importin βs or their mixtures were measured as described under “Experimental Procedures.” Shown are the averages of three assays of import of cargo EGFP-tagged ΔNLS1-Arx with different importin βs or their mixtures. A minimum of five nuclei was analyzed for each determination in each of three assays. Error bars show S.D.
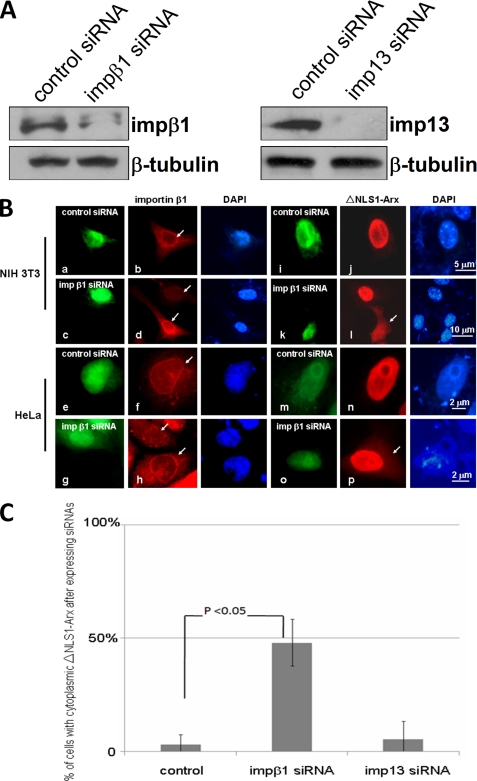
To learn which pathway(s) is important for in vivo import of ΔNLS1-Arx, we transiently expressed impβ1 siRNAs (human and mouse (53)) and imp 13 siRNAs (human (39) and mouse (46)) into HeLa and NIH3T3 cells, respectively. Expression of the siRNAs was identified by co-expression of GFP. As shown in Fig. 6A and B, d and h, both impβ1 and imp13 siRNAs were found to be able to reduce the amounts of endogenous impβ1 and imp13 in both the Western blotting analysis and the immunofluorescent staining, respectively. Interestingly, expression of impβ1 siRNAs partially relocated ΔNLS1-Arx from the nucleus into the cytoplasm (Fig. 6B, l and p). Statistically, the cytoplasmic signal of ΔNLS1-Arx was observed in nearly 50% of NIH3T3 cells expressing impβ1 siRNA. Only a few NIH3T3 cells expressing imp13 siRNA showed cytoplasmic signal of ΔNLS1-Arx. Based on these observations, we conclude that impβ1 plays a major role in nuclear import of ΔNLS1-Arx.
FIGURE 6.
Silencing of impβ1 partially mislocalizes ΔNLS1-Arx into the cytoplasm in vivo. siRNAs for impβ1 and imp13 were expressed in NIH3T3 and HeLa cells, respectively. After 48 h, endogenous impβ1 and imp13 in 3T3 cells were analyzed by Western blotting as shown in panel A. Amounts of both impβ1 and imp13 were partially reduced. Endogenous tubulin was used a loading control. In panel B, endogenous impβ1 was detected by an anti-impβ1 antibody (arrows). As shown in d and h, the siRNAs of both mouse and human impβ1 reduces the level of impβ1. By contrast, the control shows no changes of impβ1 (b and f). Note that some DsRed-tagged ΔNLS1-Arx is found in the cytoplasm when impβ1 siRNAs was expressed (l and p, arrows). No cytoplasmic signal of DsRed-tagged ΔNLS1-Arx was found in cells expressing control plasmids (j and n) or in cells without impβ1 siRNAs expression (l). The green signal in this panel represents GFP co-expressed with the siRNAs. DAPI, 4′,6-diamidino-2-phenylindole. In panel C, when impβ1 siRNA was expressed in 3T3 cells, near 50% show cytoplasmic DsRed-tagged ΔNLS1-Arx. Nevertheless, imp13 siRNA does not relocate DsRed-tagged ΔNLS1-Arx in 3T3 cells. A minimum of 25 cells coexpressing DsRed-tagged ΔNLS1-Arx and siRNA was analyzed for each determination in each of three experiments. Error bars show S.D.
Arginine Residues in BC2 of the Homeobox Are Essential to the Function of NLS2 of Arx
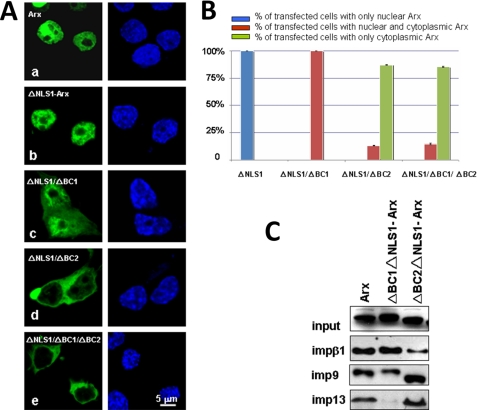
To examine the role of NLS2 more closely, we replaced positively charged amino acids in NLS2 of ΔNLS1-Arx by alanine residues (Fig. 7A). All mutants were expressed in NIH3T3, N2a, and 293T cells. As shown, none of mutations in the first basic cluster domain (BC1, aa 327–334, Fig. 7A) of the homeobox resulted in mislocalization of Arx (a–f in Fig. 7, A and B). By contrast, although mutation of lysine residue at 384 in the second basic cluster (BC2, aa 381–388, Fig. 7A) did not change the nuclear localization of Arx (i in Fig. 7, A and B), mutation of other positively charged amino acids (R381A, R382A, and R386A) in this domain mislocalized Arx (g, h, and j in Fig. 7, A and B). Interestingly, R382A-Arx caused cytoplasmic localization of Arx in more than 90% of transfected cells, and about 60% of them showed no nuclear Arx (h in Fig. 7, A–C). These observations suggest that the BC2 domain in the homeobox plays an important role in the function of NLS2. Similar results were observed in N2a and 293T cells (data not shown).
To understand why these mutations mislocalized ΔNLS1-Arx, the mutants were expressed in 293T cells, and lysates were used in GST pulldown protocols. As shown in Fig. 7D, interactions between impβ1 with mutants in the BC1 domain were not significantly reduced, in comparison to the binding between ΔNLS2-Arx and impβ1. These mutations have only modest effects on the binding between Arx and imp9 or imp13 (data not shown). By contrast, interactions between R382A-Arx and impβ1, imp9, or imp13 were almost abolished (Fig. 7E). Interactions between mutant R381A-Arx or R386A-Arx and impβ1, imp9, or imp13 are also much weaker than for wild-type Arx (Fig. 7E). Binding of K384A-Arx to impβ1, imp9, and imp13 changed very little by comparison with the binding of wild-type Arx (Fig. 7E). These data suggest that arginine residues at 381, 382, and 386 are important for the function of NLS2 in Arx and that they are involved in the recognition of NLS2 by impβ1, imp9, and imp13. When interactions between impβ1, imp9, and imp13 with NLS2 via these amino acids are abolished, nuclear import of Arx via NLS2 is impaired.
As positively charged amino acids within the BC1 or BC2 domain have a different impact on the function of NLS2, it is of interest to understand their individual contributions. To this end, we deleted BC1 and BC2 in EGFP-tagged ΔNLS1-Arx and transiently expressed them in N2a, NIH3T3, and 293T cells. As shown in Fig. 8, mutant ΔNLS1ΔBC1-Arx localized to both the cytoplasm and the nucleus of N2a cells (Fig. 8, Ac and B), and its interaction with impβ1 was only modestly reduced (Fig. 8C). By contrast, its interaction with imp13 was greatly reduced (Fig. 8C), and its binding to imp9 was reduced by about half (Fig. 8C). Interestingly, mutant ΔNLS1ΔBC2-Arx was found mainly in the cytoplasm (Fig. 8, Ad and B). Interaction between this mutant with impβ1 was significantly reduced (Fig. 8C). Surprisingly, interaction between this mutant with imp9 or imp13 was little-affected (Fig. 8C), suggesting that these importin βs bind to NLS2 through different mechanisms. Similar results were observed in NIH3T3 and 293T cells (data not shown). BC2, therefore, mediates impβ1 binding, whereas BC1 binds imp13. BC2 may regulate the binding of imp13 to NLS2, whereas imp9 seems to bind both BC1 and BC2.
FIGURE 8.
The BC2 domain is more important for the function of NLS2 than the BC1 domain. Plasmids expressing EGFP-tagged ΔBC1ΔNLS1-Arx, ΔBC2ΔNLS1-Arx, or ΔBC1ΔBC2ΔNLS1-Arx were transfected into N2a cells. As shown in panel A, both ΔBC2ΔNLS1-Arx (d) and ΔBC1ΔBC2ΔNLS1-Arx (e) show much less nuclear signal than ΔBC1ΔNLS1-Arx (c), although deletion of only BC1 caused cytoplasmic localization of partial ΔNLS1-Arx. Note in panel B that both mutants ΔBC2ΔNLS1-Arx and ΔBC1ΔBC2ΔNLS1-Arx caused about 80% of transfected cells to have only a cytoplasmic signal, but almost all of the cells expressing ΔBC1ΔNLS1-Arx had both cytoplasmic and nuclear signals. In panel C, lysates of N2a cells expressing ΔBC1ΔNLS1-Arx and ΔBC2ΔNLS1-Arx were incubated with GST-tagged impβ1, imp9, and imp13, respectively. Note that the interaction between impβ1 with ΔBC2ΔNLS1-Arx is largely abolished, but there is no obvious change for the binding of impβ1 with ΔBC1ΔNLS1-Arx. The interaction between imp13 with ΔBC1ΔNLS1-Arx is strongly reduced, whereas imp9 shows a significant binding to ΔBC1ΔNLS1-Arx. By contrast, bindings of ΔBC2ΔNLS1-Arx with imp9 and imp13 are slightly stronger than for ΔNLS1-Arx with imp9 or imp13. Error bars show S.D.
The nuclear signal of mutant ΔNLS1ΔBC2-Arx was significantly reduced (Fig. 8, Ad and B), and interaction between R382A-Arx with impβ1 is hardly detected (Fig. 7E). Moreover, impβ1 has a higher affinity for Arx than imp9 or imp13 does (Fig. 3A), impβ1 plays a dominant role in importing ΔNLS1-Arx in the in vitro nuclear import assay (Figs. 4 and 5), and impβ1 siRNA relocates ΔNLS1-Arx to the cytoplasm (Fig. 6). We, therefore, propose that impβ1 is a major receptor for import of Arx via NLS2 and that little Arx enters the nucleus via NLS2 when impβ1 cannot bind to Arx. imp9 and imp13 could also transport Arx into the nucleus via NLS2 but play a less important role or, alternatively, only function in nuclear import of Arx via NLS2 in specific situations.
DISCUSSION
Signal-dependent nuclear import of macromolecules is mediated by importin βs. The Arx transcription factor is important for development of the forebrain, pancreas, and testis (7, 8, 56–60). The mechanism of nuclear import of Arx has been unknown, although preliminary data suggested that imp13 is involved (11). We have studied Arx in N2a cells, which are of neuronal origin, as well as in NIH3T3 cells. Two nuclear localization signals in Arx were defined, NLS1 from aa 82 to 89 and NLS2 from aa 327 to 388, which largely overlaps with the homeodomain. Although other importin βs such as importin 9 and importin 13 also appear able to import Arx into the nucleus via NLS2, in vitro kinetic parameters of nuclear import of Arx, interactions of Arx with different importin βs, and in vivo siRNA interference experiments show that nuclear import of Arx via NLS2 is mediated mainly by importin β1.
Although NLS1 of Arx is structurally similar to a monopartite classical NLS, it does not bind impα1, impβ1, or other importin βs available in this laboratory, suggesting that other untested pathways contribute to nuclear import of Arx via NLS1. NLS2 includes two basic domains (BC1 and BC2) in the homeobox. Its binding to importin β1 depends on BC2, and arginine residues in BC2 are important for the function of NLS2 (Fig. 7C). Interestingly, deletion of BC1 eliminated the interaction between imp13 and Arx. By contrast, removal of BC2 improved its binding to imp13 (Fig. 8C), suggesting that BC1 is the domain for imp13 binding and that BC2 regulates the binding between NLS2 and imp13. Therefore, NLS2 of Arx interacts with different importin βs by different mechanisms.
Although impβ1 can import Arx into the nucleus and bind NLS2 in vitro, impβ1 siRNAs only partially mislocalizes ΔNLS1-Arx. This could be because of the following. 1) impβ1 has a relatively long lifespan (61, 62). We expressed these siRNAs for 48∼72 h before examining the titer of impβ1 and the subcellular distribution of ΔNLS1-Arx. In this situation, we observed that a significant amount of impβ1 was still present (Fig. 6), and this amount could have been sufficient to import Arx. Similar results have been described by others (62–64). Unfortunately, more extensive treatment was toxic (data not shown). 2) Importin βs such as imp9 and imp13 (and perhaps other unidentified importin βs) are able to import Arx (Figs. 4 and 5).
Several importin βs have been proposed to function in import of homeobox-containing proteins. Pse1/Kap121 transports Pho4 in yeast (65), importin β1 carries pancreatic and duodenal homeobox-1 in pancreatic islet beta cells (66), and Caudal in flies is carried by Moleskin (imp7) (67). All of these importin βs bind homeobox-containing proteins via a NLS that overlaps with the homeodomain. Moreover importin α1 binds the N-terminal basic domain of the Pax5 homeobox (68). Therefore, different homeobox-containing proteins can localize to the nucleus by different mechanisms, and both the classical and the non-classical pathways may function in this process. In our observations, wild-type Arx does not interact with importin α's (data not shown), suggesting that nuclear import of Arx is not mediated by the classical pathway. Because some deletion mutants of Arx do bind importin α1 via NLS2 in vitro (data not shown), alternative import pathways for Arx should still be considered. Possible modifications of Arx which promote its binding to impα1 are under investigation.
NLS2 is recognized by multiple import receptors, and these importin βs show different abilities to import Arx. We did not see an enhanced import of Arx upon the addition of either imp9 or imp13 to impβ1 in in vitro nuclear import assays. Binding assays with BC-deleted Arxs show that these different importin βs recognize NLS2 of Arx by different mechanisms. Import of Arx via NLS2, therefore, could be regulated. Some homeobox proteins such as extradenticle (69), PBX1A (70), and Prospero (71, 72) undergo a conformational change which regulates their nuclear localization. Thus, a cis-element at the N terminus of extradenticle/PBX normally binds the homeobox and blocks its function as a NLS, causing extradenticle/PBX to remain in the cytoplasm. When cells express MEIS (myeloid ecotropic viral insertion site), it interacts with the N terminus of extradenticle/PBX, which exposes the homeobox and causes this protein to localize to the nucleus (70). As a second example, the Prospero domain at the C terminus of Prospero masks the exportin function of the homeodomain to keep it in the nucleus (72). It is of central importance to learn how differential import of Arx is regulated by its interaction with different import receptors.
Whether Arx has one or more functional nuclear export signals is also an open question. Some homeodomain-containing proteins (Vsx1, Chx10, Otx1, Otx2, Oct6, and Prospro) shuttle in and out of the nucleus (71–75). The localization of most of these proteins is sensitive to leptomycin B, suggesting a Crm1-mediated export pathway (76). Moreover, Knauer et al. (74) proposed that the octapeptide domains of the paired-type homeodomain proteins Vsx1 and Chx10 are functional nuclear export signals which are recognized by Crm1 (74). The octapeptide domain sequences of Arx and Vsx1 (data not shown) do not include a classical leucine-rich nuclear export signal of the sort which is recognized by Crm1. Arguing against shuttling, ongoing heterokaryon experiments in which 293T cells expressing EGFP-tagged Arx were fused with NIH3T3 cells show no EGFP-Arx in the nucleus of NIH3T3 cells 12 h after fusion (data not shown). Whether nuclear export of Arx is regulated remains for future investigation.
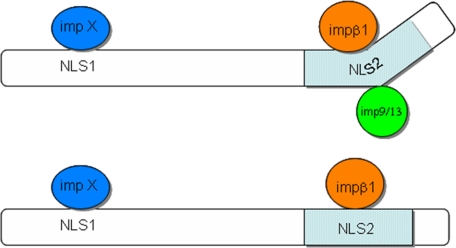
It is unclear why Arx needs two different NLSs for its nuclear localization. Although no alternative splicing sites in the ARX gene have been reported, several single nucleotide polymorphisms in the ARX gene have been identified (8). Thus, different forms of Arx may exist, and this may help explain possible regulation of nuclear import by different pathways (Fig. 9).
FIGURE 9.
Model of nuclear import of Arx. impβ1 and other importin βs such as imp9 and imp13 carry Arx into the nucleus by binding NLS2. Normally, impβ1 binds NLS2 and plays a major role in importing Arx. Under yet-unidentified circumstances, imp9 or imp13 could be function in transport Arx by binding to NLS2. Such binding is probably regulated by both BC domains. The nuclear import pathway mediated by NLS1 has not been identified (impX). The pale blue region is the homeodomain of Arx.
Proteins with multiple NLSs or a single NLS and that can be recognized by different members of the importin β superfamily have previously been reported (37, 38, 47, 48, 54, 77). Why proteins need multiple NLSs and how proteins “choose” among different import options is largely unknown. This study has provided a potential example of the complex regulatory mechanism used by one NLS with multiple receptors for nuclear import.
Supplementary Material
Acknowledgments
Plasmid importin β1 was kindly provided by Dr. K. Yamamoto of the University of California, San Franscisco. Plasmids expressing importin 4 and importin 9 were generous gifts from Dr. Dirk Görlich of Max Planck Institute for Biophysical Chemistry, Göttingen, Germany. A Plasmid expressing VDR was kindly provided by Dr. Jingzhan Zeng of Xiamen University Institute for Biomedical Researches.
This work was supported by National Natural Science Foundation of China Grants 3047085 and 90608007 and Ministry of Science and Technology of China Grant 2006AA02A310 (to T. T.). This work was also supported by National Institutes of Health Grant NS46616 through the NINDS NS46616 (to J. A. G.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental material.
- ARX
- aristaless-related homeobox
- aa
- amino acids
- GR
- glucocorticoid receptor
- GST
- glutathione S-transferase
- GFP
- green fluorescent protein
- EGFP
- enhanced GFP
- siRNA
- small interfering RNA
- NLS
- nuclear localization sequence
- cNLS
- classic NLS
- VDR
- vitamin D receptor
- DsRed
- Discosoma sp. red fluorescent protein.
REFERENCES
- 1.Affolter M., Marty T., Vigano M. A. (1999) Genes Dev. 13, 913–915 [DOI] [PubMed] [Google Scholar]
- 2.Cokol M., Nair R., Rost B. (2000) EMBO Rep. 1, 411–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miura H., Yanazawa M., Kato K., Kitamura K. (1997) Mech. Dev. 65, 99–109 [DOI] [PubMed] [Google Scholar]
- 4.Kitamura K., Miura H., Yanazawa M., Miyashita T., Kato K. (1997) Mech. Dev. 67, 83–96 [DOI] [PubMed] [Google Scholar]
- 5.Bienvenu T., Poirier K., Friocourt G., Bahi N., Beaumont D., Fauchereau F., Ben Jeema L., Zemni R., Vinet M. C., Francis F., Couvert P., Gomot M., Moraine C., van Bokhoven H., Kalscheuer V., Frints S., Gecz J., Ohzaki K., Chaabouni H., Fryns J. P., Desportes V., Beldjord C., Chelly J. (2002) Hum. Mol. Genet. 11, 981–991 [DOI] [PubMed] [Google Scholar]
- 6.Ohira R., Zhang Y. H., Guo W., Dipple K., Shih S. L., Doerr J., Huang B. L., Fu L. J., Abu-Khalil A., Geschwind D., McCabe E. R. (2002) Mol. Genet. Metab. 77, 179–188 [DOI] [PubMed] [Google Scholar]
- 7.Kitamura K., Yanazawa M., Sugiyama N., Miura H., Iizuka-Kogo A., Kusaka M., Omichi K., Suzuki R., Kato-Fukui Y., Kamiirisa K., Matsuo M., Kamijo S., Kasahara M., Yoshioka H., Ogata T., Fukuda T., Kondo I., Kato M., Dobyns W. B., Yokoyama M., Morohashi K. (2002) Nat. Genet. 32, 359–369 [DOI] [PubMed] [Google Scholar]
- 8.Gécz J., Cloosterman D., Partington M. (2006) Curr. Opin. Genet. Dev. 16, 308–316 [DOI] [PubMed] [Google Scholar]
- 9.Colasante G., Collombat P., Raimondi V., Bonanomi D., Ferrai C., Maira M., Yoshikawa K., Mansouri A., Valtorta F., Rubenstein J. L., Broccoli V. (2008) J. Neurosci. 28, 10674–10686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poirier K., Van Esch H., Friocourt G., Saillour Y., Bahi N., Backer S., Souil E., Castelnau-Ptakhine L., Beldjord C., Francis F., Bienvenu T., Chelly J. (2004) Brain Res. Mol. Brain Res. 122, 35–46 [DOI] [PubMed] [Google Scholar]
- 11.Shoubridge C., Cloosterman D., Parkinson-Lawerence E., Brooks D., Gécz J. (2007) Genomics 90, 59–71 [DOI] [PubMed] [Google Scholar]
- 12.Orr H. T., Zoghbi H. Y. (2000) Cell 101, 1–4 [DOI] [PubMed] [Google Scholar]
- 13.Ross C. A., Poirier M. A. (2004) Nat. Med. 10, (suppl.) S10–17 [DOI] [PubMed] [Google Scholar]
- 14.Kato M., Das S., Petras K., Kitamura K., Morohashi K., Abuelo D. N., Barr M., Bonneau D., Brady A. F., Carpenter N. J., Cipero K. L., Frisone F., Fukuda T., Guerrini R., Iida E., Itoh M., Lewanda A. F., Nanba Y., Oka A., Proud V. K., Saugier-Veber P., Schelley S. L., Selicorni A., Shaner R., Silengo M., Stewart F., Sugiyama N., Toyama J., Toutain A., Vargas A. L., Yanazawa M., Zackai E. H., Dobyns W. B. (2004) Hum. Mutat. 23, 147–159 [DOI] [PubMed] [Google Scholar]
- 15.Strømme P., Mangelsdorf M. E., Scheffer I. E., Gécz J. (2002) Brain Dev. 24, 266–268 [DOI] [PubMed] [Google Scholar]
- 16.Nasrallah I. M., Minarcik J. C., Golden J. A. (2004) J. Cell Biol. 167, 411–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nawara M., Szczaluba K., Poirier K., Chrzanowska K., Pilch J., Bal J., Chelly J., Mazurczak T. (2006) Am. J. Med. Genet. A 140, 727–732 [DOI] [PubMed] [Google Scholar]
- 18.Callaerts P., Halder G., Gehring W. J. (1997) Annu. Rev. Neurosci. 20, 483–532 [DOI] [PubMed] [Google Scholar]
- 19.Ploski J. E., Shamsher M. K., Radu A. (2004) Mol. Cell. Biol. 24, 4824–4834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quan Y., Ji Z. L., Wang X., Tartakoff A. M., Tao T. (2008) Mol. Cell. Proteomics 7, 1254–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tartakoff A. M., Lichtenstein M., Nanduri J., Tsao H. M. (2000) J. Struct. Biol. 129, 144–158 [DOI] [PubMed] [Google Scholar]
- 22.Terry L. J., Shows E. B., Wente S. R. (2007) Science 318, 1412–1416 [DOI] [PubMed] [Google Scholar]
- 23.Pemberton L. F., Paschal B. M. (2005) Traffic 6, 187–198 [DOI] [PubMed] [Google Scholar]
- 24.Macara I. G. (2001) Microbiol. Mol. Biol. Rev. 65, 570–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Görlich D., Kutay U. (1999) Annu. Rev. Cell Dev. Biol. 15, 607–660 [DOI] [PubMed] [Google Scholar]
- 26.Cook A., Bono F., Jinek M., Conti E. (2007) Annu. Rev. Biochem. 76, 647–671 [DOI] [PubMed] [Google Scholar]
- 27.Chook Y. M., Blobel G. (2001) Curr. Opin. Struct. Biol. 11, 703–715 [DOI] [PubMed] [Google Scholar]
- 28.Weis K. (2003) Cell 112, 441–451 [DOI] [PubMed] [Google Scholar]
- 29.Bednenko J., Cingolani G., Gerace L. (2003) Traffic 4, 127–135 [DOI] [PubMed] [Google Scholar]
- 30.Hood J. K., Silver P. A. (1999) Curr. Opin. Cell Biol. 11, 241–247 [DOI] [PubMed] [Google Scholar]
- 31.Corbett A. H., Silver P. A. (1997) Microbiol. Mol. Biol. Rev. 61, 193–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macara I. G. (1999) Curr. Biol. 9, R436–439 [DOI] [PubMed] [Google Scholar]
- 33.Conti E., Müller C. W., Stewart M. (2006) Curr. Opin. Struct. Biol. 16, 237–244 [DOI] [PubMed] [Google Scholar]
- 34.Goldfarb D. S., Corbett A. H., Mason D. A., Harreman M. T., Adam S. A. (2004) Trends Cell Biol. 14, 505–514 [DOI] [PubMed] [Google Scholar]
- 35.Picard D., Yamamoto K. R. (1987) EMBO J. 6, 3333–3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cadepond F., Gasc J. M., Delahaye F., Jibard N., Schweizer-Groyer G., Segard-Maurel I., Evans R., Baulieu E. E. (1992) Exp. Cell Res. 201, 99–108 [DOI] [PubMed] [Google Scholar]
- 37.Savory J. G., Hsu B., Laquian I. R., Giffin W., Reich T., Haché R. J., Lefebvre Y. A. (1999) Mol. Cell. Biol. 19, 1025–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freedman N. D., Yamamoto K. R. (2004) Mol. Biol. Cell 15, 2276–2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tao T., Lan J., Lukacs G. L., Haché R. J., Kaplan F. (2006) Am. J. Respir. Cell Mol. Biol. 35, 668–680 [DOI] [PubMed] [Google Scholar]
- 40.Mingot J. M., Kostka S., Kraft R., Hartmann E., Görlich D. (2001) EMBO J. 20, 3685–3694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tao T., Lan J., Presley J. F., Sweezey N. B., Kaplan F. (2004) Am. J. Respir. Cell Mol. Biol. 30, 350–359 [DOI] [PubMed] [Google Scholar]
- 42.Zhang C., Sweezey N. B., Gagnon S., Muskat B., Koehler D., Post M., Kaplan F. (2000) Am. J. Respir. Cell Mol. Biol. 22, 451–459 [DOI] [PubMed] [Google Scholar]
- 43.Su A. I., Cooke M. P., Ching K. A., Hakak Y., Walker J. R., Wiltshire T., Orth A. P., Vega R. G., Sapinoso L. M., Moqrich A., Patapoutian A., Hampton G. M., Schultz P. G., Hogenesch J. B. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 4465–4470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kahle J., Baake M., Doenecke D., Albig W. (2005) Mol. Cell. Biol. 25, 5339–5354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang J., Ke G., You W., Peng Z., Lan J., Kalesse M., Tartakoff A. M., Kaplan F., Tao T. (2008) Mol. Cell Biochem. 307, 93–100 [DOI] [PubMed] [Google Scholar]
- 46.Yamaguchi Y. L., Tanaka S. S., Yasuda K., Matsui Y., Tam P. P. (2006) Dev. Biol. 297, 350–360 [DOI] [PubMed] [Google Scholar]
- 47.Jäkel S., Görlich D. (1998) EMBO J. 17, 4491–4502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waldmann I., Wälde S., Kehlenbach R. H. (2007) J. Biol. Chem. 282, 27685–27692 [DOI] [PubMed] [Google Scholar]
- 49.Yasuhara N., Takeda E., Inoue H., Kotera I., Yoneda Y. (2004) Exp. Cell Res. 297, 285–293 [DOI] [PubMed] [Google Scholar]
- 50.Miyauchi Y., Michigami T., Sakaguchi N., Sekimoto T., Yoneda Y., Pike J. W., Yamagata M., Ozono K. (2005) J. Biol. Chem. 280, 40901–40908 [DOI] [PubMed] [Google Scholar]
- 51.Ribbeck K., Görlich D. (2001) EMBO J. 20, 1320–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tao T., Tartakoff A. M. (2001) Traffic 2, 385–394 [DOI] [PubMed] [Google Scholar]
- 53.Saijou E., Itoh T., Kim K. W., Iemura S., Natsume T., Miyajima A. (2007) J. Biol. Chem. 282, 32327–32337 [DOI] [PubMed] [Google Scholar]
- 54.Do H. J., Song H., Yang H. M., Kim D. K., Kim N. H., Kim J. H., Cha K. Y., Chung H. M., Kim J. H. (2006) FEBS Lett. 580, 1865–1871 [DOI] [PubMed] [Google Scholar]
- 55.Adam S. A., Marr R. S., Gerace L. (1990) J. Cell Biol. 111, 807–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heller R. S., Jenny M., Collombat P., Mansouri A., Tomasetto C., Madsen O. D., Mellitzer G., Gradwohl G., Serup P. (2005) Dev. Biol. 286, 217–224 [DOI] [PubMed] [Google Scholar]
- 57.Collombat P., Mansouri A., Hecksher-Sorensen J., Serup P., Krull J., Gradwohl G., Gruss P. (2003) Genes Dev. 17, 2591–2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Collombat P., Hecksher-Sørensen J., Krull J., Berger J., Riedel D., Herrera P. L., Serup P., Mansouri A. (2007) J. Clin. Invest. 117, 961–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Collombat P., Hecksher-Sørensen J., Broccoli V., Krull J., Ponte I., Mundiger T., Smith J., Gruss P., Serup P., Mansouri A. (2005) Development 132, 2969–2980 [DOI] [PubMed] [Google Scholar]
- 60.Yoshihara S., Omichi K., Yanazawa M., Kitamura K., Yoshihara Y. (2005) Development 132, 751–762 [DOI] [PubMed] [Google Scholar]
- 61.Villányi Z., Debec A., Timinszky G., Tirián L., Szabad J. (2008) Mech. Dev. 125, 196–206 [DOI] [PubMed] [Google Scholar]
- 62.Quensel C., Friedrich B., Sommer T., Hartmann E., Kohler M. (2004) Mol. Cell. Biol. 24, 10246–10255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Giri D. K., Ali-Seyed M., Li L. Y., Lee D. F., Ling P., Bartholomeusz G., Wang S. C., Hung M. C. (2005) Mol. Cell. Biol. 25, 11005–11018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hussain S., Perlman S., Gallagher T. M. (2008) J. Virol. 82, 7212–7222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaffman A., Rank N. M., O'Shea E. K. (1998) Genes Dev. 12, 2673–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guillemain G., Da Silva Xavier G., Rafiq I., Leturque A., Rutter G. A. (2004) Biochem. J. 378, 219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Han S. H., Ryu J. H., Oh C. T., Nam K. B., Nam H. J., Jang I. H., Brey P. T., Lee W. J. (2004) Insect Mol. Biol. 13, 323–327 [DOI] [PubMed] [Google Scholar]
- 68.Kovac C. R., Emelyanov A., Singh M., Ashouian N., Birshtein B. K. (2000) J. Biol. Chem. 275, 16752–16757 [DOI] [PubMed] [Google Scholar]
- 69.Rieckhof G. E., Casares F., Ryoo H. D., Abu-Shaar M., Mann R. S. (1997) Cell 91, 171–183 [DOI] [PubMed] [Google Scholar]
- 70.Saleh M., Huang H., Green N. C., Featherstone M. S. (2000) Exp. Cell Res. 260, 105–115 [DOI] [PubMed] [Google Scholar]
- 71.Demidenko Z., Badenhorst P., Jones T., Bi X., Mortin M. A. (2001) Development 128, 1359–1367 [DOI] [PubMed] [Google Scholar]
- 72.Bi X., Kajava A. V., Jones T., Demidenko Z. N., Mortin M. A. (2003) Mol. Cell. Biol. 23, 1014–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baranek C., Sock E., Wegner M. (2005) Nucleic Acids Res. 33, 6277–6286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Knauer S. K., Carra G., Stauber R. H. (2005) Mol. Cell. Biol. 25, 2573–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Y. A., Okada A., Lew C. H., McConnell S. K. (2002) Mol. Cell. Neurosci. 19, 430–446 [DOI] [PubMed] [Google Scholar]
- 76.Hutten S., Kehlenbach R. H. (2007) Trends Cell Biol. 17, 193–201 [DOI] [PubMed] [Google Scholar]
- 77.Miyamoto Y., Imamoto N., Sekimoto T., Tachibana T., Seki T., Tada S., Enomoto T., Yoneda Y. (1997) J. Biol. Chem. 272, 26375–26381 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.