Abstract
Hyperglycemia is an independent risk-factor for development of diabetic vascular complications. The molecular mechanisms that are activated by glucose in vascular cells and could explain the development of vascular complications are still poorly understood. A putative binding site for the transcription factor Aryl Hydrocarbon Receptor (AhR) was identified in the glucose-responsive fragment of the promoter of thrombospondin-1 (TSP-1), a potent anti-angiogenic and pro-atherogenic protein involved in development of diabetic vascular complications. AhR was expressed in aortic endothelial cells (EC), activated and bound to the promoter in response to high glucose stimulation of EC. The constitutively active form of AhR induced activation of the TSP-1 gene promoter. In response to high glucose stimulation, AhR was found in complex with Egr-1 and AP-2, two other nuclear transcription factors activated by glucose in EC that have not been previously detected in complex with AhR. The activity of the DNA-binding complex was regulated by glucose through the activation of hexosamine pathway and intracellular glycosylation. This is the first report of activation of AhR (a receptor for xenobiotic compounds) by a physiological stimulus. This report links the activation of AhR to the pathological effects of hyperglycemia in the vasculature.
Keywords: aryl hydrocarbon receptor, glucose, thrombospondin-1, endothelial cells
Introduction
Hyperglycemia is an independent risk factor for vascular complications of diabetes1–5. Endothelial dysfunction is the earliest sign of developing diabetic vascular complications [for recent reviews, see6–8]. Hyperlgycemia affects the expression of numerous endothelial proteins9–11, including thrombospondin-1 (TSP-1)12, a potent anti-angiogenic and pro-atherogenic protein implicated in the development of a variety of vascular diabetic complications12–15. We report here that high glucose activates Aryl Hydrocarbon Receptor (AhR) in EC, which activates transcription of the thrombospondin-1 gene (THBS1).
AhR is a transcription factor known to be activated by aromatic hydrocarbons, e.g., 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) present in industrial waste, tobacco smoke and byproducts of herbicides16–18. Although the connection between AhR expression or activity and atherogenesis has not been explored directly, multiple epidemiological and animal studies have established the association between known AhR activators and heart disease18–23. Recent reports demonstrated that AhR negatively affects angiogenesis in cancer and ischemia models24–26, further implicating this transcription factor in regulation of endothelial function.
The abnormalities observed in AhR knockout mice include cardiac hypertrophy27, 28, altered insulin regulation and responsiveness, altered glucose tolerance in pregnant females29, and immune system impairment30, 31. Although AhR is clearly required for a variety of physiological processes32–36, physiological activators of AhR are unknown, and only a few recent reports describe activation of AhR in response to pathological stimuli24, 37, 38. The mechanism of AhR transcriptional activity and the target genes have not been comprehensively studied53,57–60. There is no information on regulation of gene expression by AhR as a result of metabolic abnormalities.
Our results demonstrate that AhR is rapidly activated in EC in response to high glucose. Active AhR associates with the thrombosponsin-1 gene (THBS1) promoter and activates it. AhR forms a complex with several other transcription factors activated by glucose: AP-2, Egr-1, USF-2 and Pax-5. This complex is different from the complex formed by AhR and ARNT (HIF1β) in response to xenobiotics, and the activity of the complex is regulated by glycosylation.
This is the first report of AhR activation by high glucose that links AhR to the physiological regulation of gene expression by glucose and the pathological effects of hyperglycemia in the vasculature.
Materials and Methods
Cell stimulation with high glucose was described previously12, 15.
Antibodies used
Anti-AhR from Novus Biologicals (Littleton, CO) and Abcam (Cambridge, MA), anti-Egr-1 from Cell Signaling Technology (Danvers, MA), anti-USF-1 and anti-USF-2 from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), RL2 from Abcam (Cambridge, MA) and anti-AP2 from AbD Serotec (Raleigh, NC).
Promoter reporter constructs
The fragments −280/+66 pTHBS1 and −265/+66 pTHBS1 (ΔAhR) were generated by PCR.
Mutants: 1 - 5′AGCCCGCGAGGCGA3′, 2 −5′AGCCCGGCTGGCGA3′, 3 −5′AGCCCGGCAGGCGA3′, wt - 5′ AGCCCGCGTGGCGCA 3′.
Analysis of the binding sites for transcription factors in the THBS1 promoter region responsive to glucose
The sequence of pTHBS1 was analyzed using MatInspector 7.4.3 (Genomatix, www.genomatix.de)39.
Plasmids for the expression of AhR
The constitutively active form of AhR was prepared by constructing the AhR deletion mutant as described previously for murine AhR40.
Analysis of activation of transcription factors in glucose-stimulated HAEC was performed using TranSignal Combo Protein/DNA array (Panomics).
Immunofluorescence
Anti-AhR antibody (Novus Biologicals) and goat anti-mouse Alexa Fluor-labeled secondary antibody (Invitrogen) were used to stain sections of rat aorta12.
Treatment of EC with glycosylation inhibitors and metabolites of hexosamine pathway was done as described earlier15.
Statistical analysis
All the described experiments were performed more than 3 times and the data are presented as mean values ± S.E.M. P values were determined by T-test using Microsoft Excel. P values < 0.05 were considered statistically significant.
Results
The minimal fragment of human THBS1 gene responsive to high glucose in EC
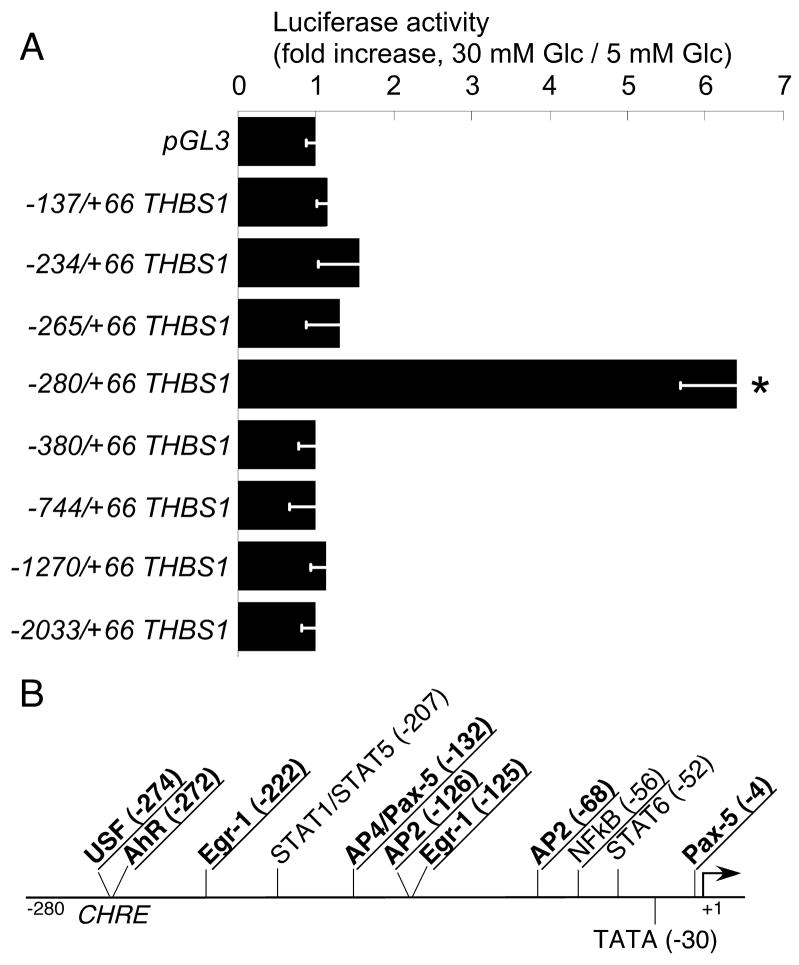
We have reported recently that the expression of thrombospondin-1 (TSP-1) is increased in response to high glucose (10 – 30 mM) in all the major vascular cell types12, and the increase in TSP-1 mRNA level is transcriptionally regulated15. The increase in mRNA levels could be detected as early as 1 hour after the start of stimulation in cultured EC and could be still detected at 72 hours in all vascular cell types12. We have analyzed the activity of TSP-1 promoter deletion constructs to identify the promoter elements responsible for this regulation in EC. The −280/+66 pTHBS1 fragment was activated in response to stimulation of HUVEC by 30 mM glucose (indicated by a 6-fold increase in activity of luciferase), and this activation was abolished by deletion of 15 base pairs in −265/+66 pTHBS1 (ΔAhR)(Fig. 1A), suggesting that a putative binding site for the transcription factor AhR predicted in this 15 bp region may control the response to high glucose. The response to glucose was inhibited in −380/+66 and longer promoter fragments, suggesting a presence of an inhibitory element in the promoter between −280 and −380, which is active in EC, but not in vascular SMC15 or mesangial cells41. We analyzed the −280/+66 fragment of THBS1 using MatInspector(Genomatix) to identify putative binding sites for transcription factors. This analysis identified several putative binding sites (Fig. 1B), including a binding site for AhR in the fragment responsible for glucose stimulation (−272, see Fig. 1B). The putative binding site for AhR overlapped with the predicted binding site for USF (−274), and this sequence was also recognized by the program as a Carbohydrate Response Element (CHRE).
Figure 1. High-glucose-responsive promoter region.
A HUVEC transfected with the promoter deletion constructs were stimulated with 30 mM glucose, luciferase activity was measured 24 hours later, n = 3, *p<0.05. B: Glucose-responsive region of the promoter was analyzed using MatInspector 7.4.3. to identify the putative binding sites for transcription factors. Transcription factors activated by high glucose are underlined, transcription factors co-precipitating with AhR in glucose-stimulated HAEC are in bold.
Identification of transcription factors activated by high glucose in EC
We focused on transcription factors (TFs) that are rapidly activated in response to high glucose and are still active at 24 hours.
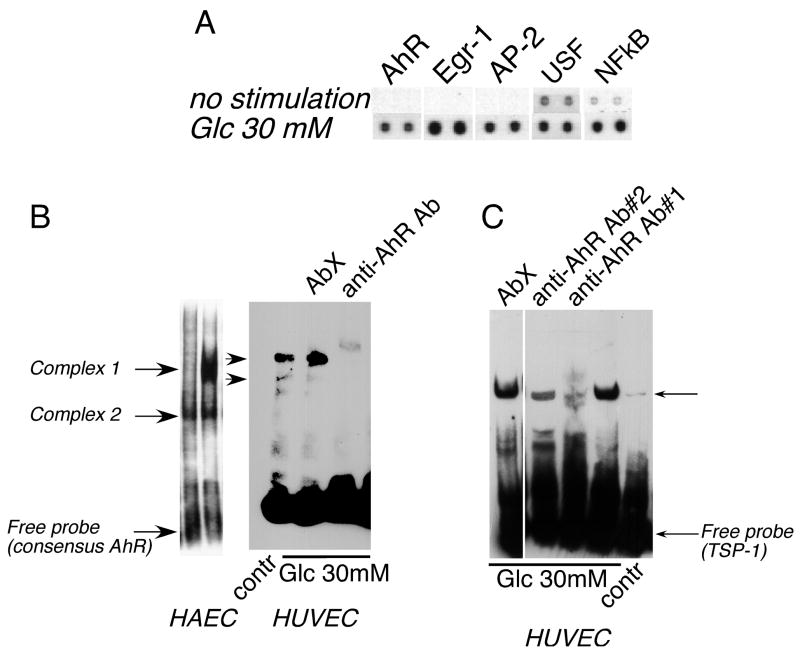
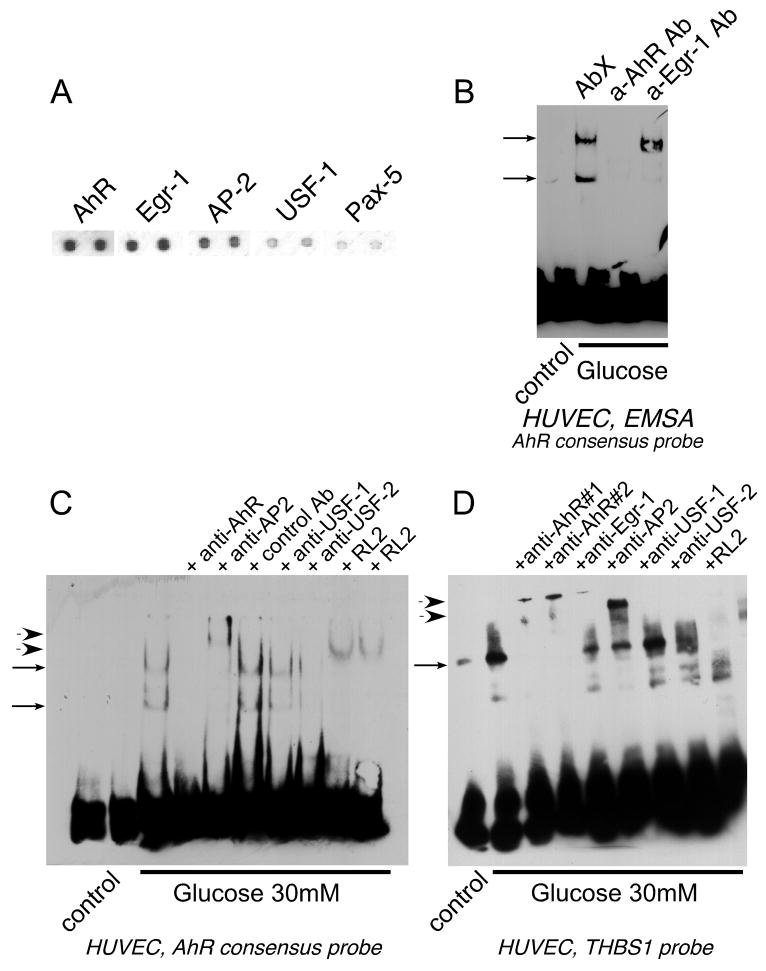
To identify the TFs rapidly activated in response to acute treatment with high glucose (1-hour stimulation with 30 mM glucose), we used four separate isolates of human aortic EC (HAEC). A targeted proteomic approach was used to identify the activated TFs. TranSignal Protein/DNA array (Panomics Inc., Fremont, CA) identifies only active TFs in nuclear extracts. Among the TFs activated in response to glucose treatment were six transcription factors whose putative binding sites have been predicted in the −280/+66 fragment of the THBS1 promoter: AhR, AP-2, Egr-1, USF, NFkB, and Pax-5 (Table 1). We have confirmed the activation of Egr-1 and AP-2 using alternative methods of detection – Northern blotting and EMSA (see online supplement). The values in Table 1 were calculated based on increase in four independent experiments. We have chosen to analyze only the values for transcription factors activated greater than 2.5 fold in at least two out of four experiments. Activation of five out of six transcription factors was statistically significant, but the values for Pax-5 did not reach statistical significance. Figure 2A demonstrates representative array results for the five transcription factors significantly stimulated in response to high glucose in HAEC. USF, NFkB, and Egr-1 are known to be activated in response to high glucose or in diabetics42, 43. However, to our knowledge, this is the first report of activation of AhR and AP2 by glucose.
Table 1.
Transcription factors with putative binding sites in −280/+66 pTHBS1 activated in response to high glucose in human aortic endothelial cells.
| Transcription factor | Activation in response to 30 mM glucose (mean fold increase ± SE, n=4) |
|---|---|
| AhR | 8.43 ± 2.3, p = 0.035* |
| AP-2 | 4.11 ± 0.57, p = 0.025* |
| Egr-1 | 3.65 ± 1.1, p = 0.035* |
| USF | 2.8 ± 0.5, p = 0.05* |
| NFkB | 3.75 ± 0.4, p = 0.01* |
| Pax-5 | 2.38 ± 1.28, p = 0.2 |
Figure 2. AhR is activated in endothelial cells in response to high glucose.
A Nuclear extracts from HAEC stimulated with glucose for 1 hour were analyzed in a Protein/DNA array (Panomics) to detect activated transcription factors. Representative results for AhR, Egr-1, AP-2, USF-1 and NFkB (transcription factors with putative binding sites in the −280/+66 fragment of the THBS1 promoter) are shown. B: Activation of AhR was confirmed in EMSA (5μg of nuclear extract) using the consensus AhR probe. Formation of complexes was prevented by anti-AhR antibody RPT1 (1 μg) but not an unrelated antibody (AbX). C: The predicted binding site for AhR in the promoter fragment responsive to glucose (−280/+66) was confirmed in EMSA using anti-AhR antibody.
Confirmation of activation of AhR in EMSA
Nuclear extracts from HAEC and human umbilical vain EC (HUVEC) were used in an electromobility shift assay (EMSA) with a consensus AhR probe to confirm the activation of AhR by high glucose (Fig. 2B). Two DNA-binding protein complexes in both EC types were activated in response to 30 mM glucose (Complex 1 and Complex 2, Fig. 2B). When anti-AhR antibodies were used, they either prevented binding of both complexes to the probe or resulted in a supershift in EMSA. Unrelated antibody (AbX) was used as a control. When the probe corresponding to the sequence of the THBS1 promoter containing the predicted binding site for AhR was used, activation and binding of AhR was also detected, and the binding was prevented by anti-AhR antibody (Fig. 2C).
AhR is expressed in EC
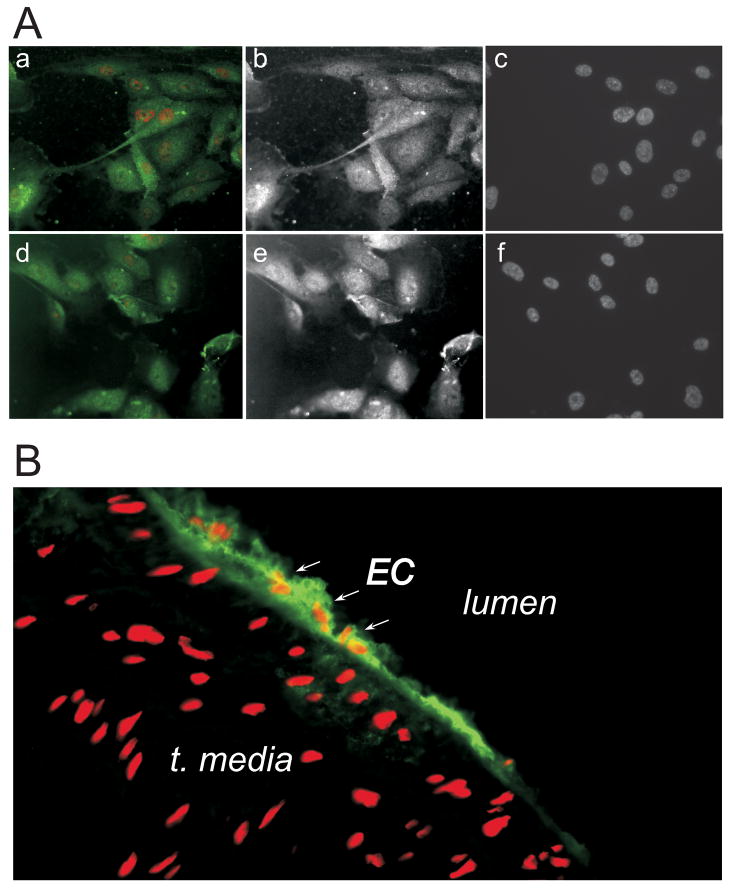
AhR is constitutively present in the cytosol and is translocated to the nucleus upon activation by its ligand44–46. We have detected AhR in both cultured HAEC and luminal EC of the rat aorta. (Fig. 3A, B).
Figure 3. AhR is expressed in EC in vitro and in vivo.
Cultured HUVEC (A) and rat aorta (B) were stained using anti-AhR antibody (green). Red – nuclei, propidium iodide staining. Aa-Ac: unstimulated HUVEC; Ad-Af: 3 hours of 30 mM glucose stimulation.
AhR associates with TSP-1 promoter in glucose-stimulated HAEC
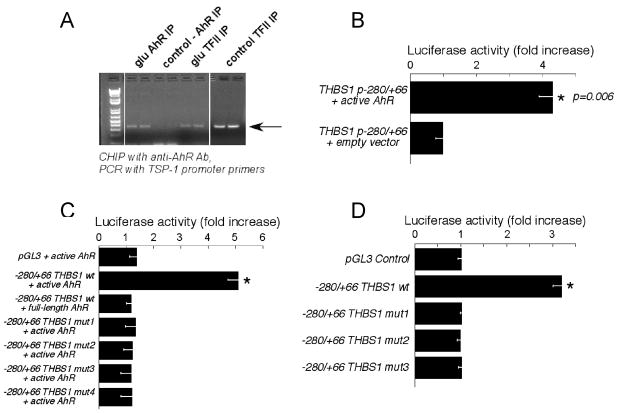
Chromatin immunoprecipitation from HAEC stimulated with 30 mM glucose for 1 hour was performed with anti-AhR antibody followed by the PCR amplification of the THBS1 promoter region. It revealed that AhR associates with TSP-1 promoter only upon glucose treatment, while the general nuclear factor TFII is associated with the promoter in both control and glucose-treated EC (Fig. 4A).
Figure 4. Active AhR binds and activates THBS1 promoter.
A HAEC (1 hour glucose) were used for chromatin immunoprecipitation with anti-AhR antibody. DNA from the precipitated fraction was used in PCR to amplify the THBS1 promoter fragment; B: HEK293 were co-transfected with the −280/+66 THBS1 fragment reporter plasmid and cDNA of constitutively active AhR, luciferase activity was measured 24 hours later. n = 3, *p < 0.05; C: HEK293 were co-transfected with indicated combinations of plasmids and the activity of luciferase was measured 24 hours later. Increase in the luciferase activity of the control, wt −280/+66 or mutant −280/+66 promoter constructs co-transfected with active or full-length AhR over the activity of the same promoter co-transfected with empty pcDNA3 is shown on the graph, n = 3, p < 0.05; D: HUVEC were transfected with the wt −280/+66 promoter fragment or mutant fragments and stimulated with 30 mM glucose for 24 hours; ratio of the luciferase activity in glucose-stimulated cells to non-stimulated cells; n = 3; *p < 0.05.
Constitutively active AhR activates the THBS1 promoter
When the constitutively active form of AhR was co-transfected with the luciferase reporter/−280/+66 pTHBS1 promoter construct in HEK293 cells, the activity of luciferase was increased more than 4 fold as compared to cells transfected with the control plasmid (Fig. 4B). The full-length AhR (inactive form) failed to induce any stimulation of the promoter (Fig. 4C).
In order to confirm that the promoter activity in response to active AhR is dependent on the binding site for this transcription factor (−272), we generated mutant −280/+66 pTHBS1 promoter constructs with one, two and three nucleotide substitutions in the core of the AhR binding site as described in Methods (Mut1, 2, and 3). The activity of these mutants remained at the basal level when co-transfected with active AhR (Fig. 4C). When HUVEC transiently transfected with these three mutants were stimulated with high glucose, none of the mutant −280/+66 pTHBS1 was activated in response to glucose (Fig. 4D), clearly confirming that the activation of −280/+66 pTHBS1 promoter by glucose depends on the AhR-binding sequence.
Complexes formed by AhR in high glucose-stimulated EC
Several transcription factors are known to form complexes with active AhR. The most common is HIF1β (ARNT) that associates with AhR in response to xenobiotic activators (reviewed in47); other proteins, including transcription factors, have been identified (reviewed in45, 48). We have used a TF/TF interaction array (Panomics) that detects only active transcription factors in complex with the transcription factor of interest and is based on the immunoprecipitation. The experiments revealed that AhR can be precipitated in complex with several transcription factors activated in response to glucose stimulation and having predicted binding sites in close proximity to the AhR binding site in the THBS1 promoter. These are AP-2, Egr-1, USF, and Pax-5 (Fig. 5A), suggesting that these transcription factors may form a complex on the promoter of THBS1 where the corresponding binding sites are in close proximity to each other in the region −280/+66. Neither of these proteins was precipitated with non-immune mouse IgG (data not shown).
Figure 5. Transcription factors activated in EC by high glucose form a complex with AhR.
A Nuclear extracts from glucose-stimulated HAEC were used for immunoprecipitation with anti-AhR antibody and associated active transcription factors were identified using TF/TF array (Panomics). B: Formation of a complex between AhR and Egr-1 in response to glucose was confirmed in EMSA using consensus AhR probe. One of the two complexes was prevented by the anti-Egr-1 antibody (1 μg). C, D: The complex of AP-2 and USF-2 with AhR was confirmed in EMSA using the antibodies against AhR (1 μg), AP-2 (1 μg), USF-1 (1 μg) and USF-2 (1 μg); C - consensus AhR probe, D - probe with the sequence of THBS1 promoter fragment. RL2 antibody was used in both C and D to prove that at least one of the proteins is O-glycosylated.
To confirm these transcription factors form a complex in response to high glucose, we used corresponding antibodies in the binding reaction with consensus AhR probe (Fig. 5B, C) and TSP-1 probe (Fig. 5D). Formation of the specific complexes was prevented by anti-AhR antibody, while an unrelated antibody did not affect the binding (Fig. 5B). To characterize the complex of transcription factors, the following antibodies were used in combination with the AhR consensus probe in EMSA: anti-AhR (Fig. 2B, C; Fig. 5B, C, D), anti-Egr-1 (Fig. 5B, C, D), anti-AP2 (Fig. 5C, D), anti-USF-1 and anti-USF-2 (Fig. 5C, D). With the exception of USF-1, all these transcription factors were found in complex with AhR. Anti-AhR, anti-Egr-1, anti-AP2 and anti-USF-2 antibodies either prevented the binding of the complex to the labeled probe, or supershifted the complex band in EMSA. Anti-Egr-1 antibody prevented the formation of lower complex (Complex 2), but did not affect the binding of Complex 1 to the AhR probe, suggesting that Egr-1 is present in association with AhR in Complex 2, but Complex 1 is formed by AhR and other transcription factors (Fig. 5B). When TSP-1 probe was used, anti-Egr-1 antibody inhibited the formation of the complex. USF-1 antibody supershifted USF-1 protein in EMSA in an unrelated experiment (not shown). Antibody against HIF1β, the most common partner of AhR, did not affect either of the two complexes (data not shown) suggesting that HIF1β is not present in the complex.
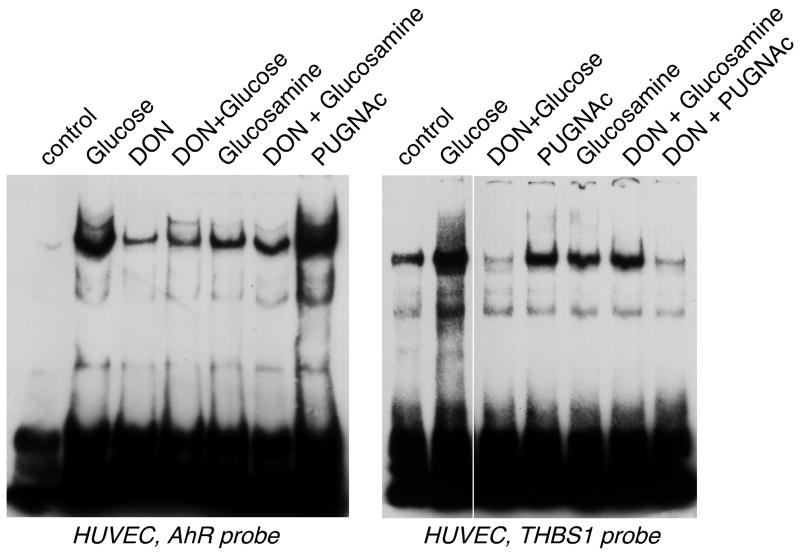
Activation of DNA-binding complex is regulated by glycosylation
We have recently reported that in vascular smooth muscle cells intracellular glycosylation is responsible for activation of THBS1 transcription15. We used RL2 antibody (anti-O-linked glucosamine N-acetyl, anti-O-GlcNAc), which recognizes glycosylated intracellular proteins, in EMSA to detect glycosylation of a protein(s) in complexes formed on the AhR consensus or TSP-1 probes (Fig. 5C, D). To confirm that glycosylation regulates the formation of the complex or its DNA-binding activity, we treated EC with: 1) 6-diazo-5-oxonorleucine (DON), which is an inhibitor of glutamine:fructose 6-phosphate amidotransferase (GFAT), an enzyme controlling the hexosamine pathway of glucose metabolism leading to formation of metabolites for glycosylation; 2) glucosamine, a glycosylation residue precursor, and a glucose metabolite entering the hexosamine pathway downstream of GFAT; 3) amino-N-phenylcarbamate (PUGNAc), known to increase O-linked protein glycosylation by effectively inhibiting β-N-acetyl-glucosaminidase (O-GlcNAcase), an enzyme responsible for cleavage of O-GlcNAc residues from intracellular proteins15. The inhibitor of GFAT prevented activation of the AhR complex (Fig. 6), and both glucosamine and PUGNAc resulted in activation of the complex without stimulation with high glucose, confirming that the activation depends on an intracellular glycosylation event. As expected, DON did not prevent complex formation in response to treatment of EC with glucosamine, which acts downstream of GFAT, confirming that the effect of DON was specific. PUGNAc did not induce the formation of the complex in the DON-treated cells, and this is consistent with our previous observations15: PUGNAc is an inhibitor of de-glycosylation, certain level of glycosylation is required in order to see its effect. However, in the DON-treated cells glycosylation is inhibited.
Figure 6. Glycosylation regulates the activity of the DNA-binding complex.
5 μg of nuclear extracts from HUVEC treated for 24 h with 30 mM glucose, 1 mM glucosamine, 100 μM DON or 40 μM PUGNAc were used in EMSA with the consensus AhR probe (left panel) or TSP-1 probe (right panel).
Discussion
Despite numerous studies on activation of AhR by xenobiotics48, 49, its physiological activators, ligands and mechanism of activation remains unknown. Multiple indications of its role in normal physiology and pathology exist, including the AhR knockout mouse that develops metabolic abnormalities and cardiac hypertrophy28–30. Recent publications have reported a new role for AhR in response to UV exposure37, modified low density lipoproteins38 and hypoxia24, further establishing its role in pathological changes.
Our results clearly demonstrate that glucose activates AhR in EC, and AhR controls the expression of TSP-1, a potent anti-angiogenic and pro-atherogenic protein. EC function determines physiological and pathological angiogenesis and initiation of atherosclerotic lesions in the large blood vessels. Therefore, activation of AhR by high glucose in diabetics or during postprandial elevation of glucose levels may initiate a series of pathological events leading to endothelial dysfunction and resulting in vascular disease. The epidemiological association between heart disease and industrial wastes and cigarette smoke that both contain activators of AhR also indicates a possible role for AhR in the development of atherosclerotic changes caused by environmental factors.
The role of AhR in the activation of the THBS1 promoter was initially established by promoter analysis. The promoter region −280/+66 responsible for the increased transcription of THBS1 was identified, and the AhR/USF binding site was predicted in this region, also recognized by MatInspector as a Carbohydrate Response Element (CHRE). The regulation of the THBS1 promoter by high glucose in EC appears to be different from the regulation in mesangial cells41 or vascular smooth muscle cells27 where a longer promoter fragment is required for the response. The activation of AhR in EC was detected by a targeted proteomic approach and confirmed using EMSA. Furthermore, active AhR bound to the promoter of the endogenous THBS1 gene in response to glucose. Overexpression of the constitutively active form of AhR but not the full-length AhR in together with the THBS1 promoter/reporter construct further confirmed the activation of the promoter by AhR. The role of the AhR-binding sequence (−272) was confirmed using the mutant constructs with substitutions in the core sequence of the AhR-binding site: the mutant promoter fragments could not be activated in response to high glucose.
Gene transcription in response to extracellular and intracellular stimuli depends both on the promoter structure and on the signal- and cell type-specific patterns of activation of transcriptional activators, co-activators and suppressors. Transcription factors and co-activators form signal- and cell type-specific multi-protein complexes on the promoters. We have identified five proteins in complex with AhR in glucose-stimulated EC: AP-2, Egr-1, USF-2 and Pax-5. Interestingly but not surprisingly, the putative binding sites for all four transcription factors were predicted in the glucose-responsive fragment of the THBS1 promoter in close proximity to the AhR binding site (MatInspector and70). Out of the multiple transcription factors with predicted binding sites in this region, only five were consistently activated (at least 2.5 fold) in response to high glucose in HAEC (AhR, AP-2, Egr-1, USF, NFkB), and four of them represented the proteins found in complex with AhR in both co-precipitation and supershift experiments with the corresponding antibodies. While the probe-based analyses did not allow to distinguish between USF-1 and USF-2 that both can bind to the same DNA sequence and form homo- and heterodimers, further analysis suggested that USF-2 is present in the complex, but not USF-1. Anti-USF-1 antibody that we used to supershift USF-1 complex in unrelated study with different cell type (data not shown) failed to change the binding of the AhR complex to both AhR concensus and AhR THBS1 probe.
While the activation of Egr-1 and NFkB in EC and the activation of USF in other cell types and tissues in response to hyperglycemia or in diabetics was reported earlier42, 50–534, this is the first report of activation of AhR and AP-2 by high glucose. Antibody against HIF1β did not affect AhR complexes in EMSA, and this transcription factor was not activated by high glucose in EC, suggesting that AhR-dependent transcriptional mechanisms activated in response to glucose differ from the well-described mechanisms activated by xenobiotics.
Our data clearly demonstrate that activation of the transcriptional complex depends on glycosylation of at least one of the proteins: RL2 antibody recognizes proteins modified by O-linked N-acetylglucosamine, and this antibody supershifted the complex. Furthermore, inhibitors of the hexosamine pathway prevent formation of intermediates for intracellular glycosylation, and these inhibitors also prevent the activation of the DNA-binding protein complex, while the inhibitors of de-glycosylation and downstream intermediates of the pathway caused formation of the complex. Further confirmation that AhR can be directly glycosylated and the identification of glycosylation sites are clearly mandated by these observations.
The activation of SP1 in response to stimulation with high glucose was previously reported in both EC54 and vascular smooth muscle cells55. SP1 undergoes post-translational modification by O-linked N-acetylglucosamine, which prevents its degradation in vascular smooth muscle cells55. Our array experiment did not detect any activation of SP1 in EC or in vascular smooth muscle cells (not shown), and our attempts to detect activated SP1 in nuclear extracts using a consensus probe were unsuccessful (data not shown). We believe that SP1 may be activated at the later time points, but not at the earlier time points analyzed in our studies. THBS1 transcription is activated rapidly in response to high glucose15, and this rapid activation suggests that only transcription factors activated before and at the onset of THBS1 activation are involved in the regulation of the promoter in response to acute glucose stimulation.
The results of this work document a novel physiological and pathological role for AhR in the response of vascular EC to hyperglycemia. Despite the rapidly accumulating evidence that EC respond to glucose by activation of a variety of genes, the transcriptional mechanisms of this regulation have not been well explored, with the exception of a few reports identifying the specific transcriptional factors mediating a change in gene expression in response to glucose42, 56–58.
This transcriptional mechanism provides a novel and unexpected link between hyperglycemia and the expression of TSP-1, a potent anti-angiogenic and pro-atherogenic protein involved in the development of multiple diabetic vascular complications.
Supplementary Material
Acknowledgments
We would like to thank Dr. Paul Bornstein (University of Washington) for cDNA of THBS1 promoter, Dr. DiCorleto and Ms. Lori Mavrakis (Cleveland Clinic) for HUVEC, Dr. Priya Raman (Cleveland Clinic) for help with preparation of the biotinylated TSP-1 probe and advice on the design of TSP-1 promoter mutants, Ms. Yana Pleshivoy and Ms. Christy Harry (Cleveland Clinic) for technical help in preparation of the constructs and plasmids, and Mr. Tim Burke (Cleveland Clinic) for help with manuscript preparation.
Sources of funding
This work was supported by National Institutes of Health Grants K01 DK62128, P50 HL077107, R01 DK067532, American Heart Association grant 0565284B and funds from the Lerner Research Institute (Cleveland Clinic Foundation) (to O.I.S.).
Footnotes
Disclosures: None
References
- 1.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. Am J Cardiol. 1995;75:894–903. doi: 10.1016/s0002-9149(99)80683-3. [DOI] [PubMed] [Google Scholar]
- 3.Nathan DM, Lachin J, Cleary P, Orchard T, Brillon DJ, Backlund JY, O’Leary DH, Genuth S. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med. 2003;348:2294–2303. doi: 10.1056/NEJMoa022314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 5.Shichiri M, Kishikawa H, Ohkubo Y, Wake N. Long-term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care. 2000;23 (Suppl 2):B21–29. [PubMed] [Google Scholar]
- 6.Singleton JR, Smith AG, Russell JW, Feldman EL. Microvascular complications of impaired glucose tolerance. Diabetes. 2003;52:2867–2873. doi: 10.2337/diabetes.52.12.2867. [DOI] [PubMed] [Google Scholar]
- 7.Skrha J. Pathogenesis of angiopathy in diabetes. Acta Diabetol. 2003;40 (Suppl 2):S324–329. doi: 10.1007/s00592-003-0113-z. [DOI] [PubMed] [Google Scholar]
- 8.Hsueh WA, Quinones MJ. Role of endothelial dysfunction in insulin resistance. Am J Cardiol. 2003;92:10J–17J. doi: 10.1016/s0002-9149(03)00611-8. [DOI] [PubMed] [Google Scholar]
- 9.Taki H, Kashiwagi A, Tanaka Y, Horiike K. Expression of intercellular adhesion molecules 1 (ICAM-1) via an osmotic effect in human umbilical vein endothelial cells exposed to high glucose medium. Life Sci. 1996;58:1713–1721. doi: 10.1016/0024-3205(96)00151-8. [DOI] [PubMed] [Google Scholar]
- 10.Park JY, Takahara N, Gabriele A, Chou E, Naruse K, Suzuma K, Yamauchi T, Ha SW, Meier M, Rhodes CJ, King GL. Induction of endothelin-1 expression by glucose: an effect of protein kinase C activation. Diabetes. 2000;49:1239–1248. doi: 10.2337/diabetes.49.7.1239. [DOI] [PubMed] [Google Scholar]
- 11.Stenina OI. Regulation of vascular genes by glucose. Curr Pharm Des. 2005;11:2367–2381. doi: 10.2174/1381612054367283. [DOI] [PubMed] [Google Scholar]
- 12.Stenina OI, Krukovets I, Wang K, Zhou Z, Forudi F, Penn MS, Topol EJ, Plow EF. Increased expression of thrombospondin-1 in vessel wall of diabetic Zucker rat. Circulation. 2003;107:3209–3215. doi: 10.1161/01.CIR.0000074223.56882.97. [DOI] [PubMed] [Google Scholar]
- 13.Daniel C, Schaub K, Amann K, Lawler J, Hugo C. Thrombospondin-1 is an endogenous activator of TGF-beta in experimental diabetic nephropathy in vivo. Diabetes. 2007;56:2982–2989. doi: 10.2337/db07-0551. [DOI] [PubMed] [Google Scholar]
- 14.Belmadani S, Bernal J, Wei CC, Pallero MA, Dell’italia L, Murphy-Ullrich JE, Berecek KH. A thrombospondin-1 antagonist of transforming growth factor-beta activation blocks cardiomyopathy in rats with diabetes and elevated angiotensin II. Am J Pathol. 2007;171:777–789. doi: 10.2353/ajpath.2007.070056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raman P, Krukovets I, Marinic TE, Bornstein P, Stenina OI. Glycosylation mediates up-regulation of a potent antiangiogenic and proatherogenic protein, thrombospondin-1, by glucose in vascular smooth muscle cells. J Biol Chem. 2007;282:5704–5714. doi: 10.1074/jbc.M610965200. [DOI] [PubMed] [Google Scholar]
- 16.Lofroth G, Rannug A. Ah receptor ligands in tobacco smoke. Toxicol Lett. 1988;42:131–136. doi: 10.1016/0378-4274(88)90070-7. [DOI] [PubMed] [Google Scholar]
- 17.Muto H, Takizawa Y. Dioxins in cigarette smoke. Arch Environ Health. 1989;44:171–174. doi: 10.1080/00039896.1989.9935882. [DOI] [PubMed] [Google Scholar]
- 18.NTP Technical Report on the Toxicity Studies of 3,3′,4,4′-Tetrachloroazoxybenzene (CAS No. 21232-47-3) Administered by Gavage to F344/N Rats and B6C3F1 Mice. Toxic Rep Ser. 1998;66:1–G4. [PubMed] [Google Scholar]
- 19.Vena J, Boffetta P, Becher H, Benn T, Bueno-de-Mesquita HB, Coggon D, Colin D, Flesch-Janys D, Green L, Kauppinen T, Littorin M, Lynge E, Mathews JD, Neuberger M, Pearce N, Pesatori AC, Saracci R, Steenland K, Kogevinas M. Exposure to dioxin and nonneoplastic mortality in the expanded IARC international cohort study of phenoxy herbicide and chlorophenol production workers and sprayers. Environ Health Perspect. 1998;106 (Suppl 2):645–653. doi: 10.1289/ehp.98106645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savouret JF, Berdeaux A, Casper RF. The aryl hydrocarbon receptor and its xenobiotic ligands: a fundamental trigger for cardiovascular diseases. Nutr Metab Cardiovasc Dis. 2003;13:104–113. doi: 10.1016/s0939-4753(03)80026-1. [DOI] [PubMed] [Google Scholar]
- 21.Bertazzi PA, Zocchetti C, Pesatori AC, Guercilena S, Sanarico M, Radice L. Mortality in an area contaminated by TCDD following an industrial incident. Med Lav. 1989;80:316–329. [PubMed] [Google Scholar]
- 22.Dalton TP, Kerzee JK, Wang B, Miller M, Dieter MZ, Lorenz JN, Shertzer HG, Nerbert DW, Puga A. Dioxin exposure is an environmental risk factor for ischemic heart disease. Cardiovasc Toxicol. 2001;1:285–298. doi: 10.1385/ct:1:4:285. [DOI] [PubMed] [Google Scholar]
- 23.Jokinen MP, Walker NJ, Brix AE, Sells DM, Haseman JK, Nyska A. Increase in cardiovascular pathology in female Sprague-Dawley rats following chronic treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin and 3,3′,4,4′,5-pentachlorobiphenyl. Cardiovasc Toxicol. 2003;3:299–310. doi: 10.1385/CT:3:4:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ichihara S, Yamada Y, Ichihara G, Nakajima T, Li P, Kondo T, Gonzalez FJ, Murohara T. A role for the aryl hydrocarbon receptor in regulation of ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2007;27:1297–1304. doi: 10.1161/ATVBAHA.106.138701. [DOI] [PubMed] [Google Scholar]
- 25.Fritz WA, Lin TM, Cardiff RD, Peterson RE. The aryl hydrocarbon receptor inhibits prostate carcinogenesis in TRAMP mice. Carcinogenesis. 2007;28:497–505. doi: 10.1093/carcin/bgl179. [DOI] [PubMed] [Google Scholar]
- 26.Fritz WA, Lin TM, Peterson RE. The Aryl Hydrocarbon Receptor (AhR) Inhibits Vanadate-Induced Vascular Endothelial Growth Factor (VEGF) Production in TRAMP Prostates. Carcinogenesis. 2008 Mar 20; doi: 10.1093/carcin/bgn069. [Epub ahead of print] PMID: 18359762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lund AK, Goens MB, Kanagy NL, Walker MK. Cardiac hypertrophy in aryl hydrocarbon receptor null mice is correlated with elevated angiotensin II, endothelin-1, and mean arterial blood pressure. Toxicol Appl Pharmacol. 2003;193:177–187. doi: 10.1016/j.taap.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Vasquez A, Atallah-Yunes N, Smith FC, You X, Chase SE, Silverstone AE, Vikstrom KL. A role for the aryl hydrocarbon receptor in cardiac physiology and function as demonstrated by AhR knockout mice. Cardiovasc Toxicol. 2003;3:153–163. doi: 10.1385/ct:3:2:153. [DOI] [PubMed] [Google Scholar]
- 29.Thackaberry EA, Bedrick EJ, Goens MB, Danielson L, Lund AK, Gabaldon D, Smith SM, Walker MK. Insulin regulation in AhR-null mice: embryonic cardiac enlargement, neonatal macrosomia, and altered insulin regulation and response in pregnant and aging AhR-null females. Toxicol Sci. 2003;76:407–417. doi: 10.1093/toxsci/kfg229. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, Nebert DW, Rudikoff S, Ward JM, Gonzalez FJ. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 31.McDonnell WM, Chensue SW, Askari FK, Moseley RH. Hepatic fibrosis in Ahr-/- mice. Science. 1996;271:223–224. [PubMed] [Google Scholar]
- 32.McMillan BJ, Bradfield CA. The Aryl Hydrocarbon Receptor sans Xenobiotics: Endogenous Function in Genetic Model Systems. Mol Pharmacol. 2007;72:487–498. doi: 10.1124/mol.107.037259. [DOI] [PubMed] [Google Scholar]
- 33.Neff-LaFord H, Teske S, Bushnell TP, Lawrence BP. Aryl hydrocarbon receptor activation during influenza virus infection unveils a novel pathway of IFN-gamma production by phagocytic cells. J Immunol. 2007;179:247–255. doi: 10.4049/jimmunol.179.1.247. [DOI] [PubMed] [Google Scholar]
- 34.Barnett KR, Tomic D, Gupta RK, Babus JK, Roby KF, Terranova PF, Flaws JA. The aryl hydrocarbon receptor is required for normal gonadotropin responsiveness in the mouse ovary. Toxicol Appl Pharmacol. 2007;223:66–72. doi: 10.1016/j.taap.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang X, Fan Y, Karyala S, Schwemberger S, Tomlinson CR, Sartor MA, Puga A. Ligand-Independent Regulation of Transforming Growth Factor {beta}1 Expression and Cell Cycle Progression by the Aryl Hydrocarbon Receptor. Mol Cell Biol. 2007;27:6127–6139. doi: 10.1128/MCB.00323-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo J, Sartor M, Karyala S, Medvedovic M, Kann S, Puga A, Ryan P, Tomlinson CR. Expression of genes in the TGF-beta signaling pathway is significantly deregulated in smooth muscle cells from aorta of aryl hydrocarbon receptor knockout mice. Toxicol Appl Pharmacol. 2004;194:79–89. doi: 10.1016/j.taap.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Fritsche E, Schafer C, Calles C, Bernsmann T, Bernshausen T, Wurm M, Hubenthal U, Cline JE, Hajimiragha H, Schroeder P, Klotz LO, Rannug A, Furst P, Hanenberg H, Abel J, Krutmann J. Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc Natl Acad Sci U S A. 2007;104:8851–8856. doi: 10.1073/pnas.0701764104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McMillan BJ, Bradfield CA. The aryl hydrocarbon receptor is activated by modified low-density lipoprotein. Proc Natl Acad Sci U S A. 2007;104:1412–1417. doi: 10.1073/pnas.0607296104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cartharius K, Frech K, Grote K, Klocke B, Haltmeier M, Klingenhoff A, Frisch M, Bayerlein M, Werner T. MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics. 2005;21:2933–2942. doi: 10.1093/bioinformatics/bti473. [DOI] [PubMed] [Google Scholar]
- 40.McGuire J, Okamoto K, Whitelaw ML, Tanaka H, Poellinger L. Definition of a dioxin receptor mutant that is a constitutive activator of transcription: delineation of overlapping repression and ligand binding functions within the PAS domain. J Biol Chem. 2001;276:41841–41849. doi: 10.1074/jbc.M105607200. [DOI] [PubMed] [Google Scholar]
- 41.Wang S, Skorczewski J, Feng X, Mei L, Murphy-Ullrich JE. Glucose up-regulates thrombospondin 1 gene transcription and transforming growth factor-beta activity through antagonism of cGMP-dependent protein kinase repression via upstream stimulatory factor 2. J Biol Chem. 2004;279:34311–34322. doi: 10.1074/jbc.M401629200. [DOI] [PubMed] [Google Scholar]
- 42.Hasan RN, Phukan S, Harada S. Differential regulation of early growth response gene-1 expression by insulin and glucose in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:988–993. doi: 10.1161/01.ATV.0000071351.07784.19. [DOI] [PubMed] [Google Scholar]
- 43.Han DC, Isono M, Hoffman BB, Ziyadeh FN. High glucose stimulates proliferation and collagen type I synthesis in renal cortical fibroblasts: mediation by autocrine activation of TGF-beta. J Am Soc Nephrol. 1999;10:1891–1899. doi: 10.1681/ASN.V1091891. [DOI] [PubMed] [Google Scholar]
- 44.Denison MS, Pandini A, Nagy SR, Baldwin EP, Bonati L. Ligand binding and activation of the Ah receptor. Chem Biol Interact. 2002;141:3–24. doi: 10.1016/s0009-2797(02)00063-7. [DOI] [PubMed] [Google Scholar]
- 45.Carlson DB, Perdew GH. A dynamic role for the Ah receptor in cell signaling? Insights from a diverse group of Ah receptor interacting proteins. J Biochem Mol Toxicol. 2002;16:317–325. doi: 10.1002/jbt.10051. [DOI] [PubMed] [Google Scholar]
- 46.Wilson CL, Safe S. Mechanisms of ligand-induced aryl hydrocarbon receptor-mediated biochemical and toxic responses. Toxicol Pathol. 1998;26:657–671. doi: 10.1177/019262339802600510. [DOI] [PubMed] [Google Scholar]
- 47.Hankinson O. The aryl hydrocarbon receptor complex. Annu Rev Pharmacol Toxicol. 1995;35:307–340. doi: 10.1146/annurev.pa.35.040195.001515. [DOI] [PubMed] [Google Scholar]
- 48.Hankinson O. Role of coactivators in transcriptional activation by the aryl hydrocarbon receptor. Arch Biochem Biophys. 2005;433:379–386. doi: 10.1016/j.abb.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 49.Mimura J, Fujii-Kuriyama Y. Functional role of AhR in the expression of toxic effects by TCDD. Biochim Biophys Acta. 2003;1619:263–268. doi: 10.1016/s0304-4165(02)00485-3. [DOI] [PubMed] [Google Scholar]
- 50.Josefsen K, Sorensen LR, Buschard K, Birkenbach M. Glucose induces early growth response gene (Egr-1) expression in pancreatic beta cells. Diabetologia. 1999;42:195–203. doi: 10.1007/s001250051139. [DOI] [PubMed] [Google Scholar]
- 51.Vaulont S, Kahn A. Transcriptional control of metabolic regulation genes by carbohydrates. Faseb J. 1994;8:28–35. doi: 10.1096/fasebj.8.1.8299888. [DOI] [PubMed] [Google Scholar]
- 52.Cuif MH, Porteu A, Kahn A, Vaulont S. Exploration of a liver-specific, glucose/insulin-responsive promoter in transgenic mice. J Biol Chem. 1993;268:13769–13772. [PubMed] [Google Scholar]
- 53.Du X, Stocklauser-Farber K, Rosen P. Generation of reactive oxygen intermediates, activation of NF-kappaB, and induction of apoptosis in human endothelial cells by glucose: role of nitric oxide synthase? Free Radic Biol Med. 1999;27:752–763. doi: 10.1016/s0891-5849(99)00079-9. [DOI] [PubMed] [Google Scholar]
- 54.Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci U S A. 2000;97:12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Han I, Kudlow JE. Reduced O glycosylation of Sp1 is associated with increased proteasome susceptibility. Mol Cell Biol. 1997;17:2550–2558. doi: 10.1128/mcb.17.5.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Asaumi S, Takemoto M, Yokote K, Ridall AL, Butler WT, Fujimoto M, Kobayashi K, Kawamura H, Take A, Saito Y, Mori S. Identification and characterization of high glucose and glucosamine responsive element in the rat osteopontin promoter. J Diabetes Complications. 2003;17:34–38. doi: 10.1016/s1056-8727(02)00189-7. [DOI] [PubMed] [Google Scholar]
- 57.Weigert C, Sauer U, Brodbeck K, Pfeiffer A, Haring HU, Schleicher ED. AP-1 proteins mediate hyperglycemia-induced activation of the human TGF-beta1 promoter in mesangial cells. J Am Soc Nephrol. 2000;11:2007–2016. doi: 10.1681/ASN.V11112007. [DOI] [PubMed] [Google Scholar]
- 58.Weigert C, Brodbeck K, Sawadogo M, Haring HU, Schleicher ED. Upstream stimulatory factor (USF) proteins induce human TGF-beta1 gene activation via the glucose-response element-1013/-1002 in mesangial cells: up-regulation of USF activity by the hexosamine biosynthetic pathway. J Biol Chem. 2004;279:15908–15915. doi: 10.1074/jbc.M313524200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.