Abstract
The γ-secretase complex plays a role in Alzheimer’s disease (AD) and cancer progression. The development of clinical useful inhibitors, however, is complicated by the role of the γ-secretase complex in regulated intramembrane proteolysis of Notch and other essential proteins. Different γ-secretase complexes containing different Presenilin or Aph1 protein subunits are present in various tissues. Here we show that these complexes have heterogeneous biochemical and physiological properties. Specific inactivation of the Aph1B γ-secretase in a murine Alzheimer’s disease model led to improvements of Alzheimer’s disease-relevant phenotypic features without any Notch-related side effects. The Aph1B complex contributes to total γ-secretase activity in the human brain, thus specific targeting of Aph1B-containing γ-secretase complexes may be helpful in generating less toxic therapies for Alzheimer’s disease.
γ-Secretase activity is responsible for the final cleavage of the Amyloid Precursor Protein (APP) releasing the Aβ peptide that accumulates in the amyloid plaques characteristic for Alzheimer’s Disease (1). The same activity cleaves Notch, N-Cadherin and other important signalling molecules. γ-Secretase activity is mediated by a multiprotein complex consisting of Presenilin (PS), Aph1, Pen2 and Nicastrin (NCT) (2). Two presenilin (PS1&2) genes and two APH1 (APH1A&B) genes, which are alternatively spliced, contribute to the heterogeneity of the complexes (3, 4). The Aph1A complexes are crucial for Notch signalling during embryogenesis (5, 6), while functional analysis of APH1B (~58% homologous to APH1A) is complicated because of the rodent-specific duplication of the gene (Aph1C). The combined inactivation of Aph1B and Aph1C (Aph1BC−/−) does not result in any overt phenotype, although disruption of Nrg1 cleavage in the brain of Aph1BC−/− mice (7) results in behavioural changes which are very similar to the ones observed in β-secretase-deficient mice (Bace1−/−) mice (8).
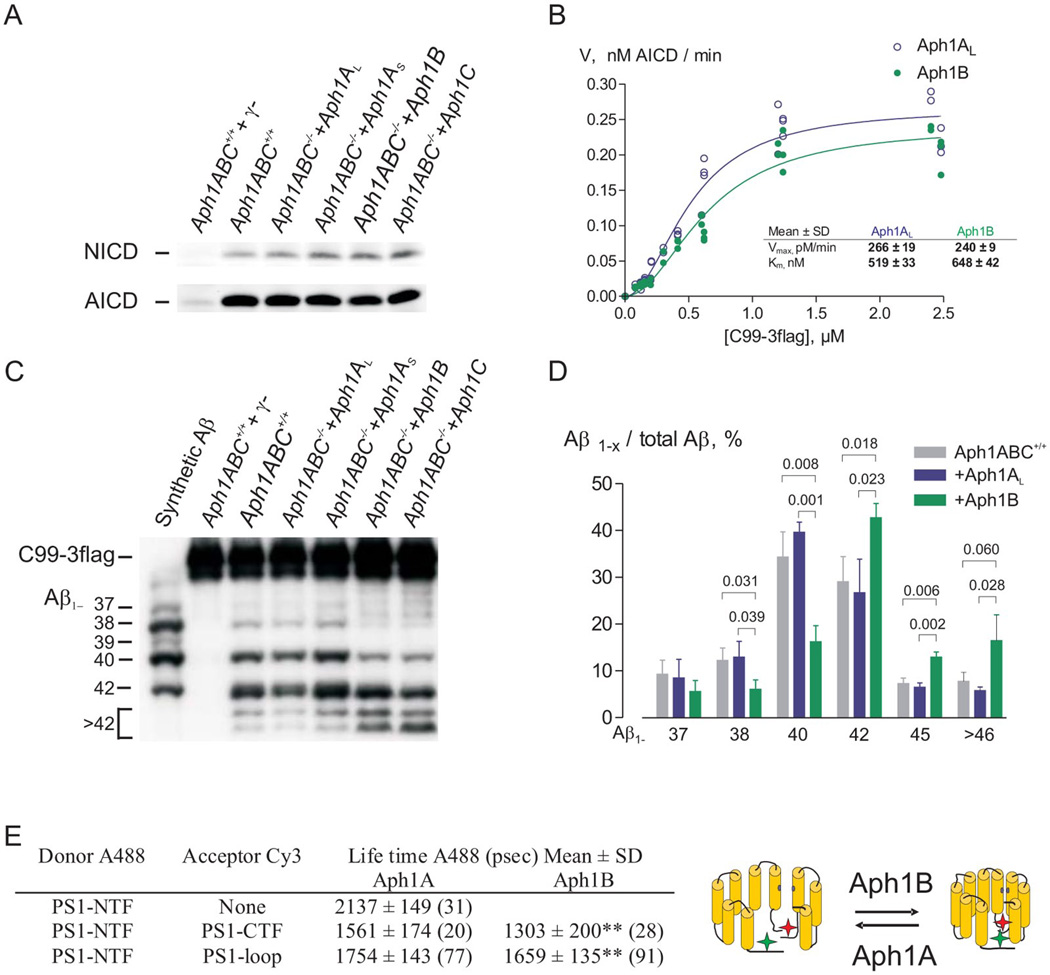
To evaluate the specific biochemical properties of the different Aph1 subunits we rescued triple deficient Aph1A−/−B−/−C−/− (Aph1ABC−/−) mouse embryonic fibroblasts (MEFs) with a single Aph1-homologue (Aph1AL, Aph1AS, Aph1B, Aph1C). They all restored complex formation as evaluated by Blue Native Polyacrylamide Gel Electrophoresis (fig. S1). Their activity was measured in vitro using the recombinant substrates APPC99-3flag and NotchΔE. All complexes supported AICD and NICD production (ε-cleavage, (9)) in vitro (Fig. 1A), and the kinetic parameters Km and Vmax for AICD production were similar for the Aph1AL and Aph1B γ-secretase complexes (Fig. 1B). Thus the “physiological” ε-cleavage was maintained. The Aph1B or Aph1C γ-secretase complexes produced however a greater proportion of longer Aβ peptide species (Aβ1–42, Aβ1–45, Aβ1–46 and Aβ1–49,) relative to shorter Aβ peptides (Aβ1–37, Aβ1–38, Aβ1–40) (Fig.1C, D and fig. S1). In an additional independent assay, specific γ-secretase pools were prepared from wild-type mouse brain by immunoprecipitation with Aph1AL-,Aph1B-, or PS1-specific antibodies or pre-immune serum. The immunoprecipitates were assessed for γ-secretase activity in both the depleted (unbound) and enriched (bound) fractions (fig. S2). Comparison of the Aβ spectra generated confirmed that production of longer Aβ species was proportionately higher in Aph1B versus Aph1AL-containing immunoprecipitates. The opposite trend was observed in the depleted fractions (unbound). Given that changes in the relative ratio of secreted Aβ1–42 to Aβ1–40 is believed to be important for AD progression (10), we determined the Aβ1–42/1–40 ratio in culture supernatants of fibroblasts and primary neurons in vitro and in hippocampal and cortical extracts from Aph1BC−/− mice in vivo. The Aβ1–42/1–40 ratio was maintained between the genotypes (table S1), confirming that Aph1B does not influence this pathological parameter directly (11). However, we observed a significant reduction in total Aβ peptide production in brain extracts from Aph1BC−/− mice, demonstrating the important contribution of the Aph1B complex to total γ-secretase activity in the mouse brain.
Fig. 1.
Aph1B γ-secretase complexes are functional and structurally distinct relative to the Aph1A γ-secretase complexes. (A,B) Immunoblot analysis of microsomal fractions of Aph1ABC+/+ and Aph1ABC−/− MEF rescued with the indicated Aph1 isoforms demonstrates that both complexes perform ε-cleavage of APP and Notch substrate. Specificity of the reaction was validated using a γ-secretase inhibitor (γ−: 10µM L L-685,458). (B) The velocity, or amount of AICD generated after 3 h, was plotted against C99-3flag concentrations. The kinetics of AICD fit a Michaelis-Menten with positive cooperation (h=2) equation. Apparent Vmax and KM values for AICD were statistically indistinguishable (mean±SD, N=5). (C, D) Urea-SDS-PAGE of solubilised Aph1B γ-secretase complexes from MEFs results in more long (Aβ1≥42) and less short (Aβ1–≤40) Aβ species (Mean±SD, N=3, p<0.05-0.01). (E) Aph1ABC−/− MEFs reconstituted with Aph1AL or Aph1B were co-transfected with wild-type human PS1 cDNA and Alexa488 lifetimes were measured in absence or presence of FRET; Aph1B γ-secretase display a significant shorter life time (mean±SD, number of cells counted in 3 to 4 independent transfections are indicated between brackets, p<0.01) implying a closer proximity between the donor and acceptor as schematically represented.
We then wanted to investigate whether the structural heterogeneity deduced from the in vitro assays was preserved in intact cells. Fluorescent Lifetime Imaging Microscopy (FLIM) (12) measures the proximity between fluorophores attached to different domains of a molecule and can detect conformational alterations in the γ-secretase complex (12). The lifetime of the donor fluorophore at the PS1 N-terminus was shortened by the presence of an acceptor fluorophore at an internal loop or at the C-terminus, demonstrating that the fluorophores are in fact in close vicinity. Importantly, complexes containing only Aph1B consistently demonstrated a significantly shorter lifetime than Aph1A-containing complexes (Fig. 1E). The shorter life time indicates a more “closed” conformation of PS1 and is similar (but milder) in effect to long-form Aβ-enhancing FAD-associated presenilin mutations (12). Thus the Aph1 component of the γ-secretase complex has a significant effect on the conformation of the PS1 subunit in situ.
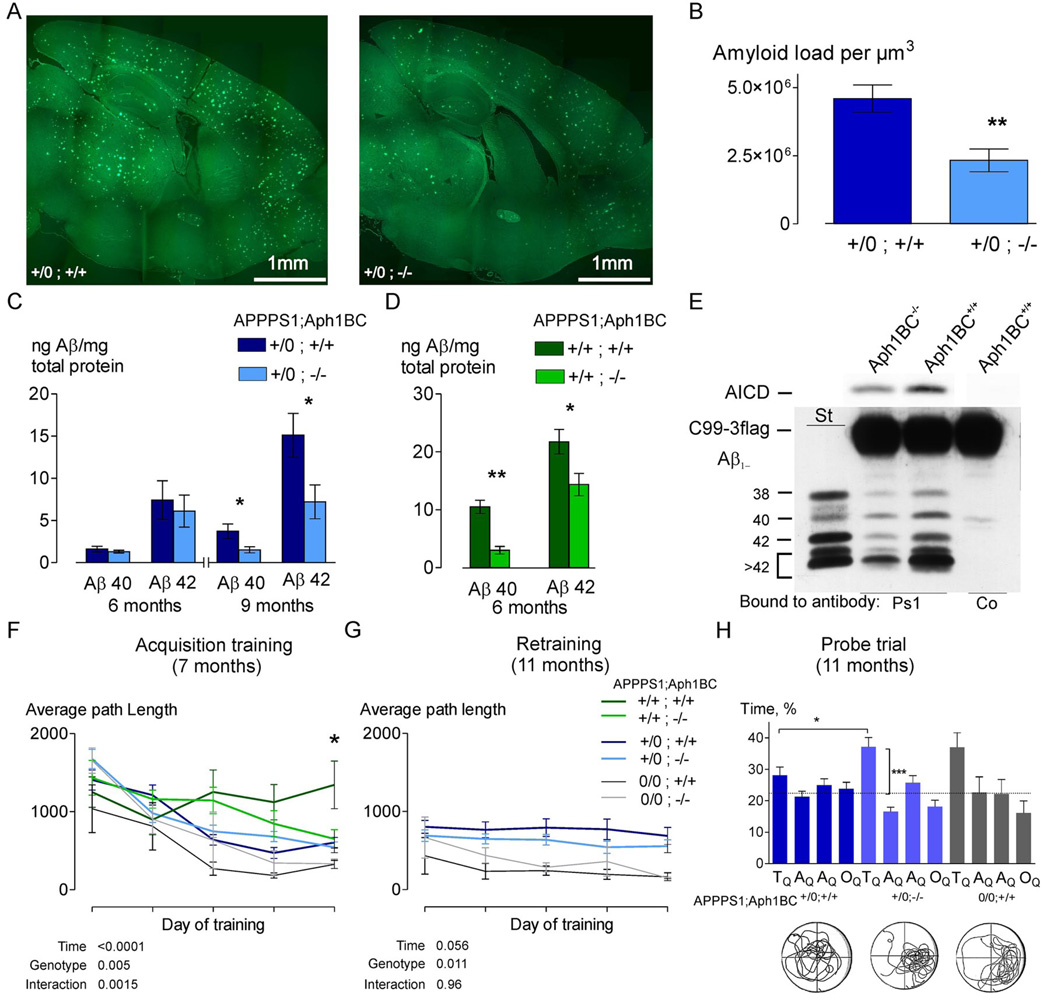
To determine whether specifically targeting Aph1B/C complexes alters the phenotype of a murine AD model overexpressing both mutated APP (APPSwe-KM670/671NL) and PS1 (PS1-L166P) from a single locus (“APPPS1”) (13), we crossed APPPS1 mice with Aph1BC−/− mice. We analyzed mice that were either homozygous or hemizygous for the APPPS1 and homozygous for the Aph1BC locus. At 9 months of age, APPPS1+/0;Aph1BC+/+ mice displayed a massive amyloid burden, which was significantly lowered in APPPS1+/0;Aph1BC−/− mice (Fig. 2A and B). In hippocampal extracts we observed a significant decrease in Aβx–40 and Aβx–42 accumulation in Aph1BC−/− mice (Fig. 2C and D).
Fig. 2.
Deletion of Aph1BC abolishes age-dependent rise in Aβ levels in the brain and rescues learning and memory deficits. (A, B) Decreased amyloid burden was evident in APPPS1+/0;Aph1BC−/− mice at 9 months of age (representative sections shown, measured using quantitative stereology (N=5, p<0.01, absolute amyloid load per unit volume, p<0.01). (C, D) Using Aβx–40 and Aβx–42-specific ELISA, Aβ-levels in the hippocampus were measured and normalized to total protein content. Accumulation of Aβx–40 and Aβx–42 were rescued in APPPS1+/0 and APPPS1+/+ mice (N=5, p<0.05). (E) Purification of the γ-secretase complexes from APPPS1+/0;Aph1BC+/+ and APPPS1+/0;Aph1BC−/− brains using the same PS1-specific antibody, followed by an in vitro activity assay for AICD and Aβ production resulted in a quantitative decrease in APPPS1+/0;Aph1BC−/− mouse brain γ-secretase activity (56±1%) and a statistically significant qualitative shift in the pattern of Aβ produced (N=4, p<0.01). (F) At a young age (median 6.5 months; range 4.8–7.6), learning, as measured by decreasing path length, occurred in all genotypes, except APPPS1+/+;Aph1BC+/+ (Mean±SEM: genotype effect p<0.01; genotype×time interaction p<0.01). This deficit was significantly improved in the APPPS1+/+;Aph1BC−/− mice (p<0.05). (G) Retraining of the same mice at 11 months of age (median 10.7 months; range 9.2–12.2 months) showed that APPPS1+/0;Aph1BC+/+ mice were significantly worse than APPPS1+/0;Aph1BC−/− mice in finding the hidden platform during all retraining days (2-way RM ANOVA between APPPS1+/0 genotypes: genotype effect p<0.01). (H) Probe trial results confirm that Aph1BC deletion prevents spatial memory deficits in hemizygous APPPS1+/0;Aph1BC+/+ mice (%Time spent in: target quadrant TQ, adjacent quadrants AQ1–AQ2 and opposite quadrant OQ, p<0.05; vertical comparison bars: t-test with hypothetical mean 25% or chance level, p<0.001).
We also evaluated the functional consequence of Aph1BC deletion. Homozygous APPPS1+/+;Aph1BC+/+ mice were underrepresented in the breeding program, probably due to a perinatal mortality associated with APPPS1 homozygosity (14–16). Mendelian ratios were restored in the Aph1BC−/− background (fig. S3). The animals also displayed abnormal cage activity (17), which improved with Aph1BC-deficiency (fig. S4). Seven-month-old APPPS1 homozygous mice displayed a profound acquisition deficit in the Morris water maze test for spatial learning and memory, and were unable to improve any performance measure by training (Fig. 2F). No overt genotypic effect on swimming velocity was observed, and visual-evoked potentials, motor coordination, and exploratory and locomotor abilities were normal in all genotypes. Deletion of Aph1BC prevented the learning deficit in APPPS1+/+ mice. Seven-month-old hemizygous APPPS1+/0 mice that were either Aph1BC+/+ or Aph1BC−/− displayed acquisition (Fig. 2F) and probe trial performance similar to control mice. Retraining at 11 months of age showed that APPPS1+/0;Aph1BC+/+ mice had largely retained their (procedural) ability to find the hidden platform but performed worse than controls during all retraining days.
APPPS1+/0;Aph1BC−/− mice performed slightly better than APPPS1+/0;Aph1BC+/+ mice, throughout the retraining (Fig. 2G). APPPS1+/0;Aph1BC−/− mice still displayed normal spatial memory, whereas their Aph1BC+/+ counterparts failed to show any preference for the target quadrant (Fig. 2H). Thus Aph1BC deletion significantly improves the AD-like phenotype of an AD mouse model.
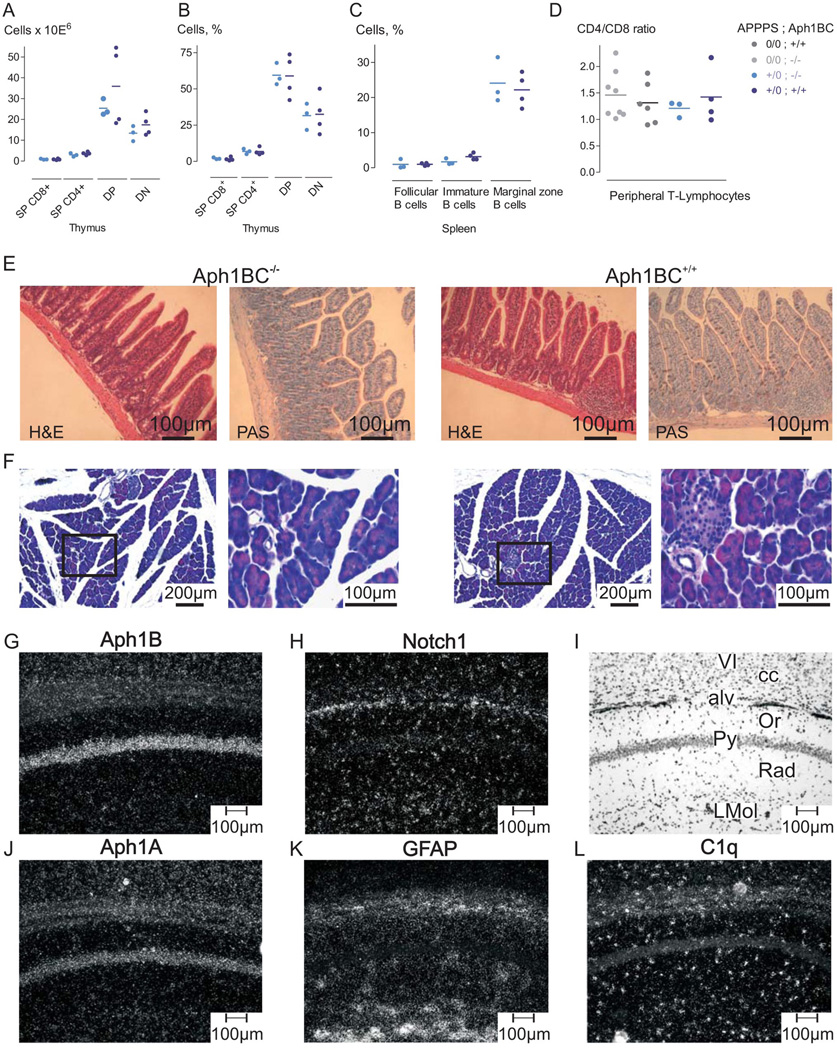
Aph1BC deficiency had little effect on murine health. Extensive behavioral and neurochemical testing revealed only a mild disturbance in prepulse inhibition which is extremely mild in comparison to the effect of γ-secretase inhibition on Notch-dependent processes (7) (Fig. 3). Aph1BC deficiency did not affect B- or T-cell maturation in thymus or spleen, nor did it alter steady-state CD4+/CD8+ ratios (18, 19). The intestinal and pancreatic morphology were also unaffected in Aph1BC−/− mice (18, 20). Finally, expression of Notch1 and its target genes (HES1, HES5, ACSL1) (21, 22), which are directly or indirectly dependent on γ-secretase activity, were similar in hippocampi of APPPS1+/0;Aph1BC−/− and APPPS1+/0;Aph1BC+/+ mice (fig. S5). We further investigated expression of the Aph1 genes in hippocampi, pancreas, spleen, gut and thymus (fig. S6). Aph1B mRNA levels are relatively high in the hippocampus and pancreas. In situ hybridization experiments using brain tissue sections confirmed the neuronal Aph1B expression in regions relevant for AD (7) (Fig. 3 and fig. S6) while Notch1 mRNA signal is predominantly expressed in non-neuronal and neuronal precursor cells, and overlapped significantly with Aph1A, but not Aph1B expression (Fig. 3). Therefore, the complete ablation of a γ-secretase subunit can be generated in an adult mouse without any Notch-related phenotypes (23).
Fig. 3.
Absence of Notch-signalling defects in Aph1BC−/− mice and expression of Aph1B/C in neurons of the adult mouse brain. (A, B, C) Sensitive T- and B-cell populations in the thymus (A, B) or in the spleen (C) were not distinguishable using FACS in age-matched Aph1BC+/+ (N=4) and Aph1BC−/− mice (N=3). (D) CD4+/CD8+ ratios of peripheral T-lymphocytes were identical in both APPPS10/0 (Aph1BC−/−: N=3; Aph1BC+/+: N=4) and APPPS1+/0 mice (Aph1BC−/−: N=8; Aph1BC+/+: N=7). (E) Intestinal mucosal and (F) pancreas morphology, evaluated in a blind study, was identical in both genotypes (N=3; H&E and PAS; representative sections). (G, H , J–L). High power dark-field micrographs of hippocampal region (CA1) hybridized with the indicated antisense probes demonstrate that Aph1B/C expression is restricted to the pyramidal cell layer of the CA1 region and neuronal cell layers of cerebral cortex (layer VI) (G). Notch1 is predominantly expressed in non-neuronal layers containing mainly glial cells (H). Aph1A is widely expressed in both neuronal- and glia-enriched regions (J). Notch1 expression is generally low and partially overlaps with the non-neuronal markers GFAP (astrocytes) (K) and C1q (microglia, perivascular macrophages) (L). Hippocampal and cortical layers are identified by bright field photography of section depicted in (I). VI: lamina six of parietal cortex, cc: corpus callosum, alv: alveus of the hippocampus (astrocytes), Or: Stratum oriens of the CA1 region, Py: contains typical pyramidal neurons, Rad: Stratum radiatum (glia), LMol: Stratum lacunosum molecolare (glia).
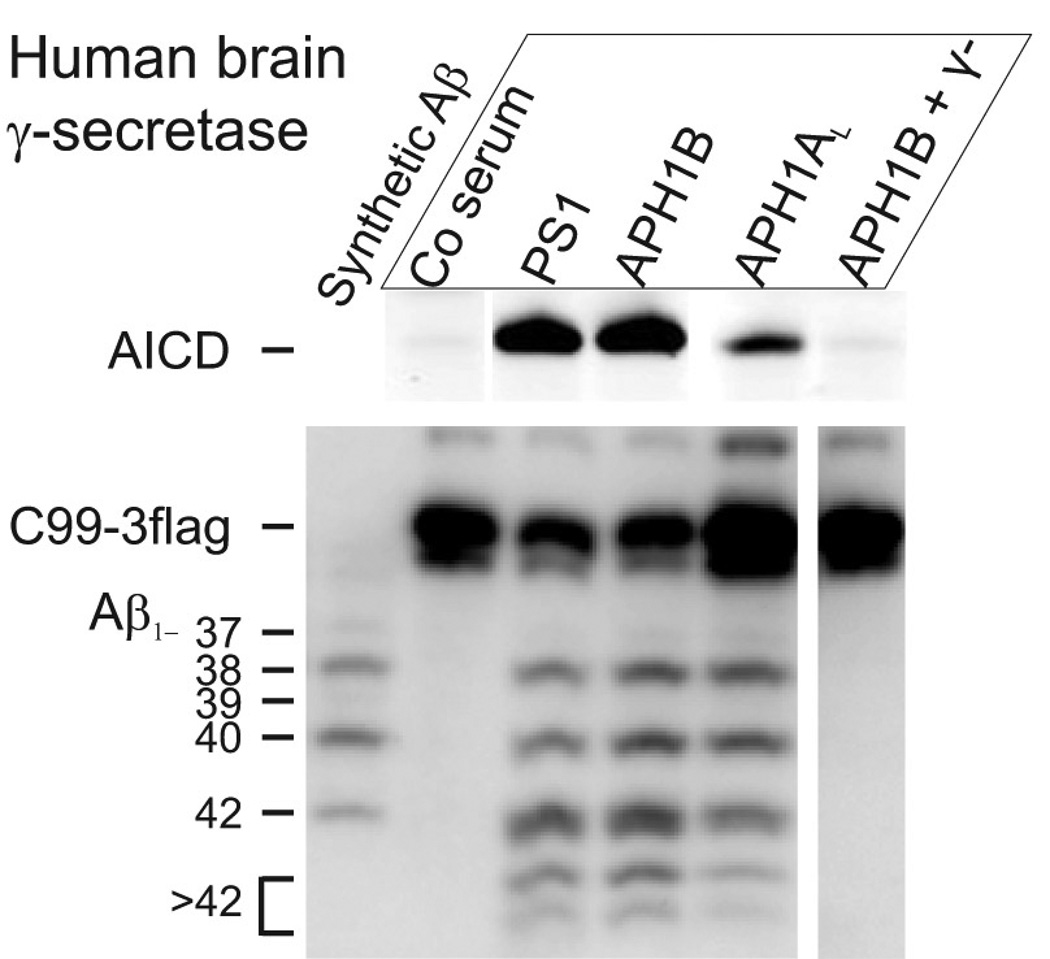
The extent to which the APH1B γ-secretase complex contributes to Aβ production in the human brain is unknown. Specific γ-secretase pools were prepared from human brain as described above using Aph1AL-, Aph1B-, PS1-specific antibodies or pre-immune serum, and then both the depleted (unbound) as well as the enriched (bound) fractions were used for in vitro cleavage assays (Fig. 4 and fig S7). Depletion of APH1B γ-secretase from the endogenous pool of human brain complexes lowered AICD- and Aβ-production considerably. Conversely, the isolated APH1B γ-secretase complex was active (Fig. 4). APH1B γ-secretase complexes (APH1B-bound) are a major contributor to total γ-secretase activity (PS1-bound) in the human brain. Furthermore, similar changes were observed in the Aβ peptide spectrum generated in vitro as seen in the murine system.
Fig. 4.
Aph1B γ-secretase contributes to Aβ-production in human brain. Different pools of γ-secretase were prepared from microsomal membranes of human brain tissue using immunoprecipitation with pre-immune serum (Co serum), or PS1-, Aph1B-, or Aph1AL-specific antibody. γ-Secretase activity was then measured in vitro. Production of AICD and Aβ species was measured using SDS-PAGE or urea-SDS-PAGE and specific antibodies. Comparison of the Aph1AL and Aph1B precipitated pools reveals a statistically significant shift in the pattern of Aβ production (mean±SD, N=3, p<0.05-0.01). Notice that Aph1AL immunoprecipitate is less active and 4x more sample was loaded. γ−: 10µM γ-secretase inhibitor L-685,458
Here we provide evidence that the Aph1 protein contributes directly to the proteolytic activity of the γ-secretase complex by influencing the conformation of the catalytic PS1 subunit in situ. Targeting specifically the Aph1B containing complexes results in significant improvements of multiple severe AD-related phenotypes in a mouse model. The lack of Notch related side effects should be compared with what was observed in other full and partial knock-outs of γ-secretase subunits (summarized in (23)). A 50% reduction in γ-secretase activity in Nct+/− heterozygous mice is associated with severe Notch side effects (24). In comparison, we have observed here the complete removal of a γ-secretase complex component without Notch-related problems and with efficient reduction of the amyloid pathology in the mouse brain. Since the Aph1B γ-secretase complex is present and active in the human brain, the selective inhibition of this complex has the potential to translate into an approach to lower Aβ peptide production in human AD with relatively few side effects. Our work has also implications for other fields, as γ-secretase is for instance involved in haematopoietic and other cancers. It might be important to analyse the role of the different complexes in these different diseases as well (25).
Supplementary Material
www.sciencemag.org/cgi/content/full/1171176/DC1
Materials and Methods
SOM Text
Figs. S1 to S7
Table S1
References
References and Notes
- 1.De Strooper B, et al. Nature. 1998 Jan 22;391:387. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- 2.De Strooper B. Neuron. 2003 Apr 10;38:9. doi: 10.1016/s0896-6273(03)00205-8. [DOI] [PubMed] [Google Scholar]
- 3.Hebert SS, et al. Neurobiology of Disease. 2004 Nov 17;17:260. doi: 10.1016/j.nbd.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Shirotani K, Edbauer D, Prokop S, Haass C, Steiner H. J. Biol. Chem. 2004 Oct 1;279:41340. doi: 10.1074/jbc.M405768200. [DOI] [PubMed] [Google Scholar]
- 5.Serneels L, et al. Proceedings of the National Academy of Sciences. 2005 Feb 1;102:1719. doi: 10.1073/pnas.0408901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma G, Li T, Price DL, Wong PC. J. Neurosci. 2005 Jan 5;25:192. doi: 10.1523/JNEUROSCI.3814-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dejaegere T, et al. Proceedings of the National Academy of Sciences. 2008 Jul 15;105:9775. doi: 10.1073/pnas.0800507105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savonenko AV, et al. Proceedings of the National Academy of Sciences. 2008 Apr 8;105:5585. doi: 10.1073/pnas.0710373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haass C, Selkoe DJ. Nat Rev Mol Cell Biol. 2007 Feb;8:101. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 10.Scheuner D, et al. Nat Med. 1996 Aug;2:864. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 11.Shirotani K, Tomioka M, Kremmer E, Haass C, Steiner H. Neurobiology of Disease. 2007 Jul;27:102. doi: 10.1016/j.nbd.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Berezovska O, et al. J. Neurosci. 2005 Mar 16;25:3009. doi: 10.1523/JNEUROSCI.0364-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radde R, et al. EMBO Rep. 2006 Sep;7:940. doi: 10.1038/sj.embor.7400784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsiao KK, et al. Neuron. 1995 Nov;15:1203. doi: 10.1016/0896-6273(95)90107-8. [DOI] [PubMed] [Google Scholar]
- 15.King DL, Arendash GW. Physiol Behav. 2002 Apr 15;75:627. doi: 10.1016/s0031-9384(02)00639-x. [DOI] [PubMed] [Google Scholar]
- 16.Pugh PL, Richardson JC, Bate ST, Upton N, Sunter D. Behav Brain Res. 2007 Mar 12;178:18. doi: 10.1016/j.bbr.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 17.Hollingworth P, et al. J Am Geriatr Soc. 2006 Sep;54:1348. doi: 10.1111/j.1532-5415.2006.00854.x. [DOI] [PubMed] [Google Scholar]
- 18.Wong GT, et al. J Biol Chem. 2004 Mar 6;279:12876. doi: 10.1074/jbc.M311652200. [DOI] [PubMed] [Google Scholar]
- 19.Tournoy J, et al. Hum Mol Genet. 2004 Jul 1;13:1321. doi: 10.1093/hmg/ddh151. [DOI] [PubMed] [Google Scholar]
- 20.Siveke JT, et al. Gastroenterology. 2008 Feb;134:544. doi: 10.1053/j.gastro.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Louvi A, Artavanis-Tsakonas S. Nat Rev Neurosci. 2006 Feb;7:93. doi: 10.1038/nrn1847. [DOI] [PubMed] [Google Scholar]
- 22.Kawai T, Takagi N, Nakahara M, Takeo S. Neuroscience Letters. 2005 Jan 21;380:17. doi: 10.1016/j.neulet.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Choi SH, Norstrom E. J. Neurosci. 2007 December 12;27:13579. doi: 10.1523/JNEUROSCI.4821-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li T, et al. J. Neurosci. 2007 October 3;27:10849. [Google Scholar]
- 25.We wish to thank C. Peeters for assistance in pathological evaluations, C. Mathieu for FACS analysis, L. Van Aerschot for assistance with behavioral testing, S. Terclavers and H. Schieb for technical assistance and A. Thathiah for MS proofreading. This work was supported by a Pioneer award from the Alzheimer’s Association, the Fund for Scientific Research Flanders (to RD and BDS), KULeuven (GOA), Federal Office for Scientific Affairs, Belgium, a Methusalem grant of the Flemish Government, MEMOSAD (F2-2007-200611) of the European Union and NIH P01AG015379, R01AG026593 and NIH AG026593 (to BH) and NIH AG 13579 (to BH and OB). BDS is a paid consultant for Eli Lilly and Envivo Pharmaceutics; BH is a paid consultant for Elan, Genentech, Pfizer, Takeda, Link and Neurophage.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
www.sciencemag.org/cgi/content/full/1171176/DC1
Materials and Methods
SOM Text
Figs. S1 to S7
Table S1
References