Abstract
The biological basis of mood is not understood. Most research on mood and affective states has focused on the roles of brain systems containing monoamines (e.g., dopamine, norepinephrine, serotonin). However, it is becoming clear that endogenous opioid systems in the brain may also be involved in regulation of mood. In this review, we focus on the potential utility of kappa-opioid receptor (KOR) ligands in the study and treatment of psychiatric disorders. Research from our group and others suggests that KOR antagonists might be useful for depression, KOR agonists might be useful for mania, and KOR partial agonists might be useful for mood stabilization. Currently available agents have some unfavorable properties that might be addressed through medicinal chemistry. The development of KOR-selective agents with improved drug-like characteristics would facilitate preclinical and clinical studies designed to evaluate the possibility that KORs are a feasible target for new medications.
Keywords: depression, antidepressant, dynorphin, kappa opioid, dopamine, model, rat, mouse
1. Introduction
The biological basis of mood is not understood. Most present day research on mood and affective states focuses on the roles of brain systems containing monoamines, such as dopamine (DA), norepinephrine (NE), and serotonin (5-hydroxytryptamine [5HT]). Drugs with mood-elevating effects have prominent interactions with these systems, in general by increasing extracellular concentrations of monoamines and prolonging their actions (Ritz et al., 1987; Di Chiara and Imperato, 1988; Koch et al., 2002). Increased DA is most often associated with rewarding (pleasurable) mood states: major drugs of abuse (including opiates, stimulants, nicotine, ethanol) have the common effect of increasing neurotransmission in midbrain DA systems (Wise and Bozarth, 1987; Di Chiara and Imperato, 1988). In contrast, antipsychotic drugs (which have prominent DA antagonist properties) tend to block reward, lower abnormally elevated mood, and produce anhedonia (Wise, 1982). Depression has often been associated with reduced NE, and one of the earliest classes of antidepressant drugs (tricyclic antidepressants [TCAs]) blocks synaptic reuptake of this transmitter (Frazer, 1997; Koch et al., 2002). The commercial success of selective serotonin reuptake inhibitors (SSRIs) as antidepressants—which appear to be safer, but possibly less clinically efficacious than TCAs (Fawcett and Barkin, 1997)—has led to discoveries of fundamental alterations in brain 5HT systems in mediating depression (e.g., the association of stress-induced depression with polymorphisms of the gene coding for the serotonin transporter may explain vulnerability to depressive disorders in some individuals) (Caspi et al., 2003; Vergne and Nemeroff, 2006). New generations of antidepressants target two or more of the monoamines. As such, studies of how brain monoamine systems regulate mood—and how they can be manipulated to control mood—have dominated the field for decades.
Endogenous opioid systems in the brain are also involved in regulation of mood, although they have received far less attention than monoamine systems. It is clear that such effects involve, at least in part, monoamines: indeed, some of the reward-related effects of opiates such as morphine appear to depend upon their ability to activate DA systems (Leone et al., 1991) via inhibition of neurons that normally inhibit the activity of midbrain DA neurons (Johnson and North, 1992). Considering that endogenous opioid systems are interwoven with monoamine systems in the brain (see Snyder and Pasternak, 2003), it should not be surprising that some effects of opioid receptor stimulation involve monoamine neurotransmitters. However, there is also evidence that rewarding effects of opiates are DA-independent (Olds, 1982). Relevant findings suggest that the major consequence of activation of endogenous opioid receptors—stimulation of inhibitory G-proteins (see Snyder and Pasternak, 2003)—could be sufficient to alter mood states. The fact that both endogenous and exogenous opioids can affect mood raises the possibility that drugs that target endogenous opioid systems could be utilized in the treatment of debilitating psychiatric conditions.
We have become particularly interested in how kappa-opioid receptors (KORs)—so-named for the prototype KOR agonist ketocyclazocine (Martin et al., 1976)—contribute to regulation of mood. Our interest in KORs has evolved from what began as disparate lines of research. The idea that KOR antagonists might be useful for the treatment of depression can be traced back to studies of the neurobiological consequences of long-term exposure to addictive drugs such as cocaine (Carlezon et al., 1998). The idea that KOR agonists might be useful for the treatment of bipolar disorder or mania can be traced back to studies of the molecular mechanisms of action of antipsychotic drugs (Ma et al., 2003), which are often used to manage the symptoms of these conditions. Studies of the antidepressant-like effects of KOR antagonists led directly to studies of the prodepressant-like effects of KOR agonists (Mague et al., 2003; Carlezon et al., 2006). In this review, we present evidence for a role of KORs in the regulation of mood, describe issues that currently hinder research in this area, and provide suggestions for how the field might be advanced.
2. KOR Antagonists
History
Until recently, the prospects for clinical applications of KOR antagonists seemed limited. One consequence of the discovery of endogenous opioid receptors (see Snyder and Pasternak, 2003) and their agonists—including dynorphin, an endogenous agonist at brain KORs (Chavkin et al., 1982)—was an interest in developing analgesic agents with reduced abuse liability. Dynorphin (Han and Xie, 1982; Han et al., 1984) and synthetic KOR agonists (e.g., bremazocine; Pazos et al., 1983) have antinociceptive effects in rats, and KOR antagonists were primarily developed and utilized initially to block and characterize these actions (Portoghese et al., 1987). There were scattered reports suggesting potential clinical uses for KOR antagonists, such as minimizing the effects of traumatic injury to the CNS (Faden et al., 1987; Vink et al., 1991) or manipulating feeding behavior (Carr et al., 1989). In general, however, these agents seemed envisioned primarily as “molecular probes” (Portoghese et al., 1987) for studies of interactions between agonists and KORs and the functional significance of KORs for behavior.
Our interest in KOR antagonists evolved from research on the ability of drugs of abuse to induce neuroadaptations within brain reward circuits, including the nucleus accumbens (NAc). Some of our early work focused on the effects of stimulant drugs on cAMP response element binding protein (CREB) (see Carlezon et al., 2005). CREB is located in the nucleus of all cells in the brain and belongs to a family of proteins called transcription factors. Transcription factors play a crucial role in translating events that occur on the cell membrane into alterations in gene and protein expression. They regulate sets of genes called “transcriptomes”; the CREB transcriptome includes genes that encode other transcription factors (e.g., Fos), enzymes (e.g., tyrosine hydroxylase), receptor subunits (e.g., GluRs), growth factors (e.g., brain-derived neurotrophic factor), stress factors (e.g., corticotropin releasing factor), and opioid peptides (e.g., dynorphin) (Carlezon et al., 2005). Alterations in the expression of these genes can “re-program” cells, leading to modification of the activity of neural circuits. In turn, these changes in activity encode behavior and, presumably, mood. Repeated administration of stimulants such as amphetamine increases the function of the transcription factor CREB within the NAc (Turgeon et al., 1997). At the time, the biological significance (if any) of this change was unclear; conceivably, it could contribute to drug tolerance or drug sensitization (reverse tolerance), two processes thought to play significant roles in the development and maintenance of addictive behaviors. In an attempt to establish causal relationships between drug-induced alterations in CREB function in the NAc and complex behaviors, we engineered viral vectors that would enable us to elevate CREB expression (thereby modeling one consequence of drug exposure) or block CREB function, specifically within this region. In tests of sensitivity to the rewarding effects of cocaine, elevated expression of CREB in the NAc caused complex alterations in behavior: it made low doses of the drug aversive, and higher doses less rewarding than normal (Carlezon et al., 1998). Only through the use of additional models did it become apparent that elevation of CREB function in the NAc was eliciting the rodent equivalent of signs of major depression: dysphoria (a state of aversion), anhedonia (a state of reduced sensitivity to pleasure), and despair (a state of hopelessness) (Pliakas et al., 2001). In addition, it became clear that stress, a common trigger for both depressive and addictive disorders (Kendler et al., 1999), also activates CREB in the NAc (Pliakas et al., 2001). Disruption of CREB function in the NAc caused opposite effects to those induced by elevated CREB, and had antidepressant-like effects that were indistinguishable from those of standard antidepressants. These findings led to the working hypothesis that activation of CREB in the NAc is a molecular trigger or mediator for aversive or depressive-like symptoms (Carlezon et al., 1998; Pliakas et al., 2001; Carlezon et al., 2005).
Because CREB is a transcription factor that regulates expression of a variety of genes, it seemed likely that the aversive effects of elevated CREB function in the NAc were mediated by CREB-regulated target genes. Evidence already in the literature implicated dynorphin: both in vitro and in vivo studies indicated that CREB regulates expression of the gene encoding the peptide precursor of the endogenous KOR agonist dynorphin (prodynorphin) (Daunais et al., 1993; Douglass et al., 1994; Cole et al., 1995). Recent work confirms that the same types of stress that activate CREB in the NAc also increase prodynorphin expression (Chartoff et al., 2009). In addition, it was already known that KOR agonists cause aversive and depressive signs (including dysphoria) in humans (Pfeiffer et al., 1986) and rodents (Bals-Kubik et al., 1989). We found that viral vector-mediated elevations in CREB function within the NAc increased prodynorphin expression, whereas disruption of CREB function reduced it (Carlezon et al., 1998). This work suggested that the ability of CREB activation in the NAc to trigger aversive states could be related to increased dynorphin and subsequent elevations in KOR activation in this brain region. This hypothesis was supported by a series of experiments demonstrating that intracerebroventricular (ICV) administration of the KOR antagonist nor-binaltorphimine (norBNI) blocked the aversive-like effects of elevated CREB on cocaine reward (Carlezon et al., 1998) as well as depressive-like behavior in the Forced Swim Test (FST) (Pliakas et al., 2001). Importantly, the effects of KOR antagonists were not limited to conditions in which CREB function had been artificially boosted using viral vectors: norBNI and related derivatives (GNTI, ANTI) had antidepressant-like effects of their own in the FST (Mague et al., 2003), a procedure that identifies in rats treatments with antidepressant efficacy in humans (Willner, 1984). The antidepressant-like effects of KOR antagonists have since been confirmed by other researchers, who have extended these finding to other protocols (Newton et al., 2002; McLaughlin et al., 2003) and structurally dissimilar drugs (Beardsley et al., 2005). More recent work has demonstrated that KOR antagonists also have acute anxiolytic-like actions (Knoll et al., 2007). Interestingly, standard antidepressant drugs often have anxiogenic effects upon acute administration (Artaiz et al., 1998; Bagdy et al., 2001; Drapier et al., 2007). Thus the behavioral profile of KOR antagonists—acute antidepressant and anxiolytic effects—is somewhat unique and suggests that this class of drugs might be particularly effective for the treatment of comorbid depressive and anxiety disorders.
Mechanisms
The mechanisms by which KOR antagonists produce antidepressant-like effects are not fully understood. There is compelling evidence that alterations in DA function within the NAc are involved; on the basis of a broad and multidisciplinary literature (see Carlezon and Thomas, 2009), we have developed a highly simplified model that enables us to test our working hypotheses (Figure 1). A key difference between KORs and both mu-opioid receptors (MORs) and delta-opioid receptors (DORs) is their anatomical localization within the NAc: KORs are located primarily on the terminals of inputs from the mesolimbic system (Svingos et al., 1999, 2002), whereas MORs and DORs are on the cell bodies of GABAergic medium spiny neurons or interneurons (Mansour et al., 1995). Thus despite common (inhibitory) effects on signal transduction, stimulation of KORs often causes effects opposite to those caused by stimulation of MORs or DORs. For example, whereas MOR and DOR agonists increase extracellular concentrations of DA in the NAc (Leone et al., 1991; Devine et al., 1993), KOR agonists decrease them (Devine et al., 1993; Carlezon et al., 2006). These effects appear to be mediated, at least in part, within the NAc: microinfusions of KOR agonists into this region decrease local DA concentrations (Donzanti et al., 1992; Spanagel et al., 1992), most likely by stimulating presynaptic KORs that inhibit DA release from ventral tegmental area (VTA) neurons (Svingos et al., 1999). Thus CREB-mediated increases in dynorphin expression within the NAc could result in local decreases in DA release, which triggers signs of depression (particularly, given the specific role of the NAc, those reflecting altered motivation). Although these effects might occur predominately in the NAc, KOR ligands could also have effects within other parts of the reward circuitry where KORs are expressed, such as the VTA and prefrontal cortex (PFC) (Svingos et al., 2002; Margolis et al., 2003). According to our model (Figure 1), KOR antagonists have antidepressant effects because they block the consequences of CREB-mediated upregulation of dynorphin function in the NAc by blocking KORs in the NAc (or other regions), leading to restored function of the mesolimbic DA system. Another possibility—which is not mutually exclusive—is that KOR antagonists reduce activation of CREB in the NAc, which normally contributes to elevated dynorphin function. There is evidence that antidepressants may, in fact, interfere with CREB function under some circumstances. A variety of antidepressants induce decreased CREB phosphorylation and CREB-mediated gene transcription in certain in vitro preparations (Schwaninger et al., 1995; Chartoff et al., 2009). In addition, chronic antidepressant treatment can lead to increased expression of cAMP phosphodiesterases (PDEs)—which metabolize cAMP—in the NAc (Takahashi et al., 1998). These findings raise the possibility that molecular processes involving CREB and KORs in the NAc play an underappreciated role in the therapeutic actions of many types of antidepressants. Yet another possibility is that KOR antagonists work through yet-to-be described interactions with 5HT or NE systems, which appear to be critically involved in the therapeutic effects of SSRIs and TCAs, the most widely prescribed classes of antidepressant drugs.
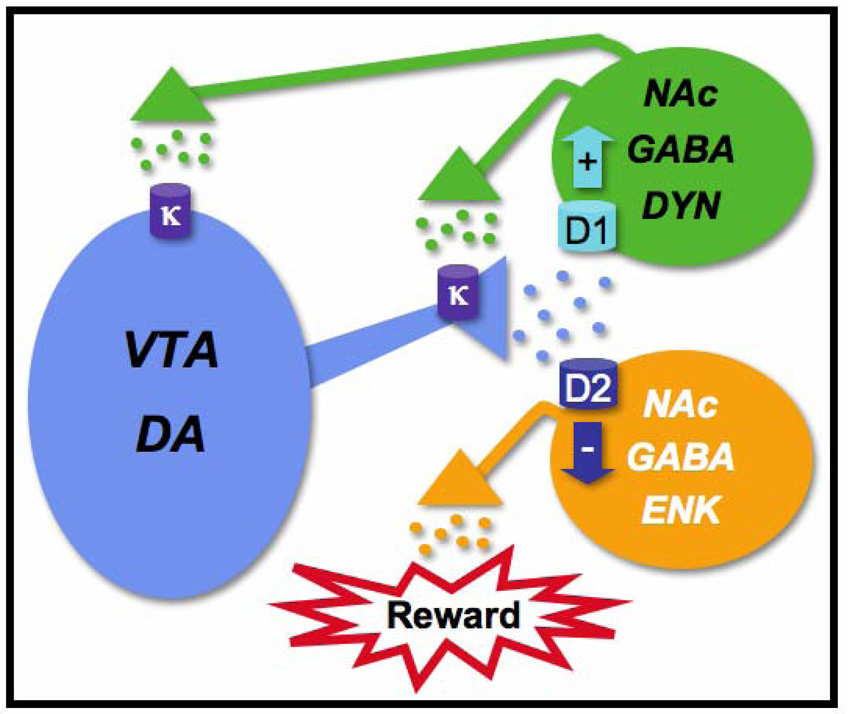
Fig.1.
A highly simplified hypothetical scheme by which kappa-opioid receptors (KORs) in the mesolimbic system might regulate mood. Dopamine (DA) neurons (blue) originating in the ventral tegmental area (VTA) project to the nucleus accumbens (NAc). Within the NAc, DA acts upon two populations of medium spiny (GABAergic) neurons. One type of neuron expresses dopamine D2 receptors and enkephalin (ENK). These neurons (orange) normally inhibit reward; DA binding at D2 receptors activates inhibitory G-proteins (Gi) and decreases the activity of these neurons, which enables reward via processes that might involve outputs to other regions (e.g., ventral pallidum). The other type of neuron expresses dopamine D1 receptors and dynorphin (DYN). These neurons (green) provide feedback regulation of the VTA; DA binding at D1 receptors activates stimulatory G-proteins (Gs) and increases the activity of these neurons. Subsequent increases in DYN-induced stimulation of Gi-coupled KORs would tend to decrease the activity of VTA DA neurons. One consequence of this effect would be reduced D2 receptor function and increased inhibition of reward, causing hallmark signs of depression (e.g., anhedonia, dysphoria). According to this scheme, administration of KOR agonists would produce signs of depression by causing acute reductions in the activity of VTA neurons, whereas exposure to stress and drugs of abuse lead to CREB-regulated increases in DYN expression and more persistent behavioral effects. KOR antagonists might normalize the function of VTA neurons by preventing overstimulation of KORs, thereby producing antidepressant effects. For additional detail, see Carlezon and Thomas (2009).
Studies in mutant mice provide genetic validation of the role of the KORs in stress-induced depressive and anxiety-like behaviors. KOR −/− mice show a complete loss of KOR transcript and receptor binding and a significant reduction in stress-induced behaviors. A series of studies by McLaughlin and colleagues (2006a) in which these mice were used has demonstrated that repeated exposure to forced swimming induces several stress-induced behaviors in wild-type mice including analgesia, increased immobility in the FST (a prodepressive-like effect), and potentiation of cocaine conditioned place preference (CPP). These stress-induced behavioral effects were completely absent in KOR −/− mice or in wild-type mice that had been treated with the KOR antagonist norBNI; that is, KOR ablation and KOR antagonists produced identical effects on these behaviors. Moreover, pretreatment of wild-type mice with the KOR agonist U50,488 mimicked the potentiation of cocaine CPP produced by forced swimming, suggesting that KOR activation is necessary and sufficient to produce this stress-induced behavior. It is important to note that initial characterizations of a separate line of KOR −/− mice under minimally stressful testing conditions did not detect differences in depression or anxiety-like behavior . Rather than reflecting an absence of a role of the KOR system in anxiety and depressive behavior, numerous lines of evidence suggest that these results reflect low levels of basal KOR signaling under non-stressful conditions. For example, studies demonstrating a reduction in depressive behavior in KOR −/− mice similarly do not see behavioral phenotypes in initial tests, but marked decreases in stress-induced behavior occur upon repeated testing . These data highlight the importance of tone in the KOR system in mediating stress-induced behaviors.
PDyn −/− mice also show reduced immobility in the FST (an antidepressant-like effect), decreased induction of stress-induced analgesia, decreased social defeat and blockade of stress-induced potentiation of cocaine CPP . A separate line of PDyn −/− mice show an anxiolytic phenotype in the elevated plus maze and open field paradigms as well as a significant decrease in serum corticosterone and corticotrophin releasing factor mRNA in the amygdala and paraventricular nucleus of the hypothalamus, brain regions involved in anxiety and depressive behaviors . In these studies, treatment of wild-type littermates with KOR antagonist mimics the anxiolytic phenotype whereas pretreatment of PDyn −/− mice with KOR agonist eliminates the anxiolytic behavioral phenotype . Taken together, the marked reduction of stress-induced behaviors in KOR −/− and PDyn −/− mice, highlights the importance of the KOR system in mediating depressive and anxiety-like behaviors.
Complexities
The possibility that the antidepressant-like effects of KOR antagonists are related to an ability to boost the function of brain DA systems adds an additional level of complexity to the development of these drugs for mood disorders. Virtually all addictive drugs facilitate brain DA function (Wise and Bozarth, 1987; Di Chiara et al., 1988), an effect often associated with their abuse liability. Standard antidepressant drugs have no abuse liability, so KOR antagonists would not represent an improved therapeutic approach if they posed a risk for misuse. To address the possibility that KOR antagonists have properties that might lead to abuse, we examined their effects in the intracranial self-stimulation (ICSS) test, which identifies drugs with reward-altering actions (Carlezon and Chartoff, 2007). We observed that the selective KOR antagonist ANTI does not alter ICSS behavior at doses 8 times higher than those with antidepressant effects in the FST (Todtenkopf et al., 2004), and we have since extended this finding to doses 32 times higher (W. A. Carlezon Jr., unpublished observations). These data suggest that although KOR antagonists may be able to reverse reductions in DA caused by increased KOR tone, they have a limited ability to enhance DA function to the degree that would make the drugs rewarding. In addition, these results suggest that KOR antagonists would not be likely to produce mania-like states, a problem seen with stimulants and, sometimes, with standard antidepressants. Indeed, the effects of KOR antagonists on DA appear to be modest: direct administration of KOR antagonists into the NAc increases local concentrations of DA to ~175% of baseline (Maisonneuve et al., 1994), whereas psychostimulants such as cocaine and amphetamine can cause increases ranging from 500–1000% of baseline (Di Chiara et al., 1988; Maisonneuve et al., 1994). Modest increases in DA concentrations within the NAc may be sufficient to cause antidepressant-like effects in the FST without producing rewarding effects. As such, it appears unlikely that KOR antagonists would have abuse liability, at least on their own. Notably, cocaine induced place preferences were not altered in KOR −/− or PDyn −/− mice or in wild-type mice treated with KOR antagonists, although these manipulations were sufficient to block stress-induced potentiation of cocaine CPP (McLaughlin et al., 2003, 2006a,b), further suggesting minimal role of KORs in establishing basal DA tone. Studies with JDTic provide no evidence of interactions between KOR antagonists and the incentive-motivational effects of cocaine (Beardsley et al., 2005).
Another factor that complicates studies of known KOR antagonists in these species is the long-time course of these agents: a single injection of these drugs can block the effects of KOR agonists for as long as 56 days (Spanagel and Shippenberg, 1993). The reasons for this extraordinarily long time course are not understood; this issue is addressed in more detail within the next section. Regardless, such a long time course can make many types of preclinical studies —particularly those designed to assess abuse liability (e.g., intravenous drug self-administration, place conditioning)—difficult because of persistent drug actions. It is also less than optimal for studies in humans, at least at the early stages of drug development, when a short duration of action or the ability to reverse unanticipated side effects would be preferable until drug safety is established.
Chemistry
In this brief section, we cannot provide a comprehensive review of the medicinal chemistry of KOR antagonists, which is available elsewhere (Beguin and Cohen, 2008). Instead, we list most brain-permeable non-peptidic selective antagonists (or inverse agonists) available for preclinical studies. We have not included selective KOR partial agonists, since, in most cases, partial agonism was detected in vitro and it is not known whether such compounds would be partial agonists in vivo.
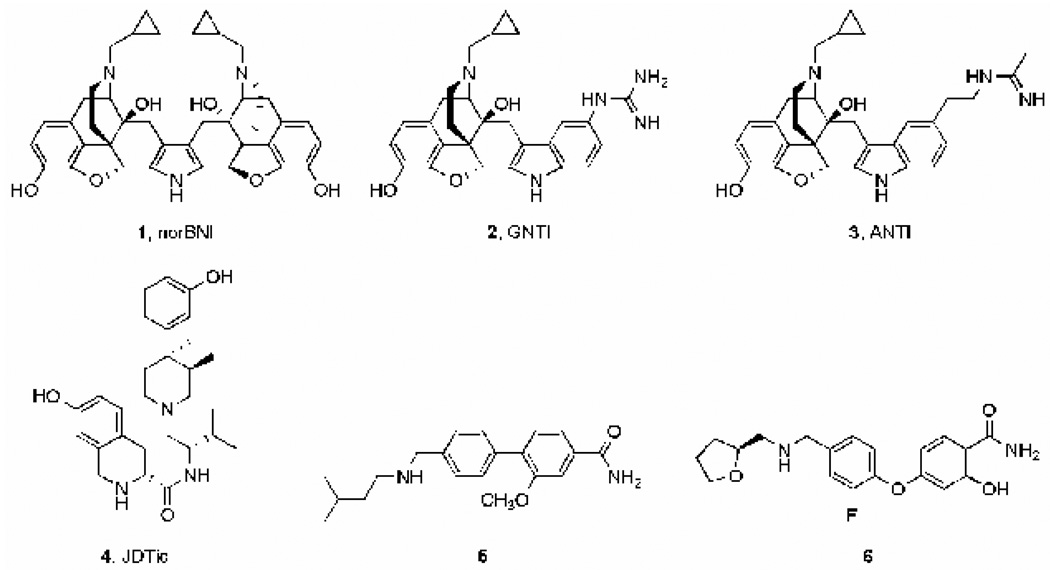
The first selective KOR antagonist (norBNI, Figure 2) was developed as a bivalent derivative of the non-selective opioid antagonist naltrexone (Portoghese et al., 1987), itself a derivative of morphine. It has been the agent of choice for many preclinical studies. However, in a few in vivo behavioral assays norBNI induced rapid and brief MOR antagonist effects in addition to its long-lasting KOR antagonist properties (Endoh et al., 1992; Broadbear et al., 1992; Spanagel et al., 1993). Structurally-simplified second-generation analogues (e.g., GNTI and ANTI; Jones et al., 1998; Stevens et al., 2000) (Figure 2) may be more selective as KOR antagonists. It is not clear if these agents act as neutral antagonists or inverse agonists (Wang et al., 2007). More recently, JDTic (Figure 2) has been developed as a potent and selective KOR antagonist (Thomas et al., 2001). JDTic is a trans-3,4-dimethyl(3-hydroxyphenyl)piperidine analogue, a class of compounds initially derived from meperidine (Zimmerman et al., 1978), a non-selective opioid agonist. Despite few similarities in structure, both the morphine analogues (norBNI, GNTI) and JDTic have a slow onset of maximal KOR antagonist action (24–48 h) and extraordinarily long-lasting effects (several weeks) in vivo (Endoh et al., 1992; Spanagel et al., 1993; Beardsley et al., 2005; Metcalf and Koop, 2005). The reasons for the long duration of action are not understood; it does not seem to be an inevitable consequence of KOR blockade, since non-selective opioid antagonists do not share this property (Bruchas et al., 2007). One potential explanation would be the so-called “depot effect”: the lipophilic properties of some molecules cause them to deposit and persist within brain membranes. However, since the KOR antagonists tested in vivo possess varying degrees of lipophilicity (logD values at pH 7 predicted using ACD/Labs: norBNI: -4.2; GNTI: -2.0; ANTI: -1.0; JDTic: 0.4), it seems unlikely that they would all deposit within brain membranes for extended periods of time. Interestingly, recent work suggests these KOR antagonists induce long-lasting changes in the function of c-Jun N-terminal kinase (JNK) (Bruchas et al., 2007), a property that might be responsible for persistent effects in vivo. These researchers also showed that pretreatment with a short-acting nonselective opioid receptor antagonist (naloxone) prevented the long-lasting effects of a single injection of norBNI, providing evidence against a depot effect. A current challenge is to determine if it is possible to design shorter-lasting potent and selective KOR antagonists, which might help to determine if the long-lasting effects of the prototypical KOR antagonists is essential for their therapeutic-like effects in animal models.
Fig.2.
Selective kappa-opioid receptor (KOR) antagonists.
One strategy might involve designing selective KOR antagonists from other classes of compounds. For example, some of the biaryl compounds (Figure 2, compounds 5 and 6) described in two recent patent applications (Magnus-Aryitey et al., 2008; Magnus-Aryitey and Ruggeri, 2008) are moderately selective KOR antagonists. If these compounds have relatively short-lasting effects, then further chemical modifications may enhance KOR selectivity. Another strategy is to base the structures on peptides, which are rapidly metabolized. Early attempts with this strategy appear to have promise (Bennett et al., 2002). Finally, there has been a growing interest in designing G-protein coupled receptors allosteric modulators (Conn et al., 2009). Negative allosteric modulation of the KOR may be an alternative way of inducing short-lasting receptor blockade. Regardless, there is a pressing need for selective KOR antagonists with improved pharmacokinetic and pharmacodynamic properties.
Potential for Clinical Use
As yet, the antidepressant-like effects of KOR antagonists have not been examined thoroughly in non-human primates or humans. Studies in non-human primates are complicated by the fact that there are no widely-accepted models of depression or antidepressant efficacy in these species. Instead, KOR antagonists have been examined in drug self-administration studies in monkeys, and mainly in the context of their ability to block the effects of KOR agonists (Negus et al., 2002; Negus, 2004). Selective KOR antagonists have not been examined in humans, although mixed agents with some ability to disrupt KOR function have been tested. One example is buprenorphine, which reportedly has antidepressant effects in certain individuals (Bodkin et al., 1995). Buprenorphine is often described as a mixed MOR agonist/KOR antagonist, although there is compelling evidence that it is actually a KOR partial-agonist: it causes low efficacy stimulation of [35S]GTP-γS in cells engineered to express human KORs, whereas true antagonists (e.g., norBNI) are without effect in this assay (Zhu et al., 1997). Thus although reports that buprenorphine can have antidepressant effects in humans are encouraging, it is difficult at this time to attribute them to antagonism of KOR receptors, considering the complex pharmacologic profile of this drug. Similarly, the mixed MOR/KOR antagonists naltrexone and nalmephene have been used in clinical trials of substance use and impulse disorders, which have a high degree of comorbid depression. At human opioid receptors, naltrexone and nalmephene have roughly equal binding affinity for MORs and KORs (Toll et al., 1997; Bart et al., 2005); nalmephene may be a weak partial agonist at KORs (Remmers et al., 1999). Naltrexone is FDA approved for use in alcohol dependence and nalmephene appears to have some efficacy for pathological gambling (Grant et al., 2006), but neither has been explicitly tested for depression, and the interpretation of their effects on depression is made difficult by their dual actions at two opioid receptors. At the present time, we are not aware of any published studies describing clinical trials of selective KOR antagonists.
Summary
The idea that KOR antagonists might be useful for the treatment of depression has emerged from basic research demonstrating that stress or repeated exposure to drugs of abuse—two stimuli that can trigger depressive conditions in humans—can activate endogenous dynorphin function. Considering that many treatments for mood disorders were discovered serendipitously over a half century ago (Nestler and Carlezon, 2006), the development of KOR antagonists for depressive conditions would represent a rare example of rational drug design in psychiatry. In rodent models, KOR antagonists block signs of anhedonia, dysphoria, and despair. The mechanisms that mediate these effects are not clear, but they may depend largely upon secondary alterations in the function of brain DA systems. Despite actions on brain DA systems, currently available evidence suggests that KOR antagonists do not cause behavioral effects that normally accompany drugs with abuse potential. A great deal of additional work is required to further characterize these agents, with particular emphasis on how chronic KOR blockade would ultimately affect behavior. Studies in rodents, non-human primates, and humans are complicated by the fact that all currently available selective KOR antagonists appear to have exceptionally long durations of action, for reasons that are not understood. The development of new classes of selective KOR antagonists with shorter durations of action is needed; such ligands would facilitate the design and performance of studies to address all of the issues described above.
3. KOR Agonists
History
Initially it was believed that KOR agonists could be utilized as non-addictive analgesics, since these drugs have antinociceptive properties but lower abuse potential than MOR agonists (Fraser and Rosenberg, 1964). Pentazocine, which is a relatively specific KOR partial agonist/MOR antagonist (Toll et al., 1999), is still used for obstetrical pain because it has a low propensity to produce respiratory depression. Administration of more selective KOR agonists (e.g., MR2033) elicit unwanted side effects in humans including dysphoria, derealization, and depersonalization (Pfeiffer et al., 1986). These observations precluded further development of these agents for clinical use. Trials of KOR agonists for psychiatric conditions have been limited and have shown only mixed success: the KOR agonist spiradoline produces sedation and decreases the frequency of tics in individuals with Tourette’s Syndrome at low doses but produces increased dysphoria and altered perception at high doses (Chappell et al., 1993). In clinical studies of substance abusers, the KOR agonist enadoline reduces some of the effects of cocaine, but also caused sedation, depersonalization, visual distortions, confusion, and (at high doses) paranoia (Walsh et al., 2001a, 2001b). Perhaps the most interesting evidence on the effects of KOR agonists in humans comes from experience with salvinorin A, the active component of the plant Salvia divinorum, used by the Mazatec peoples of Oaxaca, Mexico, in spiritual and healing rituals. Salvinorin A is currently the most selective and potent KOR agonist known (Roth et al., 2002). Its effects are highly situation dependent (Valdes, 1994), but it often induces perceptual distortions, depersonalization, and feelings of spatiotemporal dislocation (Siebert, 1994; Yan and Roth, 2004). Other reports describe derealization accompanied by brief euphoria (Gonzalez et al., 2006). Although salvinorin A is used recreationally, there is currently no evidence that it is addictive (i.e., used compulsively); notably, humans will take other substances that are aversive in other species, such as lysergic acid diethylamide (LSD). Nonetheless, despite evidence in humans and laboratory animals that KOR agonists should lower mood, there are occasional reports of salvinorin A leading to improved mood and even having antidepressant effects (Hanes, 2003). These seemingly paradoxical effects are not understood, although they may reflect individual differences in brain chemistry, effects of salvinorin A different from those of other selective KOR agonists, or homeostatic-like responses to KOR stimulation (Potter et al., 2008). Until recently there has been little consideration of the potential KOR agonists might have for the treatment of mood disorders.
The idea that KOR agonists could play an important role in the study and treatment of mood disorders is derived from two sets of preclinical studies. Antipsychotic drugs are all potent antimanic agents and all induce activation of similar populations of cells in the NAc (Cohen et al., 1998), regions that may be responsible for mediating many of the symptoms of bipolar disorder. While these studies were performed in rats, similar regional effects of antipsychotic drugs may occur in human subjects (Cohen and Yurgelun-Todd, 2001). Double-label immunohistochemistry identified the cells responding to antimanic/antipsychotic drugs as dynorphinergic/GABAergic neurons (Ma et al., 2003), implying that antipsychotic drugs may increase dynorphin release, leading to an antimanic or mood-lowering effect. The mechanism of this activation of dynorphinergic cells is likely inhibition of multiple monoamine receptors at which antipsychotic drugs are potent antagonists (Ma et al., 2006).
In parallel, the effects of KOR agonists were studied as comparison drugs in experiments designed to evaluate the antidepressant-like effects of KOR antagonists in rats. These studies demonstrated that U69,593 (a selective KOR agonist) increases immobility behavior in the FST (Mague et al., 2003). This effect is identical to that caused by elevation of CREB in the NAc (Pliakas et al., 2001), drug withdrawal (Cryan et al., 2002), or administration of antimanic agents (Tomasiewicz et al., 2006), and opposite of that caused by standard antidepressant drugs. As such, elevated immobility behavior in the FST is a putative indicator of “prodepressive” or mood-lowering effects. U69,593 also elevated ICSS thresholds (Todtenkopf et al., 2004), a prodepressive-like effect similar to that caused by drug withdrawal and antimanic agents (Markou et al., 1992; Tomasiewicz et al., 2006; Carlezon and Chartoff, 2007), and thus is a putative indicator of motivational deficits that often accompany affective disorders. An identical pattern of effects was caused by salvinorin A (Carlezon et al., 2006). When considered together, these molecular and behavior studies are consistent with the conclusion that stimulation of brain KORs causes behavioral signs that closely resemble those that characterize depressive disorders.
Mechanisms
KORs are located throughout the brain (Mansour et al., 1995), including areas such as the mesocorticolimbic system, PFC, amygdala, and septum. All of these areas have been associated with motivation and emotion, and it is easy to imagine that each plays a role in the regulation of mood and affective states (see Nestler and Carlezon, 2006). As described above, our work has tended to focus on the mesolimbic DA system, because of our strong interest in motivated behavior. KORs are expressed throughout the mesolimbic DA system and are located both on dopaminergic neurons within the VTA and on their efferent terminals in the NAc (Svingos et al., 1999; Margolis et al., 2003) (Figure 1). Stimulation of KORs in either region decreases DA function. For example, administration of KOR agonists into the NAc decreases local DA concentrations (Donzanti et al., 1992; Spanagel et al., 1992), most likely by stimulating presynaptic KORs that inhibit DA release from VTA neurons (Svingos et al., 1999). KOR agonists also have VTA-dependent inhibitory effects on the mesolimbic DA neurons, and both direct and indirect circuits may be involved (Margolis et al., 2003; Margolis et al., 2005; Margolis et al., 2006). Future studies involving brain region-specific microinfusions of KOR selective ligands will determine if stimulation of KORs in these other brain areas synergize with—or counteract—the general prodepressive consequences of systemic administration of KOR agonists (Mague et al., 2003; Todtenkopf et al., 2004; Carlezon et al., 2006).
Complexities
The effects of KOR agonists are not limited to the mesolimbic DA system. They affect other neurotransmitter systems that may be important in regulating mood. Indeed, NE release is reduced by KOR agonists, as studied in rat synaptosomes (Adamson et al., 1989) and rabbit hippocampal slices (Allgaier et al., 1989). In addition, the effects of KOR agonists on monoamine turnover may differ by brain region (Ford et al., 2006).
Although a decrease in DA neurotransmission is the immediate response to KOR agonists, less is known about the consequences of repeated or long-term drug administration. In monkeys, single doses of KOR agonists reduce cocaine intake, but this effect wanes over time (Mello and Negus, 1998). Similarly, acute administration of salvinorin A reduces the locomotor-stimulating effects of cocaine in rats, although these same effects are increased after salvinorin A is given repeatedly and then withdrawn, suggesting the development of tolerance (Chartoff et al., 2008). It is important to note that few KOR agonists are entirely selective and full agonists at KORs. Agents such as U50,488 and U69,593 are not as selective nor do they cause as full an activation of KORs as salvinorin A (Chavkin et al., 2004). Also, both U50,488 and U69,593 cause more KOR internalization than salvinorin A (Wang et al., 2005) or its derivatives (e.g., herkinorin; Groer et al., 2006). Therefore, each of these drugs may activate different intracellular signaling pathways and have different effects with repeated or long-term administration.
Sex, strain, and age differences in response to KOR agonists have also been described in rats (Barrett et al., 2002; Smith and French, 2002), and species differences have been observed with regard to receptor densities (Mansour et al., 1988) turnover of DA in response to KOR agonists (Barber and Gottschlich, 1997; Fantegrossi et al., 2005) and phosphorylation and desensitization of KORs (Li et al., 2002). Lastly, KORs may also exist as components of homomeric and heterodimeric receptor complexes , which may contribute to region-, sex-, and species-specific effects of KOR agonists (see Devi, 2001). Thus caution should to be applied in generalizing between results in rodents, monkeys, and humans.
Chemistry
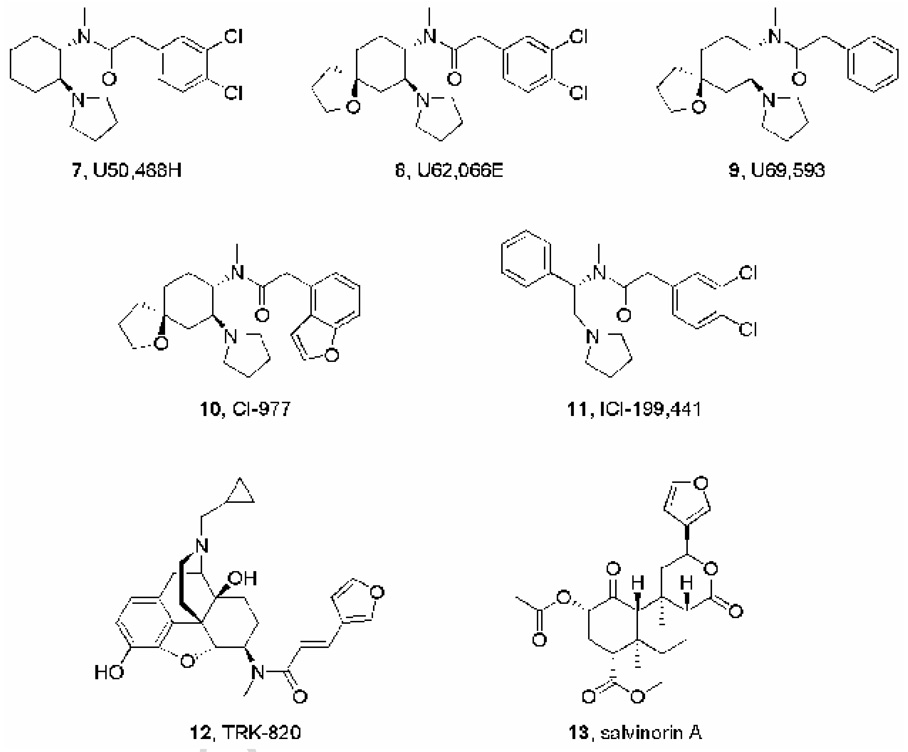
In the 1980s and 90s, several research programs focused on designing selective KOR agonists as potential treatments for pain. As noted above, such agents were predicted to possess analgesic properties and be free of the side-effects associated with MOR agonists: physical dependence and respiratory depression. Intensive medicinal chemistry efforts at Upjohn led to the identification of arylacetamide analogues as potent KOR agonists. Later, this class of compounds was studied in several other research centers. Examples include U50,488 (Szmuszkovicz and Von Voigtlander, 1982), U62,066 (spiradoline) (Wadenberg, 2003), U69,593 (Lahti et al., 1985), CI-977 (enadoline) (Wadenberg, 2003), and ICI-199441 (Costello et al., 1991) (Figure 3). These compounds are still being used in preclinical studies and a few (e.g., enadoline and spiradoline) entered clinical trials for the treatment of pain or addiction. However, the tendency of KOR agonists to produce dysphoria and other aversive effects prevented their clinical use. Structure-activity relationships have been studied in great detail for the arylacetamides, and KOR antagonist properties have never been reported for any compound belonging to this class of agents.
Fig.3.
Selective kappa-opioid receptor (KOR) agonists.
As is the case with KOR antagonists, many KOR agonists have been derived from morphine. Only a few of these analogues display some degree of selectivity for the KOR. One of them, the 4,5-epoxymorphinan TRK-820 (nalfurafine, Figure 3), is being developed as an antipruritic agent (Kawai et al., 2008).
The neoclerodane diterpenoid salvinorin A (Figure 3) is the main psychotropic component of the ethnobotanical Salvia divinorum (Roth et al., 2002; Chavkin et al., 2004). Since its identification as a potent and selective KOR agonist, salvinorin A has been characterized in multiple preclinical studies and it is being modified chemically in several laboratories (e.g., Prisinzano and Rothman, 2008) to alter its pharmacokinetic and pharmacodynamic properties. Salvinorin A is structurally unusual in the sense that it does not possess any protonated sites, a characteristic previously believed to be necessary for interaction with the KOR.
It is important to note that the crystal structure of the KOR has not been determined, making it difficult to model agonist/antagonist interactions with active sites on the receptor. However, structurally distinct full agonists appear to have different effects on secondary signaling pathways, probably due to their interaction with different conformational states of the KOR (Wang et al., 2005).
Potential for Clinical Use
Under some circumstances, the ability of KOR-agonists to decrease DA function in the basal forebrain might have clinical utility, particularly in the treatment of conditions characterized by increased motivation (e.g., drug abuse or mania). Antipsychotic drugs—which are frequently used to treat mania—activate dynorphinergic neurons (Ma et al., 2003). Also, although salvinorin A and other KOR agonists can produce psychotomimetic effects under some conditions (especially at high doses) (Pfeiffer et al., 1986; Bucheler et al., 2005), it is conceivable that administration of a derivative or related compound under more carefully controlled conditions might have useful effects. In this regard, it is notable that the effects of KOR agonists on DA turnover in the mesolimbic system are state-dependent, with reduction of release greatest when DA cells are most active (Manzanares et al., 1991), as they may be in mania or mood elevation.
With these caveats in mind, we examined the possibility that KOR agonists might have mood-normalizing effects for patients in manic episodes of bipolar disorder. The relatively KOR specific agents spiradoline and enadoline were not available for clinical studies, and salvinorin A was not initially chosen for study because it is very short acting and difficult to administer: it is often used by means that achieve absorption from the buccal or nasal mucosa, and is not reliably absorbed following ingestion. Rather, we performed our first studies with commercially available pentazocine, which has been safely given to patients and is approved for pain relief. Pentazocine is nearly a full agonist at KORs, with lower affinity for MORs, at which it is predominantly an antagonist. In an initial open-label study in ten patients with mania, two sequential doses of pentazocine (50 mg, as often used for pain) given two hours apart, reduced manic symptoms transiently but substantially and significantly in each subject without causing notable sedation or affecting concomitant psychotic symptoms (Cohen and Murphy, 2008). While preliminary, these results are consistent with the implications of studies in rats, which suggest that KOR agonists may decrease mood, especially when mood is abnormally elevated. Preclinical studies that model hallmark signs of mania provide complementary evidence that KOR agonists might be useful for treating conditions characterized by increased motivation and hyperfunction of brain reward systems (Tomasiewicz et al., 2008).
Summary
The development of KOR agonists as possible antimanic agents was suggested by the results of parallel studies in rats, one of which observed that clinically used antimanic agents increase the activity of dynorphinergic neurons (Ma et al., 2003), while the other directly documented mood lowering effects of KOR agonists (Carlezon et al., 2006; Tomasiewicz et al., 2008). Further support for this research direction comes from evidence in human subjects that both naturally occurring and synthetic KOR agonists can lower mood with acute dosing (Bucheler et al., 2005; Cohen and Murphy, 2008). Against this background, the finding that pentazocine may reduce manic symptoms is promising. However, these results should not be taken to imply that pentazocine or other KOR agonists will be useful therapeutic agents for the treatment of mania. More work is needed, including controlled blinded trials with repeated dosing to gauge the tolerability and longer term efficacy of this or similar agents.
While most synthetic chemistry has been devoted to developing KOR-specific agonists and antagonists, some agents are or will turn out to be partial agonists or mixed agonist/antagonists. These agents may also be clinically useful, especially in the treatment of bipolar disorder, as they may produce a relatively constant signal through KORs, thereby helping to restrict to a limited range the activity of DA and other monoamine neurons thought to be responsible for regulation of mood. Thus, it is possible that partial agonists will have mood-stabilizing, rather than simply antidepressant or antimanic, effects in patients with bipolar disorder.
4. Conclusions
Psychiatry needs drugs that are safer, act faster, and have fewer side effects. Virtually all existing medications for psychiatric disorders are based on serendipitous discoveries made decades ago (Ressler and Nemeroff, 2000; Manji et al., 2001; Nestler et al., 2002; Nestler and Carlezon, 2006). Despite great effort and expense, the field has not succeeded in developing fundamentally new treatments—with distinct mechanisms of action—for mood disorders. Much current research is “drug-centric” (focusing on the mechanisms by which currently available psychotropic drugs act) rather than “brain-centric” (focusing on the abnormal states that are treated by these drugs). In turn, much of what is believed about the molecular basis of mood disorders is based upon our understanding of the most prominent and immediate effects of standard medications (Nestler and Carlezon, 2006). Regardless of whether KOR ligands are ever utilized in the treatment of mood disorders, the study of endogenous KOR systems has increased our understanding of some of molecular events in the brain that cause dysregulation of mood states. Our findings have contributed to the development of a simple “brain-centric” hypothesis that treatments that reduce the excitability of the NAc elevate mood, whereas treatments that increase its excitability depress mood (Figure 1) (Carlezon and Thomas, 2009). Rigorous tests of this hypothesis will have important implications for our understanding of the biological basis of motivation, which is abnormal in mood disorders. As has been the case with other disease states, understanding how the organs of the body function normally can be the first step toward designing innovative treatments that prevent or reverse the pathophysiology underlying debilitating disorders.
Acknowledgment
Supported by the National Institutes of Health (MH063266, to WC; MH078473, to ATK) and The Stanley Medical Research Institute (to CB and BMC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors disclose that Dr. Carlezon has a US patent covering the use of kappa antagonists in the treatment of depression (Assignee: McLean Hospital), and Dr. Carlezon, Dr. Béguin, and Dr. Cohen are members of a collaborative group that has submitted a patent application covering the synthesis and use of salvinorin derivatives (Assignees: McLean Hospital and Temple University).
References
- Adamson P, Mantzouridis T, Xiang J-Z, Hajimohammadreza I, Brammer MJ, Campbell IC. α2-adrenergic, κ-opiate, and P1-purinergic autoreceptors have mutually antagonistic effects: a new regulatory mechanism? J Neurochem. 1989;53:1077–1082. doi: 10.1111/j.1471-4159.1989.tb07398.x. [DOI] [PubMed] [Google Scholar]
- Allgaier C, Daschmann B, Sieverling J, Hertting G. Presynaptic KOR-opioid receptors on noradrenergic nerve terminals couple to G proteins and interact with the alpha 2-adrenoceptors. J Neurochem. 1989;53:1629–1635. doi: 10.1111/j.1471-4159.1989.tb08561.x. [DOI] [PubMed] [Google Scholar]
- Artaiz I, Zazpe A, Del Rio J. Characterization of serotonergic mechanisms involved in the behavioural inhibition induced by 5-hydroxytryptophan in a modified light-dark test in mice. Behav Pharmacol. 1998;9:103–112. [PubMed] [Google Scholar]
- Bagdy G, Graf M, Anheuer ZE, Modos EA, Kantor S. Anxiety-like effects induced by acute fluoxetine, sertraline or m-CPP treatment are reversed by pretreatment with the 5-HT2C receptor antagonist SB-242084 but not the 5-HT1A receptor antagonist WAY-100635. Int J Neuropsychopharmacol. 2001;4:399–408. doi: 10.1017/S1461145701002632. [DOI] [PubMed] [Google Scholar]
- Bals-Kubik R, Herz A, Shippenberg TS. Evidence that the aversive effects of µ-opioid antagonists and κ-agonists are centrally mediated. Psychopharmacol. 1989;98:203–220. doi: 10.1007/BF00444692. [DOI] [PubMed] [Google Scholar]
- Barber A, Gottschlich R. Novel developments with selective, non-peptidic KOR-opioid receptor agonists. Expert Opin Investig Drugs. 1997;6:1351–1368. doi: 10.1517/13543784.6.10.1351. [DOI] [PubMed] [Google Scholar]
- Barrett AC, Smith ES, Picker MJ. Sex-related differences in mechanical nociception and antinociception produced by mu- and kappa-opioid receptor agonists in rats. Eur J Pharmacol. 2002;452:163–173. doi: 10.1016/s0014-2999(02)02274-4. [DOI] [PubMed] [Google Scholar]
- Bart G, Schluger JH, Borg L, Ho A, Bidlack JM, Kreek MJ. Nalmefene induced elevation in serum prolactin in normal human volunteers: partial kappa opioid agonist activity? Neuropsychopharmacol. 2005;30:2254–2262. doi: 10.1038/sj.npp.1300811. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Howard JL, Shelton KL, Carroll FI. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacol. 2005;183:118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- Beguin C, Cohen BM. Medicinal chemistry of kappa opioid receptor antagonists. In: Dean RL III, Bilsky EJ, Negus SS III, editors. Opioid Receptors and Antagonists: From Bench to Clinic. Humana Press; 2008. [Google Scholar]
- Beguin C, Richards MR, Wang YL, Chen Y, Liu-Chen LY, Ma ZZ, Lee DYW, Carlezon WA, Jr, Cohen BM. Synthesis and in vitro pharmacological evaluation of salvinorin A analogues modified at C(2) Bioorgan Med Chem Lett. 2005;15:2761–2765. doi: 10.1016/j.bmcl.2005.03.113. [DOI] [PubMed] [Google Scholar]
- Bennett MA, Murray TF, Aldrich JV. Identification of arodyn, a novel acetylated dynorphin A-(1–11) analogue, as a kappa opioid receptor antagonist. J Med Chem. 2002;45:5617–5619. doi: 10.1021/jm025575g. [DOI] [PubMed] [Google Scholar]
- Bodkin JA, Zornberg GL, Lukas SE, Cole JO. Buprenorphine treatment of refractory depression. J Clin Psychopharmacol. 1995;15:49–57. doi: 10.1097/00004714-199502000-00008. [DOI] [PubMed] [Google Scholar]
- Broadbear JH, Negus SS, Butelman ER, de Costa BR, Woods JH. Differential effects of systemically administered nor-binaltorphimine (nor-BNI) on kappa-opioid agonists in the mouse writhing assay. Psychopharmacol. 1994;115:311–319. doi: 10.1007/BF02245071. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Yang T, Schreiber S, DeFino M, Kwan SC, Li S, Chavkin C. Long-acting κ opioid antagonists disrupt receptor signaling and produce noncompetitive effects by activating c-Jun N-terminal kinase. J Biol Chem. 2007;282:29803–29811. doi: 10.1074/jbc.M705540200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucheler R, Gleiter CH, Schwoerer P, Gaertner I. Use of nonprohibited hallucinogenic plants: increasing relevance for public health? A case report and literature review on the consumption of Salvia divinorum (Diviner's Sage) Pharmacopsychiatry. 2005;38:1–5. doi: 10.1055/s-2005-837763. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Beguin C, DiNieri J, Baumann MH, Richards M, Todtenkopf MS, Rothman RB, Ma Z, Lee DY, Cohen BM. Depressive-like effects of the κ-opioid receptor agonist Salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther. 2006;314:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nature Protocols. 2007;2:2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thomas M. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56:122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, Nestler EJ. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- Carr KD, Bak TH, Simon EJ, Portoghese PS. Effects of the selective kappa opioid antagonist, nor-binaltorphimine, on electrically-elicited feeding in the rat. Life Sci. 1989;45:1787–1792. doi: 10.1016/0024-3205(89)90518-3. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chartoff EH, Papadopoulou M, Barhight MF, MacDonald M, Parsegian A, Potter D, Konradi C, Carlezon WA., Jr Desipramine reduces stress-activated dynorphin expression and CREB phosphorylation in nucleus accumbens tissue. Molecular Pharmacology. 2009 doi: 10.1124/mol.108.051417. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff EH, Potter D, Damez-Werno D, Cohen BM, Carlezon WA., Jr Exposure to the selective kappa agonist salvinorin A modulates the behavioral and molecular effects of cocaine in rats. Neuropsychopharmacology. 2008;33:2676–2687. doi: 10.1038/sj.npp.1301659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell PB, Leckman JF, Scahill LD, Hardin MT, Anderson G, Cohen DJ. Neuroendocrine and behavioral effects of the selective kappa agonist spiradoline in Tourette's syndrome: a pilot study. Psychiatry Res. 1993;47:267–280. doi: 10.1016/0165-1781(93)90084-t. [DOI] [PubMed] [Google Scholar]
- Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215:413–415. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- Chavkin C, Sud S, Jin W, Stewart J, Zjawiony JK, Siebert DJ, Toth BA, Hufeisen SJ, Roth BL. Salvinorin A, an active component of the hallucinogenic sage salvia divinorum is a highly efficacious kappa-opioid receptor agonist: structural and functional considerations. J Pharmacol Exp Ther. 2004;308:1197–1203. doi: 10.1124/jpet.103.059394. [DOI] [PubMed] [Google Scholar]
- Cohen BM, Murphy B. The effects of pentazocine, a kappa agonist, in patients with mania. Int J Neuropsychopharmacol. 2008;11:243–247. doi: 10.1017/S1461145707008073. [DOI] [PubMed] [Google Scholar]
- Cohen BM, Wan W, Froimowitz MP, Ennulat DJ, Cherkerzian S, Konieczna H. Activation of midline thalamic nuclei by antipsychotic drugs. Psychopharmacol. 1998;135:37–43. doi: 10.1007/s002130050483. [DOI] [PubMed] [Google Scholar]
- Cohen BM, Yurgelun-Todd D. Alterations of thalamic activity in schizophrenia and in response to antipsychotic drugs: studies in the legacy of Seymour S. Kety. Neuropsychopharmacol. 2001;25:305–312. doi: 10.1016/S0893-133X(01)00277-9. [DOI] [PubMed] [Google Scholar]
- Cole RL, Konradi C, Douglass J, Hyman SE. Neuronal adaptation to amphetamine and dopamine: molecular mechanisms of prodynorphin gene regulation in rat striatum. Neuron. 1995;14:813–823. doi: 10.1016/0896-6273(95)90225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Christopoulos A, Lindsley CW. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov. 2009;8:41–54. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello GF, James R, Shaw JS, Slater AM, Stutchbury NC. 2-(3,4-Dichlorophenyl)-N-methyl-N-[2-(1-pyrrolidinyl)-1-substituted- ethyl]-acetamides: the use of conformational analysis in the development of a novel series of potent opioid kappa agonists. J Med Chem. 1991;34:181–189. doi: 10.1021/jm00105a027. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Daunais JB, Roberts DC, McGinty JF. Short-term cocaine self administration alters striatal gene expression. Brain Res Bull. 1995;37:523–527. doi: 10.1016/0361-9230(95)00049-k. [DOI] [PubMed] [Google Scholar]
- Devi LA. Heterodimerization of G-protein-coupled receptors: pharmacology, signaling and trafficking. Trends Pharmacol Sci. 2001;22(10):532–537. doi: 10.1016/s0165-6147(00)01799-5. [DOI] [PubMed] [Google Scholar]
- Devine DP, Leone P, Pocock D, Wise RA. Differential involvement of ventral tegmental mu, delta and kappa opioid receptors in modulation of basal mesolimbic dopamine release: in vivo microdialysis studies. J Pharmacol Exp Ther. 1993;266:1236–1246. [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donzanti BA, Althaus JS, Payson MM, Von Voigtlander PF. Kappa agonist-induced reduction in dopamine release: site of action and tolerance. Res Commun Chem Pathol Pharmacol. 1992;78:193–210. [PubMed] [Google Scholar]
- Douglass J, McKinzie AA, Pollock KM. Identification of multiple DNA elements regulating basal and protein kinase A-induced transcriptional expression of the rat prodynorphin gene. Mol Endocrinol. 1994;8:333–344. doi: 10.1210/mend.8.3.8015551. [DOI] [PubMed] [Google Scholar]
- Drapier D, Bentue-Ferrer D, Laviolle B, Millet B, Allain H, Bourin M, Reymann JM. Effects of acute fluoxetine, paroxetine and desipramine on rats tested on the elevated plus-maze. Behav Brain Res. 2007;176:202–209. doi: 10.1016/j.bbr.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Endoh T, Matsuura H, Tanaka C, Nagase H. Nor-binaltorphimine: a potent and selective kappa-opioid receptor antagonist with long-lasting activity in vivo. Arch Int Pharmacodyn Ther. 1992;316:30–42. [PubMed] [Google Scholar]
- Faden AI, Takemori AE, Portoghese PS. Kappa-selective opiate antagonist nor-binaltorphimine improves outcome after traumatic spinal cord injury in rats. Cent Nerv Syst Trauma. 1987;4:227–237. doi: 10.1089/cns.1987.4.227. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Kugle KM, Valdes LJ, 3rd, Koreeda M, Woods JH. Kappa-opioid receptor-mediated effects of the plant-derived hallucinogen, salvinorin A, on inverted screen performance in the mouse. Behav Pharmacol. 2005;16:627–633. doi: 10.1097/00008877-200512000-00005. [DOI] [PubMed] [Google Scholar]
- Fawcett J, Barkin RL. Efficacy issues with antidepressants. J. Clin. Psychiatry. 1997;58:32–39. [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gaveriaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer BL. Mice deficient for delta-and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- Ford CP, Mark GP, Williams JT. Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J Neurosci. 2006;26:2788–2797. doi: 10.1523/JNEUROSCI.4331-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser HF, Rosenberg DE. Studies on the human addiction liability of 2’-hydroxy-5, 9-dimethyl-2-(3,3-dimethylally)-6, 7-benzomorphan (WIN 20, 228): A weak narcotic antagonist. J Pharmacol Exp Ther. 1964;143:149–156. [PubMed] [Google Scholar]
- Frazer A. Pharmacology of antidepressants. J. Clin. Psychopharmacol. 1997;17 Suppl 1:2S–18S. doi: 10.1097/00004714-199704001-00002. [DOI] [PubMed] [Google Scholar]
- Gonzalez D, Riba J, Bouso JC, Gomes-Jarabo G, Barbanoj MJ. Pattern of use and subjective effects of Salvia divinorum among recreational users. Drug Alcohol Depend. 2006;85:157–162. doi: 10.1016/j.drugalcdep.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Grant JE, Potenza MC, Hollander E, Cunningham-Williams R, Nurminen T, Smits G, Kallio A. Multicenter investagation of the opioid antagonist nalmefene in the treatment of pathological gambling. Am J Psychiatry. 2006;163:303–312. doi: 10.1176/appi.ajp.163.2.303. [DOI] [PubMed] [Google Scholar]
- Groer CE, Tidgewell K, Moyer RA, Harding WW, Rothman RB, Prisinzano TE, Bohn LM. An Opioid Agonist that does not induce mu opioid receptor-arrestin interactions or receptor internalization. Mol Pharmacol. 2006 doi: 10.1124/mol.106.028258. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JS, Xie CW. Dynorphin: potent analgesic effect in spinal cord of the rat. Life Sci. 1982;31:1781–1784. doi: 10.1016/0024-3205(82)90209-0. [DOI] [PubMed] [Google Scholar]
- Han JS, Xie GX, Goldstein A. Analgesia induced by intrathecal injection of dynorphin B in the rat. Life Sci. 1984;34:1573–1579. doi: 10.1016/0024-3205(84)90612-x. [DOI] [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Chen Y, Schuller A, Zhu Y, Zhang J, Menge WM, Leurs R, Timmerman H, Pintar JE. Improgan, a cimetidine analog, induces morphine-like antinociception in opioid receptor-knockout mice. Brain Res. 2000;880:102–108. doi: 10.1016/s0006-8993(00)02776-1. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RM, Hjorth SA, Schwartz TW, Portoghese PS. Mutational evidence for a common kappa antagonist binding pocket in the wild-type kappa and mutant mu[K303E] opioid receptors. J Med Chem. 1998;41:4911–4914. doi: 10.1021/jm9805182. [DOI] [PubMed] [Google Scholar]
- Kawai K, Hayakawa J, Miyamoto T, Imamura Y, Yamane S, Wakita H, Fujii H, Kawamura K, Matsuura H, Izumimoto N, Kobayashi R, Endo T, Nagase H. Design, synthesis, and structure-activity relationship of novel opioid kappa-agonists. Bioorg Med Chem. 2008;16:9188–9201. doi: 10.1016/j.bmc.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA., Jr Anxiolytic-like effects of κ-opioid receptor antagonists in behavioral models of unlearned and learned fear in rats. J Pharmacol Exp Ther. 2007;323:838–845. doi: 10.1124/jpet.107.127415. [DOI] [PubMed] [Google Scholar]
- Koch S, Perry KW, Nelson DL, Conway RG, Threlkeld PG, Bymaster FP. R-fluoxetine increases extracellular DA, NE, as well as 5-HT in rat prefrontal cortex and hypothalamus: an in vivo microdialysis and receptor binding study. Neuropsychopharmacol. 2002;27:949–959. doi: 10.1016/S0893-133X(02)00377-9. [DOI] [PubMed] [Google Scholar]
- Lahti RA, Mickelson MM, McCall JM, Von Voigtlander PF. [3H]U-69593 a highly selective ligand for the opioid kappa receptor. Eur J Pharmacol. 1985;109:281–284. doi: 10.1016/0014-2999(85)90431-5. [DOI] [PubMed] [Google Scholar]
- Leone P, Pocock D, Wise RA. Morphine-dopamine interaction: ventral tegmental morphine increases nucleus accumbens dopamine release. Pharmacol Biochem Behav. 1991;39:469–472. doi: 10.1016/0091-3057(91)90210-s. [DOI] [PubMed] [Google Scholar]
- Li J, Li J-G, Chen C, Zhang F, Liu-Chen L-Y. Molecular basis of differences in (−)(trans)-3,4-Dichloro-N-methyl-N-[2-(1-pyrrolidiny)-cyclohexyl]benzeneacetamide-induced desensitzation and phosphorylation between human and rat κ-opioid receptors expressed in Chinese hamster ovary cells. Mol Pharmacol. 2002;61:73–84. doi: 10.1124/mol.61.1.73. [DOI] [PubMed] [Google Scholar]
- Ma J, Ye N, Lange N, Cohen BM. Dynorphinergic GABA neurons are a target of both typical and atypical antipsychotic drugs in the nucleus accumbens shell, central amygdaloid nucleus and thalamic central medial nucleus. Neurosci. 2003;121:991–998. doi: 10.1016/s0306-4522(03)00397-x. [DOI] [PubMed] [Google Scholar]
- Ma J, Ye N, Cohen BM. Expression of noradrenergic α1, serotoninergic 5HT2a and dopaminergic D2 receptors on neurons activated by typical and atypical antipsychotic drugs. Prog Neuro-Psychopharmacol Biol Psych. 2006;30:647–657. doi: 10.1016/j.pnpbp.2005.11.040. [DOI] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, Jones RM, Portoghese PS, Carlezon WA., Jr Antidepressant-like effects of κ-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- Maisonneuve IM, Archer S, Glick SD. U50,488, a κ-opioid receptor agonist, attenuates cocaine-induced increases in extracellular dopamine in the nucleus accumbens of rats. Neurosci Lett. 1994;181:57–60. doi: 10.1016/0304-3940(94)90559-2. [DOI] [PubMed] [Google Scholar]
- Mansour A, Watson SJ, Akil H. Opioid receptors: past, present and future. Trends Neurosci. 1995;18:69–70. [PubMed] [Google Scholar]
- Manzanares J, Lookingland KJ, Moore KE. Kappa opioid receptor-mediated regulation of dopaminergic neurons in the rat brain. J Pharmacol Exp Ther. 1991;256:500–505. [PubMed] [Google Scholar]
- Margolis EB, Hjelmstad GO, Bonci A, Fields HL. Kappa opioid agonists directly inhibit midbrain dopaminergic neurons. J Neurosci. 2003;23:9981–9986. doi: 10.1523/JNEUROSCI.23-31-09981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Hjelmstad GO, Bonci A, Fields HL. Both kappa and mu opioid agonists inhibit glutamatergic input to ventral tegmental area neurons. J Neurophysiol. 2005;93:3086–3093. doi: 10.1152/jn.00855.2004. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Chefer VI, Shippenberg TS, Hjelmstad GO, Fields HL. Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc Natl Acad Sci USA. 2006;103:2938–2942. doi: 10.1073/pnas.0511159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Hauger RL, Koob GF. Desmethylimipramine attenuates cocaine withdrawal in rats. Psychopharmacol. 1992;109:305–314. doi: 10.1007/BF02245878. [DOI] [PubMed] [Google Scholar]
- Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- Magnus-Aryitey GT, Ruggeri RB. Diaryl ether derivatives as opioid receptors antagonists and their preparation, pharmaceutical compositions and use in the treatment of obesity. Patent application WO2008032156. 2008 [Google Scholar]
- Magnus-Aryitey GT, Ruggeri RB, Thuma BA. Biaryl, bipyridinyl and arylpyridinyl derivatives as mu-, kappa-, and delta-opioid receptor antagonist and their preparation and use in the treatment of opioid receptor-mediated diseases. Patent application WO 2008059335. 2008 [Google Scholar]
- Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Anatomy of CNS opioid receptors. Trends Neurosci. 1988;11:308–314. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C. Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacol. 2006a;31:787–794. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacol. 2006b;31:1241–1248. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Negus SS. Effects of kappa opioid agonists on cocaine- and food-maintained responding by rhesus monkeys. J Pharmacol Exp Ther. 1998;286:812–824. [PubMed] [Google Scholar]
- Metcalf MD, Coop A. Kappa opioid antagonists: past successes and future prospects. Aaps J. 2005;7:E704–E722. doi: 10.1208/aapsj070371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Linsenmayer DC, Jones RM, Portoghese PS. Kappa opioid antagonist effects of the novel KOR antagonist 5'-guanidinonaltrindole (GNTI) in an assay of schedule-controlled behavior in rhesus monkeys. Psychopharmacol. 2002;163:412–419. doi: 10.1007/s00213-002-1038-x. [DOI] [PubMed] [Google Scholar]
- Negus SS. Effects of the kappa opioid agonist U50,488 and the kappa opioid antagonist nor-binaltorphimine on choice between cocaine and food in rhesus monkeys. Psychopharmacol. 2004;176:204–213. doi: 10.1007/s00213-004-1878-7. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Gould E, Manji H, Buncan M, Duman RS, Greshenfeld HK, Hen R, Koester S, Lederhendler I, Meaney M, Robbins T, Winsky L, Zalcman S. Preclinical models: status of basic research in depression. Biol Psychiatry. 2002;52:503–528. doi: 10.1016/s0006-3223(02)01405-1. [DOI] [PubMed] [Google Scholar]
- Newton SS, Thome J, Wallace T, Shirayama Y, Dow A, Schlesinger L, Duman CH, Sakai N, Chen JS, Neve R, Nestler EJ, Duman RS. Inhibition of CREB or dynorphin in the nucleus accumbens produces an antidepressant-like effect. J Neurosci. 2002;22:10883–10890. doi: 10.1523/JNEUROSCI.22-24-10883.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds ME. Reinforcing effects of morphine in the nucleus accumbens. Brain Res. 1982;237:429–440. doi: 10.1016/0006-8993(82)90454-1. [DOI] [PubMed] [Google Scholar]
- Pazos A, Tristan C, Florez J. A comparative study of the respiratory depressant and analgesic effects of bremazocine, a kappa-agonist. Life Sci. 1983;33 Suppl 1:579–581. doi: 10.1016/0024-3205(83)90569-6. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Pliakas AM, Carlson R, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element binding protein expression in nucleus accumbens. J Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portoghese PS, Lipkowski AW, Takemori AE. Binaltorphimine and nor-binaltorphimine, potent and selective kappa-opioid receptor antagonists. Life Sci. 1987;40:1287–1292. doi: 10.1016/0024-3205(87)90585-6. [DOI] [PubMed] [Google Scholar]
- Potter D, Roitman MF, Carlezon WA, Jr, Cohen BM, Chartoff EH. Biphasic effects of the kappa opioid receptor agonist salvinorin A on hedonic state. Soc Neurosci Abstracts. 2008;34:564.14. [Google Scholar]
- Prisinzano TE, Rothman RB. Salvinorin A analogs as probes in opioid pharmacology. Chem Rev. 2008;108:1732–1743. doi: 10.1021/cr0782269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmers AE, Clark MJ, Mansour A, Akil H, Woods JH, Medzihradsky F. Opioid efficacy in a C6 glioma cell line stably expressing the human kappa opioid receptor. J Pharmacol Exp Ther. 1999;288:827–833. [PubMed] [Google Scholar]
- Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12 Suppl 1:2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Roth BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg S, Ernsberger P, Rothman RB. Salvinorin A: a potent naturally occurring nonnitrogenous kappa opioid selective agonist. Proc Natl Acad Sci U S A. 2002;99:11934–11939. doi: 10.1073/pnas.182234399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaninger M, Schofl C, Blume R, Rosswig L, Knepel W. Inhibition by antidepressant drugs of cyclic AMP response element binding protein/cyclic AMP response element-directed gene transcription. Mol Pharmacol. 1995;47:1112–1118. [PubMed] [Google Scholar]
- Shirayama Y, Ishida H, Iwata M, Hazama GI, Kawahara R, Duman RS. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J Neurochem. 2004;90:1258-126. doi: 10.1111/j.1471-4159.2004.02589.x. [DOI] [PubMed] [Google Scholar]
- Siebert DJ. Salvia divinorum and Salvinorin A: new pharmacologic findings. J Ethnopharmacol. 1994;43:53–55. doi: 10.1016/0378-8741(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Simonin F, Valverde O, Smadja C, Slowe S, Kitchen I, Dierich A, Le Meur M, Roques BP, Maldonado R, Kieffer BL. Disruption of the kappa-opioid receptor gene in mice enhances sensitivity to chemical visceral pain, impairs pharmacological actions of the selective kappa-agonist U-50,488H and attenuates morphine withdrawal. Embo J. 1998;17:886–897. doi: 10.1093/emboj/17.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, French AM. Age-related differences in sensitivity to the antinociceptive effects of kappa opioids in adult male rats. Psychopharmacol. 2002;162:255–264. doi: 10.1007/s00213-002-1102-6. [DOI] [PubMed] [Google Scholar]
- Snyder SH, Pasternak GW. Historical review: Opioid receptors. Trends Pharmacol Sci. 2003;24:198–205. doi: 10.1016/S0165-6147(03)00066-X. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci USA. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Shippenberg TS. Modulation of morphine-induced sensitization by endogenous kappa opioid systems in the rat. Neurosci Lett. 1993;153:232–236. doi: 10.1016/0304-3940(93)90329-j. [DOI] [PubMed] [Google Scholar]
- Stevens WC, Jr, Jones RM, Subramanian G, Metzger TG, Ferguson DM, Portoghese PS. Potent and selective indolomorphinan antagonists of the kappa-opioid receptor. J Med Chem. 2000;43:2759–2769. doi: 10.1021/jm0000665. [DOI] [PubMed] [Google Scholar]
- Svingos AL, Colago EE, Pickel VM. Cellular sites for dynorphin activation of kappa-opioid receptors in the rat nucleus accumbens shell. J Neurosci. 1999;19:1804–1813. doi: 10.1523/JNEUROSCI.19-05-01804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svingos AL, Colago EE. Kappa-opioid and NMDA glutamate receptors are differentially targeted within rat medial prefrontal cortex. Brain Res. 2002;946:262–271. doi: 10.1016/s0006-8993(02)02894-9. [DOI] [PubMed] [Google Scholar]
- Szmuszkovicz J, Von Voigtlander PF. Benzeneacetamide amines: structurally novel non-m mu opioids. J Med Chem. 1982;25:1125–1126. doi: 10.1021/jm00352a005. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Terwilliger R, Lane C, Menzes PS, Conti M, Duman RS. Chronic antidepressant administration increases the expression of cAMP phosphodiesterase 4A and 4B isoforms. J Neurosci. 1998;19:610–618. doi: 10.1523/JNEUROSCI.19-02-00610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JB, Atkinson RN, Rothman RB, Fix SE, Mascarella SW, Vinson NA, Xu H, Dersch CM, Lu Y, Cantrell BE, Zimmerman DM, Carroll FI. Identification of the first trans-(3R,4R)- dimethyl-4-(3-hydroxyphenyl)piperidine derivative to possess highly potent and selective opioid kappa receptor antagonist activity. J Med Chem. 2001;44:2687–2690. doi: 10.1021/jm015521r. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr Effects of κ-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacol. 2004;172:463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- Toll L, Berzetal-Gurske IP, Polgar WE, Brandt SR, Adapa D, Rodrigues L, Schwarts RW, Haggart D, O’Brien A, White A, Kennedy JM, Craymer K, Farrington L, Auh JS. Standard binding and functional assays related to medications development division testing for potential cocaine and opiate narcotic treatment medications. NIDA Res Mono. 1997;178:440–466. [PubMed] [Google Scholar]
- Tomasiewicz HC, Mague SD, Cohen BM, Carlezon WA., Jr Behavioral effects of acute and sub-acute administration of lithium and valproic acid in rats. Brain Res. 2006;1093:83–94. doi: 10.1016/j.brainres.2006.03.102. [DOI] [PubMed] [Google Scholar]
- Tomasiewicz HC, Todtenkopf MS, Chartoff EH, Cohen BM, Carlezon WA., Jr The kappa-opioid agonist U69,593 blocks cocaine-induced enhancement of brain stimulation reward. Biological Psychiatry. 2008;64:982–988. doi: 10.1016/j.biopsych.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon SM, Pollack AE, Fink JS. Enhanced CREB phosphorylation and changes in c-Fos and FRA expression in striatum accompany amphetamine sensitization. Brain Res. 1997;749:120–126. doi: 10.1016/s0006-8993(96)01316-9. [DOI] [PubMed] [Google Scholar]
- Valdes LJ., 3rd Salvia divinorum and the unique diterpene hallucinogen, Salvinorin (divinorin) A. J Psychoactive Drugs. 1994;26:277–283. doi: 10.1080/02791072.1994.10472441. [DOI] [PubMed] [Google Scholar]
- Vergne DE, Nemeroff CB. The interaction of serotonin transporter gene polymorphisms and early adverse life events on vulnerability for major depression. Curr Psychiatry Reports. 2006;8:452–457. doi: 10.1007/s11920-006-0050-y. [DOI] [PubMed] [Google Scholar]
- Vink R, Portoghese PS, Faden AI. Kappa-opioid antagonist improves cellular bioenergetics and recovery after traumatic brain injury. Am J Physiol. 1991;261:R1527–R1532. doi: 10.1152/ajpregu.1991.261.6.R1527. [DOI] [PubMed] [Google Scholar]
- Wadenberg ML. A review of the properties of spiradoline: a potent and selective kappa-opioid receptor agonist. CNS Drug Rev. 2003;9:187–198. doi: 10.1111/j.1527-3458.2003.tb00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Geter-Douglas B, Strain EC, Bigelow GE. Enadoline and butorphanol: evaluation of kappa-agonists on cocaine pharmacodynamics and cocaine self-administration in humans. J Pharmacol Exp Ther. 2001a;299:147–158. [PubMed] [Google Scholar]
- Walsh SL, Strain EC, Abreu ME, Bigelow GE. Enadoline, a selective kappa opioid agonist: comparison with butorphanol and hydromorphone in humans. Psychopharmacol. 2001b;157:151–162. doi: 10.1007/s002130100788. [DOI] [PubMed] [Google Scholar]
- Wang D, Sun X, Sadee W. Different effects of opioid antagonists on mu-, delta-, and kappa-opioid receptors with and without agonist pretreatment. J Pharmacol Exp Ther. 2007;321:544–552. doi: 10.1124/jpet.106.118810. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tang K, Inan S, Siebert D, Holzgrabe U, Lee DY, Huang P, Li JG, Cowan A, Liu-Chen LY. Comparison of pharmacological activities of three distinct KOR ligands (Salvinorin A, TRK-820 and 3FLB) on kappa opioid receptors in vitro and their antipruritic and antinociceptive activities in vivo. J Pharmacol Exp Ther. 2005;312:220–223. doi: 10.1124/jpet.104.073668. [DOI] [PubMed] [Google Scholar]
- Wittmann W, Schunk E, Rosskothen I, Gaburro S, Singewald N, Herzog H, Schwarzer C. Prodynorphin-derived peptides are critical modulators of anxiety and regulate neurochemistry and corticosterone. Neuropsychopharmacol. 2009;34:775–785. doi: 10.1038/npp.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. The validity of animal models of depression. Psychopharmacol. 1984;83:1–16. doi: 10.1007/BF00427414. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psych Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Wise RA. Neuroleptics and operant behavior: The anhedonia hypothesis. Behav Brain Sci. 1982;5:39–87. [Google Scholar]
- Yan F, Roth BL. Salvinorin A: a novel and highly selective kappa-opioid receptor agonist. Life Sci. 2004;75:2615–2619. doi: 10.1016/j.lfs.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Zhu J, Luo LY, Li JG, Chen C, Liu-Chen LY. Activation of the cloned human kappa opioid receptor by agonists enhances [35S]GTPgammaS binding to membranes: determination of potencies and efficacies of ligands. J Pharmacol Exp Ther. 1997;282:676–684. [PubMed] [Google Scholar]
- Zimmerman DM, Nickander R, Horng JS, Wong DT. New structural concepts for narcotic antagonists defined in a 4-phenylpiperidine series. Nature. 1978;275:332–334. doi: 10.1038/275332a0. [DOI] [PubMed] [Google Scholar]