Abstract
Primarily, rats have served as subjects in Δ9-tetrahydrocannabinol's (THC) discrimination studies although other species such as monkeys and pigeons have been used. While the introduction of the knockout and transgenic mice has vastly stimulated the study of the discriminative stimulus effects of drugs there is only a single published report of mice trained to discriminate THC. Thus, this study extended those results by providing a systematic replication that THC serves as an effective discriminative stimulus in mice and by further investigating the mechanisms of action involved in the THC discrimination model in the mouse. Male C57BL/6J mice were trained to discriminate 10 mg/kg THC from vehicle in 2-lever drug discrimination. THC fully and dose dependently substituted for itself. Cannabinoid indoles, except one with low cannabinoid CB1 receptor affinity, substituted for THC. Anandamide failed to substitute for THC when administered alone but completely substituted when administered with the non-specific fatty acid amide hydrolase inhibitor, phenylmethylsulphonyl fluoride. As expected, nicotine failed to substitute for THC. Lastly, the cannabinoid CB1 receptor antagonist rimonabant blocked THC's discriminative stimulus effects. Taken together these studies demonstrate THC's ability to produce discriminative stimulus effects as well as demonstrate its pharmacological specificity and mechanism of action in a two-lever drug discrimination mouse model.
Keywords: THC, drug discrimination, anandamide, mice, cannabinoid
1. Introduction
Mapping of the mouse genome has allowed rapid advances in the development of techniques for mutagenesis of specific genes, as seen in the creation of knockout and transgenic mice (see Picciotto and Wickman, 1998). Although genotype is relatively easy to verify, determination of phenotypic differences is often more difficult, particularly if expression of the mutant phenotype primarily involves behavior. Consequently, recent years have seen increased emphasis on development of novel behavioral techniques for mice as well as alteration of existing procedures (often developed in rats) for use in mice. Since differences in the functional consequences of manipulation of genes that encode for specific receptors or steps in neurotransmission processes may not be evident through use of strictly observational measures, more complicated operant procedures have often been used to measure constructs such as learning and memory. In addition, pharmacological challenge often is employed.
Drug discrimination is increasingly being used in mice as a method to tease out pharmacologically selective differences in behavioral phenotypes. In drug discrimination, a subject is trained to make one response if administered a specific psychoactive drug (i.e., the training drug) and to make another response if administered vehicle or a drug that does not share the same psychoactive properties. In mice, the typical response requirement is a lever press or a head poke on a drug- or vehicle-associated side, respectively, with correct responses resulting in delivery of reinforcement (an appetitive reinforcer such as food pellet or sweetened milk). Previous studies have demonstrated that mice can be trained to discriminate a variety of psychoactive drugs, including clozapine (Philibin et al., 2005), ethanol (Bowen and Balster, 1997), pentobarbital (Balster and Moser, 1987), and nicotine (Varvel et al., 1999). Moreover, knockout mice have been used to gain further insights regarding the discriminative stimulus effects of drugs such as nicotine with alpha 7 knockout mice (Stolerman et al., 2004) and cocaine with dopamine D5 knockout mice (Elliot et al., 2003).
Δ9-Tetrahydrocannabinol (THC), the principal psychoactive substituent of the marijuana plant (Cannabis sativa), has also been used as a discriminative stimulus in laboratory animals, including rats (Burkey and Nation, 1997; Järbe et al., 1998; Solinas et al., 2007; Vann et al., 2007; Wiley et al., 1995a), rhesus monkeys (Wiley et al., 1995c), pigeons (Henriksson et al., 1975), and gerbils (Järbe et al., 1975). Collectively, these studies have revealed that the discriminative stimulus effects of THC are pharmacologically selective for psychoactive cannabinoids (Wiley et al., 1995b) and are blocked by administration of rimonabant and other antagonists of cannabinoid (CB1) receptors in the brain, but not by an antagonist of cannabinoid CB2 receptors (Järbe et al., 2001; Järbe et al., 2006; Wiley et al., 1995c). Further, centrally active cannabinoids of various structural classes substitute for THC with strong correlation between potency for substitution and affinity for cannabinoid CB1 receptors (Compton et al., 1993). Moreover, the discriminative stimulus effects of cannabinoids in animals correspond to the subjective effects of cannabinoids that have been assessed in humans (Chait et al., 1988; Lile et al., 2008); hence, THC discrimination represents an animal model of marijuana intoxication (Balster and Prescott, 1992).
Recently, McMahon and colleagues (2008) reported the first successful training of a 2-nose poke THC versus vehicle discrimination in C57BL/6J mice. In this initial manuscript, the structurally dissimilar cannabinoids, CP 55940 and WIN 55212-2, substituted for THC in mice trained whereas the metabolically stable anandamide analog methanandamide and noncananbinoid drugs (ketamine, cocaine and ethanol) did not. The present study extends the findings of that study and provides additional findings regarding the mechanisms of action of a 2-lever THC versus vehicle discrimination in C57BL/6J mice through a variety of techniques, including co-administration of antagonists or metabolic inhibitors, substitution tests with a noncannabinoid compound (nicotine), and preliminary assessment of structure-activity relationship with indole-derived cannabinoids (Table 1).
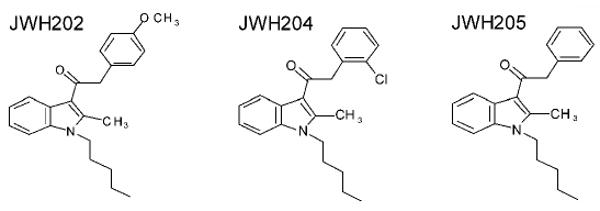
Table 1. 1-Pentyl-3-phenylacetylindoles: Relationship between cannabinoid CB1 receptor affinity and potency for producing THC-like discriminative stimulus effects.
 | ||||
|---|---|---|---|---|
| Compound | Molecular weight | CB1 Affinity Ki(nM) ± S.E.M. | ED50(95% CL) μmol/kg | |
| Δ9-THC | Group 1 | 314 | 41 ± 2 a | 12 (6-24) |
| Group 2 | 11 (7-18) | |||
| JWH-202 | ||||
| 1-pentyl-3-(4-methoxyphenylacetyl)indole | 350 | 1678 ± 62 b | No substitution | |
| JWH-204 | ||||
| 1-pentyl-3-(2-chlorophenylacetyl)indole | 353 | 13 ± 1 b | 8 (2-28) | |
| JWH-205 | ||||
| 1-pentyl-3-phenylacetylindole | 319 | 134 ± 23 b | 92 (23-ucl out of range) | |
2. Method and materials
2.1 Subjects
Male C57B1/6J mice (20-25 g) obtained from Jackson Laboratories (Bar Harbor, ME) were housed individually in clear plastic cages (18 × 29 × 13 cm) with steel wire fitted tops and wood-chip bedding in a temperature-controlled (20-22°C) vivarium. Water was available ad libitum except while the mice were in the operant chambers. Training and test sessions were conducted at similar times during the light phase of a 12-h light/dark cycle. Completion of the entire study required sequential training of two groups of mice (due to the novelty of training this procedure in mice combined with the short lifespan of this species). Mice were maintained at 85 - 90% of free-feeding body weights by restricting daily ration of standard rodent chow. Animals used in this study were cared for in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Virginia Commonwealth University and the ‘Guide for the Care and Use of Laboratory Animals’ (National Research Council, 1996).
2.2 Apparatus
Eight standard mice operant conditioning chambers that were sound- and light-attenuated (MED Associates, St. Albans, VT) were used for behavioral training and testing. Each operant conditioning chamber (18 × 18 × 18 cm) was equipped with a house light, two levers (left and right), and a recessed dipper receptacle centered between the levers. A dipper arm delivered sweetened milk in a 0.05-ml cup, which was available for 5 s. Fan motors provided ventilation and masking noise for each chamber. House lights were illuminated during training and testing sessions. A computer with Logic ‘1’ interface (MED Associates) and MED-PC software (MED Associates) was used to control schedule contingencies and to record data.
2.3 Procedures
2.31 Lever press training
Each mouse was placed in a standard operant chamber and trained to lever press according to a fixed ratio (FR) 1 schedule of reinforcement. Milk reinforcement was delivered after every lever press. The FR value was gradually increased to the final FR10 schedule of reinforcement in which 10 consecutive responses were required for delivery of milk reinforcement. After mice were trained on one lever, contingency requirements for milk delivery were switched to the other lever. Lever press training at this second lever proceeded identically to that at the first lever. When responding on the second lever under a FR10 schedule was acquired, discrimination training began.
2.32 Discrimination training
Mice were trained to press one lever following administration of 10 mg/kg THC and to press the other lever following vehicle injection according to a FR10 schedule of milk reinforcement. Each response on the incorrect lever reset the response requirement on the correct lever. Daily injections were administered on a double alternation sequence of THC and vehicle (e.g., drug, drug, vehicle, vehicle). Daily 15-min training sessions were held Monday-Friday until the mice had met two criteria during 8 of 10 consecutive sessions: (1) the first completed FR-10 (e.g. consecutive correct responses ≥ 10) and (2) ≥ 80% of the total responding occurred on the correct lever. When these two criteria were met, acquisition of the discrimination was established and substitution testing began.
2.33 Substitution and combination tests
Following successful acquisition of the discrimination, stimulus substitution tests were conducted on Tuesdays and Fridays during 15-min test sessions. Training continued on Mondays, Wednesdays, and Thursdays. During test sessions, responses on either lever delivered reinforcement according to an FR-10 schedule. To be tested, mice must have completed the first FR10 on the correct lever and ≥ 80% of the total responding must have occurred on the correct lever on the preceding day. In addition, the mouse must have met these same criteria during previous training sessions with the alternate training compound (training drug or vehicle). Prior to substitution tests generalization curve for THC were generated with all mice. Then, substitution tests were conducted with several indole-derived cannabinoids synthesized in our laboratories (see Huffman et al., 2005), namely compounds JWH202, 204, 205 (n = 5). In a separate group of mice (n=6), anandamide alone and in combination with the non-specific amidase inhibitor, phenylmethylsulphonyl fluoride (PMSF), and the non-cannabinoid, nicotine were tested. PMSF was injected 15 min prior to administration of anandamide. For antagonism studies, mice (n=4) were injected i.p. with 1 mg/kg rimonabant 10 min prior to administration of 10 mg/kg THC. To ensure that THC's discriminative stimulus effects were effectively maintained through out these studies control tests with the training dose of THC and with vehicle were re-determined prior to conducting all substitution or combination tests.
2.4 Drugs
THC (National Institute on Drug Abuse; NIDA, Rockville, MD), anandamide (Organix, Inc., Woburn, MA), PMSF (Sigma Chemicals Co., St. Louis, MO, USA), and rimonabant base (NIDA) were dissolved in a vehicle of 7.8% tween80 and 92.2% saline. Indole-derived cannabinoids were synthesized in our laboratories (Clemson University, Clemson, S.C.) and were dissolved in the cannabinoid vehicle. Nicotine hydrogen tartrate salt (Sigma Chemicals Co., St. Louis, MO, USA) was dissolved in saline and administered 5 min prior to testing. Nicotine doses are expressed in terms of the base. THC, PMSF, and indole-derived cannabinoids were administered 30 min prior to testing. Anandamide and nicotine were administered 15 min prior to testing. Injections times used in this study are based on our extensive research experience with cannabinoids in drug discrimination model in rats (Vann et al., 2007) or with the tetrad in mice (Wiley et al., 2006). Except when noted all drugs were administered s.c. at a volume of 0.1 ml/10 g.
2.5 Data Analysis
Acquisition indices were the percentage of animals that pressed the first FR on the correct lever and achieved ≥ 80% of the total responding on the correct lever during the course of the session. For each test session, percentage of responses on the drug lever and response rate (responses/s) were calculated. ED50 values were calculated for percentage of responses on the drug lever using least squares linear regression analysis followed by calculation of 95% confidence limits (Tallarida and Murray, 1987). Since mice that responded less than 10 times during a test session did not press either lever a sufficient number of times to earn a reinforcer, their data were excluded from analysis of drug lever selection, but their response rate data were included. Response-rate suppression (relative to rates after vehicle administration) was determined by separate analyses of variance (ANOVA) using GBSTAT statistical software (GB-STAT software; Dynamic Microsystems, Silver Spring, MD). Significant ANOVAs were further analyzed with Tukey post hoc tests (α = 0.05) to specify differences between means.
3. Results
3.1 THC discrimination
Acquisition to criteria of the THC discrimination required an average of 78.2 (range = 53-93) and 68.8 training sessions (range = 53-91) for the mice tested with JWH compounds and for nicotine-anandamide group, respectively. THC fully and dose-dependently substituted for itself with similar patterns of generalization and with nearly identical ED50 values (Table 1) in both groups of THC-trained mice (Fig. 1 and 2, top panels). Repeated measures ANOVA conducted on the response rate data from the THC dose-effect curves resulted in significant differences as a function of dose for group 2 [F (4,20)=3.6, P < 0.05; Fig. 2 bottom panel] but not for group 1 (P >.05, Fig. 1 bottom panel). Compared with responding following vehicle injections, response rates were significantly increased by 1 mg/kg THC (P < 0.05) in group 2. No other significant changes in response rates for THC-treated mice were observed.
Fig. 1.
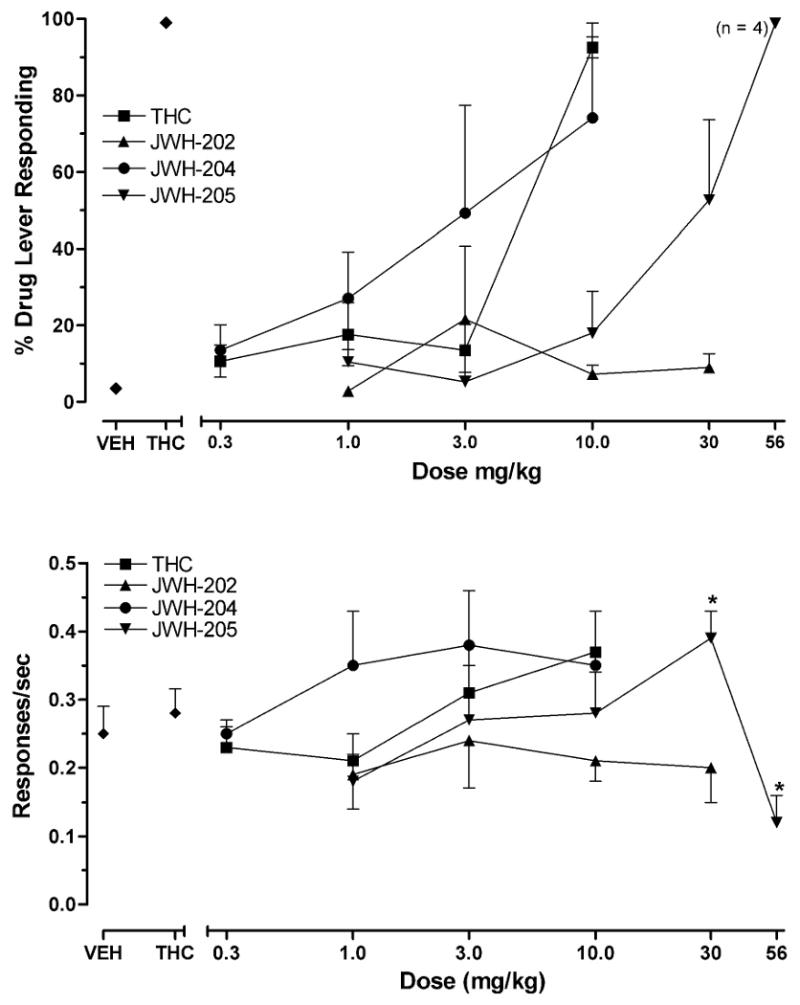
Effects of THC and JWH compounds 202, 204, and 205 on percentage of THC-lever responding (upper panels) and response rates (lower panels) in mice trained to discriminate 10 mg/kg THC from vehicle. Points above VEH and THC represent the results of control tests with vehicle and 10 mg/kg THC conducted before each dose-effect determination. Asterisks (*) represents significant decreases or increases in rates of responding compared to vehicle (P < 0.05). For each dose-effect curve determination, values represent the mean (±S.E.M.) of 5 mice.
Fig. 2.

Effects of THC, nicotine, anandamide alone, and anandamide administered with 30 mg/kg PMSF on percentage of THC -lever responding (upper panels) and response rates (lower panels) in mice trained to discriminate 10 mg/kg THC from vehicle(n = 6). All other details are the same as Fig 1.
3.2 Substitution tests with cannabinoid indoles
In substitution tests with the cannabinoid indoles (Fig. 1, top panel), JWH-205 produced full dose-dependent substitution, but was less potent than THC (Table 1). Repeated measures ANOVA conducted on the response rate data from the JWH-205 dose-effect curves resulted in significant differences as a function of dose [F (4,25)=5.1, P < 0.05]. Post hoc tests revealed that JWH-205 significantly decreased response rates compared to vehicle at the 56 mg/kg dose and increased response rates at the 30 mg/kg dose (P < 0.05, Fig. 1, bottom panel). Similar to JWH-205, JWH-204 increased responding on the THC-associated lever in a dose-dependent manner (Fig. 1, top panel). Although it completely substituted in three (of four) mice at the 10 mg/kg dose, this compound could not be tested at higher doses because of limited availability. ED50 values for JWH-204 substitution were similar to those of THC (see Table 1). In contrast with results for the other two indole-derived cannabinoids, JWH-202 did not substitute for THC, producing a maximum of only 21.7 % THC-lever responding at doses up to 30 mg/kg (Fig. 1, top panel). Since response rates were not affected by JWH-202 (Fig. 1, bottom panel) it could be argued that higher doses may have substituted. It should be noted that at the high dose of JWH-202 none of the mice responded at percentage levels other than those associated with vehicle responding.
3.21 Substitution, combination, and antagonism tests
Fig. 2 (top panel) shows that neither anandamide administered alone nor nicotine substituted for THC. Concomitant administration of PMSF and anandamide, however, produced full dose-dependent substitution. Whereas response rates for anandamide (with or without PMSF) were not altered (P>0.05), nicotine significantly decreased response rates at the highest dose tested, 0.08 mg/kg [F (3,12)=4.0, P < 0.05; Fig. 2 bottom panel].
Fig. 3 shows the results of antagonism tests with 1 mg/kg rimonabant and 10 mg/kg THC (i.e., training dose). Rimonabant blocked the THC-like discriminative stimulus effects exhibited by this dose [F (3,8)=10.04, P < 0.05].
Fig. 3.

Effects of rimonabant challenges on THC-like responding produced by the THC training dose on percentage of THC -lever responding in mice trained to discriminate 10 mg/kg THC from vehicle. Bars above VEH & SR and VEH represent the results of control tests with co-administration of either vehicle and 1 mg/kg rimonabant or vehicle and 10 mg/kg THC. The bar above SR represents the antagonism test with 1 mg/kg rimonabant 10 min prior to 10 mg/kg THC. The asterisk (*) represents significant blockade by the cannabinoid CB1 receptor antagonist, rimonabant of THC-like discriminative stimulus effects exhibited by 10 mg/kg THC (P < 0.05).Values represent the mean (±S.E.M) of 4 mice.
4. Discussion
Rats have been the chosen species for the majority of previous drug discrimination studies in rodent models; however, the importance of training drug discrimination in mice has greatly increased in recent years due, in part, to the growing body of research using genetically modified mice. Basic characterization of mouse models of discrimination with different drug classes is necessary in order to determine how best to maximize benefits of models and to facilitate the most effective comparisons across species and within species (e.g., different strains, wildtype versus knockout). To this end, successful training of THC discrimination in mice has been reported only in a single recently published study (McMahon et al., 2008). Similar to the findings of that study, the present study demonstrated that THC produced full and dose-dependent substitution, with nearly equal ED50 values for THC, in two separate groups of C57BL/6J mice using a 2-lever drug discrimination paradigm. THC discrimination in mice shares other characteristics with the rat model, including cross-substitution with CP 55940 and WIN 55212-2 (McMahon et al., 2008; Wiley, 1999; Wiley et al., 1995a) and antagonism by rimonabant (Wiley et al., 1995c). A limited degree of support for the pharmacological selectivity of this mouse model of THC discrimination has also been demonstrated in these studies, although a thorough evaluation has not yet been performed. To date, several other abused drugs have failed to substitute for THC, including nicotine, in the present study, and ketamine, cocaine and ethanol in the previous study (McMahon et al., 2008). In each case, behaviorally active doses were evaluated, as indicated by response rate suppression at higher doses. These results are consistent with those of previous studies in rats (Wiley et al., 1995a) and monkeys (Wiley et al., 1995b) that have demonstrated that non-cannabinoid drugs of various pharmacological classes fail to generalize to THC.
In the present study, evaluation of three indole-derived cannabinoids (see, Huffman et al., 2005) revealed a systematic relationship between cannabinoid CB1 receptor affinity and potency for producing THC-like discriminative stimulus effects, as has been reported previously in rats (Wiley et al., 1998a). JWH-204 and JWH-205 had strong and moderate cannabinoid CB1 receptor affinities with Ki values of 13 and 134 (nM) respectively, and produced dose-dependent substitution for THC. In contrast, JWH-202 had low affinity (Ki = 1678 nM) and failed to increase THC-lever responding at doses up to 30 mg/kg. Although maximal mean substitution for JWH-204 was slightly less than full (80%) substitution, the 10 mg/kg dose substituted for THC in all but one of the mice tested. Moreover, the mouse that failed to substitute also failed to generalize to THC at doses lower than the training dose. This suggests that complete substitution in all mice may have been achieved with an increase in dose; however, a limited supply of the compound prevented its further evaluation. Of the compounds that substituted for THC, rank order potency for THC-like discriminative stimulus effects (JWH-204 ≥ THC > JWH-205) corresponded with cannabinoid CB1 receptor affinities (see Table 1). These results are consistent with the strong structure-activity relationship that has been demonstrated between cannabinoid CB1 receptor affinity and potency of cannabinoids for producing a characteristic tetrad of in vivo pharmacological effects in mice, including suppression of spontaneous activity, antinociception, hypothermia and catalepsy (Compton et al., 1993; Martin et al., 1991).
The relationship between cannabinoid CB1 receptor affinity and potency in the tetrad tests in mice and in THC discrimination in rats is less strong for anandamide and its analogs (Adams et al., 1995). When administered alone, anandamide failed to substitute for THC in mice in the present study, as was the case in previous studies in rats (Burkey and Nation, 1997; Wiley et al., 1998b) and monkeys (Wiley et al., 1997). In contrast, concomitant administration of anandamide and PMSF produced full, dose-dependent THC-like discriminative stimulus effects. Although its inhibitory action on amidases is non-specific, previous research has shown that PMSF penetrates the blood-brain barrier and increases anandamide levels (Wiley et al., 2000), inhibits the action of fatty acid amide hydrolyses (FAAH) (Desarnaud et al., 1995), and enhances anandamide's cannabinoid effects in the tetrad (Wiley et al., 2006). Together, these results suggest that the initial lack of anandamide substitution was due to its rapid metabolism by FAAH, a process that was prevented by co-administration of PMSF. In support of this hypothesis, a recent study in rats demonstrated that anandamide produced THC-like discriminative stimulus effects upon concomitant administration of a more selective FAAH inhibitor, URB597, but not when administered alone (Solinas et al., 2007). Additional support is provided by the finding that intravenously administered anandamide will substitute for THC in monkeys at a dose 10 fold higher than is required for THC to generalize to itself (McMahon, 2008). In contrast, the effects of metabolically stable anandamide analogs in rodent THC discrimination are variable.
Methanandamide, the only anandamide analog that has been evaluated in this procedure in mice, failed to substitute for THC and did not alter the discriminative stimulus effects of THC when co-administered (McMahon et al., 2008). In rats, methanandamide substitution for THC was dependent upon training dose, with full substitution at a THC training dose of 1.8 mg/kg and partial substitution at a THC training dose of 5.6 mg/kg (Järbe et al., 2000; Järbe et al., 1998). Other methylated anandamide analogs also substituted to varying degrees for THC in rats, but did so primarily at doses that significantly reduced response rates (Wiley et al., 1997; Wiley et al., 2004).
Despite the similarities in THC discrimination in mice and rats that have been described above, one difference was particularly notable: i.e., the training dose used in this mouse study and the one other published study (McMahon et al., 2008) was higher than the dose used in rats (3.0 mg/kg) in our laboratory (Barrett et al., 1995; Vann et al., 2007; Wiley et al., 2004) as well as others (Burkey and Nation, 1997, 2 mg/kg; Järbe and McMillan, 1980, 3 mg/kg). Similar species differences in training doses of other drugs have been reported. For example, 0.4 mg/kg is a frequently used training dose for nicotine in rats in this laboratory and others (Robinson et al., 2007; Smith et al., 2007; Wright et al., 2006; Zaniewska et al., 2006). Mice trained with nicotine doses of 0.4 and 0.6 mg/kg, however, only reach incomplete levels of acquisition (Shoaib et al., 2002; Stolerman et al., 2004; Stolerman et al., 1999) whereas doses of 0.8 and 1.2 mg/kg are fully trainable (Shoaib et al., 2002; Stolerman et al., 2004; Stolerman et al., 1999). Higher training doses have been stipulated in other drug discrimination studies in mice using drugs such as lysergic acid diethylamide (LSD) (Benneyworth et al., 2005) and 2, 5-dimethoxy-4-iodo-amphetamine (DOI) (Smith et al., 2003).
In conclusion, the results of this and other studies show that THC discrimination in mice shares a number of characteristics with THC discrimination in rats, including mediation via cannabinoid CB1 receptors, pharmacological selectivity, dose-dependent substitution of psychoactive cannabinoids of several classes (bicyclic cannabinoids, aminoalkylindoles, and the endogenous ligand anandamide), and correspondence between affinity for cannabinoid CB1 receptors and potency for THC-like discriminative stimulus effects. These findings offer support for the validity of the mouse model of THC discrimination and suggest that it may provide a useful tool for research with genetically manipulated mice, including cannabinoid CB1 receptor knockout mice that lack the gene for encoding cannabinoid CB1 receptors (Zimmer et al., 1999) and FAAH knockout mice that lack the primary enzyme for anandamide metabolism (Cravatt et al., 2001). Future discrimination studies with these and other knockout mice might show more precisely the role of the endogenous cannabinoid system in THC's discriminative stimulus effects, and perhaps, eventually, marijuana's subjective effects in humans.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams IB, Ryan W, Singer M, Thomas BF, Compton DR, Razdan RK, Martin BR. Evaluation of cannabinoid receptor binding and in vivo activities for anandamide analogs. J Pharmacol Exp Ther. 1995;273:1172–1181. [PubMed] [Google Scholar]
- Balster RL, Moser VC. Pentobarbital discrimination in the mouse. Alcohol Drug Res. 1987;7:233–242. [PubMed] [Google Scholar]
- Balster RL, Prescott WR. Δ9-Tetrahydrocannabinol discrimination in rats as a model for cannabis intoxication. Neurosci Biobehav Rev. 1992;16:55–62. doi: 10.1016/s0149-7634(05)80051-x. [DOI] [PubMed] [Google Scholar]
- Barrett RL, Wiley JL, Balster RL, Martin BR. Pharmacological specificity of delta 9-tetrahydrocannabinol discrimination in rats. Psychopharmacology. 1995;118:419–424. doi: 10.1007/BF02245942. [DOI] [PubMed] [Google Scholar]
- Benneyworth MA, Smith RL, Barrett RJ, Sanders-Bush E. Complex discriminative stimulus properties of (+)lysergic acid diethylamide (LSD) in C57Bl/6J mice. Psychopharmacology. 2005;179:854–862. doi: 10.1007/s00213-004-2108-z. [DOI] [PubMed] [Google Scholar]
- Bowen SE, Balster RL. Desflurane, enflurane, isoflurane and ether produce ethanol-like discriminative stimulus effects in mice. Pharmacol Biochem Behav. 1997;57:191–198. doi: 10.1016/s0091-3057(96)00308-5. [DOI] [PubMed] [Google Scholar]
- Burkey RT, Nation JR. (R)-methanandamide, but not anandamide, substitutes for delta 9-THC in a drug-discrimination procedure. Exp Clin Psychopharmacol. 1997;5:195–202. doi: 10.1037//1064-1297.5.3.195. [DOI] [PubMed] [Google Scholar]
- Chait LD, Evans SM, Grant KA, Kamien JB, Johanson CE, Schuster CR. Discriminative stimulus and subjective effects of smoked marijuana in humans. Psychopharmacology. 1988;94:206–212. doi: 10.1007/BF00176846. [DOI] [PubMed] [Google Scholar]
- Compton DR, Rice KC, De Costa BR, Razdan RK, Melvin LS, Johnson MR, Martin BR. Cannabinoid structure-activity relationships: Correlation of receptor binding and in vivo activities. J Pharmacol Exp Ther. 1993;265:218–226. [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desarnaud F, Cadas H, Piomelli D. Anandamide amidohydrolase activity in rat brain microsomes. Identification and partial characterization. J Biol Chem. 1995;270:6030–6035. doi: 10.1074/jbc.270.11.6030. [DOI] [PubMed] [Google Scholar]
- Elliot EE, Sibley DR, Katz JL. Locomotor and discriminative-stimulus effects of cocaine in dopamine D5 receptor knockout mice. Psychopharmacology. 2003;169:161–168. doi: 10.1007/s00213-003-1494-y. [DOI] [PubMed] [Google Scholar]
- Henriksson BG, Johansson JO, Järbe TU. Delta 9-tetrahydrocannabinol produced discrimination in pigeons. Pharmacol Biochem Behav. 1975;3:771–774. doi: 10.1016/0091-3057(75)90105-7. [DOI] [PubMed] [Google Scholar]
- Huffman JW, Szklennik PV, Almond A, Bushell K, Selley DE, He H, Cassidy MP, Wiley JL, Martin BR. 1-Pentyl-3-phenylacetylindoles, a new class of cannabimimetic indoles. Bioorg Med Chem Lett. 2005;15:4110–4113. doi: 10.1016/j.bmcl.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Johansson JO, Henriksson BG. Delta9-tetrahydrocannabinol and pentobarbital as discriminative cues in the Mongolian Gerbil (Meriones unguiculatus) Pharmacol Biochem Behav. 1975;3:403–410. doi: 10.1016/0091-3057(75)90048-9. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Lamb RJ, Lin S, Makriyannis A. Delta9-THC training dose as a determinant for (R)-methanandamide generalization in rats: a systematic replication. Behavioural pharmacology. 2000;11:81–86. doi: 10.1097/00008877-200002000-00009. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Lamb RJ, Lin S, Makriyannis A. (R)-methanandamide and Delta 9-THC as discriminative stimuli in rats: tests with the cannabinoid antagonist SR-141716 and the endogenous ligand anandamide. Psychopharmacology. 2001;156:369–380. doi: 10.1007/s002130100730. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Lamb RJ, Makriyannis A, Lin S, Goutopoulos A. Delta9-THC training dose as a determinant for (R)-methanandamide generalization in rats. Psychopharmacology. 1998;140:519–522. doi: 10.1007/s002130050797. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Liu Q, Makriyannis A. Antagonism of discriminative stimulus effects of delta(9)-THC and (R)-methanandamide in rats. Psychopharmacology. 2006;184:36–45. doi: 10.1007/s00213-005-0225-y. [DOI] [PubMed] [Google Scholar]
- Järbe TU, McMillan DE. delta 9-THC as a discriminative stimulus in rats and pigeons: generalization to THC metabolites and SP-111. Psychopharmacology. 1980;71:281–289. doi: 10.1007/BF00433063. [DOI] [PubMed] [Google Scholar]
- Lile JA, Kelly TH, Pinsky DJ, Hays LR. Substitution profile of Delta(9)-tetrahydrocannabinol, triazolam, hydromorphone, and methylphenidate in humans discriminating Delta(9)-tetrahydrocannabinol. Psychopharmacology. 2008 doi: 10.1007/s00213-008-1393-3. epub 10.1007/s00213-00008-01393-00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BR, Compton DR, Thomas BF, Prescott WR, Little PJ, Razdan RK, Johnson MR, Melvin LS, Mechoulam R, Ward SJ. Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacol Biochem Behav. 1991;40:471–478. doi: 10.1016/0091-3057(91)90349-7. [DOI] [PubMed] [Google Scholar]
- McMahon LR. Apparent affinity estimates of rimonabant in combination with anandamide and chemical analogs of anandamide in rhesus monkeys discriminating Delta(9)-tetrahydrocannabinol. Psychopharmacology. 2008 doi: 10.1007/s00213-008-1230-8. DOI 10.1007/s00213-00008-01230-00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LR, Ginsburg BC, Lamb RJ. Cannabinoid agonists differentially substitute for the discriminative stimulus effects of Delta(9)-tetrahydrocannabinol in C57BL/6J mice. Psychopharmacology. 2008;198:487–495. doi: 10.1007/s00213-007-0900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, D.C.: 1996. [Google Scholar]
- Philibin SD, Prus AJ, Pehrson AL, Porter JH. Serotonin receptor mechanisms mediate the discriminative stimulus properties of the atypical antipsychotic clozapine in C57BL/6 mice. Psychopharmacology. 2005;180:49–56. doi: 10.1007/s00213-005-2147-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Wickman K. Using knockout and transgenic mice to study neurophysiology and behavior. Physiological reviews. 1998;78:1131–1163. doi: 10.1152/physrev.1998.78.4.1131. [DOI] [PubMed] [Google Scholar]
- Robinson SE, Vann RE, Britton AF, O'Connell MM, James JR, Rosecrans JA. Cellular nicotinic receptor desensitization correlates with nicotine-induced acute behavioral tolerance in rats. Psychopharmacology. 2007;192:71–78. doi: 10.1007/s00213-006-0687-6. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Gommans J, Morley A, Stolerman IP, Grailhe R, Changeux JP. The role of nicotinic receptor beta-2 subunits in nicotine discrimination and conditioned taste aversion. Neuropharmacology. 2002;42:530–539. doi: 10.1016/s0028-3908(01)00194-0. [DOI] [PubMed] [Google Scholar]
- Smith JW, Mogg A, Tafi E, Peacey E, Pullar IA, Szekeres P, Tricklebank M. Ligands selective for alpha4beta2 but not alpha3beta4 or alpha7 nicotinic receptors generalise to the nicotine discriminative stimulus in the rat. Psychopharmacology. 2007;190:157–170. doi: 10.1007/s00213-006-0596-8. [DOI] [PubMed] [Google Scholar]
- Smith RL, Barrett RJ, Sanders-Bush E. Discriminative stimulus properties of 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane [(+/-)DOI] in C57BL/6J mice. Psychopharmacology. 2003;166:61–68. doi: 10.1007/s00213-002-1252-6. [DOI] [PubMed] [Google Scholar]
- Solinas M, Tanda G, Justinova Z, Wertheim CE, Yasar S, Piomelli D, Vadivel SK, Makriyannis A, Goldberg SR. The endogenous cannabinoid anandamide produces delta-9-tetrahydrocannabinol-like discriminative and neurochemical effects that are enhanced by inhibition of fatty acid amide hydrolase but not by inhibition of anandamide transport. J Pharmacol Exp Ther. 2007;321:370–380. doi: 10.1124/jpet.106.114124. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Chamberlain S, Bizarro L, Fernandes C, Schalkwyk L. The role of nicotinic receptor alpha 7 subunits in nicotine discrimination. Neuropharmacology. 2004;46:363–371. doi: 10.1016/j.neuropharm.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Naylor C, Elmer GI, Goldberg SR. Discrimination and self-administration of nicotine by inbred strains of mice. Psychopharmacology. 1999;141:297–306. doi: 10.1007/s002130050837. [DOI] [PubMed] [Google Scholar]
- Tallarida R, Murray R. Springer Verlag; New York: 1987. [Google Scholar]
- Vann RE, Cook CD, Martin BR, Wiley JL. Cannabimimetic properties of ajulemic acid. J Pharmacol Exp Ther. 2007;320:678–686. doi: 10.1124/jpet.106.111625. [DOI] [PubMed] [Google Scholar]
- Varvel SA, James JR, Bowen S, Rosecrans JA, Karan LD. Discriminative stimulus (DS) properties of nicotine in the C57BL/6 mouse. Pharmacol Biochem Behav. 1999;63:27–32. doi: 10.1016/s0091-3057(98)00262-7. [DOI] [PubMed] [Google Scholar]
- Wiley JL. Cannabis: discrimination of “internal bliss”? Pharmacol Biochem Behav. 1999;64:257–260. doi: 10.1016/s0091-3057(99)00059-3. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Barrett RL, Lowe J, Balster RL, Martin BR. Discriminative stimulus effects of CP 55,940 and structurally dissimilar cannabinoids in rats. Neuropharmacology. 1995a;34:669–676. doi: 10.1016/0028-3908(95)00027-4. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Compton DR, Dai D, Lainton JA, Phillips M, Huffman JW, Martin BR. Structure-activity relationships of indole- and pyrrole-derived cannabinoids. J Pharmacol Exp Ther. 1998a;285:995–1004. [PubMed] [Google Scholar]
- Wiley JL, Dewey MA, Jefferson RG, Winckler RL, Bridgen DT, Willoughby KA, Martin BR. Influence of phenylmethylsulfonyl fluoride on anandamide brain levels and pharmacological effects. Lif Sci. 2000;67:1573–1583. doi: 10.1016/s0024-3205(00)00749-9. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Golden KM, Ryan WJ, Balster RL, Razdan RK, Martin BR. Evaluation of cannabimimetic discriminative stimulus effects of anandamide and methylated fluoroanandamide in rhesus monkeys. Pharmacol Biochem Behav. 1997;58:1139–1143. doi: 10.1016/s0091-3057(97)00327-4. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Huffman JW, Balster RL, Martin BR. Pharmacological specificity of the discriminative stimulus effects of delta 9-tetrahydrocannabinol in rhesus monkeys. Drug Alcohol Depend. 1995b;40:81–86. doi: 10.1016/0376-8716(95)01193-5. [DOI] [PubMed] [Google Scholar]
- Wiley JL, LaVecchia KL, Karp NE, Kulasegram S, Mahadevan A, Razdan RK, Martin BR. A comparison of the discriminative stimulus effects of delta(9)-tetrahydrocannabinol and O-1812, a potent and metabolically stable anandamide analog, in rats. Exp Clin Psychopharmacol. 2004;12:173–179. doi: 10.1037/1064-1297.12.3.173. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Lowe JA, Balster RL, Martin BR. Antagonism of the discriminative stimulus effects of delta 9-tetrahydrocannabinol in rats and rhesus monkeys. J Pharmacol Exp Ther. 1995c;275:1–6. [PubMed] [Google Scholar]
- Wiley JL, Razdan RK, Martin BR. Evaluation of the role of the arachidonic acid cascade in anandamide's in vivo effects in mice. Life Sci. 2006;80:24–35. doi: 10.1016/j.lfs.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Ryan WJ, Razdan RK, Martin BR. Evaluation of cannabimimetic effects of structural analogs of anandamide in rats. Eur J Pharmacol. 1998b;355:113–118. doi: 10.1016/s0014-2999(98)00502-0. [DOI] [PubMed] [Google Scholar]
- Wright MJ, Jr, Vann RE, Gamage TF, Damaj MI, Wiley JL. Comparative effects of dextromethorphan and dextrorphan on nicotine discrimination in rats. Pharmacol Biochem Behav. 2006;85:507–513. doi: 10.1016/j.pbb.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaniewska M, McCreary AC, Przegalinski E, Filip M. Evaluation of the role of nicotinic acetylcholine receptor subtypes and cannabinoid system in the discriminative stimulus effects of nicotine in rats. Eur J Pharmacol. 2006;540:96–106. doi: 10.1016/j.ejphar.2006.04.034. [DOI] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]


