Abstract
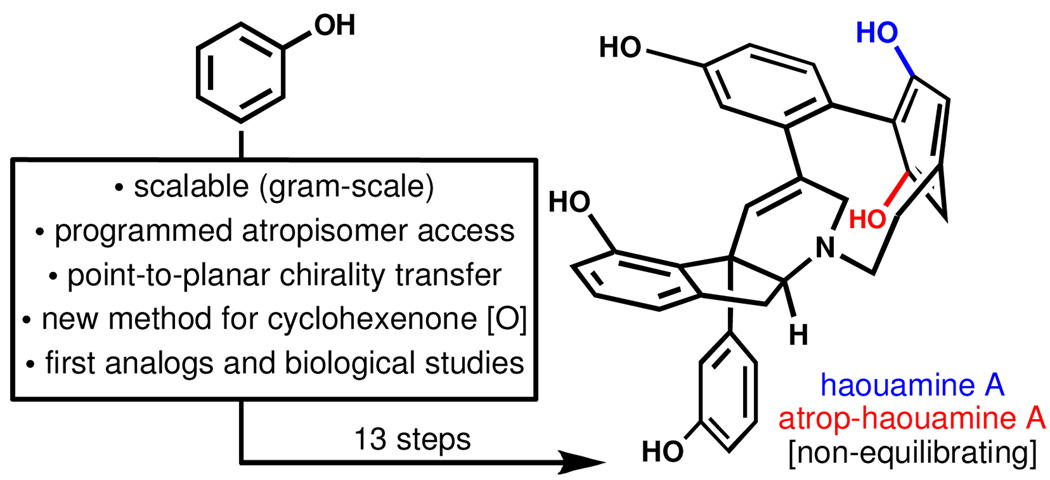
A total synthesis of the complex, bent aromatic ring-containing marine alkaloid haouamine A is achieved through a route in which every step (with the exception of the final deprotection) is performed on a gram-scale. This is accomplished through the development of a method for the dehydrogenation of cyclohexenones that allows for point-to-planar chirality transfer. This strategy makes it possible to program the desired atropisomeric outcome from a simple chiral cyclohexenone. By synthesizing atrop-haouamine A, this work has firmly established that natural haouamine exists as a single, non-equilibrating atropisomer. Finally, biological investigations demonstrate that the bent aromatic ring of this natural product is critical for anti-cancer activity against PC3 cells.
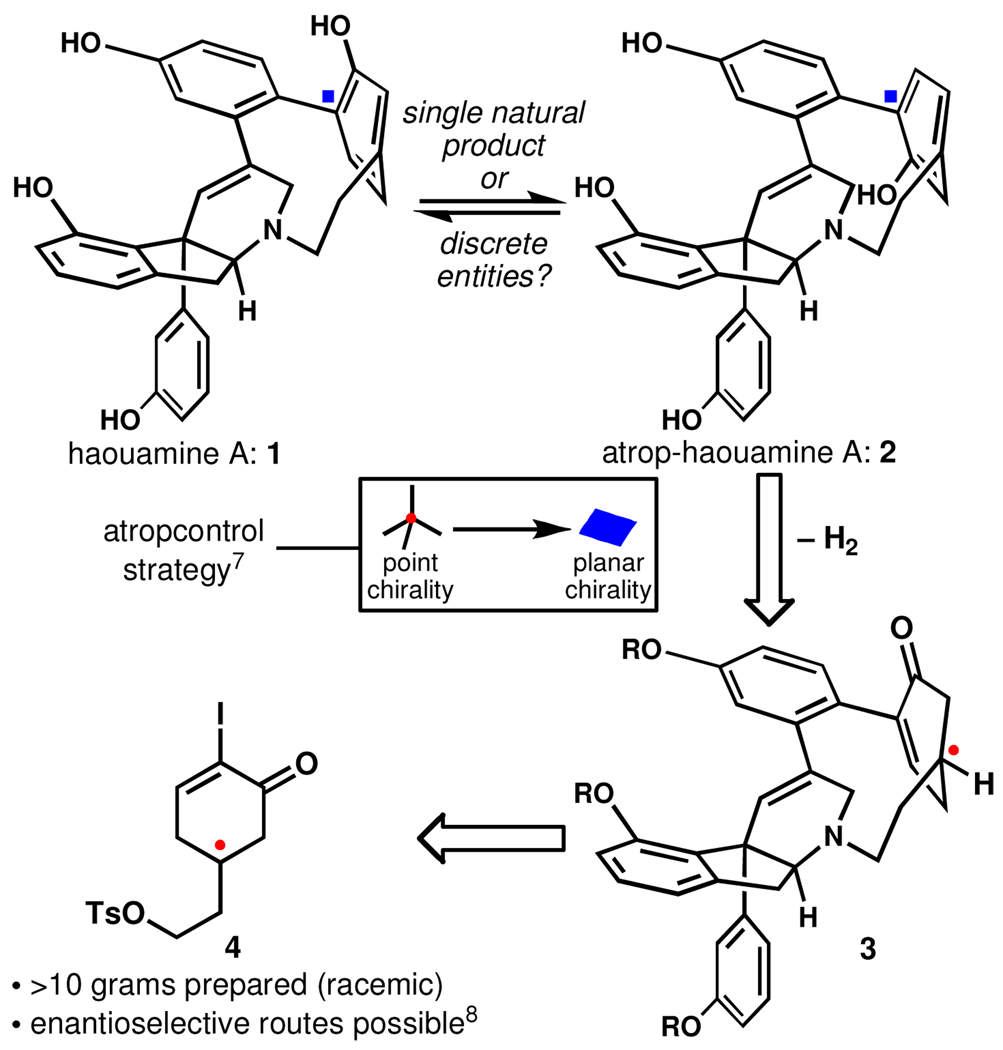
Haouamine A1 (1, Figure 1) is a biologically active and architecturally unique alkaloid whose striking feature is a [7]-azaparacyclophane macrocycle containing a highly deformed non-planar aromatic ring. Somewhat mysteriously, 1 was discovered to exist as a mixture of two rapidly interconverting isomers in solution, a quality attributed to either atropisomerism of the bent arene or slowed pyramidal inversion at nitrogen. Recent computational work2 supported a theory coupling the latter process with conformational reorganization of the tetrahydropyridine ring, but could not rule out atropisomerism.3 The total synthesis of 1 from this lab4 also did not answer this question unequivocally due to an inability to control the atropisomer formed in the low-yielding cyclophane-forming step which fortuitously favored the natural planar stereochemistry.5,6
Figure 1.
Synthetic strategy and an unanswered structural question.
In this Communication we report a scalable and controllable route to 1 and its atropisomer (2) that features point-to-planar chirality transfer7 via a one-step, chemoselective cyclohexenone to phenol oxidation to introduce strain within the macrocycle – a reaction that may find future use in strained chiral cyclophane synthesis. We also demonstrate that the strained cyclophane in 1 is crucial for anti-cancer activity in PC3 cells.
In order to program planar chirality within the cyclophane macrocycle a reduced compound (3, Figure 1) was selected as the bent phenol precursor. Molecular models suggested that rehybridization of one of the para carbons of the cyclophane from sp2 to sp3 should significantly reduce the strain present within the macrocycle and thus make for an accesible intermediate5c (numerous attempts to form the macrocycle with a preinstalled phenol in this4 and other5,6 labs have all failed). Furthermore, it was surmised that the point chirality introduced by such a change in hybridization could be used to select for the planar chirality present in 1 and 2. Saturated cyclophane 3 could then be traced back to the simple functionalized cyclohexenone 4, utilized as the racemate in this study.8
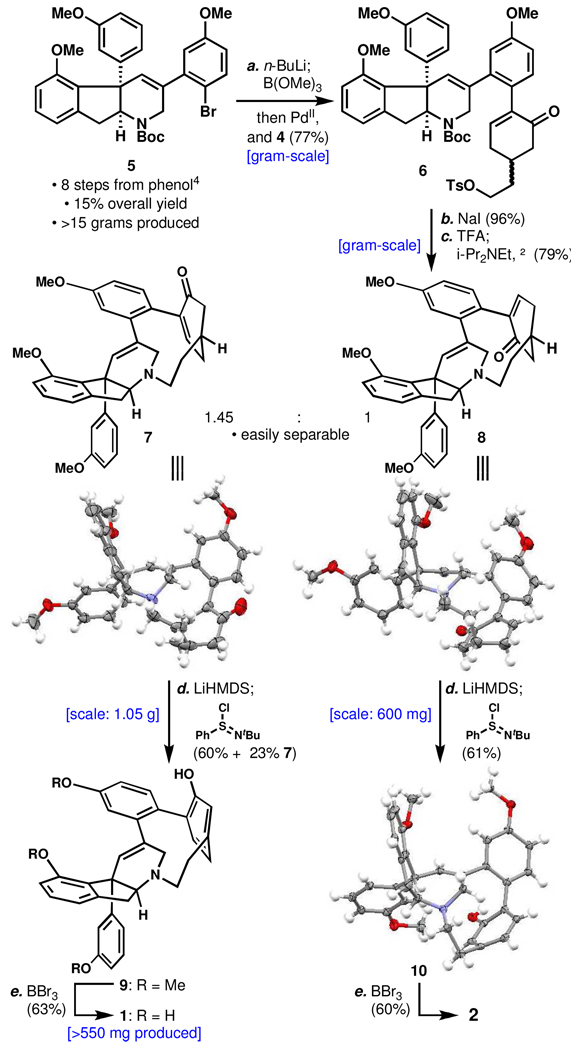
To commence this plan, racemic bromo-indenotetrahydropyridine 54a (Scheme 1) was cross-coupled with racemic tosyloxy-iodocyclohexenone 49 in a one-step procedure involving lithium-halogen exchange, quenching with B(OMe)3, addition of water, and direct transfer of the resulting boronic acid to 4 in the presence of palladium. The product 6 (77% yield on gram-scale) was isolated as an inseparable mixture of diastereomers that was converted to a mixture of primary iodides in high yield. N-Boc deprotection and heating of the unpurified amine-TFA salt (after removal of excess TFA) in dilute acetonitrile with Hünig’s base then delivered macrocycles 7 and 8 (79% combined yield, gram-scale) as a 1.45:1 readily separable mixture (this ratio implies a small amount of selectivity in either the macrocyclization or the previous coupling step). Interestingly, each of these compounds was found to exist as two isomers in solution providing early evidence that 1 is not a mixture of atropisomers. X-ray crystallographic analysis of both 7 and 8 identified their correspondance to haouamine A (1) and atrop-haouamine A (2), respectively.
Scheme 1.
Scalable, Programmed Syntheses of 1 and 2.a
aReagents and conditions: (a) n-BuLi (1.1 equiv), THF, −78 °C, 10 min; B(OMe)3, −78 to 23 °C, 1 h; H2O; 4 (1.0 equiv), (PhCN)2PdCl2 (0.1 equiv), Ph3As (0.2 equiv), Ag2O (1.6 equiv), 8:1 THF/H2O, 18 h, 23 °C, 77%; (b) NaI (10 equiv), acetone, 23 °C, 7 h, 96%; (c) 20:1 CH2Cl2/TFA, 5 °C, 24 h; i-Pr2NEt (10.0 equiv), CH2CN (0.002 M), reflux, 26 h, 79%; (d) LiHMDS (2.0 equiv), LiCl (5.0 equiv), THF, −78 to 0 °C, 20 min; PhSClNt-Bu (1.3 equiv), −78 °C (for 7) or −95 °C (for 8), 1 min, 60% of 9 + 23% 7, or 61% of 10; (e) BBr3 (7.0 equiv), CH2Cl2, −78 to 5 °C, 20 h, 63% for 1, or 60% for 2.
Efforts were then focused towards the key aromatization step. Initial attempts to oxidize the silyl dienol ether of 7 and 8 with palladium10 or MnO211 saw competitive oxidation of the indeno-tetrahydropyridine core. Several other attempts to oxidize the enolate or cyclohexenone directly also failed.9 Taking inspiration from the use of N-t-butylbenzenesulfinimidoyl chloride by Mukaiyama12 to introduce α,β-unsaturation to ketones in one step, it was discovered that treatment of the lithium dienolates of 7 and 8 with this reagent rapidly affected the desired oxidation to deliver the bent phenol macrocycles 9 and 10 as isomeric mixtures in respective 60% and 61% yield (23% of the starting material could be recovered in reaction of 7 to 9). This represents the first use of such a reagent to generate aromatic systems, and it should find future applicability to do so particularly in strained systems of this type due to its high oxidation potential and the possibility to introduce asymmetry into the starting cyclohexenone.8 A low reaction temperature (−78 °C for 7 and −95 °C for 8), the addition of lithium chloride, and a very short (1 minute) reaction time were necessary in order to prevent subsequent reaction of the phenol product with the reagent. This transformation has proved to be highly practical and scalable as it has been conducted on 1.05 g of 7 and 600 mg of 8 with no yield diminishment. BBr3-mediated removal of the methyl ethers in 9 then delivered haouamine A (1) in 63% yield. As a testament to the practicality of this route, its utilization has allowed for the production of over 550 mg of (±)-1 to date. Syntheses of enantiopure 1 and 2 (ca. 10 mg) have also been accomplished from enantiopure 5.4b,9
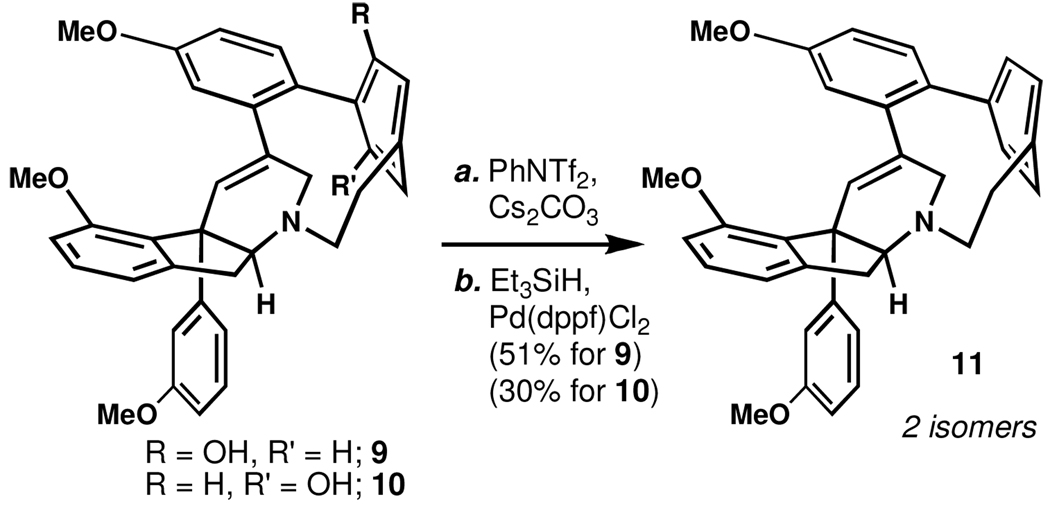
X-ray crystallographic analysis of 10 secured its identity as the atropisomer of 9. Interestingly, the isomer of 10 in this crystal structure displayed an inversion at nitrogen and an alternative tetrahydropyridine conformation as compared to the crystal structure of 11 providing physical substantiation to computational results.2 Ether cleavage then delivered atrophaouamine A (2) whose solution isomeric behavior is similar to that of 1. As a further confirmation of this phenomenon, both intermediates were found to converge on cyclophane 11 (Scheme 2), a compound that also exists as two isomers.
Scheme 2.
Reductive Convergence of 9 and 10 to 11.
While an initial bioassay was reported along with the isolation of 1, the large amounts of material that are now made available with this chemistry has enabled a more thorough investigation into its bioactivity. Initial results have shown that 1 exhibits high activity against PC3 human prostate cancer cells with IC50 = 29 ± 2 µM. atrop-Haouamine A (2) also shows high activity (IC50 = 32 ± 3 µM), however des-methyl 7 and des-methyl 8 (dihydro-1 and dihydro-2) are much less active (IC50 > 180 µM and IC50 > 75 µM, respectively) indicating that the presence of the cyclophane is necessary for activity in PC3 cells. Our findings on the biological activity of 1 are different than those reported by Zubía and co-workers.1,9
Thus, a scalable route to haouamine A (1) and atrophaouamine A (2) has been developed, allowing for the synthesis of ample quantities of each. In the case of 1, all steps (with the exception of the final methyl ether removal) have been conducted on gram-scale. This synthesis of 1 and 2 has put to rest the question of whether 1 exists as a mixture of atropisomers and was enabled by the development of a method for the chemoselective aromatization of cyclohexenones that allows for point-to-planar chirality transfer; application of this strategy to other chiral strained cyclophane-containing natural products is underway. As a result of this work, the haouamine material supply is no longer an issue, and extensive biological studies (including determination of the mechanism of action of 1) are taking place and will be reported shortly.
Supplementary Material
Detailed experimental procedures, copies of all spectral data and full characterization. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgement
We thank Drs. D.-H. Huang and L. Pasternack for NMR spectroscopic assistance, Dr. G. Siuzdak and Dr. Arnold L. Rheingold (UCSD) for mass spectrometric and X-ray crystallographic assistance, respectively. Funding for this work was provided by Bristol-Myers Squibb, the NIH/NCI (CA134785), the Skaggs Institute for Chemical Biology, and the ARCS Foundation (predoctoral fellowship to N.Z.B.).
References
- 1.Garrido L, Zubía E, Ortega MJ, Salvá J. J. Org. Chem. 2003;68:293. doi: 10.1021/jo020487p. [DOI] [PubMed] [Google Scholar]
- 2.Belostotskii AM. J. Org. Chem. 2008;73:5723. doi: 10.1021/jo702766x. [DOI] [PubMed] [Google Scholar]
- 3.For an example of surprising atropisomerism in a natural product, see: Nicolaou KC, Harrison ST. Angew. Chem. Int. Ed. 2006;45:3256. doi: 10.1002/anie.200601116.
- 4.Racemic: Baran PS, Burns NZ. J. Am. Chem. Soc. 2006;128:3908. doi: 10.1021/ja0602997. Enantioselective: Burns NZ, Baran PS. Angew. Chem. Int. Ed. 2008;47:205. doi: 10.1002/anie.200704576.
- 5.For model studies, see: Smith ND, Hayashida J, Rawal VH. Org. Lett. 2005;7:4309. doi: 10.1021/ol0512740. Grundl MA, Trauner D. Org. Lett. 2006;8:23. doi: 10.1021/ol052262h. Wipf P, Furegati M. Org. Lett. 2006;8:1901. doi: 10.1021/ol060455e.
- 6.For formal syntheses, see: Jeong JH, Weinreb SM. Org. Lett. 2006;8:2309. doi: 10.1021/ol060556c. Fürstner A, Ackerstaff J. Chem. Commun. 2008:2870. doi: 10.1039/b805295f. Taniguchi T, Zaimoku H, Ishibashi H. J. Org. Chem. 2009;74:2624. doi: 10.1021/jo802787j.
- 7.For examples of point-to-planar chirality transfer, see: Evans DA, Dinsmore CJ, Watson PS, Wood MR, Richardson TI, Trotter BW, Katz JL. Angew. Chem. Int. Ed. 1998;37:2704. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2704::AID-ANIE2704>3.0.CO;2-1. Layton ME, Morales CA, Shair MD. J. Am. Chem. Soc. 2002;124:773. doi: 10.1021/ja016585u. Nicolaou KC, Boddy CNC. J. Am. Chem. Soc. 2002;124:10451. doi: 10.1021/ja020736r. For examples of planar-to-point chirality transfer, see: Fu GC.Acc. Chem. Res 200639853.and references therein.
- 8.For an enantioselective route to the starting material for 4, see: Jiricek J, Blechert S. J. Am. Chem. Soc. 2004;126:3534. doi: 10.1021/ja0399021.
- 9.See Supporting Information for details.
- 10.Bierling B, Kirschke K, Oberender H. J. Prakt. Chem. 1972;314:170. [Google Scholar]
- 11.Corey EJ, Lazerwith SE. J. Am. Chem. Soc. 1998;120:12777. [Google Scholar]
- 12.Mukaiyama T, Matsuo J, Kitagawa H. Chem. Lett. 2000;29:1250. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed experimental procedures, copies of all spectral data and full characterization. This material is available free of charge via the Internet at http://pubs.acs.org.