Abstract
The sarcoglycans are known as an integral subcomplex of the dystrophin glycoprotein complex, the function of which is best characterized in skeletal muscle in relation to muscular dystrophies. Here we demonstrate that the white adipocytes, which share a common precursor with the myocytes, express a cell-specific sarcoglycan complex containing β-, δ-, and ϵ-sarcoglycan. In addition, the adipose sarcoglycan complex associates with sarcospan and laminin binding dystroglycan. Using multiple sarcoglycan null mouse models, we show that loss of α-sarcoglycan has no consequence on the expression of the adipocyte sarcoglycan complex. However, loss of β- or δ-sarcoglycan leads to a concomitant loss of the sarcoglycan complex as well as sarcospan and a dramatic reduction in dystroglycan in adipocytes. We further demonstrate that β-sarcoglycan null mice, which lack the sarcoglycan complex in adipose tissue and skeletal muscle, are glucose-intolerant and exhibit whole body insulin resistance specifically due to impaired insulin-stimulated glucose uptake in skeletal muscles. Thus, our data demonstrate a novel function of the sarcoglycan complex in whole body glucose homeostasis and skeletal muscle metabolism, suggesting that the impairment of the skeletal muscle metabolism influences the pathogenesis of muscular dystrophy.
Muscle fat infiltration is recognized as a hallmark pathological feature in dystrophin glycoprotein complex (DGC)3-related muscular dystrophies (1) that include dystrophinopathies (2, 3) and sarcoglycanopathies (LGMD2C-F) (4). In agreement, magnetic resonance imaging measurements of fat infiltration allow accurate assessments of disease severity in Duchenne muscular dystrophy patients (3). Association of adipose tissue development with degenerative/regenerative or atrophic changes in skeletal muscle is also supported by the finding that adipogenesis-competent cells within the skeletal muscle are activated during muscle regeneration (5). However, the molecular mechanism(s) underlying muscle fatty metamorphosis remain unclear.
Ectopic fat deposition in skeletal muscles is primarily described in animals and humans with lipodystrophy and sarcopenia. In these conditions, the accumulation of lipids and adipocytes in skeletal muscle is often accompanied by hyperglycemia and insulin resistance (6–11), both of which are strong indicators of muscle metabolic defects (12, 13) and deregulated adipogenesis (14). Furthermore, both adipose-derived and muscle-derived stem cells differentiate into adipocytes upon exposure to high levels of glucose (15), linking impaired muscle metabolism with muscle fat deposition.
It is long held that the biogenesis of a basement membrane takes place in the earliest steps of adipogenesis and that extensive extracellular matrix (ECM) remodeling occurs throughout adipogenesis (16, 17). The concept that cell surface receptors play a role in the regulation of adipogenesis and thus may underlie metabolic disorders just recently emerged with a study of the integrin complexes (18). Given that the DGC in its capacity as an ECM receptor is critical for muscle integrity (19, 20) and that white adipocytes and skeletal muscle cells originate from the same mesenchymal precursor cells (21, 22), we set out to determine whether components of the skeletal muscle DGC are expressed in white adipocytes. Herein, we describe a unique adipose sarcoglycan (SG) complex that includes β-, δ-, and ϵ-SG. This complex is tightly associated with sarcospan (Sspn) and dystroglycan (DG). Moreover, we show that DG functions as a novel ECM receptor in white adipocytes. Because adipose tissue and skeletal muscle play critical roles in the maintenance of normal glucose homeostasis and whole body insulin sensitivity (23), we examined the metabolic consequences of the SG complex disruption in both adipose tissue and skeletal muscle. Using in vivo approaches, we observed that the β-SG null mouse (24), a mouse model of muscular dystrophy, is glucose-intolerant and exhibits whole body insulin resistance specifically due to impaired insulin-stimulated glucose uptake in skeletal muscle.
EXPERIMENTAL PROCEDURES
Animals
Animal care and procedures were approved and performed in accordance with the standards set forth by the National Institutes of Health and the Animal Care Use and Review Committee at the University of Iowa.
Biochemical Analysis
White adipocytes were isolated from wild-type gonadal white adipose tissue (25). Total RNA extraction using RNA-STAT60TM (IsoTex Diagnostics Inc., Friendswood, TX) and preparation of total membrane extracts (26) were performed from isolated adipocytes. Sucrose gradient purification of the DGC components (27) was performed from isolated adipocytes and whole WT gonadal adipose tissue.
Physiological Analysis
Glucose tolerance tests (GTTs) were performed on 16-h fasted male mice following intraperitoneal injection of d-glucose (1 g/kg). Blood glucose was measured from tail vein using OneTouch Ultra test strips (LifeScan). Several measurements were done at each time point to ensure reproducibility, and the lowest value was used. Fasting insulin was quantified in serum using enzyme-linked immunosorbent assay (Millipore Corp.). Euglycemic-hyperinsulinemic (EU) clamps were conducted on male mice according to the slightly modified protocol (28). Basal rates of whole body glucose clearance were assessed using a continuous infusion of [3H]glucose for 2 h prior to the start of the clamp. Insulin was infused continuously at 4 milliunits/kg/min. Forty-five min before the end of the clamp, 2-deoxy-d-[1-14C]glucose was administrated as a bolus (10 μCi) to estimate insulin-stimulated glucose uptake in individual tissues.
Statistics
GTT data were analyzed with two-way repeated measures analysis of variance, and all pairwise multiple comparison procedures were done with the Bonferroni t test. Other data were analyzed with Student's unpaired t test.
RESULTS
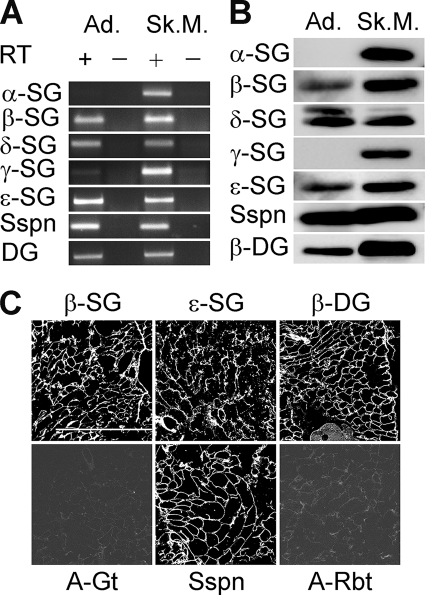
To determine whether known components of the DGC are expressed in white adipose tissue while ensuring that our results would not reflect contamination from other tissues, we chose freshly isolated white adipocytes as starting material. RT-PCR data revealed the presence of β-, δ-, and ϵ-SG, Sspn and DG transcripts in adipocytes (Fig. 1A and supplemental Table 1s). The identity of these transcripts was confirmed by BLAST analysis. In contrast to skeletal muscle, there was a conspicuous absence of α-SG and γ-SG transcript. The detection of a transcript correlated with the detection of the corresponding protein (Fig. 1B). Results were confirmed by immunofluorescence analysis of adipose tissue sections (Fig. 1C and data not shown). Of the known DGC proteins, DG is the focus of most research interest due to its widespread expression, association with the basal lamina, and the complex and heterogeneous tissue-specific O-glycosylation of its extracellular α-subunit. Post-translational modification of α-DG is critical for its interaction with a broad range of ECM proteins, and loss of this interaction is a common factor in the biogenesis of complex diseases (19). To better characterize the binding properties of α-DG in adipose tissue, we chose to make use of a well characterized mouse model, the myodystrophy mice (Largemyd or myd) (29). These mice have a spontaneous loss-of-function mutation in Large, which encodes a putative glycosyltransferase involved in α-DG O-glycosylation, leading to brain and skeletal muscle α-DG hypoglycosylation and pathology (30). We found adipocyte α-DG reactivity using antibodies directed against either the α-DG core (sheep5) or its sugar moieties (IIIH11), albeit at a lower relative molecular mass (120 kDa) than that in skeletal muscle (supplemental Fig. 1s, A and B, lanes 1 and 3). The absence of Large led to a loss of α-DG glycosylated epitope, with preservation of the α-DG protein core in adipocytes (supplemental Fig. 1s, A and B, lanes 2 and 4). Agrin and laminin-1 overlay experiments conclusively demonstrated that the adipose-specific glycosylation was sufficient for α-DG to function as an ECM ligand receptor (supplemental Fig. 1s, C and D, lanes 1 and 3). As expected and consistent with the observations made in myd brain and skeletal muscle, laminin and agrin binding to α-DG were abolished in absence of Large (supplemental Fig. 1s, C and D, lanes 2 and 4), with the level of expression of the SGs and Sspn being preserved (data not shown). These data demonstrate that Large-mediated post-translational processing of α-DG takes place in white adipocytes. In support of this conclusion, expression of Large and other α-DG post-translational processing enzymes, i.e. Pomt1, Pomt2, PomGnt1, and Fukutin, was observed in adipocytes by RT-PCR (supplemental Fig. 2s), confirming a previous gene profiling study performed in white adipocytes (31).
FIGURE 1.
Expression of DGC components in white adipocytes. A, transcripts of known DGC components were amplified from isolated adipocytes (Ad.) and skeletal muscle (Sk.M.) by RT-PCR in the presence (+) or absence (−) of reverse transcriptase (RT). B, expression of the corresponding proteins in total membrane fraction from white adipocytes (100 μg) and KCl-washed microsomes from skeletal muscle (50 μg), as determined by Western blotting. C, expression of DGC proteins in epididymal white adipose tissue, analyzed by confocal microscopy. Negative controls using cyanine 3-labeled anti-goat (A-Gt) and Alexa Fluor 555-labeled anti-rabbit (A-Rbt) were performed by omitting the primary antibody. Scale bar, 500 μm.
To test whether the DGC components expressed in the white adipocytes associate in a fashion similar to that described for skeletal muscle (19, 20), we analyzed their co-fractionation on sucrose gradient (supplemental Fig. 3sA). We found that Sspn migrated in the denser gradient fractions, along with the β-, δ-, and ϵ-SG and β-DG. The tight association of these proteins upon solubilization indicates that these proteins are integral components of a multisubunit complex that via DG can function as a novel ECM receptor in the white adipocytes. Control experiments in differentiated 3T3-L1 cells supported this conclusion as well (data not shown).
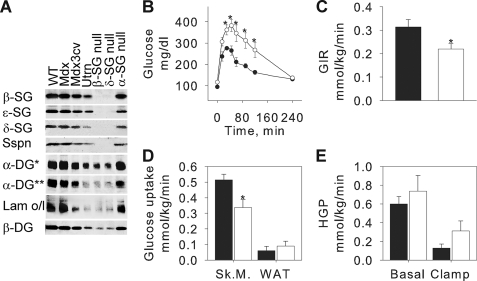
To determine whether the adipose SG complex functions as a single unit, we tested whether the loss of a single component will influence the expression of the others and impair α-DG function. We took advantage of existing mouse models of muscular dystrophy, each with a systemic genetic ablation of one skeletal DGC component (32). Fig. 2A shows the composition of the SG complex and its associated Sspn and DG in mdx and mdx3cv mice, utrophin (utrn), β-SG, δ-SG, and α-SG null mice. As expected, loss of either full-length dystrophin (dys) or α-SG, whose expression are restricted to skeletal muscle, had no consequence on the expression of the SGs, Sspn, and DG at the adipocyte membrane nor on the ability of α-DG to bind laminin. The loss of all dys isoforms in mdx3cv mice or of utrn in the utrn null mice was accompanied by modest decreased expression of all the proteins tested and a reduced laminin binding to α-DG. Therefore, although one cannot rule out that a short dys isoform and utrophin could be expressed in the adipocytes, their expression does not appear to be critical for proper SG complex expression at the adipocyte plasma membrane. However, loss of either β-SG or δ-SG led to a complete loss of the other SGs as well as Sspn. Moreover, only residual expression of the glycosylated form of α-DG could be detected, which correlated with weak residual laminin binding. Altogether, our data suggest that the adipose SG complex should be considered as a single unit that relies on all its individual components for full functionality.
FIGURE 2.
β-SG null mice are glucose-intolerant and insulin-resistant. A, whole adipose tissue from various mouse models was analyzed by Western blotting and laminin-1 overlay (Lam o/l) after digitonin solubilization and glycoprotein enrichment. *, anti-core antibody sheep5; **, anti-carbohydrate antibody IIIH11. B, intraperitoneal GTTs performed on 3–4-month-old male mice. ●, WT, n = 14; ○, β-SG null mice, n = 7. Mean ± S.E., *, p < 0.05. C–E, conscious EU clamps were performed on 3–4-month-old male mice. Whole body glucose infusion rate (GIR) (C) and glucose uptake in soleus (Sk.M.) and white adipose tissue (WAT) (D) were measured during clamp conditions. Hepatic glucose production was measured in both basal and clamp conditions (E). Filled columns, WT, n = 7; open columns, β-SG null mice, n = 8. Mean ± S.E., *, p < 0.05.
Because both skeletal muscle and adipose tissue play important roles in glucose homeostasis, we took advantage of the β-SG null mice due to the concomitant loss of expression of the entire adipose and skeletal SG complexes. Upon dissection, we observed that the β-SG null mice had virtually no subcutaneous adipose tissue (data not shown). Furthermore, Table 1 demonstrates that the β-SG null had a significant decrease of their visceral white adipose tissue mass (epididymal and retroperitoneal fat expressed as a percentage of total body mass) as compared with age-matched WT mice. Histological analysis of the white adipose tissue revealed conspicuous changes such as those that characterize lipodystrophy (supplemental Fig. 4s). Specifically, unilocular cells were heterogeneous in size and shape; numerous small multilocular cells were present, and large blood vessels and fibrous strands coursed throughout the adipose tissue.
TABLE 1.
Organ weights expressed as a percentage of total weight
Organs were carefully dissected and weighed. Results are expressed as mean (S.E.). All experiments were performed in double-blind fashion. IBAT, intrascapular brown adipose tissue. *, p < 0.001.
| Organ | WT (n = 15) | β-SG null mice (n = 11) |
|---|---|---|
| Quadriceps muscle | 1.54 (0.02) | 1.79 (0.06)* |
| Calf muscle | 1.09 (0.03) | 1.43 (0.03)* |
| Cardiac muscle | 0.56 (0.01) | 0.57 (0.03) |
| Kidney | 1.24 (0.02) | 1.22 (0.03) |
| Liver | 4.14 (0.09) | 5.92 (0.11)* |
| Epididymal fat | 1.57 (0.07) | 1.01 (0.06)* |
| Retroperitoneal fat | 0.39 (0.02) | 0.22 (0.03)* |
| Lungs | 0.56 (0.02) | 0.66 (0.05) |
| Spleen | 0.27 (0.01) | 0.39 (0.04) |
| Pancreas | 0.53 (0.02) | 0.56 (0.04) |
| IBAT | 0.22 (0.02) | 0.27 (0.02) |
| Testis | 0.69 (0.02) | 0.67 (0.03) |
Functionally, β-SG null mice were glucose-intolerant (Fig. 2B) and had a trend to have higher fasting insulin levels than their WT counterparts (data not shown), suggesting an alteration of their whole body insulin sensitivity. To address this last question, we performed conscious EU clamps coupled with [3H]glucose infusion in β-SG null and WT mice (Fig. 2, C–E). EU clamps indicated that the glucose clearance was significantly diminished in the β-SG null mice as compared with the WT controls (22% decrease with p = 0.029, data not shown), confirming the glucose tolerance test data. The whole body glucose infusion rate was 30% lower (p = 0.03) in the β-SG null mice than in the controls (Fig. 2C), demonstrating that the null animals were insulin-resistant. Interestingly, insulin resistance was accompanied by decreased insulin sensitivity in skeletal muscle only (35% decrease in insulin-stimulated glucose uptake in skeletal muscle of β-SG null mice as compared with that of WT age-matched controls, p = 0.016) (Fig. 2D). Insulin sensitivity remained normal in both white adipose tissue (Fig. 2D) and liver (Fig. 2E).
DISCUSSION
We have demonstrated here that white adipocytes express at their plasma membrane an SG complex unique to this cell type. It includes β-, δ-, and ϵ-SG but lacks α- and γ-SG as determined by RT-PCR and immunoblotting. Both β-SG and δ-SG proteins can seed the formation of the SG complexes (α-βδγ and ϵ-βδγ) found in skeletal and smooth muscle, respectively (33, 34), and a recent model proposed that γ-SG links the β/δ-SG precomplex to α-SG in skeletal muscle (35). As ζ-SG, the latest SG identified is the closest γ-SG homolog (36), it is possible that ζ-SG functionally replaces γ-SG in white adipocytes. We also showed that Sspn co-purifies with the above SG complex and that its expression is gone in the adipose tissue of SG-null mice. It was previously suggested that the assembly of a complete SG complex was a prerequisite for proper expression of Sspn at the sarcolemma (37). Likewise, our data suggest that expression of Sspn at the adipocyte membrane is dependent on proper SG complex expression.
The role played by the ECM receptors (18) and the importance of the cell-cell and cell-matrix interactions in adipose tissue are just emerging concepts (38–40). Here, we found that decreased expression of DG and a loss of extracellular ligand binding to α-DG were associated with a loss of SG complex expression in white adipocytes. This suggests that the SG complex is necessary for functional DG expression as an ECM receptor at the adipocyte plasma membrane. The physiological characterization of β-SG null mice suggests that these mice, primarily known as a genetic model of muscular dystrophy (24, 32), could also be a new genetic model of lipodystrophy (9). It is well established that adipose tissue serves as a crucial integrator of glucose homeostasis (12, 28) and that alterations of its physiology can have a wide range of metabolic consequences (41, 42). Thus, it is tempting to speculate that the alteration of the adipose SG complex could represent a new molecular mechanism underlying phenotypes of altered adiposity, abnormal fat distribution, and metabolic disorders, likely via an alteration of the cell-cell and/or cell-matrix interactions in adipose tissue. However, since the global ablation of β-SG affects both skeletal muscle and adipocytes, future studies using tissue-specific knock-out animals will be necessary to specifically resolve the tissue-specific roles of the SG complex in glucose metabolism.
Nevertheless, these findings raise novel questions that have important implications for clinical studies of muscular dystrophy. DGC-related muscular dystrophies are often accompanied by progressive muscle fat replacement (2–4), and the ECM imparts specific characteristics to each tissue. Thus, we speculate that the SG complex, along with Sspn and DG, plays a role in regulating the adipogenesis/myogenesis cell fate decisions potentially involved in the mechanism of muscle fat replacement. Furthermore, muscular dystrophies are complex diseases likely to involve more than the skeletal muscles (19). However, the efficiency of the therapeutic strategies are so far solely based on the recovery of the skeletal DGC and/or muscle strength (43, 44). Our data indicate that a cell-specific SG complex is expressed in the white adipocytes. Furthermore, we for the first time bring evidence of metabolic defects in the skeletal muscles and whole body of the β-SG null mice. We speculate that the severity of the metabolic symptoms is likely to depend on whether the skeletal SG complex alone (i.e. in α-SG null mice) or both adipose and skeletal SG complexes (i.e. in β-SG null mice) are disrupted and on the relative functional contribution of the primary missing protein. Although the association of metabolic defects with the progression of muscular dystrophy is not documented yet in patients, it is possible that the recovery of skeletal muscle function in treated patients might uncover new health problems due to defective SG complexes in non-muscle tissues, such as adipose tissue.
Supplementary Material
Acknowledgments
We thank Sarah Anderson and Keith Garringer for maintaining the mouse colonies, Sally J. Prouty for technical assistance, and Campbell laboratory members for the critical reading of the manuscript. We also acknowledge The University of Iowa DNA Facility, which is supported in part by the Holden Comprehensive Cancer Center (HCCC), as well as the University of Iowa Hybridoma Facility.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 AR051199-01 through the NIAMS. This work was also supported by a Paul D. Wellstone Muscular Dystrophy Cooperative Research Center grant and by a grant from the Muscular Dystrophy Association.

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Experimental Procedures,” supplemental Table 1, and supplemental Figs. 1s–4s.
- DG
- dystroglycan
- DGC
- dystrophin glycoprotein complex
- SG
- sarcoglycan
- Sspn
- sarcospan
- RT
- reverse transcriptase
- ECM
- extracellular matrix
- WT
- wild type
- GTT
- glucose tolerance test
- EU
- euglycemic-hyperinsulinemic
- utrn
- utrophin
- dys
- dystrophin.
REFERENCES
- 1.McNally E. M., Pytel P. (2007) Annu. Rev. Pathol. 2, 87–109 [DOI] [PubMed] [Google Scholar]
- 2.Tyler K. L. (2003) Muscle Nerve 28, 402–422 [DOI] [PubMed] [Google Scholar]
- 3.Wren T. A., Bluml S., Tseng-Ong L., Gilsanz V. (2008) AJR Am. J. Roentgenol. 190, W8–12 [DOI] [PubMed] [Google Scholar]
- 4.Lodi R., Muntoni F., Taylor J., Kumar S., Sewry C. A., Blamire A., Styles P., Taylor D. J. (1997) Neuromuscul. Disord. 7, 505–511 [DOI] [PubMed] [Google Scholar]
- 5.Yamanouchi K., Yada E., Ishiguro N., Hosoyama T., Nishihara M. (2006) Exp. Cell Res. 312, 2701–2711 [DOI] [PubMed] [Google Scholar]
- 6.Garg A., Peshock R. M., Fleckenstein J. L. (1999) J. Clin. Endocrinol. Metab. 84, 170–174 [DOI] [PubMed] [Google Scholar]
- 7.Simha V., Garg A. (2002) J. Clin. Endocrinol. Metab. 87, 776–785 [DOI] [PubMed] [Google Scholar]
- 8.Capeau J., Magré J., Lascols O., Caron M., Béréziat V., Vigouroux C., Bastard J. P. (2005) Biochem. Soc. Trans. 33, 1073–1077 [DOI] [PubMed] [Google Scholar]
- 9.Reue K., Phan J. (2006) Curr. Opin. Clin. Nutr. Metab. Care 9, 436–441 [DOI] [PubMed] [Google Scholar]
- 10.Hegele R. A., Joy T. R., Al-Attar S. A., Rutt B. K. (2007) J. Lipid Res. 48, 1433–1444 [DOI] [PubMed] [Google Scholar]
- 11.Abbatecola A. M., Paolisso G. (2008) Curr. Pharm. Des. 14, 405–410 [DOI] [PubMed] [Google Scholar]
- 12.Rosen E. D., Spiegelman B. M. (2006) Nature 444, 847–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Herpen N. A., Schrauwen-Hinderling V. B. (2008) Physiol. Behav. 94, 231–241 [DOI] [PubMed] [Google Scholar]
- 14.Yang X., Jansson P. A., Nagaev I., Jack M. M., Carvalho E., Sunnerhagen K. S., Cam M. C., Cushman S. W., Smith U. (2004) Biochem. Biophys. Res. Commun. 317, 1045–1051 [DOI] [PubMed] [Google Scholar]
- 15.Aguiari P., Leo S., Zavan B., Vindigni V., Rimessi A., Bianchi K., Franzin C., Cortivo R., Rossato M., Vettor R., Abatangelo G., Pozzan T., Pinton P., Rizzuto R. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 1226–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornelius P., MacDougald O. A., Lane M. D. (1994) Annu. Rev. Nutr. 14, 99–129 [DOI] [PubMed] [Google Scholar]
- 17.Atanassova P. K. (2003) Folia Med. (Plovdiv) 45, 31–35 [PubMed] [Google Scholar]
- 18.Liu J., DeYoung S. M., Zhang M., Zhang M., Cheng A., Saltiel A. R. (2005) Cell Metab. 2, 165–177 [DOI] [PubMed] [Google Scholar]
- 19.Barresi R., Campbell K. P. (2006) J. Cell Sci. 119, 199–207 [DOI] [PubMed] [Google Scholar]
- 20.Kanagawa M., Toda T. (2006) J. Hum. Genet. 51, 915–926 [DOI] [PubMed] [Google Scholar]
- 21.Gregoire F. M., Smas C. M., Sul H. S. (1998) Physiol. Rev. 78, 783–809 [DOI] [PubMed] [Google Scholar]
- 22.Sordella R., Jiang W., Chen G. C., Curto M., Settleman J. (2003) Cell 113, 147–158 [DOI] [PubMed] [Google Scholar]
- 23.Tomas E., Kelly M., Xiang X., Tsao T. S., Keller C., Keller P., Luo Z., Lodish H., Saha A. K., Unger R., Ruderman N. B. (2004) Proc. Nutr. Soc. 63, 381–385 [DOI] [PubMed] [Google Scholar]
- 24.Durbeej M., Cohn R. D., Hrstka R. F., Moore S. A., Allamand V., Davidson B. L., Williamson R. A., Campbell K. P. (2000) Mol. Cell 5, 141–151 [DOI] [PubMed] [Google Scholar]
- 25.Hertzel A. V., Sanders M. A., Bernlohr D. A. (2000) J. Lipid Res. 41, 1082–1086 [PubMed] [Google Scholar]
- 26.Carpéné C. (2001) Methods Mol. Biol. 155, 129–140 [DOI] [PubMed] [Google Scholar]
- 27.Durbeej M., Campbell K. P. (1999) J. Biol. Chem. 274, 26609–26616 [DOI] [PubMed] [Google Scholar]
- 28.Bastie C. C., Zong H., Xu J., Busa B., Judex S., Kurland I. J., Pessin J. E. (2007) Cell Metab. 5, 371–381 [DOI] [PubMed] [Google Scholar]
- 29.Grewal P. K., Holzfeind P. J., Bittner R. E., Hewitt J. E. (2001) Nat. Genet. 28, 151–154 [DOI] [PubMed] [Google Scholar]
- 30.Michele D. E., Barresi R., Kanagawa M., Saito F., Cohn R. D., Satz J. S., Dollar J., Nishino I., Kelley R. I., Somer H., Straub V., Mathews K. D., Moore S. A., Campbell K. P. (2002) Nature 418, 417–422 [DOI] [PubMed] [Google Scholar]
- 31.Henegar C., Tordjman J., Achard V., Lacasa D., Cremer I., Guerre-Millo M., Poitou C., Basdevant A., Stich V., Viguerie N., Langin D., Bedossa P., Zucker J. D., Clement K. (2008) Genome Biol. 9, R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Durbeej M., Campbell K. P. (2002) Curr. Opin. Genet. Dev. 12, 349–361 [DOI] [PubMed] [Google Scholar]
- 33.Barresi R., Moore S. A., Stolle C. A., Mendell J. R., Campbell K. P.49 (2000) J. Biol. Chem. 275, 38554–38560 [DOI] [PubMed] [Google Scholar]
- 34.Ozawa E., Mizuno Y., Hagiwara Y., Sasaoka T., Yoshida M. (2005) Muscle Nerve 32, 563–576 [DOI] [PubMed] [Google Scholar]
- 35.Shi W., Chen Z., Schottenfeld J., Stahl R. C., Kunkel L. M., Chan Y. M. (2004) Muscle Nerve 29, 409–419 [DOI] [PubMed] [Google Scholar]
- 36.Shiga K., Yoshioka H., Matsumiya T., Kimura I., Takeda S., Imamura M. (2006) Exp. Cell Res. 312, 2083–2092 [DOI] [PubMed] [Google Scholar]
- 37.Crosbie R. H., Lim L. E., Moore S. A., Hirano M., Hays A. P., Maybaum S. W., Collin H., Dovico S. A., Stolle C. A., Fardeau M., Tomé F. M., Campbell K. P.13 (2000) Hum. Mol. Genet. 9, 2019–2027 [DOI] [PubMed] [Google Scholar]
- 38.Fischbach C., Spruss T., Weiser B., Neubauer M., Becker C., Hacker M., Göpferich A., Blunk T. (2004) Exp. Cell Res. 300, 54–64 [DOI] [PubMed] [Google Scholar]
- 39.Piasecki J. H., Moreno K., Gutowski K. A. (2008) Aesthet. Surg. J. 28, 306–312 [DOI] [PubMed] [Google Scholar]
- 40.Mojallal A., Lequeux C., Auxenfans C., Braye F., Damour O. (2008) Biomed. Mater. Eng. 18, 187–192 [PubMed] [Google Scholar]
- 41.Blüher M. (2005) Best Pract. Res. Clin. Endocrinol Metab 19, 605–623 [DOI] [PubMed] [Google Scholar]
- 42.Asterholm I. W., Halberg N., Scherer P. E. (2007) Drug. Discov. Today Dis. Models 4, 17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Deutekom J. C., Bremmer-Bout M., Janson A. A., Ginjaar I. B., Baas F., den Dunnen J. T., van Ommen G. J. (2001) Hum. Mol. Genet. 10, 1547–1554 [DOI] [PubMed] [Google Scholar]
- 44.Davies K. E., Grounds M. D. (2006) Cell 127, 1304–1306 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.