Abstract
Recent studies have indicated that direct intestinal secretion of plasma cholesterol significantly contributes to fecal neutral sterol loss in mice. The physiological relevance of this novel route, which represents a part of the reverse cholesterol transport pathway, has not been directly established in vivo as yet. We have developed a method to quantify the fractional and absolute contributions of several cholesterol fluxes to total fecal neutral sterol loss in vivo in mice, by assessing the kinetics of orally and intravenously administered stable isotopically labeled cholesterol combined with an isotopic approach to assess the fate of de novo synthesized cholesterol. Our results show that trans-intestinal cholesterol excretion significantly contributes to removal of blood-derived free cholesterol in C57Bl6/J mice (33% of 231 μmol/kg/day) and that pharmacological activation of LXR with T0901317 strongly stimulates this pathway (63% of 706 μmol/kg/day). Trans-intestinal cholesterol excretion is impaired in mice lacking Abcg5 (−4%), suggesting that the cholesterol transporting Abcg5/Abcg8 heterodimer is involved in this pathway. Our data demonstrate that intestinal excretion represents a quantitatively important route for fecal removal of neutral sterols independent of biliary secretion in mice. This pathway is sensitive to pharmacological activation of the LXR system. These data support the concept that the intestine substantially contributes to reverse cholesterol transport.
Reverse cholesterol transport (RCT)3 is defined as the flux of excess cholesterol from peripheral tissues toward the liver followed by biliary secretion and subsequent disposal via the feces (1). Accumulation of cholesterol in macrophages in the vessel wall is considered a primary event in the development of atherosclerosis and, therefore, removal of excess cholesterol from these cells is of crucial importance for prevention and/or treatment of atherosclerotic cardiovascular diseases. It is generally accepted that HDL is the obligate transport vehicle in RCT and that plasma HDL levels reflect the capacity to accommodate this flux. In line herewith, HDL-raising therapies are currently considered as a promising strategy for prevention and treatment of atherosclerotic cardiovascular diseases (2). In the “classical” scenario, the liver has a central role in RCT (3). Biliary secretion of free cholesterol, facilitated by the heterodimeric ABC-transporter ABCG5/ABCG8 (4), and hepatic conversion of cholesterol into bile acids followed by fecal excretion are referred to as the main routes for quantitatively important elimination of cholesterol from the body. Fecal excretion of sterols is stimulated upon whole body activation of the liver X receptor (LXR, NR1H2/3), a member of the nuclear receptor family for which oxysterols have been identified as natural ligands (5). LXR regulates expression of several genes involved in RCT and activation of LXR by synthetic agonists leads to elevated plasma HDL-cholesterol levels, increased hepatobiliary cholesterol secretion, reduced fractional intestinal cholesterol absorption and increased fecal sterol loss (6). LXR is thus considered an attractive target for therapeutic strategies aimed at stimulation of RCT, which, however, will require approaches to circumvent potential detrimental consequences of LXR activation such as induction of lipogenesis.
Recent studies indicate that the classical concept of RCT may require reconsideration. Studies in apoA-I-deficient mice revealed that the magnitude of the centripetal cholesterol flux from the periphery to the liver is not related to the concentration of HDL-cholesterol or apoA-I in plasma (7). Furthermore, Abca1−/− mice that completely lack plasma HDL show unaffected rates of hepatobiliary cholesterol secretion and fecal sterol loss (8). Additionally, mice lacking both Abcg5 and Abcg8 do not show a reduction in fecal neutral sterol excretion to the extent expected on the basis of their strongly reduced hepatobiliary cholesterol secretion (9). Recent studies by Plösch et al. (6) have revealed that increased fecal neutral sterol loss upon general LXR activation cannot be attributed to the increased hepatobiliary cholesterol secretion only, suggesting a major contribution of the intestine in excretion of cholesterol. This potential role of the intestine in cholesterol removal from the body has been corroborated by Kruit et al. (10), who showed that fecal sterol loss is not affected in Mdr2−/− (Abcb4−/−) mice that have a dramatic reduction in biliary cholesterol secretion (11). Moreover, intravenously administered [3H]cholesterol could be recovered in the neutral sterol fraction of the feces in these mice and fecal excretion of neutral sterols was stimulated upon treatment with an LXR agonist (10). However, the exact quantitative contribution of the direct intestinal pathway under physiological conditions has not directly been determined so far. Very recently, intestinal perfusion studies in mice revealed that, in the presence of mixed micelles as cholesterol acceptors in the intestinal lumen, murine enterocytes indeed have a high capacity to secrete cholesterol via a specific process that is most active in the proximal part of the small intestine (12). In addition, it was shown that direct trans-intestinal cholesterol excretion (TICE) could be stimulated by a high fat diet. The existence of a non-biliary route for fecal neutral sterol excretion is further supported by very recent studies by Brown et al. (13) in mice with targeted deletion of hepatic ACAT2.
The present study provides insight into the relative and absolute contributions of several cholesterol fluxes relevant to total fecal sterol loss in mice, making use of a panel of stable isotope tracers. Our results show that TICE is a major route for removal of blood-derived free cholesterol and that pharmacological LXR activation strongly stimulates this arm of the reverse cholesterol transport pathway.
MATERIALS AND METHODS
Animals
Male C57Bl/6J mice (Charles River, L'Arbresle Cedex, France) as well as male Abcg5−/− mice and their wild-type littermates (14) were kept in a light- and temperature-controlled environment and fed a standard rodent diet (RMH-B, Abdiets, Woerden, The Netherlands) and water ad libitum. All experimental procedures were approved by the local Ethical Committee for Animal Experiments of the University of Groningen. For the experiments with triglyceride (TG)-rich particles, C57Bl/6J mice were fed a standard rodent diet. The procedures for these experiments were approved by the Ethical Committee for Animal Experiments of the Academic Medical Center (University of Amsterdam).
Preparation of Emulsion Particles
TG-rich emulsion particles (80-nm sized) were prepared according to the sonication and ultracentrifugation procedure of Redgrave and Maranhao (15) as modified by Rensen et al. (16). Briefly, a mixture of 100 mg of total lipid was dispersed in NaCl buffer of density 1.10 g/ml. The lipid mixture consisted of triolein (∼99%), egg yolk phosphatidylcholine, (99%), l-α-lysophosphatidylcholine (99%), cholesteryl oleate, cholesterol (>99%; Sigma-Aldrich) and at a weight ratio of, respectively, 70.0:22.7:2.3:3.0:2.0. In addition, 80 μCi of [3H]cholesteryl oleate ([3H]CO) or [3H]cholesterol oleoyl ether ([3H]COEth; Amersham Biosciences) was added to the lipid mixture. After sonification for 30 min at 54 °C, the particles were obtained via density gradient ultracentrifugation.
Hepatic and Intestinal Uptake
Mice were anaesthesized intraperitoneal with 100 μl of FFD (Hypnorm (1 ml/kg) and diazepam (10 mg/kg)) per 5 g of body weight and received radiolabeled TG-rich particles (2 μCi/mouse) via tail vein injection. Bile was diverted via cannulation of the bile duct via the gallbladder. Plasma samples were collected through tail-bleeding at 1, 10, and 30 min after injection. Subsequently, after 3 h, mice were sacrificed, and liver and intestine were collected. The total amount of radioactivity in the plasma was calculated based on the estimated total plasma volume (4.5% of body weight, Refs. 17, 18). To determine liver uptake, the livers were weighed, and tissue samples were treated with soluene-350 (PerkinElmer Life Sciences). Total cholesterol in intestine samples was extracted using the Bligh and Dyer method (19), and its concentration was determined with a kit from Biomerieux. For all samples, radioactivity was determined using liquid scintillation counter.
Experimental Procedures
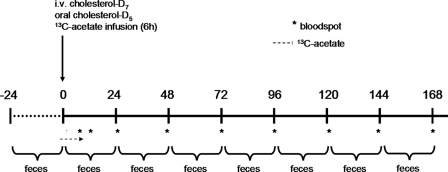
C57Bl6/J mice were fed either standard laboratory chow or chow supplemented with the LXR agonist T0901317 (0.015%, w/w; Cayman Chemicals, Ann Arbor, MI) from 3 days before the onset of experiments (see below) until termination of the experiments at day 8. Abcg5−/− mice and their littermate controls were fed standard laboratory chow only. Mice were fitted with a permanent catheter in the heart via the right jugular vein as previously described (20) and were allowed to recover from surgery for at least 4 days. Mice received an intravenous dose of 0.3 mg (0.763 μmol) cholesterol-D7 dissolved in Intralipid® (20%, Fresenius Kabi, Den Bosch, The Netherlands) and an oral dose of 0.6 mg (1.535 μmol) cholesterol-D5 dissolved in medium chain triglyceride oil. Immediately thereafter, mice received a constant intravenous infusion of [1-13C]acetate (Isotec, Miamisburg, OH) at a rate of 0.24 mmol/h for 6 h. The start of the experiment (t = 0) was defined by the start of [1-13C]acetate infusion (at 8:00 pm). Blood spots were collected from the tail on filter paper before administration of labeled cholesterol and acetate and at hourly intervals during the infusion period. After the infusion period, mice were individually housed for 8 days during which feces and blood spots were collected every 24 h. At the end of the experiment, mice were sacrificed by cardiac puncture and livers and small intestines were excised. A schematic representation of the administration and sampling protocol is given in Fig. 1.
FIGURE 1.
Schematic representation of the administration and sampling protocol. The asterisk indicates sampling of bloodspots. The vertical arrow indicates the time point at which cholesterol-D7 and cholesterol-D5 are administered immediately followed by the start of [1-13C]acetate infusion. The horizontal arrow indicates the period of [1-13C]acetate infusion.
Separate groups of animals (n = 6) received an intravenous dose of labeled cholesterol as described above. At 24 h after administration, mice were anesthetized by intraperitoneal injection with Hypnorm (fentanyl/fluanisone, 1 ml/kg) and Diazepam (10 mg/kg). Bile was collected by cannulation of the gallbladder for 30 min, during which body temperature was stabilized using a humidified incubator.
Analytical Procedure
Cholesterol was extracted from blood spots with 1 ml of 95% ethanol/acetone (1:1, v/v) for gas chromatography/mass spectrometric (GC/MS) analysis according to Neese et al. (21). Biliary lipids were extracted according to Bligh and Dyer (19). Unesterified cholesterol from both blood spots and bile was derivatized using N,O-bis-(trimethyl)trifluoroacetamide with 1% trimethylchlorosilane at room temperature. Fecal neutral sterols were extracted as described by Arca et al. (22) and were derivatized using N,O-bis-(trimethyl)trifluoroacetamide/pyridine (1:1 v/v) with 1% trimethylchlorosilane at room temperature. Total biliary and plasma concentrations of cholesterol were determined (6). For Abcg5−/− mice and their wild-type littermates, plasma cholesterol concentrations were determined by gas chromatography (23). Enrichments of fecal neutral sterols were measured in the cholesterol fraction, whereas total fecal neutral sterols were determined as the sum of fecal cholesterol and its bacterial metabolites, which were assumed to have similar specific enrichments as cholesterol. GC/MS measurement of mass isotopomer distribution is described under supplemental Materials and Methods.
Calculations of Kinetic Parameters
From the decay curves of iv-administered D7-cholesterol in blood spots, several kinetic parameters were calculated. Equations used for these calculations are given under supplemental Materials and Methods.
Fractional Cholesterol Absorption Measurement
Fractional cholesterol absorption was measured using an adapted plasma dual isotope ratio method (24) using blood spots obtained at 72 h after intravenous and oral administration of stable isotopically labeled cholesterol (25) (equation given in supplemental Materials and Methods).
Mass Isotopomer Distribution Analysis (MIDA)
To determine de novo cholesterol synthesis, the MIDA approach was used. The theoretical background of this technique has been described in detail elsewhere (21, 26, 27). Calculation of the synthesis rate of cholesterol is explained in supplemental Materials and Methods.
Determination of the Sources of Fecal Neutral Sterols
To calculate fecal excretion of blood-derived cholesterol, we adapted the method described for use in humans by Férézou et al. (28). For this calculation, we measured the mean enrichment of cholesterol-D7 in feces from day 2–5 of the experiment, which was divided by the enrichment of cholesterol-D7 in blood spots 24 h before the midpoint of the feces collection (i.e. day 2), to take into account the intestinal transit.
To determine the fraction of biliary cholesterol that is derived from the blood compartment, we measured the enrichment of intravenously administered cholesterol-D7 in blood spots and bile samples at 24 h after administration. The ratio of these enrichments represents the fraction of biliary cholesterol that is derived from the blood compartment. The remaining fraction of biliary cholesterol consists of cholesterol excreted from the liver without having entered the circulation and is assumed to be a newly synthesized fraction. Correcting these values for the fractional cholesterol (re)absorption gives the amount of blood-derived cholesterol that is excreted via the biliary pathway. The difference between total blood-derived cholesterol in the feces and the fecal blood-derived cholesterol secreted via bile gives the amount of cholesterol in the feces that is excreted from the blood compartment directly into the intestinal lumen.
Enrichments of newly synthesized cholesterol in blood-spots and feces, combined with the calculations mentioned above, enabled us to calculate the amounts of newly synthesized cholesterol excreted into feces from different sources, i.e. from the blood compartment, directly from the liver or directly from enterocytes.
Based on the dietary intake of cholesterol and values of fractional cholesterol absorption, the mass of cholesterol that is not absorbed and eventually ends up in the feces can be calculated. The fraction of total fecal neutral sterol content that is not excreted via one of the above mentioned pathways must be derived from shedding of enterocytes or other sources.
RNA Isolation and Measurement of mRNA Levels by Quantitative Real-time PCR
RNA isolation, cDNA synthesis, and real-time quantitative PCR were performed as described by Plösch et al. (6). PCR results of liver and intestine were normalized to β-actin mRNA levels. Primer and probe sequences are listed in supplemental Table S1.
Statistics
The statistical significance was assessed by using the Mann-Whitney-U test. The level of significance was set at p < 0.05. Analyses were performed using SPSS version 12 for Windows software (SPSS, Chicago, IL).
RESULTS
TG-rich Particles Are Rapidly Taken Up by the Liver but Not by the Intestine
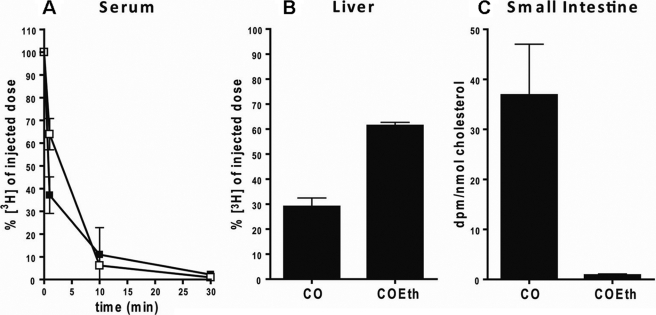
To investigate the fate of cholesterol in intravenous injected TG-rich particles, they were labeled with non-degradable [3H]cholesterol-oleoyl-ether or [3H]cholesterol-oleate. Upon intravenous injection, the TG-rich particles were rapidly cleared from the circulation with a half-life of less than 5 min (Fig. 2A). As has been reported before (16), over 60% of these TG-rich particles are taken up by the liver as is demonstrated here by the percentage of the non-hydrolyzable [3H]COEth of injected dose found in the liver (Fig. 2B). After uptake of TG-rich particles labeled with [3H]CO by the liver, [3H]cholesterol can be liberated by hydrolysis and released back into circulation. Hence, less activity was recovered in the liver compared with animals treated with [3H]COEth-containing particles. Part of this re-distributed [3H]cholesterol derived from [3H]CO is taken up by the intestine (Fig. 2C). In contrast, almost no [3H]COEth activity could be detected in the intestine, indicating that these TG-rich particles are not taken up directly.
FIGURE 2.
Plasma decay (A), hepatic uptake (B), and small intestinal uptake (C) of emulsions. [3H]CO (■)- or [3H]COEt (□)-labeled TG-rich particles were injected into mice (n = 4). The plasma decay was determined by taking plasma samples at 1, 10, and 30 min. After three and a half hours, the animals were sacrificed, and radioactivity was determined in the liver and intestine. Values are means ± S.D.
LXR Activation Increases Pool Size and Turnover of the Free Cholesterol Pool, while Abcg5 Deficiency Has the Opposite Effects
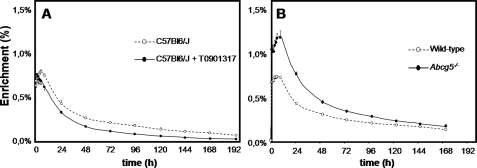
Plasma cholesterol turnover was studied by injecting cholesterol-D7 in intralipid intravenously. As shown above, triglyceride-rich particles are rapidly taken up in the liver (29) and subsequently, the labeled cholesterol is resecreted into the circulation. Fig. 3, A and B show the mean curves of the fractional enrichments in blood spots of intravenously administered cholesterol-D7. For each individual mouse, kinetic parameters of cholesterol turnover were estimated by curve-fitting using SAAMII software (Table 1). In accordance with previous studies (14, 30), treatment with T0901317 increased plasma cholesterol concentrations because of elevation of plasma HDL-cholesterol (30), whereas these concentrations were lower in Abcg5-deficient mice compared with their controls. The apparent volume of distribution of cholesterol-D7 was reduced upon LXR activation, and the total rapidly exchangeable unesterified cholesterol pool, consisting of plasma erythrocyte- and hepatic-free cholesterol, was increased by 20%. The absence of Abcg5 had opposite effects, i.e. was associated with a markedly increased volume of distribution and a 28% decrease in pool size. Importantly, the calculated pool sizes are in the same order of magnitude as the estimation proposed by Neese et al. (21). The turnover of plasma cholesterol was higher in mice treated with T0901317. This increase was mainly ascribed to an increase in R2, which represents disposal of cholesterol. The combination of an increased turnover and elevated plasma cholesterol concentration led to a reduced metabolic clearance rate upon LXR activation. In mice lacking Abcg5, cholesterol turnover was lower than in their wild-type littermates. Combined with the lower plasma cholesterol levels, this led to similar metabolic clearance rates in mice of both genotypes. All kinetic parameters showed comparable values in both control groups, i.e. untreated C57Bl/6J mice and the wild-type littermates of Abcg5−/− mice.
FIGURE 3.
Fractional enrichment of free cholesterol in blood spots upon intravenous administration of cholesterol-D7 during the course of the experiments in untreated (open circles) and T0901317-treated (filled circles) C57Bl6/J mice (A) and in wild-type (open circles) and Abcg5−/− (filled circles) mice (B). n = 5–6 per group. Values represent means ± S.E.
TABLE 1.
Kinetic parameters of cholesterol
Kinetic parameters of cholesterol were calculated by curve-fitting of the decay curves of D7-cholesterol using SAAM II. Values are expressed as means ± S.D. (n = 5 or 6 per group). Abbreviations: Ctot, total plasma cholesterol concentration; CIV, concentration of administered D7-cholesterol in blood; DIV, dose of intravenously administered cholesterol; V, volume of distribution; Atot, total rapidly exchangeable free cholesterol pool; t½, half-life time; R, turnover rate; MCR, metabolic clearance rate.
| Parameter | C57Bl6/J | T0901317 | Abcg5+/+ | Abcg5−/− |
|---|---|---|---|---|
| Bodyweight (g) | 26.9 ± 0.4 | 25.8 ± 0.8 | 26.0 ± 0.8 | 24.8 ± 0.3 |
| Ctot (μmol/l) | 2281 ± 254 | 4730 ± 192a | 2522 ± 136 | 1153 ± 72a |
| CIV (μmol/l) | 26.3 ± 3.9 | 46.5 ± 2.1a | 27.0 ± 1.4 | 17.9 ± 1.3a |
| DIV (μmol/kg) | 11.8 ± 0.2 | 12.4 ± 0.4 | 12.3 ± 0.4 | 12.9 ± 0.2 |
| V (l/kg) | 0.487 ± 0.065 | 0.268 ± 0.011a | 0.464 ± 0.036 | 0.733 ± 0.058a |
| Atot (μmol/kg) | 1046 ± 39 | 1262 ± 34a | 1148 ± 31 | 831 ± 37a |
| t½(1) (h) | 10.5 ± 0.7 | 10.6 ± 0.4 | 10.2 ± 0.0 | 14.7 ± 0.5a |
| t½ (2) (h) | 94.4 ± 9.0 | 59.1 ± 0.8a | 136.3 ± 4.5 | 110.0 ± 6.8 |
| R1 (μmol/kg/h) | 69.7 ± 4.5 | 82.8 ± 2.3 | 77.7 ± 2.1 | 39.4 ± 2.2a |
| R2 (μmol/kg/h) | 8.0 ± 0.9 | 14.8 ± 0.3a | 5.9 ± 0.3 | 5.3 ± 0.2 |
| Rtot (μmol/kg/h) | 77.7 ± 5.0 | 97.6 ± 2.4a | 83.6 ± 3.2 | 44.7 ± 2.1a |
| MCR (l/kg/h) | 0.035 ± 0.004 | 0.021 ± 0.000a | 0.034 ± 0.003 | 0.040 ± 0.004 |
a Significant difference between treated and non-treated C57Bl6/J mice or between wild-type and Abcg5−/− mice.
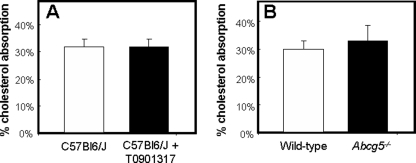
No Effect of LXR Activation or Abcg5 Deficiency on Fractional Cholesterol Absorption Assessed by the Plasma Dual Isotope Technique
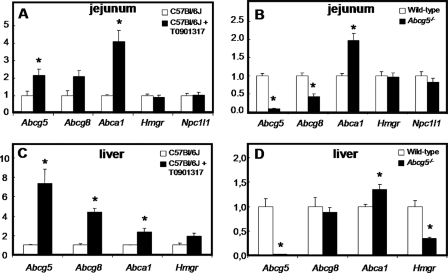
To calculate fractional cholesterol absorption (31), we used the ratio in blood spots of the orally and intravenously administered labels (cholesterol-D5 and -D7) at t = 72 h. Although intestinal expression levels of Abcg5 and Abcg8 were increased upon treatment with T0901317 and were absent or reduced, respectively, in Abcg5−/− mice (Fig. 4, A and B), neither LXR activation by T0901317 nor the absence of Abcg5 affected fractional intestinal cholesterol absorption in mice fed a standard chow (Fig. 5). Corresponding to the unchanged cholesterol absorption rate, intestinal expression of Npc1l1 was unaffected by either T0901317 treatment or Abcg5 deficiency (Fig. 4A). For mice treated with T0901317, this unchanged absorption was in contrast to previous studies in which the fecal dual isotope ratio methodology was used (10, 14, 32). Calculation of the absolute amounts of cholesterol being absorbed, based on a dietary cholesterol intake of 58 μmol/kg/day and the actually measured biliary secretion rates (see Table 2), revealed that C57Bl6/J mice treated with T0901317 actually absorbed ∼44% more cholesterol than their untreated controls (54.1 ± 6.84 μmol/kg/day versus 37.7 ± 3.68 μmol/kg/day, respectively). On the other hand, Abcg5−/− mice absorbed ∼15% less cholesterol than their wild-type littermates (20.8 ± 0.73 μmol/kg/day versus 24.6 ± 1.24 μmol/kg/day, respectively). These differences in the absolute amounts of absorbed cholesterol were attributable to differences in hepatobiliary cholesterol secretion rates. As expected (6), hepatic expression of Abcg5 and Abcg8 was strongly increased in mice treated with T0901317 (Fig. 4, C and D), leading to increased hepatobiliary secretion of cholesterol in these mice. It should be noted that hepatobiliary cholesterol secretion rates were significantly higher in control C57Bl6/J mice (57.4 ± 13.0 μmol/kg/day) compared with the wild-type littermates of Abcg5−/− mice with a mixed background (27.9 ± 3.26 μmol/kg/day).
FIGURE 4.
Intestinal (A and B) and hepatic (C and D) gene expression levels in untreated (open bars) and T0901317-treated (filled bars) C57Bl6/J mice (A and C) and in wild-type (open bars) and Abcg5−/− (filled bars) mice (B and D), measured by real-time PCR. mRNA was prepared form individual mice (n = 5–6 per group), and data are presented as means of 5–6 animals performed in duplicate ± S.E. Expression values are normalized to β-actin, and expression in untreated C57Bl6/J and Abcg5+/+ mice were both set at 1.00. The asterisk indicates significant differences (Mann-Whitney-U test, p < 0.05). Abcg5/g8/a1, Abc-transporter g5, g8, and a1; Hmgr, 3-hydroxy-3-methylglutaryl-coenzyme A reductase.
FIGURE 5.
Fractional cholesterol absorption in untreated (open bars) and T0901317-treated (filled bars) C57Bl6/J mice (A) and in wild-type (open bars) and Abcg5−/− (filled bars) mice (B). Fractional cholesterol absorption was measured using the adapted plasma dual isotope method making use of blood spots collected on filter paper (n = 5–6 per group). Mice received an intravenous injection of cholesterol-D7 and an oral dose of cholesterol-D5. Blood spots obtained 72 h after administration were used for the calculation of fractional cholesterol absorption. Values represent means ± S.E.
TABLE 2.
Secretion rates and enrichments of biliary cholesterol
Secretion rates and enrichments of cholesterol were measured in treated and non-treated C57Bl/6J mice and in wild-type and Abcg5−/− mice. Values are expressed as means ± S.D. (n = 5–7 per group).
| Parameter | C57Bl6/J | T0901317 | Abcg5+/+ | Abcg5−/− |
|---|---|---|---|---|
| Biliary cholesterol(μmol/kg/day) | 57.4 ± 13.0 | 108 ± 21.6 | 27.9 ± 3.26a | 7.61 ± 1.28b |
| f (D7) blood spot (%) | 0.458 ± 0.051 | 0.400 ± 0.040 | 0.469 ± 0.020 | 0.672 ± 0.047 |
| f (D7) bile (%) | 0.474 ± 0.058 | 0.398 ± 0.035 | 0.431 ± 0.015 | 0.503 ± 0.052 |
| Bile/blood spot | 1.03 ± 0.04 | 1.00 ± 0.02 | 0.92 ± 0.01 | 0.75 ± 0.05b |
a Significant difference between untreated C57Bl6/J mice and wild-type littermates of Abcg5−/− mice.
b Significant difference between treated and non-treated C57Bl6/J mice or between wild-type and Abcg5−/− mice.
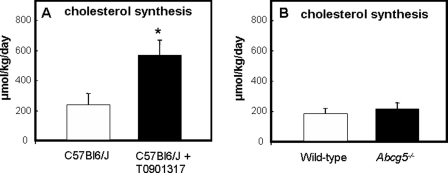
Activation of LXR Stimulates de Novo Cholesterol Synthesis in Mice
To quantify cholesterol synthesis in vivo, we infused [1-13C]acetate and calculated the fractional and absolute cholesterol synthesis rates employing MIDA. We observed a 2.5-fold increase in de novo cholesterol synthesis, which was secreted into plasma upon treatment with T0901317 (236 ± 75.8 μmol/kg/day in controls versus 568 ± 103 μmol/kg/day in T0901317-treated mice, Fig. 6A), corresponding to a 2-fold increase in hepatic mRNA levels of Hmgr in these mice (Fig. 4C). There was no difference in cholesterol synthesis between Abcg5−/− mice and their wild-type littermates (217 ± 38.6 versus 183 ± 38.4 μmol/kg/day, respectively, Fig. 6B), although hepatic Hmgr expression was reduced in Abcg5−/− mice (Fig. 3D). Neither treatment with T0901317 nor the absence of Abcg5 affected the enrichments (p values) of the acetyl-CoA precursor pools from which de novo synthesized cholesterol was derived (all ∼8%, data not shown).
FIGURE 6.
de novo cholesterol synthesis rates in untreated (open bars) and T0901317-treated (filled bars) C57Bl6/J mice (A) and in wild-type (open bars) and Abcg5−/− (filled bars) mice (B). [1-13C]Acetate was infused, and the absolute cholesterol synthesis rates were calculated employing MIDA. Values represent means ± S.E. (n = 5–6 per group). The asterisk indicates significant differences (Mann-Whitney-U test, p < 0.05).
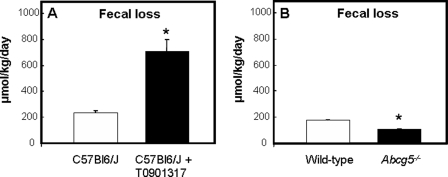
Direct Cholesterol Excretion via the Intestine Is Increased upon LXR Activation and Reduced in Abcg5−/− Mice
In accordance with previous studies (6), fecal excretion of neutral sterols, ∼80% of which comprised of cholesterol, was 3-fold increased upon activation of LXR (231 ± 18.9 μmol/kg/day in control versus 706 ± 90.0 μmol/kg/day in T0901317-treated mice, Fig. 7A). Fecal bile acid excretion was slightly increased (+25%) upon LXR activation (177 ± 14.7 μmol/kg/day in control versus 220 ± 53.4 μmol/kg/day in T0901317-treated mice, data not shown). Deficiency of Abcg5 led to a 39% reduction in fecal neutral sterol loss (175 ± 6.12 μmol/kg/day in control versus 106 ± 7.28 μmol/kg/day in Abcg5−/− mice, Fig. 7B). Fecal excretion of bile acids was slightly increased (+25%) in mice lacking Abcg5 (141 ± 29.2 μmol/kg/day in control versus 176 ± 26.5 μmol/kg/day in Abcg5−/− mice, data not shown).
FIGURE 7.
Fecal loss of neutral sterols in untreated (open bars) and T0901317-treated (filled bars) C57Bl6/J mice (A) and in wild-type (open bars) and Abcg5−/− (filled bars) mice (B). Feces were collected every 24 h during the course of the experiment. Values represent means ± S.E. (n = 5–6 per group). The asterisk indicates significant differences (Mann-whitney-U test, p < 0.05).
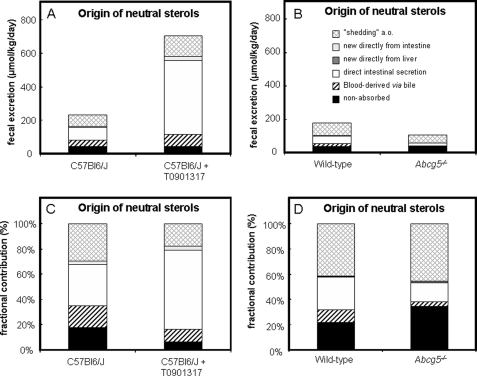
Fig. 8 summarizes the contribution of different sources of cholesterol to total fecal cholesterol output in both absolute (A and B) and relative values (C and D). Calculation of the different fractions revealed that fecal loss of cholesterol derived from the circulation was 49.8% (115 μmol/kg/day) in C57Bl6/J mice and increased to 73.0% (515 μmol/kg/day) upon LXR activation. In Abcg5−/− mice, this fraction was reduced to 18.7% (19.9 μmol/kg/day) compared with 35.7% (62.4 μmol/kg/day) in their wild-type controls. The portion of blood-derived fecal cholesterol that is delivered via the biliary pathway was increased after treatment with T0901317 (+88%; 39.02 μmol/kg/day in control versus 73.51 μmol/kg/day in T0901317-treated mice) and decreased in mice lacking Abcg5 (−79%; 17.94 μmol/kg/day in wild-type versus 3.82 μmol/kg/day in Abcg5−/− mice). In untreated and treated C57Bl6/J mice, there was no preferential secretion of newly synthesized cholesterol into bile, as indicated by the ratios of cholesterol-D7 enrichment in bile and blood spots approaching a value of 1 (Table 2). These ratios were 0.92 and 0.75 for wild-type and Abcg5−/− mice, respectively, indicating that ∼8 and ∼25% of biliary cholesterol was newly synthesized in the liver and directly secreted into bile. This direct secretion by the liver accounted for only 0.89% (1.56 μmol/kg/day) in wild-type and 1.20% (1.28 μmol/kg/day) in Abcg5−/− mice of total fecal neutral sterol loss. Comparably low values were obtained for direct excretion of newly synthesized cholesterol from the enterocyte into the intestinal lumen: 2.9% (6.65 μmol/kg/day) and 3.4% (24.1 μmol/kg/day) in non-treated and T0901317-treated C57Bl6/J mice, respectively, whereas this fraction was virtually absent in Abcg5−/− and their wild-type littermates. The most striking results were observed for trans-intestinal cholesterol excretion. This flux represents the movement of blood-derived cholesterol into the feces directly via the intestine. This flux accounted for 32.9% (76.0 μmol/kg/day) of total fecal sterol excretion in C57Bl6/J mice and was massively increased to 62.6% (442 μmol/kg/day) upon treatment with T0901317. In Abcg5−/− mice, the fecal contribution of this direct intestinal excretion pathway was reduced to 15.1% (16.1 μmol/kg/day) compared with 25.4% (44.5 μmol/kg/day) in their wild-type controls. The remaining part of total fecal neutral sterol excretion is likely derived from shedding of enterocytes, among others, and varied between 17.7–45.7% in the different groups.
FIGURE 8.
Fractional contribution of different sources of cholesterol to total fecal cholesterol output in untreated and T0901317-treated C57Bl6/J mice and Abcg5−/− mice and their wild-type littermates. Absolute values are presented in A and B and relative values in C and D.
DISCUSSION
The results of the current study enforce the notion that the classical concept of RCT, i.e. HDL-mediated transport of excess cholesterol from peripheral tissues to the liver followed by its hepatobiliary secretion and disposal into the feces, requires modification, at least for the situation in mice (6, 10, 12). The use of differentially labeled cholesterol molecules and [1-13C]acetate allowed us to determine the relative and absolute contributions of different cholesterol fluxes to total fecal neutral sterol loss. For this purpose, we have adapted the method described for use in humans by Férézou et al. (28) and combined this method with the MIDA approach (21, 27) to quantify de novo cholesterol synthesis. Using this kinetic approach, we were able to show that trans-intestinal excretion of blood-derived cholesterol strongly contributes to fecal neutral sterol loss, the obligatory end-point of RCT, in mice and that this process is sensitive to pharmacological interference.
Our data demonstrate that pharmacological activation of LXR increases the turnover of cholesterol in mice. T0901317-treated mice excreted more cholesterol and converted more cholesterol into bile acids or steroid hormones per day than their untreated controls. The absence of Abcg5 had opposite effects: turnover of free cholesterol was reduced compared with their wild-type littermates. Additionally, the volume of distribution was reduced upon treatment with T0901317, indicating that the uptake of cholesterol into tissues is lower in these mice.
In accordance with previous studies (14, 32), the absence of Abcg5 did not influence fractional intestinal cholesterol absorption. However, these earlier studies showed that activation of LXR by T0901317 reduced fractional intestinal cholesterol absorption (10, 14, 32), whereas the present study revealed no effect of this treatment. This discrepancy may be explained by the fact that in the aforementioned studies, fractional cholesterol absorption was determined using the fecal dual isotope method (33), while we made use of an adapted plasma dual isotope method. Using the fecal dual isotope method, the recovery in feces of labeled cholesterol relative to an unabsorbable plant sterol during 3–4 days after oral administration of the sterol mixture, reflects the fractional cholesterol absorption. However, labeled cholesterol that has been absorbed and subsequently excreted either via bile or directly via the intestine will be recovered in the feces as well. Especially in the situation of LXR activation by T0901317, in which the trans-intestinal excretion of cholesterol appears to be strongly stimulated, underestimation of absorption efficiency may be of major significance.
In line with our earlier work (6), pharmacological activation of LXR led to elevated plasma HDL-cholesterol levels and a 3-fold increase in fecal neutral sterol loss in C57Bl6/J mice, i.e. an increase by ∼475 μmol/kg/day. Additionally, we observed a 2.5-fold increase in cholesterol synthesis upon LXR activation, which means that, in absolute terms, T0901317-treated mice synthesized at least 332 μmol/kg/day cholesterol more than untreated controls did. It is of importance to note that this value is in the same order of magnitude as the increase in fecal sterol loss measured by independent methodology. Because dietary intake as well as fractional intestinal absorption was not affected upon treatment with T0901317, this means that body stores of cholesterol are maintained. Yet, it should be realized that cholesterol synthesis may occur in pools that do not equilibrate with plasma within the experimental timeframe. The increased de novo synthesis of cholesterol coincides with a ∼2-fold increase in hepatic expression of Hmgr, while no effects on intestinal Hmgr mRNA levels were found.
To address the proposed role of the intestine in RCT (6, 10, 12), we have developed a method to analyze the origin of fecal neutral sterols. To our opinion, the most important finding of this work is the major contribution of trans-intestinal excretion of blood-derived cholesterol to total fecal neutral sterol loss in the in vivo situation. In C57Bl6/J mice, this route accounted for 33% of fecal neutral sterol excretion, a value that is in the same order of magnitude previously estimated for FVB mice (∼20%) (10). Notably, this trans-intestinal cholesterol excretion was massively increased upon LXR activation. In T0901317-treated mice, this pathway contributed up to 63% percent of total fecal sterol loss, which means that in absolute terms 442 μmol/kg of cholesterol was excreted via this route every day. Obviously, the pathways involved in this process now need to be identified: the protein(s) that mediate this trans-intestinal cholesterol excretion are currently unknown. Presumably, the last step of this flux is in part mediated by Abcg5/Abcg8, because the presence of this transporter heterodimer is required for increased fecal neutral sterol excretion upon LXR activation (32). Yet Abcg5/Abcg8 are not fully responsible for TICE activity. In Abcg5−/− mice, a significant amount of TICE could still be measured. Interestingly, intestinal perfusion studies revealed no distinct effect of Abcg8 deletion on TICE (12). Differences in experimental set-up may be responsible for these deviating results. In the in vivo situation, the intestinal lumen contains a complex mixture of diet- and bile-derived lipids and bile salts (34). During the intestinal perfusions, however, a selected intestinal segment is isolated, rinsed with saline to remove food and bile components and then perfused with a perfusate containing taurocholate and phosphatidylcholine (12). Both the absence of food components and the distinct micellar composition of the perfusate may influence the excretory capacity of enterocytes. The question arises which transporter/receptor is responsible for uptake of cholesterol at the basolateral pole of the enterocyte. So far, the carrier for cholesterol in this process is not known. However, our data as well as previous studies (16) show that the majority of radiolabeled CO in TG-particles is cleared by the liver. Attenuated liver uptake of TG-rich particles in animals pretreated with lactoferrin, resulted in reduced serum decay. However this prolonged circulation of TG particles did not result in increased uptake of [3H]CO by the intestine (16). These results are indicative for no direct uptake TG particles by intestine. If the carrier for TICE would be HDL, one possible candidate could be SR-BI, which is implicated in bi-directional flux of cholesterol and phospholipids (35). SR-BI is localized both at the apical and basolateral membrane along the entire length, but mostly in the proximal part, of the small intestine (36). However, recent studies by van der Velde et al. (37) show no reduction of TICE in mice lacking SR-BI, suggesting that this transporter is not involved in trans-intestinal cholesterol excretion. Recently, Brown et al. (13) confirmed the existence of a non-biliary route for fecal sterol loss in mice with a targeted deletion of hepatic ACAT2. They observed that recovery of lipoprotein-associated [3H]cholesterol in intestinal wall and lumen was higher when lipoproteins were derived from mice with low levels of ACAT2 compared with when derived from control mice, while recovery of [3H]cholesterol in liver and bile was similar.
Cholesterol absorption by enterocytes has been shown to be mediated by a vesicular pathway involving Nieman-Pick C1 like 1 protein (38). Rab proteins maybe involved in vesicular cholesterol transport as well; Rab8a has been implicated in control of apical protein localization in intestinal cells (39), and Rab9 has been shown to modulate transport of cholesterol from the late endosomes to the trans-Golgi network (40). Overexpression of Rab9 in vitro induced transport of free cholesterol from late endosomes/lysosomes to the endoplasmic reticulum where cholesterol is esterified and also induced efflux of cellular sterols into the medium (41). We observed no change in expression of Rab8a but an increase (∼80%) in intestinal Rab9 expression was observed upon T0901317 administration (data not shown). This might indicate its involvement in induction of trans-intestinal cholesterol excretion.
In summary, this study describes a method to measure the fractional and absolute contributions of distinct cholesterol fluxes to total fecal neutral sterol loss in vivo and revealed that trans-intestinal cholesterol excretion is a major route for removal of blood-derived free cholesterol. Because stable isotopes are used, a simplified version of this method will also be suitable for studies in human subjects. This novel pathway appears to contribute significantly to the process of reverse cholesterol transport and to be sensitive to pharmacological manipulation, as demonstrated for the LXR agonist T0901317. Activation of LXR specifically aimed at the intestine could thus be an attractive approach to stimulate reverse cholesterol transport without inducing undesirable systemic side effects, such as hepatic steatosis (30).
Supplementary Material
Acknowledgment
We thank Dr. Patrick C. N. Rensen, Department of Endocrinology and Metabolic Diseases, Leiden University Medical Center, Leiden, The Netherlands, for the synthesis of TG-rich particles.
This work was supported by Grant 912-02-063 from the Netherlands Organization for Scientific Research.

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Materials and Methods,” equations, and Table S1.
- RCT
- reverse cholesterol transport
- Abcg5/g8/a1
- Abc-transporter g5, g8, and a1
- Hmgr
- 3-hydroxy-3-methylglutaryl-coenzyme A reductase
- LXR
- liver X receptor
- MIDA
- mass isotopomer distribution analysis
- TG
- triglyceride
- TICE
- trans-intestinal cholesterol excretion
- HDL
- high density lipoprotein.
REFERENCES
- 1.Fielding C. J., Fielding P. E. (1995) J. Lipid Res. 36, 211–228 [PubMed] [Google Scholar]
- 2.Rader D. J. (2007) Nat. Clin. Pract. Cardiovasc. Med. 4, 102–109 [DOI] [PubMed] [Google Scholar]
- 3.Dietschy J. M., Turley S. D., Spady D. K. (1993) J. Lipid Res. 34, 1637–1659 [PubMed] [Google Scholar]
- 4.Berge K. E., Tian H., Graf G. A., Yu L., Grishin N. V., Schultz J., Kwiterovich P., Shan B., Barnes R., Hobbs H. H. (2000) Science 290, 1771–1775 [DOI] [PubMed] [Google Scholar]
- 5.Janowski B. A., Willy P. J., Devi T. R., Falck J. R., Mangelsdorf D. J. (1996) Nature 383, 728–731 [DOI] [PubMed] [Google Scholar]
- 6.Plōsch T., Kok T., Bloks V. W., Smit M. J., Havinga R., Chimini G., Groen A. K., Kuipers F. (2002) J. Biol. Chem. 277, 33870–33877 [DOI] [PubMed] [Google Scholar]
- 7.Jolley C. D., Woollett L. A., Turley S. D., Dietschy J. M. (1998) J. Lipid Res. 39, 2143–2149 [PubMed] [Google Scholar]
- 8.Groen A. K., Bloks V. W., Bandsma R. H., Ottenhoff R., Chimini G., Kuipers F. (2001) J. Clin. Invest. 108, 843–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu L., Hammer R. E., Li-Hawkins J., Von Bergmann K., Lutjohann D., Cohen J. C., Hobbs H. H. (2002) Proc. Natl. Acad. Sci. U. S. A. 99, 16237–16242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kruit J. K., Plösch T., Havinga R., Boverhof R., Groot P. H., Groen A. K., Kuipers F. (2005) Gastroenterology 128, 147–156 [DOI] [PubMed] [Google Scholar]
- 11.Smit J. J., Schinkel A. H., Oude Elferink R. P., Groen A. K., Wagenaar E., van Deemter L., Mol C. A., Ottenhoff R., van der Lugt N. M., van Roon M. A., et al. (1993) Cell 75, 451–462 [DOI] [PubMed] [Google Scholar]
- 12.van der Velde A. E., Vrins C. L., van den Oever K., Kunne C., Oude Elferink R. P., Kuipers F., Groen A. K. (2007) Gastroenterology 133, 967–975 [DOI] [PubMed] [Google Scholar]
- 13.Brown J. M., Bell T. A., 3rd, Alger H. M., Sawyer J. K., Smith T. L., Kelley K., Shah R., Wilson M. D., Davis M. A., Lee R. G., Graham M. J., Crooke R. M., Rudel L. L. (2008) J. Biol. Chem. 283, 10522–10534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plösch T., Bloks V. W., Terasawa Y., Berdy S., Siegler K., Van Der Sluijs F., Kema I. P., Groen A. K., Shan B., Kuipers F., Schwarz M. (2004) Gastroenterology 126, 290–300 [DOI] [PubMed] [Google Scholar]
- 15.Redgrave T. G., Maranhao R. C. (1985) Biochim. Biophys. Acta 835, 104–112 [DOI] [PubMed] [Google Scholar]
- 16.Rensen P. C., van Dijk M. C., Havenaar E. C., Bijsterbosch M. K., Kruijt J. K., van Berkel T. J. (1995) Nat. Med. 1, 221–225 [DOI] [PubMed] [Google Scholar]
- 17.Herz J., Qiu S. Q., Oesterle A., DeSilva H. V., Shafi S., Havel R. J. (1995) Proc. Natl. Acad. Sci. U. S. A. 92, 4611–4615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rensen P. C., Herijgers N., Netscher M. H., Meskers S. C., van Eck M., van Berkel T. J. (1997) J. Lipid Res. 38, 1070–1084 [PubMed] [Google Scholar]
- 19.Bligh E. G., Dyer W. J. (1959) Can. J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 20.Kuipers F., Havinga R., Bosschieter H., Toorop G. P., Hindriks F. R., Vonk R. J. (1985) Gastroenterology 88, 403–411 [DOI] [PubMed] [Google Scholar]
- 21.Neese R. A., Faix D., Kletke C., Wu K., Wang A. C., Shackleton C. H., Hellerstein M. K. (1993) Am. J. Physiol. 264, E136–E147 [DOI] [PubMed] [Google Scholar]
- 22.Arca M., Montali A., Ciocca S., Angelico F., Cantafora A. (1983) J. Lipid Res. 24, 332–335 [PubMed] [Google Scholar]
- 23.Kuksis A., Myher J. J., Marai L., Little J. A., McArthur R. G., Roncari D. A. (1986) J. Chromatogr. 381, 1–12 [DOI] [PubMed] [Google Scholar]
- 24.Turley S. D., Herndon M. W., Dietschy J. M. (1994) J. Lipid Res. 35, 328–339 [PubMed] [Google Scholar]
- 25.Bosner M. S., Lange L. G., Stenson W. F., Ostlund R. E., Jr. (1999) J. Lipid Res. 40, 302–308 [PubMed] [Google Scholar]
- 26.Bandsma R. H., Stellaard F., Vonk R. J., Nagel G. T., Neese R. A., Hellerstein M. K., Kuipers F. (1998) Biochem. J. 329, 699–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hellerstein M. K., Neese R. A. (1992) Am. J. Physiol. 263, E988–E1001 [DOI] [PubMed] [Google Scholar]
- 28.Férézou J., Coste T., Chevallier F. (1981) Digestion 21, 232–243 [DOI] [PubMed] [Google Scholar]
- 29.Redgrave T. G., Vassiliou G. G., Callow M. J. (1987) Biochim. Biophys. Acta 921, 154–157 [DOI] [PubMed] [Google Scholar]
- 30.Grefhorst A., Elzinga B. M., Voshol P. J., Plösch T., Kok T., Bloks V. W., van der Sluijs F. H., Havekes L. M., Romijn J. A., Verkade H. J., Kuipers F. (2002) J. Biol. Chem. 277, 34182–34190 [DOI] [PubMed] [Google Scholar]
- 31.Zilversmit D. B., Hughes L. B. (1974) J. Lipid Res. 15, 465–473 [PubMed] [Google Scholar]
- 32.Yu L., York J., von Bergmann K., Lutjohann D., Cohen J. C., Hobbs H. H. (2003) J. Biol. Chem. 278, 15565–15570 [DOI] [PubMed] [Google Scholar]
- 33.Borgström B. (1968) J. Lipid Res. 9, 473–481 [PubMed] [Google Scholar]
- 34.Hernell O., Staggers J. E., Carey M. C. (1990) Biochemistry 29, 2041–2056 [DOI] [PubMed] [Google Scholar]
- 35.Ji Y., Jian B., Wang N., Sun Y., Moya M. L., Phillips M. C., Rothblat G. H., Swaney J. B., Tall A. R. (1997) J. Biol. Chem. 272, 20982–20985 [DOI] [PubMed] [Google Scholar]
- 36.Cai S. F., Kirby R. J., Howles P. N., Hui D. Y. (2001) J. Lipid Res. 42, 902–909 [PubMed] [Google Scholar]
- 37.van der Velde A. E., Vrins C. L., van den Oever K., Seemann I., Oude Elferink R. P., van Eck M., Kuipers F., Groen A. K. (2008) Am. J. Physiol. Gastrointest. Liver Physiol. 295, G203–G208 [DOI] [PubMed] [Google Scholar]
- 38.Davies J. P., Scott C., Oishi K., Liapis A., Ioannou Y. A. (2005) J. Biol. Chem. 280, 12710–12720 [DOI] [PubMed] [Google Scholar]
- 39.Sato T., Mushiake S., Kato Y., Sato K., Sato M., Takeda N., Ozono K., Miki K., Kubo Y., Tsuji A., Harada R., Harada A. (2007) Nature 448, 366–369 [DOI] [PubMed] [Google Scholar]
- 40.Novick P., Zerial M. (1997) Curr. Opin. Cell Biol. 9, 496–504 [DOI] [PubMed] [Google Scholar]
- 41.Narita K., Choudhury A., Dobrenis K., Sharma D. K., Holicky E. L., Marks D. L., Walkley S. U., Pagano R. E. (2005) Faseb J. 19, 1558–1560 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.