Abstract
Protein ubiquitylation is essential for many events linked to intracellular protein trafficking. Despite the significance of this process, the molecular mechanisms that govern the regulation of ubiquitylation remain largely unknown. Plasma membrane transporters are subjected to tightly regulated endocytosis, and ubiquitylation is a key signal at several stages of the endocytic pathway. The yeast monocarboxylate transporter Jen1 displays glucose-regulated endocytosis. We show here that casein kinase 1-dependent phosphorylation and HECT-ubiquitin ligase Rsp5-dependent ubiquitylation are required for Jen1 endocytosis. Ubiquitylation and endocytosis of Jen1 are induced within minutes in response to glucose addition. Jen1 is modified at the cell surface by oligo-ubiquitylation with ubiquitin-Lys63 linked chain(s), and Jen1-Lys338 is one of the target residues. Ubiquitin-Lys63-linked chain(s) are also required directly or indirectly to sort Jen1 into multivesicular bodies. Jen1 is one of the few examples for which ubiquitin-Lys63-linked chain(s) was shown to be required for correct trafficking at two stages of endocytosis: endocytic internalization and sorting at multivesicular bodies.
Ubiquitylation is one of the most prevalent protein post-translational modifications in eukaryotes. In addition to its role in promoting proteasomal degradation of target proteins, ubiquitylation has been shown to regulate multiple processes, including DNA repair, signaling, and intracellular trafficking. Ubiquitylation serves as a key signal mediating the internalization of plasma membrane receptors and transporters, followed by their intracellular transport and subsequent recycling or lysosomal/vacuolar degradation (1, 2). In Saccharomyces cerevisiae, transporters usually display both constitutive and accelerated endocytosis regulated by factors such as excess substrate, changes in nutrient availability, and stress conditions. Ubiquitylation of these cell surface proteins acts as a signal triggering their internalization (1). A single essential E34 ubiquitin ligase, Rsp5, has been implicated in the internalization of most, if not all, endocytosed proteins (3). Rsp5 is the unique member in S. cerevisiae of the HECT (homologous to E6AP COOH terminus)-ubiquitin ligases of the Nedd4/Rsp5 family (4). In a few cases, Rsp5-dependent cell surface ubiquitylation was shown to involve PY-containing adapters that bind to Rsp5 (5–7). Rsp5-mediated ubiquitylation is also required for sorting into multivesicular bodies (MVBs) of endosomal membrane proteins that come from either the plasma membrane (through endocytosis) or the Golgi (through vacuolar protein sorting (VPS) pathway) (8). Although much progress has been made in elucidating the mechanistic basis of various steps in protein trafficking, the precise requirement for a specific type and length of Ub chains at various stages of the endocytic pathway remains to be addressed.
The ubiquitin profile needed for proper internalization has been established for some yeast membrane proteins (1). The α-factor receptor Ste2 was described as undergoing monoubiquitylation on several lysines (multimonoubiquitylation). The a-factor receptor, Ste3p; the general transporter of amino acids, Gap1; the zinc transporter, Ztr1; and the uracil transporter, Fur4, have been shown to be modified by short chains of two to three ubiquitins, each attached to one, two, or more target lysine residues (oligo-ubiquitylation). Among them, Fur4 and Gap1 were the only transporters demonstrated to undergo plasma membrane oligo-ubiquitylation with ubiquitin residues linked via ubiquitin-Lys63 (9, 10). In addition, the two siderophore transporters Arn1 and Sit1 were also shown to undergo Lys63-linked cell surface ubiquitylation (11, 12). Whether these four transporters are representative of a larger class of plasma membrane substrates remains to be determined. Little is known about the type of ubiquitylation involved and/or required for sorting to MVBs. Some MVB cargoes appear to undergo monoubiquitylation (8), whereas Sna3, an MVB cargo of unknown function, undergoes Lys63-linked ubiquitylation (13). Lys63-linked ubiquitin chains were also recently reported to be required, directly or indirectly, for MVB sorting of the siderophore transporter, Sit1, when trafficking through the VPS pathway in the absence of its external substrate (11). In agreement with the possibility that additional membrane-bound proteins might undergo Lys63-linked ubiquitylation, a proteomic study aiming to uncover ubiquitylated yeast proteins showed that Lys63-ubiquitin chains are far more abundant than previously thought (14).
The transport of monocarboxylates, such as lactate and pyruvate, as well as ketone bodies across the plasma membrane is essential for the metabolism of cells of various organisms. A family of monocarboxylate transporters has been reported that includes mainly mammalian members (15). In S. cerevisiae, two monocarboxylate-proton symporters have been described, Jen1 and Ady2 (16, 17). These transporters exhibit differences in their mechanisms of regulation and specificity. Jen1 is a lactate-pyruvate-acetate-propionate transporter induced in lactic or pyruvic acid-grown cells (18). Ady2, which accepts acetate, propionate, or formate, is present in cells grown in non-fermentable carbon sources (19). Jen1 has unique regulatory characteristics and has been extensively studied. It was the first secondary porter of S. cerevisiae characterized by heterologous expression in Pichia pastoris at both the cell and the membrane vesicle levels (20). The addition of glucose to lactic acid-grown cells very rapidly triggers loss of Jen1 activity and repression of JEN1 gene expression (21, 22). Newly synthesized Jen1-GFP fusion protein is sorted to the plasma membrane in an active and stable form, and loss of Jen1-GFP activity upon glucose addition is the result of its endocytosis followed by vacuolar degradation (23). Data from large scale analyses based on mass spectrometry approaches led to the detection of two sites of ubiquitylation for Jen1, one located in the N terminus of the protein and the second in the central loop (14), and several sites of phosphorylation in the N terminus, central loop, and C terminus of the protein (14, 24). In the present study, we aimed at further characterizing the internalization step of endocytosis of the transporter Jen1 and the potential role of the phosphorylation and ubiquitylation events required for its correct endocytic trafficking.
EXPERIMENTAL PROCEDURES
Media and Growth Conditions
Complex medium with 1% yeast extract and 1% peptone (YP medium) or a synthetic minimal medium with 0.67% yeast nitrogen base (Difco), supplemented with amino acids to meet auxotrophic requirements (YNB medium), was used for submerged culture at 30 °C. Carbon sources were either 2% glucose, 2% raffinose, 2% galactose, or lactic acid (0.5%, pH 5.0). Cells were always harvested during exponential growth phase. Glucose (2%)-containing medium was used for growth of cells encoding JEN1 from its own promoter under repression conditions. For derepression conditions, glucose-grown cells were collected by centrifugation, washed twice in ice-cold deionized water, and grown in fresh medium with lactic acid (0.5%, pH 5.0). Raffinose-containing medium was used for growth of cells encoding JEN1 from the GAL promoter, under non-induction conditions. For induction conditions, 2% galactose was added to raffinose-grown cells.
Strains and Plasmid Constructions
The S. cerevisiae strains used in this study are listed in Table 1. Yeast cells were transformed as described in Ref. 25. The JEN1 gene was C-terminally tagged on the chromosome with a His6 tag and three consecutive hemagglutinin (HA) epitope tags by integration of a KanMX4 PCR product obtained from plasmid pU6H-3HA, following the strategy described in Ref. 26. The PCR product was used to transform strains 27064b and LRB346, containing deletions or mutations for RSP5/NPI1 (27) and YCK1 YCK2 (28), respectively, strains 27061b and LBR351, the corresponding wild-types, and strain W303-1A (29). With the transformants obtained, a colony PCR was performed to confirm correct integration. In this way, we constructed strains SP1, SP2, SP5, SP6, and SP7, harboring JEN1-3HA in the chromosome. For construction of the pJEN1-JEN1-GFP fusion expression plasmid, 1 kb of the promoter region immediately upstream of JEN1 (excluding the start codon) was amplified with a primer introducing homology with the plasmid p416-GPD (30) and another with 56 bp homologous to the 5′ part of the JEN1 coding sequence. Plasmid p416-GPD-JEN1 (20), containing the JEN1 coding sequence, under the control of the GPD promoter, was digested with the enzymes SacI and BamHI, removing the promoter. The linearized plasmid was co-transformed with the JEN1 promoter PCR product described above into the S. cerevisiae W303-1A jen1Δ mutant strain. The resulting plasmid pSP1, obtained by gap repair, expresses the JEN1 gene under control of the native promoter. Correct clones were identified by colony PCR. In a similar fashion, plasmid pSP2 was obtained by gap repair between EcoRI-linearized pSP1 and a PCR product containing the GFP open reading frame. The PCR product was obtained from the vector pFA6a-GFP-S65T-kanMX6 (31) using primers introducing regions of homology to the 3′ of JEN1 and the CYC1 terminator of p416-GPD. Correct clones were identified by colony PCR. The pSP2 plasmid expresses a C-terminal Jen1-GFP fusion protein under the control of the native JEN1 promoter. Mutations that substitute JEN1 lysine codons for arginine were introduced in the plasmid pSP2, using site-directed mutagenesis as described (32). The resulting plasmids pSP3 to -5 express the resulting mutant alleles of JEN1 (Table 1). All of the mutations were confirmed by DNA sequencing. For construction of the JEN1-GFP-6HIS fusion expression plasmid, a PCR product containing the GFP coding sequence fused in the 3′-end with a 6HIS coding sequence, was digested with BamHI and XhoI and cloned into the plasmid p416-GAL (33) digested with the same enzymes; this resulted in the plasmid p416-GFP-6HIS. The JEN1 coding sequence, excluding the stop codon, was amplified by PCR and digested with BamHI and EcoRI. The fragment obtained was cloned into p416-GFP-6HIS digested with the same enzymes, resulting in plasmid pJEN1-GFP-6HIS.
TABLE 1.
Strains and plasmids used in this study
| Genotype | Reference/Source | |
|---|---|---|
| Strain | ||
| W303-1Ajen1Δ | MATaura3-52 trp1-1 leu2-3-112 his3-11 ade2-1 can1-100 jen1 ::KanMX4 | Ref. 17 |
| BLC 493 | MATahis4 leu2 ura3 ar1-1 end3-1 JEN1-GFP-KanMX4 | Ref. 23 |
| 27061b | MATaura3 trp1 | Ref. 27 |
| MOB52 | MATaura3 trp1 end3 ::KanMX4 | Ref. 42 |
| 27064b | MATaura3 trp1 npi1 | Ref. 27 |
| SP1 | MATaura3 trp1 JEN1-6His-3HA-loxP-KanMX4-loxP | This study |
| SP2 | MATaura3 trp1 npi1 JEN1-6His-3HA-loxP-KanMX4-loxP | This study |
| SP5 | MATahis3 leu2 ura3-52 JEN1-6His-3HA-loxP-KanMX4-loxP | This study |
| SP6 | MATahis3 leu2 ura3-52 Δyck1 yck2-1ts JEN1-6His-3HA-loxP-KanMX4-loxP | This study |
| SP7 | MATaura3-52 trp1-1 leu2-3-112 his3-11 ade2-1 can1-100 JEN1-6His-3HA-loxP-KanMX4-loxP | This study |
| BY4741 | MATahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | Euroscarf |
| BY4741end3Δ | MATahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 end3 ::KanMX4 | Euroscarf |
| BY4741npi1 | MATahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 npi1 | R. H.-T. laboratory |
| SUB280 | MATalys2-801 leu2-3 112 ura3-52 his3-Δ200 trp1-1 ubi1-Δ1 ::TRP1 ubi2-Δ2:ura3 ubi3-Δub-2 ubi4-Δ2 ::LEU2 (pUB39 Ub, LYS2) (pUB100, HIS3) | Ref. 40 |
| SUB413 | MATalys2-801 leu2-3,112 ura3-52 his3-Δ200 trp1-1(am) ubi1-Δ1 ::TRP1 ubi2-Δ2 ::ura3 ubi3-ΔUb-2, ubi4-Δ2 ::LEU2 (pUB39 UbK63R, LYS2) (pUB100, HIS3) | Ref. 40 |
| Plasmid | ||
| pSP2 | CEN, URA3, pJEN1-JEN1-GFP | This study |
| pSP3 | CEN, URA3, pJEN1-JEN-K9R-GFP | This study |
| pSP4 | CEN, URA3 pJEN1-JEN-K338R-GFP | This study |
| pJEN-GFP-6HIS | CEN, URA3, pGAL-JEN1-GFP-6×HIS | This study |
Transport Assays
Transport assays, using labeled [U-14C]lactic acid (sodium salt; Amersham Biosciences) (4000 dpm/nmol), at pH 5.0, were carried out as described (23).
Fluorescence Microscopy
Cells grown to exponential growth phase in YNB medium were concentrated by a factor of 10 by centrifugation. The cells were viewed immediately, without fixation, under a fluorescence microscope (type BY61; Olympus, Tokyo, Japan), and images were captured with a digital camera.
Cell Extracts and Immunoblotting
Total protein extracts were prepared by the NaOH-TCA lysis technique (34). Lysates were prepared from cells harvested by centrifugation at 4 °C in the presence of 10 mm sodium azide. The cells were washed once and resuspended in cold lysis buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl plus a mixture of EDTA-free protease inhibitors: Complete from Roche Applied Science and 25 mm freshly prepared N-ethylmaleimide to prevent artifactual deubiquitylation). They were then disrupted in a “One Shot” Cell Disrupter (Constant Systems LDT, Daventry, UK) at a maximum pressure of 2.7 kilobars. The disrupted cells were centrifuged twice (3000 × g for 3 min at 4 °C) to remove unbroken cells. Proteins from either total extracts or lysates were resuspended in sample buffer, heated at 37 °C, and resolved by SDS-PAGE in 10% acrylamide gels using Tricine buffer and transferred to nitrocellulose membranes. The membranes were probed with monoclonal antibodies anti-HA (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), anti-GFP antiserum (Roche Applied Science), anti-phosphoglycerol kinase (Molecular Probes), or anti-Ub (clone P4D1)-HRP conjugate (Santa Cruz Biotechnology). Horseradish peroxidase-conjugated anti-mouse immunoglobulin G was used as the secondary antibody (Sigma) and was detected by enhanced chemiluminescence.
His6-tagged Jen1-GFP Purification
His6-tagged Jen1-GFP purification experiments were performed essentially as previously described (35), except that Ni2+-NTA resin was used in batch rather than in column. 6–7 × 108 cells were harvested, and lysate was prepared. The lysate was subjected to centrifugation at 13,000 × g for 30 min to generate the supernatant and pellet fractions. The pellet was resuspended in 300 μl of buffer A (lysis buffer supplemented with 5 mm imidazole, 0.1% SDS, and 1% Triton X-100). The suspension was incubated on ice for 30 min and then diluted by adding 300 μl of buffer B (lysis buffer supplemented with 5 mm imidazole and 1% Triton X-100) and centrifuged for 10 min at 13,000 × g to remove the remaining insoluble material. The supernatant was added to 200 μl of Ni2+-NTA Superflow resin (Qiagen Inc., Hilden, Germany) and incubated with mixing for 1 h at 4 °C. The unbound fraction was collected, and the resin was washed three times with 200 μl of buffer B. Jen1-GFP-6His was eluted three times with 200 μl of elution buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 200 mm imidazole). Aliquots of different fractions were prepared for Western blot analysis.
RESULTS
The Casein Kinase I Activity Is Required for Jen1 Turnover
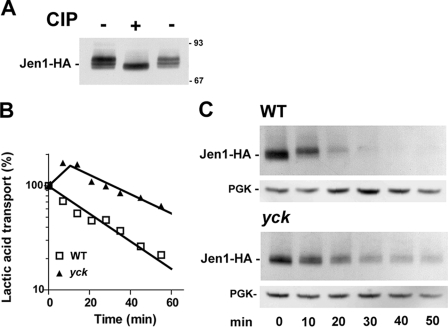
The ubiquitylation and internalization of the Fur4 and Ste2 membrane proteins depend on their prior phosphorylation at several Ser residues, directly or indirectly by the casein kinase 1 isoforms Yck1/Yck2 (36–38). The potential state of phosphorylation of Jen1-HA on extracts of whole cells grown on lactic acid was initially tested (Fig. 1A). The electrophoretic pattern of Jen1-HA displayed several bands with different mobilities on an immunoblot. Treatment with alkaline phosphatase increased the electrophoretic mobility of the slower running bands, suggesting that Jen1-HA is constitutively phosphorylated. A mutant strain lacking the YCK1 gene and carrying a temperature-sensitive allele of the YCK2 gene, yck2-2 (hereafter referred to as yck-ts), was used to investigate the potential role of Yck activity in controlling Jen1-HA phosphorylation and internalization. The transporter activity was followed after glucose addition to lactic acid-induced wild-type and yck-ts cells, grown at 24 °C and shifted to 37 °C, for 30 min (Fig. 1B). The addition of glucose caused a sharp decrease in lactic acid uptake in wild-type cells incubated at 37 °C with an approximate half-life time of 25 min. The decrease in lactic acid uptake was less severe in yck-ts cells shifted to the restrictive temperature (approximate half-life time of 60 min). The relative protection against loss of transport activity indicated that the defect in Yck activity stabilized the transporter at the plasma membrane. Extracts from cells withdrawn at various times after glucose addition were analyzed by immunoblotting (Fig. 1C). Significant protection against degradation was observed in yck-ts cells; therefore, the glucose-induced internalization and subsequent degradation of the transporter are dependent on Jen1 phosphorylation, which directly or indirectly requires Yck kinase activity.
FIGURE 1.
Endocytosis of Jen1-HA at restrictive temperature in Casein Kinase I (yck-ts) mutant cells. A, protein extracts from SP7 cells harboring chromosomally encoded Jen1-HA and induced for the production of Jen1-HA in lactic acid for 4 h were incubated at 37 °C for 1 h in the presence (+) or absence (−) of 50 units of calf intestinal phosphatase (CIP). The samples were then separated by SDS-PAGE and analyzed for Jen1-HA by Western immunoblotting with an anti-HA antibody. The sizes of molecular weight markers are indicated. B, parental SP5 (YCK) and SP6 (yck) cells harboring chromosomally encoded Jen1-HA were induced for the production of Jen1-HA in lactic acid for 3.5 h at permissive temperature (23 °C), followed by 30 min at restrictive temperature (37 °C). Lactic acid uptake was measured at the times indicated after the addition of glucose (2%). Results are percentages of initial activities. □, wild type; ▲, yck cells. C, protein extracts were prepared at the same time points and analyzed for Jen1-HA by Western immunoblotting with an anti-HA antibody. Blots were reprobed with an anti-phosphoglycerol kinase (PGK) antibody to provide loading controls.
The Ubiquitin Ligase Rsp5 Is Required for Jen1 Turnover
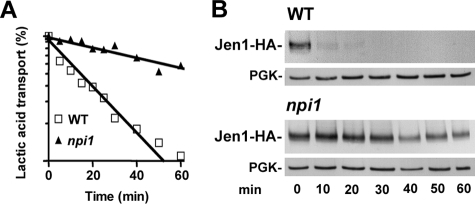
The ubiquitin ligase Rsp5 is involved in the internalization step of endocytosis of numerous transporters (3). Since Rsp5 is essential for cell viability, we investigated its potential role on the turnover of Jen1 in a hypomorphic allele of rsp5, npi1 (39). The fully viable rsp5/npi1 mutant strain displays low levels of RSP5 expression, allowing production of less than one-tenth the amount of Rsp5 present in wild-type cells (35, 39). Jen1 transport activity (Fig. 2A) and stability (Fig. 2B) were followed after a pulse of glucose to lactic acid-induced cells. In wild-type cells, both Jen1 activity and the Jen1 immunodetection signal decreased rapidly, reflecting internalization and subsequent vacuolar degradation of the transporter. In contrast, Jen1 activity and the Jen1 signal were greatly stabilized in rsp5/npi1 cells (relative protection against loss of transport activity, 3-fold). Thus, the protection against degradation of Jen1 in rsp5/npi1 cells is mainly due to stabilization of Jen1 at the cell surface.
FIGURE 2.
Endocytosis of Jen1-HA in rsp5/npi1 mutant cells. Parental SP1 (WT) and SP2 (rsp5/npi1) cells harboring chromosomally encoded Jen1-HA were induced for 4 h in lactic acid before glucose addition. A, lactic acid uptake was measured at the times indicated after the addition of glucose (2%). The results are percentages of initial activities. □, wild-type; ▲, rsp5/npi cells. B, protein extracts were prepared at the same time points and analyzed by Western immunoblotting with an anti-HA antibody. The blots were reprobed with an anti-phosphoglycerol kinase (PGK) antibody to provide loading controls.
Cell Surface Rsp5-dependent Oligo-ubiquitylation of Jen1
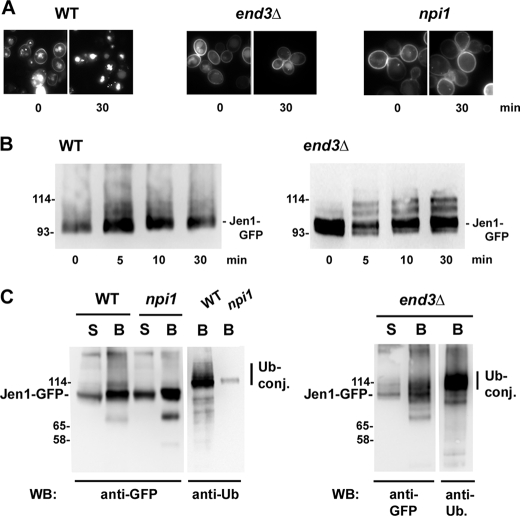
The involvement of Rsp5 in destabilizing Jen1 suggested that ubiquitylation of the transporter is required for transporter degradation. Parental rsp5/npi1 together with end3Δ cells impaired in the internalization step of endocytosis were transformed with plasmid pJEN1-GFP-6HIS, induced in galactose for the production of Jen1-GFP-6His, and then treated with glucose to trigger endocytosis of the protein. First, the localization of the protein was followed by fluorescence microscopy before and after the addition of glucose for 30 min (Fig. 3A). Fluorescence was detected both at the plasma membrane and in the vacuolar lumen in WT cells before the addition of glucose. In contrast, Jen1-GFP-6His only stained the plasma membrane of end3Δ cells, and the vacuolar signal was hardly detectable. This suggests that constitutive endocytosis rather than direct, premature sorting of Jen1-GFP to the VPS pathway might be the cause of the vacuolar staining in WT cells. Jen1-GFP-6HIS stained essentially the plasma membrane of rsp5/npi1 cells, and the vacuolar fluorescent signal was less bright when compared with WT cells, suggesting that some constitutive endocytosis may occur in these mutant cells. The fluorescent signal stained the vacuolar lumen of parental cells 30 min after glucose addition, whereas fluorescence was only detectable at the cell surface of rsp5/npi1 and end3Δ cells.
FIGURE 3.
Ubiquitylation of Jen1-GFP-6His in end3Δ and rsp5/npi1 mutant cells. Parental 27061b (WT), rsp5/npi1, and end3Δ cells were transformed with pJEN1-GFP-6HIS. The cells were induced for the production of Jen1-GFP-6His in galactose for 2 h (30 °C). A, microscopy images of Jen1-GFP-6His in living cells before and after the addition of 2% glucose for 30 min. B, protein extracts from induced parental and end3Δ cells were prepared before and at the indicated times after the addition of 2% glucose and were then analyzed by Western immunoblotting with an anti-GFP antibody. C, lysates of cells incubated for 10 min with glucose were fractionated, as described under “Experimental Procedures.” All experiments were conducted in identical conditions of growth and cell fractionation. Solubilized membranes were incubated with Ni2+-NTA beads. The bound fraction corresponding to His6-tagged ubiquitylated proteins were eluted by buffer containing 200 mm imidazole. Aliquots of solubilized membranes and bound fractions were resolved by electrophoresis and analyzed by Western immunoblotting (WB) with anti-GFP and anti-Ub antibodies. Note that this particular anti-Ub antibody hardly recognizes mono- and diubiquitins. S, solubilized membrane fractions; B, purified His6-tagged proteins. The sizes of molecular weight markers are indicated.
We used the end3Δ mutant strain to uncover possible ubiquitylation of Jen1 triggered by glucose addition. WT and end3Δ cells expressing pJEN1-GFP-6HIS were induced in galactose and then treated with glucose to trigger endocytosis of the protein. Fig. 3B shows immunoblots of cell extracts revealed with anti-GFP antibody. Two to three more slowly migrating bands of immunoreactive material were observed above the main Jen1-GFP signal of end3Δ cell extracts. These bands were hardly visible at time 0 of the experiment (i.e. before glucose addition). This ladder of bands was far less clear in WT cells, in which internalization was not blocked. An immunoblot of end3Δ cell extracts in which chromosomally encoded Jen1-GFP was induced in lactic acid and treated with glucose to induce endocytosis was also performed (Fig. S1). Immunoreactive material displayed the same profile with two to three more slowly migrating bands above the main Jen1-GFP signal. Hence, the post-translational modifications of Jen1 were identical with or without an additional His6 tag and with different levels of expression. Strikingly, in both experiments, Jen1 modifications occurred within minutes after the glucose addition.
A biochemical characterization of the Jen1-GFP-6His more slowly migrating species was attempted. Aliquots of WT, rsp5/npi1, and end3Δ cells were withdrawn 10 min after glucose addition (i.e. under conditions where a high proportion of Jen1-GFP was still present at the plasma membrane), and the corresponding protein extracts were subjected to cell fractionation (Fig. 3C). Membrane-enriched fractions were solubilized and incubated with Ni2+-NTA beads. Aliquots from the solubilized pellet together with corresponding aliquots of bound material were resolved by electrophoresis and subjected to Western blotting to identify the immunoreactive transporter. Jen1-GFP-6His was specifically retained on nickel beads, as visible with anti-GFP antibodies. The slower migrating bands were detected with anti-ubiquitin antibodies in WT cells, indicating that they correspond to Jen1-GFP-6His conjugated with ubiquitin. The slowly migrating bands detected with anti-ubiquitin antibodies were far less abundant in the Ni2+-NTA-retained fractions of rsp5/npi1 cells, showing that Rsp5 is involved in ubiquitylation of the transporter. The slowly migrating bands detected with anti-ubiquitin antibodies were also present in the Ni2+-NTA-retained fractions of end3Δ cells, showing that ubiquitylation of the transporter occurs at the plasma membrane. These experiments indicate that the Jen1 transporter is a target for ubiquitylation by Rsp5 at the cell surface. The profile of ubiquitin conjugates corresponding to Jen1-GFP purified on nickel beads appeared more like a smear (Fig. 3C), suggesting that Jen1 ubiquitylation may correspond to the conjugation of more ubiquitin species than the two to three Ub bands visualized directly on cell extracts (Fig. 3B).
Lys to Arg Substitution at Lys338 Extends the Half-life of Jen1
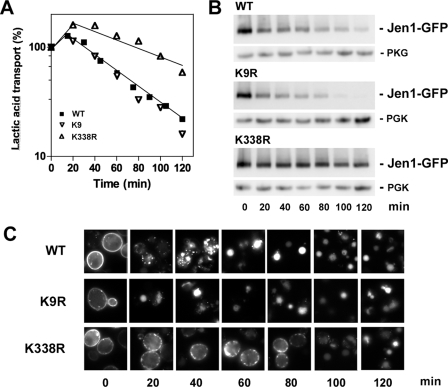
The hydrophilic parts of Jen1 harbor numerous lysines that could be potential targets for ubiquitylation of the protein. We focused our analysis on Lys9 and Lys338, which were identified by a proteomic approach, as target sites for ubiquitylation (14). Lys9 is located in the N-terminal hydrophilic part of the transporter, whereas Lys338 is located in its third predicted cytoplasmic loop. To define whether ubiquitylation of these two lysines plays a role in Jen1 endocytosis, they were replaced by conservative but non-ubiquitylatable arginine residues. The corresponding variant proteins were tested for their cellular trafficking and stability (Fig. 4). Ectopic Jen1-GFP and the corresponding Lys to Arg variants were expressed under the JEN1 promoter. The variant transporters were expressed in lactic acid-induced cells, and endocytosis was triggered by glucose addition. At time 0, fluorescence was mainly detected at the plasma membrane, in all types of cells, showing that all of the variant proteins were correctly targeted to the plasma membrane (Fig. 4C). Following a pulse of glucose, plasma membrane fluorescence corresponding to wild-type Jen1-GFP disappeared progressively, and intracellular dots were detected. These dots, heterogeneous in size, might correspond to early and late endosomes. Some staining of the vacuole was also observed. After 60 min, the fluorescence was only detected in the vacuolar lumen. Trafficking of the protein from the cell surface to the vacuole appeared more efficient for the K9R variant, since staining was exclusively detected in the vacuole 20 min after glucose addition. In contrast, the K338R variant still stained the plasma membrane 80 min after glucose addition.
FIGURE 4.
Effect of Lys to Arg mutations on the stability of Jen1-GFP. W303-1A jen1Δ cells transformed with either pSP2 (JEN1-GFP), pSP3 (JEN1-K9R-GFP), or pSP4 (JEN1-K338R-GFP) were induced for the production of Jen1-GFP in lactic acid for 4 h at 30 °C. A, acid lactic uptake was measured at the times indicated after the addition of 2% glucose. The results are percentages of initial activities. ■, Jen1-GFP; ▿, Jen1-K9R-GFP; ▵, Jen1-K338R-GFP; B, protein extracts collected at the same time points were analyzed by Western immunoblotting with an anti-GFP antibody. The blots were reprobed with an anti-phosphoglycerol kinase (PGK) antibody to provide loading controls. C, microscopy images of Jen1-GFP in living cells at the time points indicated after the addition of 2% glucose. WT, wild type (Jen1-GFP).
To follow more quantitatively the fate of the Lys to Arg variants relative to wild-type Jen1-GFP, their stability was tested at the plasma membrane by monitoring Jen1 transport activity after the addition of glucose to lactic acid-induced cells. Glucose treatment caused a drop in lactic acid uptake in wild-type cells, with a half-life time of roughly 70 min (Fig. 4A). Transporter immunoreactivity declined in parallel to the drop in transporter activity (Fig. 4B). The same drop in transport activity was observed with the K9R variant transporter. However, the amount of K9R variant immunoreactive species appeared to decline more rapidly than the amount of the wild-type species, confirming the fluorescent observations. In contrast, the decrease in transport activity was far less severe in cells producing the K338R variant, with a half-life time higher than 120 min. In agreement, the K338R variant transporter was more slowly degraded than the wild-type protein. The relative protection (2-fold) against loss of transport activity (Fig. 4A) and the relative stabilization of the K338R variant protein at the plasma membrane (Fig. 4, B and C) suggested that Lys388 is a major target site for ubiquitylation of Jen1 at the cell surface. In contrast, the absence of stabilization observed when Lys9 was substituted for Arg (Fig. 4A) is unexpected and may argue against Lys9 being a target for ubiquitylation at the plasma membrane. However, it may be possible that other Lys residue(s) of Jen1 may be ubiquitylated in place of Lys9 when the latter is mutated. Surprisingly, the internalized K9R variant is more quickly targeted to the vacuole for degradation than the WT protein (Fig. 4, B and C). It might indicate that, for unknown reasons, Jen1-K9R-GFP is a more efficient substrate for sorting of the protein into the MVB than Jen1-GFP or is unable to undergo putative recycling from endosomes to the plasma membrane.
We monitored the ubiquitylation status of the Jen1-GFP variants produced in end3Δ cells after ubiquitylation was triggered by glucose addition. Immunoblots of all of the corresponding lysates displayed a similar ladder of ubiquitin-permease conjugates with three minor bands with slower mobilities than the main transporter signal (data not shown). This result most probably suggests that Jen1 may be ubiquitylated on more than one lysine and is in agreement with the partial stabilization observed at the cell surface for the K338R variant.
These overall data indicate that Jen1-Lys338 is probably one of the targets for cell surface ubiquitylation, involved in Jen1 internalization, in agreement with proteomic data (14). The situation appears more complex for Jen1-Lys9.
Ubiquitin Lys63 Is Involved in Trafficking of Jen1
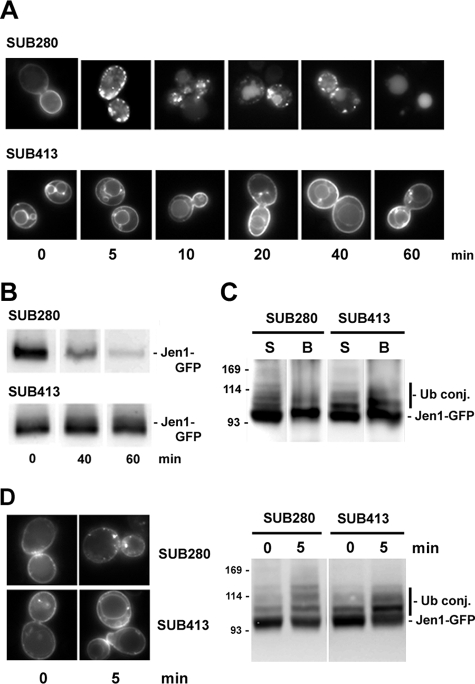
We analyzed the localization and ubiquitin status of Jen1-GFP-6His during endocytosis in a collection of SUB strains, deleted in the four natural ubiquitin genes and expressing different ubiquitin Lys to Arg variants, as their sole source of ubiquitin. It was previously shown that all ubiquitin Lys residues can be used for the formation of ubiquitin chains in yeast (14) and that the unique lysine in ubiquitin required for viability is Lys48, which is the favorite lysine involved in the formation of chains used for proteasome degradation (40, 41). Jen1-GFP was correctly internalized and sorted to the vacuolar lumen over time, after glucose addition to galactose-induced SUB280 cells producing wild-type ubiquitin (Fig. 5A). Jen1-GFP was also internalized and sorted to the vacuolar lumen in cells producing K6R, K11R, K27R, K29R, and K33R variants of ubiquitin (data not shown). In contrast, Jen1-GFP was stabilized at the plasma membrane after glucose addition to SUB413 cells, producing the Ub-K63R variant. An immunoblot of cell extracts prepared at different times after glucose addition confirmed that Jen1-GFP was protected from degradation in SUB413 cells (Fig. 5B). We therefore examined the ubiquitylation pattern of Jen1-GFP. Lysates from SUB280 and SUB413 cells producing Jen1-GFP-6His were subjected to cell fractionation just after glucose addition (i.e. under conditions where a high proportion of Jen1-GFP was still present at the plasma membrane). Solubilized membrane-bound Jen1-GFP-6His was retained on Ni2+-NTA beads, as visualized with an anti-GFP antibody (Fig. 5C). In wild-type cells, up to four bands of higher molecular weight than the main unmodified Jen1-GFP species were visible. In SUB413 cells, almost all of these species could also be detected, with high enrichment of a species that probably corresponds to monoubiquitylated Jen1-GFP.
FIGURE 5.
Ubiquitylation and stability of Jen1-GFP in SUB280 and SUB413 cells. SUB280 and SUB413 cells producing WT or Ub-K63R ubiquitin, respectively, were transformed with pJEN1-GFP-6HIS. Cells were induced for the production of Jen1-GFP-6His in galactose for 2 h at 30 °C. A, microscopy images of Jen1-GFP-6His in living cells at the time points indicated after the addition of 2% glucose. B, protein extracts collected at the indicated times were analyzed by Western immunoblotting with an anti-GFP antibody. C, lysates of 5-min glucose-incubated cells were fractionated, as described under “Experimental Procedures.” All experiments were conducted in identical conditions of growth and cell fractionation. Solubilized membranes were incubated with Ni2+-NTA beads. The bound fraction corresponding to His6-tagged ubiquitylated proteins was eluted by 200 mm imidazole-containing buffer. Aliquots of solubilized membranes and bound fractions were resolved by electrophoresis and analyzed by Western immunoblotting with an anti-GFP antibody. S, solubilized membrane fractions; B, purified His6-tagged proteins. D, SUB280 and SUB413 cells were transformed with pSP2 (JEN1-GFP). Cells were induced for the production of Jen1-GFP in lactic acid for 4 h at 30 °C before the addition of glucose for 5 min. Left, microscopy images of Jen1-GFP in living cells at the time points indicated after the addition of 2% glucose. Right, protein lysates collected at the indicated times were analyzed by Western immunoblotting with an anti-GFP antibody. The sizes of molecular weight markers are indicated.
We then analyzed the localization and ubiquitin status of Jen1-GFP expressed under its own promoter during endocytosis in SUB280 and SUB413 (Fig. 5D). Jen1-GFP was stabilized at the plasma membrane after glucose addition to SUB413 cells. Immunoreactive material in SUB413 cell lysates also displayed high enrichment of a species that probably corresponds to monoubiquitylated Jen1-GFP.
These observations could suggest that Jen1-GFP carries one main target lysine and that it is modified by a Lys63-linked ubiquitin chain. These results are in agreement with the fact that partial stabilization of Jen1-K338R was observed, and they also show that Jen1-GFP is probably modified on several target lysines that could be monoubiquitylated in SUB413 cells. Importantly, endocytosis was not entirely blocked in SUB413 cells, and some Jen1-GFP also stained the vacuolar rim even at time 0. This is the typical localization observed for membrane proteins coming from the Golgi apparatus, when MVB sorting is inhibited as a result of either defective ubiquitylation before or at the MVB (11, 42) or defective MVB sorting machinery (i.e. in vps class E mutants) (43). The deficiency in vacuolar delivery of Jen1-GFP in SUB413 cells could result either from impaired ubiquitylation of Jen1 itself or from a deficiency in MVB sorting machineries. Whatever the case, this indicates that formation of Ub-Lys63-linked chains plays an important role for MVB sorting of Jen1-GFP.
DISCUSSION
In the present report, we provide critical data demonstrating rapid glucose-triggered ubiquitylation and subsequent endocytosis of Jen1 and the involvement of casein kinase 1 in this process. We also demonstrate that the HECT-ubiquitin ligase Rsp5 modifies Jen1 at the cell surface by oligo-ubiquitylation. Additionally, Jen1-Lys338 was identified as one of the targets for ubiquitylation, confirming the data obtained by a proteomic approach. Results concerning the other potential target, Jen1-Lys9, are more enigmatic. Finally, our data suggest that Lys63-linked ubiquitin chains are required for Jen1 endocytic internalization and directly or indirectly for Jen1 sorting at the MVB.
Most plasma membrane receptors and transporters display regulated ubiquitylation and internalization in yeast. For instance, Gap1, Fur4, Tat2, and Ctr1 ubiquitylation and internalization are triggered by ammonium, high uracil, high tryptophan, and high copper concentration, respectively (44, 45). The presence of Jen1 at the cell surface is tightly controlled by glucose. Jen1 is one of the few documented examples in which ubiquitylation and endocytosis of a transporter are induced within minutes, as a response to a metabolic change. It is tempting to hypothesize that phosphorylation events may play a role in these processes. It was previously shown that yeast casein kinase 1 phosphorylation of Ste2 (triggered by its ligand, α-factor) and of Fur4 drive their subsequent ubiquitylation and internalization (36–38). We show here that phosphorylation of Jen1 is also required prior to its internalization. The yeast casein kinase 1 isoforms, Yck1/Yck2, two plasma membrane anchored proteins (46), are involved in this process.
Rsp5-mediated ubiquitylation and subsequent internalization appears to be a general feature of the vast majority of plasma membrane proteins in yeast. Ubiquitin-dependent endocytosis also occurs in mammalian cells, mediated both by Nedd4/Nedd4-like ligases and by RING finger ligases (1, 2). This has raised questions concerning substrate recognition and mode of ubiquitylation. All members of the Nedd4 family, including Rsp5, display three to four WW modules that interact with PY motifs. But no Rsp5 plasma membrane endocytic substrates carry such PY motifs. However, Bul1 and Bul2, two PY-containing proteins, regulate endocytosis of a subset of plasma membrane transporters (7, 45, 47). Very recently, PY-containing arrestin-like proteins were also shown to play the role of Rsp5-substrate adaptors at the cell surface (5, 6). Whether glucose-triggering phosphorylation of Jen1 may result in a conformational change rendering certain Lys residues directly accessible for ubiquitylation or whether such a conformational change may trigger the interaction of Rsp5 with Jen1 via an arrestin-like adaptor that remains to be discovered still needs to be further investigated.
Jen1 harbors numerous cytoplasmic lysines. We and others previously suggested that Lys residues lying in (D/E)XK(S/T) motifs are probably primary targets for ubiquitylation of plasma membrane proteins, at least in yeast (1, 48). A proteomic approach identified two ubiquitylation targets for Jen1, Lys9 and Lys388 (14). These two lysine-surrounding sequences are DEK9IS and DAVK388AN. We demonstrate here that at least Jen1-Lys388 must be a target for ubiquitylation at the plasma membrane, even if the sequence surrounding Lys388 does not exactly fit the established consensus. In contrast, the sequence around Lys9 does fit, but the results obtained for the K9R variant protein were unexpected. The rapid destabilization of the internalized Jen1-K9R variant could not be easily explained. One possibility is that the amount of Jen1 observed at the plasma membrane could result from its endocytosis, followed by its recycling to the cell surface, since plasma membrane redistribution of endosomal targeted transporters was described in the case of the vps class E mutants (49, 50) and corresponds to the physiological fate of some transporters in WT cells (51). In that case, the instability of the internalized K9R variant, upon glucose addition, would be due to an unexpected increase in internalization into MVBs or to a defect in recycling. This would allow its rapid clearance from the plasma membrane into the vacuole for degradation that could mask a potential stabilization at the plasma membrane linked to the absence of the Lys9 ubquitylation site. This hypothesis raises new questions that will constitute the basis of future investigations.
Little is known so far about the ubiquitylation features associated with the internalization step of endocytosis. Ubiquitylation of yeast plasma membrane proteins, when defined, occurs via conjugation of ubiquitin monomers (multimonoubiquitylation) or of short chains of ubiquitin (oligo-ubiquitylation) (1). Fur4 and Gap1 transporters are modified by Lys63-linked oligo-ubiquitylation for efficient endocytosis (9, 10). It was recently shown that the siderophore transporters Arn1 and Sit1 are also modified with Ub-Lys63-linked ubiquitin chains at the plasma membrane (11, 12). The same holds true in mammalian cells, where a number of plasma membrane transporters and receptors were shown to undergo modification by Lys63-linked ubiquitin chains, a process carried out by Nedd4 in the case of the dopamine DAT transporter (2). Our data indicate that Ub-Lys63 is also essential for internalization of Jen1 and that Jen1 is most probably modified by oligo-ubiquitylation. But whether Jen1 is modified at the cell surface by Ub-Lys63-linked ubiquitin chains remains to be further documented. However, this modification is likely to occur given both our data and the recent report that Rsp5 preferentially assembles this type of ubiquitin chains in vitro and in vivo (52, 53).
Far less is known about the type of ubiquitylation associated with and required for MVB sorting. Ubiquitin fused in frame to lysineless MVB cargoes trafficking through the VPS pathway is sufficient for correct MVB sorting of these cargoes (35, 54, 55). However, Sna3 is modified with a unique and long Ub-Lys63-linked ubiquitin chain (13). These proteins are all substrates of Rsp5 at the Golgi/MVB level (13, 35, 54, 55). The precise identification of the ubiquitylation status of other proteins at the level of MVB is still poorly documented. Nevertheless, it was reported that the Sit1 transporter diverted from the Golgi to the VPS pathway for premature degradation in the absence of its substrate displays deficient MVB sorting in cells unable to form Ub-Lys63-linked ubiquitin chains (11). Similarly, Jen1 coming from the plasma membrane was recovered at the vacuolar rim instead of the vacuolar lumen of cells producing Ub-K63R as sole source of ubiquitin. This implies that Lys63-linked ubiquitin chains are needed directly (ubiquitylation of the cargo) or indirectly (ubiquitylation of the MVB sorting machinery) or both, for proper Jen1 sorting at the MVB. A requirement for this type of ubiquitin chains at MVB fits neatly with the knowledge that the mammalian deubiquitylating enzyme AMSH, which specifically disassembles Ub-Lys63 chains, associates with Vps class E proteins (56, 57) and the observation that the ubiquitin binding domain of the Vps class E protein Hrs/Vps27 displays specific or increased affinity for Lys63-linked ubiquitin chains (58). If the requirement for MVB sorting depends on direct modification of cargo by Ub-Lys63-linked chains, it would imply that transporters undergo a step of deubiquitylation (allowing potential recycling to the cell surface), followed by appropriate Rsp5-dependent reubiquitylation for proper entry of endocytic cargoes into the MVB. Whether such a process exists is currently speculative. Further studies on transporter trafficking, including Jen1, will certainly clarify this hypothesis.
Supplementary Material
Acknowledgment
We thank members of the R. H.-T. laboratory for comments on the manuscript and B. Johansson and A. L. Haenni for editorial assistance. We are grateful to Z. Erpapazoglou for sharing unpublished results and N. Buisson for providing the BY4741npi1 strain.
Note in Proof
While this manuscript was in revision, it was reported that MVB sorting of Gap1 and CPS1 also depends on Lys63-linked ubiquitin (Lauwers, E., Jacob, C., and André, B. (2009) J. Cell Biol. 185, 493–502).
Experiments performed in the laboratory of R. H.-T. were supported by CNRS-University Paris Diderot Paris 7, by Association pour la Recherche sur le Cancer Grant 3298, a grant from the Ministère de l'Enseignement et de la Recherche and the Action Concertée Incitative (BCMS; contrat 183), and a grant from the European Network VI Framework (RUBICON; contract 018683). Experiments performed in the laboratory of M. C. were supported by Portuguese Grant POCI/BIA-BCM/57812/2004 (Eixo 2, Medida 2.3, QCAIII-FEDER).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- E3
- ubiquitin-protein isopeptide ligase
- MVB
- multivesicular body
- GFP
- green fluorescent protein
- NTA
- nitrilotriacetic acid
- VPS
- vacuolar protein sorting
- Ub
- ubiquitin
- HA
- hemagglutinin
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine.
REFERENCES
- 1.Dupré S., Urban-Grimal D., Haguenauer-Tsapis R. (2004) Biochim. Biophys. Acta 1695, 89–111 [DOI] [PubMed] [Google Scholar]
- 2.Miranda M., Sorkin A. (2007) Mol. Interv. 7, 157–167 [DOI] [PubMed] [Google Scholar]
- 3.Horák J. (2003) Biochim. Biophys. Acta 1614, 139–155 [DOI] [PubMed] [Google Scholar]
- 4.Rotin D., Staub O., Haguenauer-Tsapis R. (2000) J. Membr. Biol. 176, 1–17 [DOI] [PubMed] [Google Scholar]
- 5.Lin C. H., MacGurn J. A., Chu T., Stefan C. J., Emr S. D. (2008) Cell 135, 714–725 [DOI] [PubMed] [Google Scholar]
- 6.Nikko E., Sullivan J. A., Pelham H. R. (2008) EMBO Rep. 9, 1216–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soetens O., De Craene J. O., Andre B. (2001) J. Biol. Chem. 276, 43949–43957 [DOI] [PubMed] [Google Scholar]
- 8.Katzmann D. J., Odorizzi G., Emr S. D. (2002) Nat. Rev. Mol. Cell Biol. 3, 893–905 [DOI] [PubMed] [Google Scholar]
- 9.Galan J. M., Haguenauer-Tsapis R. (1997) EMBO J. 16, 5847–5854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Springael J. Y., Galan J. M., Haguenauer-Tsapis R., André B. (1999) J. Cell Sci. 112, 1375–1383 [DOI] [PubMed] [Google Scholar]
- 11.Erpapazoglou Z., Froissard M., Nondier I., Lesuisse E., Haguenauer-Tsapis R., Belgareh-Touzé N. (2008) Traffic 9, 1372–1391 [DOI] [PubMed] [Google Scholar]
- 12.Kim Y., Deng Y., Philpott C. C. (2007) Mol. Biol. Cell 18, 1790–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stawiecka-Mirota M., Pokrzywa W., Morvan J., Zoladek T., Haguenauer-Tsapis R., Urban-Grimal D., Morsomme P. (2007) Traffic 8, 1280–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng J., Schwartz D., Elias J. E., Thoreen C. C., Cheng D., Marsischky G., Roelofs J., Finley D., Gygi S. P. (2003) Nat. Biotechnol. 21, 921–926 [DOI] [PubMed] [Google Scholar]
- 15.Casal M., Paiva S., Queirós O., Soares-Silva I. (2008) FEMS Microbiol. Rev. 32, 974–994 [DOI] [PubMed] [Google Scholar]
- 16.Casal M., Paiva S., Andrade R. P., Gancedo C., Leão C. (1999) J. Bacteriol. 181, 2620–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paiva S., Devaux F., Barbosa S., Jacq C., Casal M. (2004) Yeast 21, 201–210 [DOI] [PubMed] [Google Scholar]
- 18.Cássio F., Leão C., van Uden N. (1987) Appl. Environ. Microbiol. 53, 509–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casal M., Cardoso H., Leão C. (1996) Microbiology 142, 1385–1390 [DOI] [PubMed] [Google Scholar]
- 20.Soares-Silva I., Schuller D., Andrade R. P., Baltazar F., Cássio F., Casal M. (2003) Biochem. J. 376, 781–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andrade R. P., Casal M. (2001) Fungal Genet. Biol. 32, 105–111 [DOI] [PubMed] [Google Scholar]
- 22.Andrade R. P., Kötter P., Entian K. D., Casal M. (2005) Biochem. Biophys. Res. Commun. 332, 254–262 [DOI] [PubMed] [Google Scholar]
- 23.Paiva S., Kruckeberg A. L., Casal M. (2002) Biochem. J. 363, 737–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reinders J., Wagner K., Zahedi R. P., Stojanovski D., Eyrich B., van der Laan M., Rehling P., Sickmann A., Pfanner N., Meisinger C. (2007) Mol. Cell Proteomics 6, 1896–1906 [DOI] [PubMed] [Google Scholar]
- 25.Gietz D., St Jean A., Woods R. A., Schiestl R. H. (1992) Nucleic Acids Res. 20, 1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Antoni A., Gallwitz D. (2000) Gene 246, 179–185 [DOI] [PubMed] [Google Scholar]
- 27.Galan J. M., Moreau V., Andre B., Volland C., Haguenauer-Tsapis R. (1996) J. Biol. Chem. 271, 10946–10952 [DOI] [PubMed] [Google Scholar]
- 28.Robinson L. C., Menold M. M., Garrett S., Culbertson M. R. (1993) Mol. Cell. Biol. 13, 2870–2881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas B. J., Rothstein R. (1989) Genetics 123, 725–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mumberg D., Müller R., Funk M. (1995) Gene 156, 119–122 [DOI] [PubMed] [Google Scholar]
- 31.Wach A., Brachat A., Pöhlmann R., Philippsen P. (1994) Yeast 10, 1793–1808 [DOI] [PubMed] [Google Scholar]
- 32.Ansaldi M., Lepelletier M., Méjean V. (1996) Anal. Biochem. 234, 110–111 [DOI] [PubMed] [Google Scholar]
- 33.Mumberg D., Müller R., Funk M. (1994) Nucleic Acids Res. 22, 5767–5768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Volland C., Urban-Grimal D., Géraud G., Haguenauer-Tsapis R. (1994) J. Biol. Chem. 269, 9833–9841 [PubMed] [Google Scholar]
- 35.Morvan J., Froissard M., Haguenauer-Tsapis R., Urban-Grimal D. (2004) Traffic 5, 383–392 [DOI] [PubMed] [Google Scholar]
- 36.Hicke L., Zanolari B., Riezman H. (1998) J. Cell Biol. 141, 349–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marchal C., Haguenauer-Tsapis R., Urban-Grimal D. (1998) Mol. Cell. Biol. 18, 314–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marchal C., Haguenauer-Tsapis R., Urban-Grimal D. (2000) J. Biol. Chem. 275, 23608–23614 [DOI] [PubMed] [Google Scholar]
- 39.Hein C., Springael J. Y., Volland C., Haguenauer-Tsapis R., André B. (1995) Mol. Microbiol. 18, 77–87 [DOI] [PubMed] [Google Scholar]
- 40.Spence J., Sadis S., Haas A. L., Finley D. (1995) Mol. Cell. Biol. 15, 1265–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu P., Duong D. M., Seyfried N. T., Cheng D., Xie Y., Robert J., Rush J., Hochstrasser M., Finley D., Peng J. (2009) Cell 137, 133–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blondel M. O., Morvan J., Dupré S., Urban-Grimal D., Haguenauer-Tsapis R., Volland C. (2004) Mol. Biol. Cell 15, 883–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katzmann D. J., Babst M., Emr S. D. (2001) Cell 106, 145–155 [DOI] [PubMed] [Google Scholar]
- 44.Haguenauer-Tsapis R., André B. (2004) Top. Curr. Genet. 9, 273–323 [Google Scholar]
- 45.Liu J., Sitaram A., Burd C. G. (2007) Traffic 8, 1375–1384 [DOI] [PubMed] [Google Scholar]
- 46.Vancura A., Sessler A., Leichus B., Kuret J. (1994) J. Biol. Chem. 269, 19271–19278 [PubMed] [Google Scholar]
- 47.Umebayashi K., Nakano A. (2003) J. Cell Biol. 161, 1117–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Catic A., Collins C., Church G. M., Ploegh H. L. (2004) Bioinformatics 20, 3302–3307 [DOI] [PubMed] [Google Scholar]
- 49.Bugnicourt A., Froissard M., Sereti K., Ulrich H. D., Haguenauer-Tsapis R., Galan J. M. (2004) Mol. Biol. Cell 15, 4203–4214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rubio-Texeira M., Kaiser C. A. (2006) Mol. Biol. Cell 17, 3031–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strochlic T. I., Setty T. G., Sitaram A., Burd C. G. (2007) J. Cell Biol. 177, 115–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kee Y., Lyon N., Huibregtse J. M. (2005) EMBO J. 24, 2414–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kee Y., Muñoz W., Lyon N., Huibregtse J. M. (2006) J. Biol. Chem. 281, 36724–36731 [DOI] [PubMed] [Google Scholar]
- 54.Dunn R., Klos D. A., Adler A. S., Hicke L. (2004) J. Cell Biol. 165, 135–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Katzmann D. J., Sarkar S., Chu T., Audhya A., Emr S. D. (2004) Mol. Biol. Cell 15, 468–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clague M. J., Urbé S. (2006) Trends Cell Biol. 16, 551–559 [DOI] [PubMed] [Google Scholar]
- 57.McCullough J., Clague M. J., Urbé S. (2004) J. Cell Biol. 166, 487–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barriere H., Nemes C., Du K., Lukacs G. L. (2007) Mol. Biol. Cell 18, 3952–3965 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.