Abstract
Phospholipase A2 catalyzes the specific hydrolysis of the sn-2 acyl bond of various glycerophospholipids, producing fatty acids and lysophospholipids. Phospholipase A2s (PLA2s) constitute a large superfamily of enzymes whose products are important for a multitude of signal transduction processes, lipid mediator release, lipid metabolism, development, plant stress responses, and host defense. The crystal structure of rice (Oryza sativa) isoform 2 phospholipase A2 has been determined to 2.0 Å resolution using sulfur SAD phasing, and shows that the class XIb phospholipases have a unique structure compared with other secreted PLA2s. The N-terminal half of the chain contains mainly loop structure, including the conserved Ca2+-binding loop, but starts with a short 310-helix and also includes two short anti-parallel β-strands. The C-terminal half is folded into three anti-parallel α-helices, of which the two first are also present in other secreted PLA2s and contain the conserved catalytic histidine and calcium liganding aspartate residues. The structure is stabilized by six disulfide bonds. The water structure around the calcium ion binding site suggests the involvement of a second water molecule in the mechanism for hydrolysis, the water-assisted calcium-coordinate oxyanion mechanism. The octanoate molecule in the complex structure is bound in a hydrophobic pocket, which extends to the likely membrane interface and is proposed to model the binding of the product fatty acid. Due to the differences in structure, the suggested surface for binding to the membrane has a different morphology in the rice PLA2 compared with other phospholipases.
Phospholipase A2 (PLA2)3 catalyzes the specific hydrolysis of the sn-2 acyl bond of various glycerophospholipids, producing fatty acids and lysophospholipids. PLA2s are widely distributed in nature and constitute a large superfamily of enzymes whose products are important for a multitude of signal transduction processes, lipid mediator release, lipid metabolism, and host defense (1). In plants they are implicated in plant growth, development, stress responses, and defense signaling (2–7). Translocation, secretion, and catalytic activation by many different stimuli control the activities of PLA2s. Based on sequence, and specific characteristics, 15 distinct groups of PLA2s are defined, and these are divided into 5 main clades within the superfamily: secreted sPLA2s, cytosolic cPLA2s, calcium-independent iPLA2s, platelet-activating factor acetylhydrolases, and the lysosomal PLA2s (8). The sPLA2s are small secreted proteins of 14–18 kDa that usually contain 5–8 disulfide bonds and an active site His/Asp dyad and are dependent on binding of Ca2+ ions for activity (1). The sPLA2s display different tissue distribution patterns and distinct physiological functions. The sPLA2s contain PLA2 groups I–III, V, and IX–XIV, including the snake venoms PLA2s (9) and the mammalian pancreatic PLA2s (10). Members of this family were first studied nearly 100 years ago, and a wealth of information on their structures and molecular action is available; to date, searching the Protein Data Bank for phospholipase A2 yields 230 structure hits, the majority of which refer to sPLA2s.
The eukaryotic sPLA2s all contain highly conserved Ca2+-binding loop (XCGXGG) and catalytic site (DXCCXXHD) motifs. The catalytic mechanism was originally proposed by Verheij et al. in 1980 (11) and subsequently modified from available crystal structures (12, 13). In the catalytic cycle, substrate hydrolysis proceeds through the activation and orientation of a water molecule by hydrogen bonding to the active site histidine. Adjacent to this histidine there is a conserved aspartate residue, which, together with main-chain carbonyl-oxygen atoms from the Ca2+-binding loop, acts as ligands for Ca2+. The calcium ion assists by polarizing the scissile bond and by stabilizing the negative charge developing in the transition state during phospholipid hydrolysis (14). More recently, a second water molecule, bridging the Ca2+-coordinated catalytic water to the active site histidine, has been inferred to be involved in catalysis, the water-assisted calcium-coordinate oxyanion mechanism of PLA2 (15–18). An interesting aspect of PLA2 catalysis is interfacial activation; i.e. the activity on monomeric substrates is low but hugely increases on aggregated substrates (19).
The three-dimensional structures of class I, II, and X PLA2s are very similar and are mainly folded into three long α-helices, a two-stranded β-sheet referred to as the β-wing, and a conserved calcium-binding loop (9–10, 12, 20–21). The same motifs are also present in the class III enzymes (13), although these proteins are more divergent. A structure of a prokaryotic sPLA2 (class XIV) showed a different, all α-helical fold (22), whereas structures of PLA2s from the other sPLA2 families have not yet been determined.
Only a few plant sPLA2s have been characterized (23–28), and these have been assigned as subfamilies XIa and XIb based on sequence alignments (7–8). In rice (Oryza sativa) a minimum of three isoforms of sPLA2 are present (24). Isoform 2 (class XIb) contains 128 amino acids preceded by a 25-amino acid signal peptide and contains the conserved active site and calcium ion binding motifs of sPLA2s but otherwise shows low homology to other classes of sPLA2 (24). Here we present the crystal structure of the mature isoform 2 PLA2 from rice (rPLA2) as well as that of a complex with octanoic acid.
EXPERIMENTAL PROCEDURES
Cloning and Expression
A synthetic cDNA coding for the predicted mature rPLA2 protein, amino acids 1–128 without the signal peptide, was designed with optimized codons for high level expression in Escherichia coli. The cDNA was generated by a sequence of five PCR reactions using ten synthesized single-stranded oligonucleotides, each about 60 nucleotides in length. These are listed as supplemental material in Table IS, and the cDNA sequence obtained for the mature rPLA2 is shown in supplemental Fig. S1a. The amplification started with the two most central oligonucleotides, 5′-5 and 3′-1, designed with 20 nucleotides overlap, being mixed and amplified with a 1 to 1 mixture of Taq and Pfu DNA polymerases. For each PCR cycle the growing fragment was extended by ∼40 nucleotides in each direction. After each PCR reaction the obtained DNA fragment was separated on agarose gel, excised, and purified, before use as template in the next PCR amplification. In the second PCR oligonucleotides 5′-4 and 3′-2 were used, in the third PCR 5′-3 and 3′-3, in the fourth PCR 5′-2 and 3′-4, and finally in the fifth PCR oligonucleotides 5′-1 and 3′-5 were used. The final DNA fragment was then amplified using two different 5′ PCR primers together with a 3′ PCR primer (supplemental Table IS) to obtain two constructs with slightly different N-terminal amino acid sequences. The A construct has a start methionine and an alanine in front of Asn-2 of the rPLA2 sequence, and the B construct has a start methionine and a glycine in front of Leu-1, see supplemental Fig. S1b. The two cDNA constructs were gel-purified, blunt-end-polished with T4 DNA polymerase, phosphorylated, and then blunt-end-cloned into the EcoRV-digested and dephosphorylated pETBlue-1 vector. The sequences of the inserts and the cloning borders with the plasmid were verified by sequence analysis.
The E. coli strain Tuner (DE3) LacI was used as host for the expression of the rPLA2 constructs. A preculture of 30 ml of Luria broth media containing 100 μg/ml ampicillin and 34 μg/ml chloramphenicol was inoculated from fresh single colonies and grown at 37 °C until the A600 reached ∼0.5. The preculture was used to inoculate 1 liter of the same media, which was grown until A600 reached 0.5–0.8 before isopropyl 1-thio-β-d-galactopyranoside was added to a final concentration of 1 mm. The culture was then grown for an additional 4 h after which the cells were harvested by 15-min centrifugation at 3000 × g at 4 °C, frozen, and stored at −80 °C until further use.
Refolding and Purification
Purification, solubilization, and refolding were done according to Valentin et al. (29) with some modifications. The cell pellet was thawed and resuspended in 40 ml of lysis buffer (50 mm Tris-HCl, pH 8.0, 50 mm NaCl, 2 mm EDTA, 0.1 mm phenylmethylsulfonyl fluoride, 1% Triton X-100, and 1% deoxycholate) by stirring for 1 h at 4 °C. Cells were broken by passing the cell slurry twice through a pre-cooled French press and an inclusion body pellet was obtained by centrifugation at 10,000 × g for 20 min. The inclusion body pellet was washed by four cycles of resuspension in lysis buffer (two times with and two times without detergents), followed by sonication for 10 intervals of 15 s on ice with 15 s of cooling in between. After each cycle the inclusion bodies were pelleted by centrifugation at 10,000 × g for 20 min. The washed inclusion body pellet was either used directly or frozen at −80 °C.
Cysteine residues of the rPLA2 were then sulfonated. The washed inclusion body pellet was dissolved to a concentration of ∼10 mg of wet pellet/ml in 50 mm Tris-HCl, pH 8.0, 6 m guanidine chloride, 0.3 m Na2SO3 by stirring at room temperature. A 0.05 volume of Thannhauser reagent (30) was added, and the solution was stirred at room temperature for a further 1 h. The extract was then dialyzed overnight against 50 volumes of 1% acetic acid at 4 °C. Precipitated sulfonated protein was collected by centrifugation at 10,000 × g for 20 min.
The protein pellet was dissolved to a protein concentration of 10 mg/ml in 50 mm Tris-HCl, pH 8.0, 6 m guanidine chloride at room temperature by end-over-end rotation for 1 h. The protein solution was added dropwise at one drop per second to refolding buffer (0.9 m guanidine chloride, 50 mm Tris-HCl, pH 8.0, 10 mm CaCl2, and 5 mm freshly added l-cysteine, 30% acetonitrile) to a final concentration of ∼0.02 mg/ml with constant stirring at room temperature. The refolding solution was then stirred for a few minutes before it was allowed to stand at room temperature for 2–3 days. The refolding was monitored by measuring PLA2 activity.
Lubrol PX 0.01% and 1 mm of methionine were added to the solution, which was then loaded into an Amicon-stirred cell with a YM-3 filter (Millipore) and concentrated ∼100-fold. The concentrate was dialyzed (cut-off of 10 kDa) overnight against 10 mm Tris/HCl, pH 8.0, containing 1 mm methionine. A freshly packed 10-ml Q-Sepharose Fast Flow (Amersham Biosciences) column was run with buffer A (20 mm Tris/HCl, pH 8.0, with 1 mm methionine and acetonitrile 20%) and buffer B (as buffer A plus 1 m NaCl) and eluted with a 30-ml gradient from 0 to 25% B. During elution 1-ml fractions were collected, and the fractions with peaking PLA2 activity were pooled.
The pooled Q-Sepharose Fast Flow eluate was injected onto a C4 reversed-phase high-performance liquid chromatography column (0.46 × 10.0 cm, Vydac, Hesperia, CA), previously equilibrated with 0.1% trifluoroacetic acid. The column was developed at 1.0 ml/min with a 30-min gradient (35–41% of acetonitrile in 0.1% trifluoroacetic acid). Absorbance was monitored at 214 and 280 nm, peak fractions were collected manually, and their pH was adjusted by adding 10 μl of Tris base per ml of eluate. The acetonitrile content was reduced by evaporation in a SpeedVac concentrator. The pooled fractions from several high-performance liquid chromatography runs were dialyzed against 10 mm Tris-HCl, pH 8.0, overnight at 4 °C, divided into aliquots, and stored at −80 °C. Purity of the sample was confirmed by SDS-PAGE and the protein concentration was calculated from the absorbance measured at 280 nm of a small volume of sample diluted in 20 mm sodium phosphate, pH 6.5, 6 m guanidine chloride, using the extinction coefficient 5120 m−1 cm−1. Prior to crystallization the protein was concentrated to 7–9 mg/ml in 10 mm Tris-HCl, pH 8.0, and filtered through a 0.1-μm centrifugal filter.
PLA2 Assay
PLA2 assays were performed according to Ståhl et al. (23). In brief, 10 nmol of 1-palmitoyl-2-[14C]oleoylphosphocholine dissolved in assay buffer (50 mm Tris-HCl, pH 8.0, 10 mm CaCl2, and 0.06% (w/v) Lubrol PX) was incubated with enzyme fractions (0.5–10 μl) in a total volume of 50 μl at 30 °C for 5 min. The reaction was stopped, and the lipids were extracted according to the method of Bligh and Dyer (31). The lipid-containing chloroform phases from PLA2 assays were applied to mini-columns of silica gel, and the chloroform eluates containing the 14C-labled free fatty acids were collected and their radioactivity was determined.
Crystallization
The B construct of the rPLA2 was crystallized by the sitting drop, vapor-diffusion method. The well contained a mixture of two solutions, A and B, with best results obtained by using 750 μl of A and 250 μl of B. Solution A contained 16–18% polyethylene glycol 3350, 0.1 m BisTris-propane, pH 6.5, and 0.2 m potassium thiocyanate. Solution B contained 0.1 m sodium acetate buffer, pH 4.6, and 2 m sodium chloride. The drop was comprised of 1.5 μl of well solution mixed with 1.5 μl of protein solution. After incubation for 24–48 h at 20 °C, crystallization was initiated by streak seeding, and incubation was continued at the same temperature. Crystals typically began to grow in seeded drops after 2–3 weeks and reached maximum size after ∼6 weeks. The A construct of the protein was crystallized in the same manner, with seeding performed using previously grown crystals of the B protein. In this case thin plate-like crystals grew after ∼8 weeks. Prior to data collection, both crystal forms were cryoprotected using either 20% glycerol or 25% ethylene glycol and were flash-frozen in liquid nitrogen.
The octanoic acid derivative was prepared by adding 0.5 μl of octanoic acid directly to a crystallization drop after crystal growth. After 8-h soaking the crystals from the drop were harvested, cryoprotected with 20% glycerol in the presence of 5 mm octanoic acid, and frozen as before.
Data Collection and Processing
All x-ray diffraction data were collected at the European Synchrotron Radiation Facility, Grenoble, France. The structure of the rice PLA2 was solved by sulfur-SAD phasing using data collected to 2.2-Å resolution from a single hexagonal crystal on beamline BM14, which is equipped with a Mar225 charge-coupled device detector. 395 degrees of data were collected at a wavelength of 1.771 Å using a 1° oscillation angle, to give a single highly redundant dataset. Higher resolution native data were later collected to a resolution of 2.0 Å on the micro focus beamline ID23-2, using a second crystal. Native data from the orthorhombic crystal form were collected on beamline ID29 to a resolution of 2.3 Å. In each case the data were processed using the HKL2000 suite (32). Where necessary, the scaled intensities were converted to CCP4 format using Scalepack2MTZ from the CCP4 suite (33), and structure factors were calculated using TRUNCATE. Data from the octanoic acid soaked crystals were collected on beamline ID14-1 at European Synchrotron Radiation Facility and processed using Mosflm (34) and SCALA from the CCP4 suite (33). Table 1 shows a summary of the statistics for each of the four datasets.
TABLE 1.
Data collection statistics
Data in parentheses are for the highest resolution shell.
| Data set | Sulfur SAD | High resolution | Octanoic acid soak | Crystal form 2 |
|---|---|---|---|---|
| Space group | P62 | P62 | P62 | P21212 |
| Unit cell (Å) | 108.5, 108.5, 41.54 | 108.7, 108.7, 41.65 | 109.2, 109.2, 41.39 | 169.1, 41.49, 53.12 |
| Molecules in asymmetric unit | 2 | 2 | 2 | 3 |
| Resolution (Å) | 50-2.2 (2.28-2.2) | 30-2.0 (2.07-2.0) | 30-2.0 (2.11-2.0) | 30-2.3 (2.42-2.3) |
| Rsym | 0.092 (0.320) | 0.079 (0.334) | 0.074 (0.237) | 0.090 (0.326) |
| 〈I〉/〈σI〉 | 39.1 (4.3) | 13.91 (1.96) | 12.6 (4.2) | 17.4 (5.4) |
| Completeness (%) | 99.1 (93.5) | 99.7 (98.0) | 98.4 (90.8) | 95.0 (93.2) |
| Multiplicity | 22.7 (12.5) | 3.4 (2.9) | 3.8 (2.4) | 4.8 (4.9) |
| Anomalous completeness (%) | 99.0 | |||
| Anomalous multiplicity | 12.4 |
Phasing, Model Building, and Refinement
Phases were obtained by sulfur-SAD phasing using the program SHELX (35). The protein contains 12 cysteines and 2 methionines, and there are two subunits in the asymmetric unit, thus it was not trivial to locate the sulfur positions. We performed numerous trials in SHELX with different resolution intervals and varying number of sulfurs and super-sulfurs to search for and different resolutions for refinement of the sites. Finally, searching for 18 sites using the data to 3.8 Å, we obtained a solution that showed a substantial difference between the two possible enantiomorphs in contrast (0.57 versus 0.48) and connectivity (0.91 versus 0.88) as well as map correlation upon refinement to 2.8 Å. However, the non-crystallographic symmetry relation between the sites could not readily be determined, and averaging could thus not be used for improving the un-interpretable electron density map obtained. The sites were then refined in PHASER_EP (36), and the phases obtained were modified with PIRATE (37). All versions of ARP/wARP (38), including quick fold, were run at different resolutions, but no sensible structure fragments were obtained. However, while looking at an electron density map at 3.2 Å we observed some electron density that vaguely resembled a stretch of α-helix, which we built with COOT (39). Fortunately, in the vicinity there were three sulfur sites that could correspond to a Cys-Cys-Met sequence in the protein and that allowed us to put in some sequence and define the direction of the helix. After this began an iterative process, using the CCP4 program suite (33), consisting of model building in COOT, refinement with REFMAC5 (40), phase-combination of the partial model and refined sulfur sites using PHASER_EP, and then phase improvement with PIRATE and, once the non-crystallographic symmetry operator became known, DM (41) with NCS. Tight NCS restraints were applied during refinement. After a few rounds, the electron density maps became of excellent quality allowing unambiguous chain tracing and sequence fitting. Final refinement with inclusion of water molecules was performed using the high resolution data set to 2.0 Å with medium main-chain and loose side-chain NCS restraints. A final check of the model building was performed by calculating a composite omit map in CNS (42).
The structure of the second crystal form was determined by molecular replacement in MOLREP (43), using the monomer of the refined P62 structure as the search model. The initial search placed two monomers in the asymmetric unit, but manual inspection of the resulting maps showed that a third monomer was present, albeit with weaker density. A second MOLREP search, performed after fixing the positions of both previously identified monomers, was successful in placing the final copy of the protein. The structure was refined with alternating cycles of restrained refinement in REFMAC5 (40) and manual inspection and model building in COOT. Initial refinement included the use of medium NCS restraints on all monomers, but this was later relaxed for chain C, in which crystal-packing effects result in slight differences for the other two chains. In the final stages of refinement, atomic displacement parameters were refined in REFMAC by the TLS (translation, libration, and screw) method (44) with each of the three monomers in the asymmetric unit treated as a single TLS group.
The structure of the octanoic acid complex was solved by molecular replacement, using the monomer of the refined P62 structure as the search model. Restrained refinement with NCS was performed in REFMAC5 and model building in COOT.
The geometry of each model was checked using the validation functions of COOT in addition to a final analysis in PROCHECK (45), and the fit between the data and model was assessed in SFCHECK (46). The results from refinement are detailed in Table 2. Structure alignments were carried out using the SSM superposition function in COOT, and LSQ in the program O (47). The figures were prepared by using PyMOL.4
TABLE 2.
Refinement and model building statistics
| Data set | Crystal form P62 | Crystal form P21212 | Octanoic acid soak |
|---|---|---|---|
| Refinement statistics | |||
| Reflections in working set | 18,172 | 15,596 | 18,061 |
| Reflections in test set | 986 | 846 | 963 |
| R-factor/R-free | 0.185/0.230 | 0.190/0.245 | 0.186/0.220 |
| Number of protein atoms | 1,826 | 2,477 | 1,757 |
| Number of calcium ions | 2 | 3 | 2 |
| Number of waters | 176 | 206 | 176 |
| Average B-factor | 23.68 | 26.49 | 21.67 |
| r.m.s.d. values from ideal | |||
| Bond lengths (Å) | 0.008 | 0.009 | 0.008 |
| Bond angles (°) | 1.021 | 1.195 | 1.005 |
| Ramachandran plot | |||
| Preferred regions | 95.98% | 96.24% | 95.35% |
| Allowed regions | 4.04% | 3.76% | 4.65% |
| Outliers | 0.0% | 0.0% | 0.0% |
Structure factors and the coordinates of the final models have been deposited in the Protein Data Bank (http://www.rcsb.org) with accession codes native P62: 2wg7; native P21212: 2wg8; and octanoic acid complex: 2wg9.
RESULTS AND DISCUSSION
Cloning, Refolding, and Purification
To produce enough material for crystallization trials of a plant sPLA2 a synthetic gene approach, with optimized codons for high levels of expression, was used to produce large quantities of recombinant mature rPLA2 enzyme in E. coli. This strategy has been successfully used for several animal sPLA2 enzymes (49–53). The synthetic gene (supplemental Fig. S1a) was generated by five consecutive PCR reactions using ten oligonucleotides (supplemental Table IS). To optimize the chance of obtaining correct refolding of the protein, removal of the start methionine was desired, and this is known to occur more likely with a small amino acid in the second position of the sequence (51). To achieve this, two different variants were generated with slightly different N-terminal amino acid sequences (supplemental Fig. S1b). In construct A, Leu-1 of rPLA2 was mutated to an alanine, whereas in construct B an extra glycine was inserted between the start methionine and Leu-1.
The proteins were produced in E. coli as inclusion bodies and therefore easily purified to a high level by simple washing of the inclusion body pellet. After solubilization in guanidine hydrochloride, and reduction and sulfonation of cysteine residues, the protein was refolded and purified. The yield of refolded and purified protein was ∼10 mg/liter E. coli expression culture, and both were shown to be homogeneous by SDS-PAGE. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry confirmed that the start methionine was removed from both constructs. The A and B variants of the mature rPLA2 showed similar specific activity after refolding, at best ∼33 μmol/min/mg.
Crystallization and Data Collection
Initial crystallization trials failed to produce crystals but did yield spherulites in a single condition for the B-construct. Crystals were ultimately achieved by using Hampton Crystal Screen HT as an additive screen, which resulted in the unusual crystallization condition described under “Experimental Procedures.” From the 96 conditions of the Hampton screen, 1 gave a single, small, cluster of crystals when mixed with the original well solution at a 1 to 3 ratio. Streak seeding gave the single crystals that were used for data collection. The hexagonal, rod-shaped crystals grew to approximate maximum dimensions of 20 × 20 × 80 μm and diffracted in space group P62 or P64 with unit cell dimensions of a = 108.67 Å, b = 108.67 Å, and c = 41.65 Å. The asymmetric unit contained two monomers, and the solvent content of the crystals has been estimated at 52%.
Crystals of the A construct of the protein were obtained from cross-seeding with the hexagonal crystals obtained with the B construct and using the same crystallization conditions. The crystals grew to approximate maximum dimensions of 80 × 40 × 5 μm and diffracted in space group P21212 with unit cell dimensions of a = 169.09Å, b = 41.49 Å, and c = 53.12 Å. The asymmetric unit contained three monomers, and the solvent content of the crystals has been estimated at 45%.
Quality of the Electron Density Map and Model
The final electron density maps from the 2.0-Å resolution data in the P62 space group, containing two subunits in the asymmetric unit, allowed unambiguous tracing of residues 13–125 in both subunits with the exception of some flexible side chains at the surface. In addition, the positions of the main chain of residues 2–7 at the N terminus could be defined. Two calcium ions, 176 bound water molecules, and 1 sodium ion on the surface of the molecule were modeled. The resulting R and Rfree values were 18.4 and 23.0, respectively. The geometry of the model is good with 96% of the residues in the most favored regions and none in the disallowed regions of the Ramachandran plot. The two subunits superimpose with an r.m.s.d. of 0.28 Å for all main-chain atoms. In the orthorhombic space group, containing three subunits in the asymmetric unit, residues 15–121 in all three subunits are defined and very similar to those in the hexagonal lattice (r.m.s.d. of ∼0.5 Å for the main-chain atoms, except subunit C, which is less ordered and distorted by packing interactions in the crystal) and the N-terminal residues 1–8 could be modeled in subunit B. Three calcium ions and 206 water molecules were modeled. The subunit structures in the octanoic acid complex were virtually identical to the unliganded structure (r.m.s.d. of ∼0.23 Å for the main-chain atoms), but the N-terminal residues were not defined. The refinement results are shown in (Table 2). Due to the higher resolution of the data in the hexagonal space group, we will use this in the description of the structure.
Overall Structure
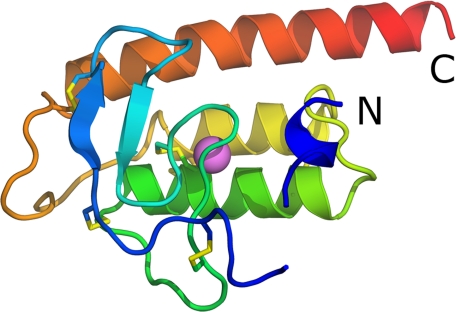
A schematic picture of the structure of rPLA2 is shown in Fig. 1. The chain starts with a weakly defined 310-helix (residues 2–7), which makes few interactions to the rest of the molecule and which is stabilized by crystal contacts. After a five-residue gap, for which no electron density is visible, the chain continues with a loop (residues 12–21) held in place by two disulfide bonds, Cys17–Cys45 and Cys21–Cys51. Following two short anti-parallel β-strands (residues 22–25 and 32–35 respectively), the chain forms the Ca2+-binding loop (residues 36–44). After a surface loop, the remainder of the protein chain is folded into three anti-parallel α-helices (residues 52–69, 74–88 and 99–125 respectively) with connecting loops. The last of these helices is particularly long and its C-terminal protrudes from the core of the structure. All 12 cysteines participate in disulfide bonds; Cys17-Cys45, Cys21–Cys51, Cys26–Cys98, which connect the N- and C-terminal part of the protein, Cys38–Cys58 anchors the Ca2+-binding loop to α-helix 1, and Cys57–Cys84 and Cys64–Cys77, which tether α-helices 1 and 2. In the crystal, the two monomers in the asymmetric unit form a dimer burying a surface area of about 1000 Å2. This positions the openings to the two active sites at opposite ends of the dimer. Because there are no hints from gel-filtration experiments that the enzyme is dimeric, it does not seem likely that the dimer observed in the asymmetric unit is physiologically relevant. Another subunit-subunit interaction, observed in the hexagonal lattice and for one subunit in the orthorhombic crystal, is obtained by crystal symmetry and is made by the hydrophobic surface of the amphiphilic N-terminal 310-helix of one subunit and the hydrophobic tunnel leading to the active site of a neighboring subunit. At high concentrations of rPLA2, this interaction might become of importance by blocking the catalytic site. This N-terminal 310-helix is obviously quite dynamic, and we believe it is involved in anchoring the rPLA2 to the membrane interface as discussed below.
FIGURE 1.
Schematic image of rPLA2 colored from blue at the N terminus to red at the C terminus. Disulfides are shown as sticks, and the calcium ion is shown as a pink sphere.
Comparison to Other PLA2 Structures
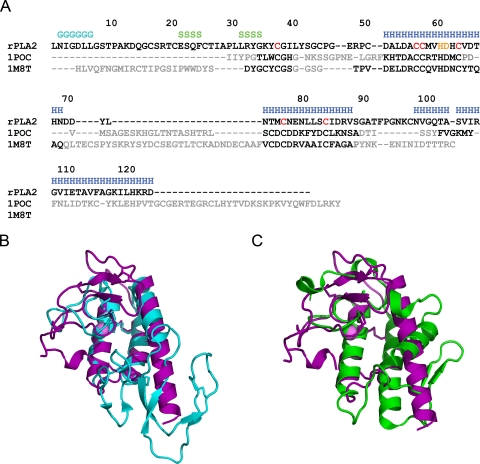
The structure of rPLA2 is distinct from those of other sPLA2s. A DALI search (54) against the Protein Data Bank gives the highest scores to vacuolar protein sorting factor 4a, toxin B, and vacuolar protein sorting-associated protein 4 due to the similar orientations and lengths of the three α-helices between these structures. In the fourth position is a group III PLA2, bee venom PLA2 (PDB id 1poc) (13), which has an r.m.s.d. from DALI of 2.9 Å for 80 superposed Cα atoms with 15% sequence identity. Number 13 in the list is PLA2 from the venom of Ophiophagus hannah (PDB id 1m8t) (55) with an r.m.s.d. of 3.0 Å for 62 superposed Cα atoms with 29% sequence identity. In both cases α-helices 1 and 2 superpose well, and the general location of the Ca2+ binding loop is conserved (Fig. 2). Although the Ca2+-binding loop of rPLA2 contains a one-residue insertion compared with other eukaryotic PLA2s, and thus has a different conformation, the Ca2+-ion is bound in the same position. The 30 first residues of rPLA2 are not present in the class III bee venom PLA2. The C-terminal third helix is present in both proteins but is much longer in rPLA2, whereas the bee PLA2 instead continues with a very long β-hairpin-like structure (Fig. 2B). The C-terminal α-helix 3 of rPLA2 is in the same location as the shorter N-terminal α-helix in the class I, II, and X sPLA2s but is inthe reverse direction and with different orientation of the axis (Fig. 2C). This arrangement positions the N and C termini of rPLA2 on opposite sides of the active site compared with these sPLA2s. Furthermore, the so-called β-wing, present in other eukaryotic sPLA2s, is absent from rPLA2. The structure of rPLA2 is thus different from the known sPLA2s. Three disulfides are conserved in all three proteins, Cys38–Cys58 anchoring the Ca2+-binding loop to α-helix 1 and Cys57–Cys84 and Cys64–Cys77 between α-helices 1 and 2. The only other conserved residues between all three proteins are the catalytic His61, the Ca2+-ligand Asp62, and Gly39 and Asp55, which are important for maintaining the structure of the Ca2+-binding loop.
FIGURE 2.
A, a structure-based alignment of rPLA2 with bee venom PLA2 (1poc) and O. hannah (1m8t). Secondary structure assignments are given for the rPLA2, and residues with pairwise r.m.s.d. of <2.5 Å are colored black in the 1poc and 1m8t sequences. The cysteines that comprise the three conserved disulfide bonds are highlighted in red, and the catalytic histidine and aspartic acid are in orange. B, superposition of rPLA2 in purple with PDB id 1poc in cyan. Disulfides are shown as sticks. C, superposition of rPLA2 in purple with PDB id 1m8t in green. Disulfide are shown as sticks.
Active Site and Substrate Channel
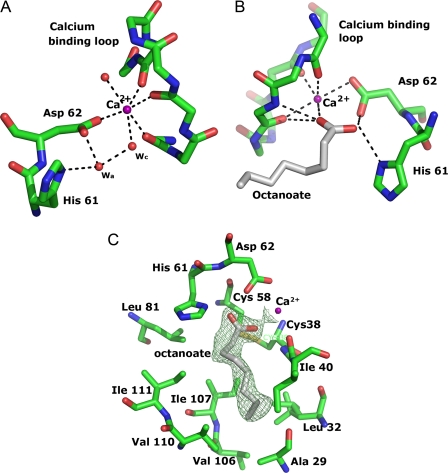
Besides the conserved Asp62, the Ca2+-ion is ligated by the main-chain carbonyl oxygen atoms of Tyr37, Gly39, and Tyr41 from the Ca2+-binding loop, and two water molecules (Fig. 3A). The Ca2+-bound water molecule, which acts as a nucleophile in the reaction, is in turn hydrogen-bonded to three other water molecules. The positions of these waters are similar in the two monomers of the asymmetric unit, but the density indicates that their occupancies differ. Strong density is observed in both subunits for the Ca2+-bound water and for another that is bound to it. In subunit A, clear density is observed for a third, which interacts with the Ca2+-bound water, the side chains of His61 and Asp62 and the main-chain carbonyl of Cys58, whereas weak density suggests the presence of a fourth water molecule interacting only with the other waters. In subunit B the latter has strong density, whereas very little density is seen for the water coordinating His61, Asp62, and Cys58. The water at this position is interesting, because it corresponds to the proposed assisting water molecule, and its presence supports the water-assisted calcium-coordinate oxyanion mechanism of PLA2 (15–18). However, this assisting water has been assumed to be present only in the activated form of sPLA2s (56) and to be the result of the interfacial activation process (57). In rPLA2, His61 is held in its proper orientation by interaction with the side-chain oxygen atom of Asn78, which substitutes the aspartate residue in the catalytic Asp/His dyad of other eukaryotic sPLA2s. Asn78 is replaced by a serine residue in some of the other plant sPLA2 enzymes, and substitution of this serine to alanine or aspartate in the Arabidopsis thaliana PLA2-α resulted in considerable loss of activity (58) confirming the importance of this interaction. The hydrophobic part of the active site is lined by the disulfide Cys38–Cys58, together with residues Leu32, Ile40, Leu81, Ile85, Val106, Ile107, Val110, and Ile111 (Fig. 3C) at the inner surface. It is then extended by a hydrophobic channel (Fig. 4A), comprising residues Ala29, Pro30, Val65, Tyr72, Leu41, and Ala114, that leads to what we propose is the interfacial binding site. The hydrophobic character of the residues in this crevice and lining the active site is conserved in the whole class XIb, but their identities vary, which might give rise to differences in substrate preference. In other eukaryotic sPLA2s there is a “flap” residue, a tyrosine or a lysine, that is suggested to facilitate the dynamic transfer of substrates from the membrane to the active site and to bind the phospholipid phosphate head-group in the transition state (48). In a corresponding spatial position in rPLA2 is Tyr72, which is conserved in the class XIB sPLA2s. In the ligand-free enzyme it is well defined but with some disorder in the side chain, stabilized by interactions with the N-terminal 310-helix of a symmetry related subunit that binds with its hydrophobic face to the channel (Fig. 4A). Changing the rotamer conformation of Tyr72 to that most commonly observed in structures of sPLA2s would put the hydroxyl group in the same position as in the transition state analogue complex PDB id 1mkv (48), interacting with the phospholipid head-group. In the octanoate complex, where this 310-helix is disordered, the Tyr72 side chain is also more or less disordered.
FIGURE 3.
A, the catalytic residue His61 and bound Ca2+ with ligands. Only the main-chain atoms are shown for the Ca2+-binding loop. Polar interactions are shown with dashed lines. The catalytic water and the assisting water are labeled wc and wa, respectively. B, octanoate bound to Ca2+. Polar interactions are shown with dashed lines. C, the hydrophobic walls of the active-site cleft with bound octanoate. The unbiased 2Fo − Fc map at 1.2σ is shown as green mesh.
FIGURE 4.
A, transparent surface of the hydrophobic crevice in rPLA2, colored according to atom type with carbon in gray, oxygen in red, nitrogen in blue, sulfur in yellow, and Ca2+ in green. Side chains in are shown as sticks. The N-terminal tail from a neighboring subunit, with green carbon atoms, is packing against the opening of the crevice. B, surface of the suggested i-face of rPLA2 viewed from the membrane and colored according to atom type with carbon in gray, oxygen in red, nitrogen in blue, sulfur in yellow, and Ca2+ in green. Residues suggested to reside at the interface are shown as sticks. The octanoate molecule is shown in green sticks for orientation.
Octanoic Acid Complex
Unbiased 2Fo − Fc electron density maps clearly defined the binding mode of octanoate in the crystals of the complex (Fig. 3C). Binding of the product molecule octanoic acid does not introduce any large structural changes in the protein. The N-terminal residues are not defined in the complex structure, although the resolution and packing are the same as for the unbound structure, the binding of octanoate might interfere with this subunit interaction. The octanoate molecule binds in the active site (Fig. 3B), with one carboxylate oxygen replacing the catalytic water as ligand to the Ca2+-ion and the second carboxylate oxygen substituting the assisting water molecule (Fig. 3B), which is in accordance with the mechanism with the Ca2+-bound water molecule as the nucleophile attacking the ester bond. The position of the carboxylate group of octanoate is very similar to the phosphate group of the transition state analog in PDB id 1mkv (48), and in PDB id 1pob (12), and to the inhibitor MJ33 in PDB id 1poc (13), but different from that in PDB id 1fxp (56).
The hydrophobic tail of octanoate makes favorable interactions to the hydrophobic walls of the active site, residues Ala29, Gly39, Ile40, Cys58, Leu81, Ile107, and Ile111, replacing several water molecules in the structure of the unbound active site crevice (Fig. 3C). It seems likely that the structure represents the true conformation of a bound fatty acid product.
The Membrane Interface Site
The hydrophobic crevice described above allows the transfer of phospholipid substrates from the membrane to the active site. The channel leads to a predominantly hydrophobic outer surface, which is likely to face the lipid-aqueous interface of the membrane or substrate aggregates. The flexible N-terminal 310-helix, part of the α-helical C-terminal, Tyr72 and Leu73 in the loop between α-helix 1 and 2, and Phe25, Ile28, and Leu31 in the loop between the two anti-parallel β-strands, constitute the most probable interacting surface, the i-face (Fig. 4B). These parts of the structure also display the highest flexibility as indicated by the main-chain B-factors. The surface formed by these residues is not flat; it is rather concave with Tyr72 in the middle of the surface. The conserved residues Tyr72, the “flap residue,” and Arg33, basic residues at the C terminus, also present in the whole class, and conserved polar residues of the dynamic N-terminal 310-helix, might be the residues anchoring the enzyme to the polar head-groups at the membrane surface. Upon close contact of the i-face to the membrane, desolvation, and perhaps curvature of the membrane, allows for tight association, which permits the substrate to diffuse into the active site. Due to the different architectures of the rPLA2 and other sPLA2s, this i-face is somewhat different from that defined by e.g. the crystal structure of the anion-assisted dimer of porcine pancreatic PLA2 (56). It has been suggested that the allosteric coupling between the i-face and the active site is mediated by hydrogen-bond networks (57) and that the resulting higher activity at the interface is due to the presence of the “assisting water” (56). Similar hydrogen-bond networks are present in rPLA2, however, in rPLA2, the assisting water appears to be present already in the un-activated form, and the activation process remains unknown, unless the interaction with the neighboring subunit N terminus (Fig. 4B) induces an activated state.
Supplementary Material
Acknowledgments
We gratefully acknowledge the European Synchrotron Radiation Facility (ESRF) for beam time allocation and the staff of ESRF beamline BM14 for assistance with sulfur-SAD data collection.
This work was supported by a grant from the Swedish Research Council (to Y. L.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1 and Table IS.
The atomic coordinates and structure factors (codes 2wg7, 2wg8 and 2wg9) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
W. L. DeLano (2002) PyMOL, DeLano Scientific, San Carlos, CA.
- PLA2
- phospholipase A2
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- SAD
- single-wavelength anomalous dispersion
- sPLA2
- secretory PLA2.
REFERENCES
- 1.Six D. A., Dennis E. A. (2000) Biochim. Biophys. Acta 1488, 1–19 [DOI] [PubMed] [Google Scholar]
- 2.Chapman K. D. (1998) Trends Plant Sci. 3, 419–426 [Google Scholar]
- 3.Munnik T., Irvine R. F., Musgrave A. (1998) Biochim. Biophys. Acta 1389, 222–272 [DOI] [PubMed] [Google Scholar]
- 4.Scherer G. F. E. (2002) Plant Mol. Biol. 49, 357–372 [PubMed] [Google Scholar]
- 5.Ryu S. B. (2004) Trends Plant Sci. 9, 229–235 [DOI] [PubMed] [Google Scholar]
- 6.Wang X. (2004) Curr. Opin. Plant Biol. 7, 329–336 [DOI] [PubMed] [Google Scholar]
- 7.Lee H. Y., Bahn S. C., Shin J. S., Hwang I., Back K., Doelling J. H., Ryu S. B. (2005) Prog. Lipid Res. 44, 52–67 [DOI] [PubMed] [Google Scholar]
- 8.Schaloske R. H., Dennis E. A. (2006) Biochim. Biophys. Acta 1761, 1246–1259 [DOI] [PubMed] [Google Scholar]
- 9.Brunie S., Bolin J., Gewirth D., Sigler P. B. (1985) J. Biol. Chem. 260, 9742–9749 [PubMed] [Google Scholar]
- 10.Dijkstra B. W., Kalk K. H., Hol W. G., Drenth J. (1981) J. Mol. Biol. 147, 97–123 [DOI] [PubMed] [Google Scholar]
- 11.Verheij H. M., Slotboom A. J., de Haas G. H. (1981) Rev. Physiol. Biochem. Pharmacol. 91, 91–203 [DOI] [PubMed] [Google Scholar]
- 12.White S. P., Scott D. L., Otwinowski Z., Gelb M. H., Sigler P. B. (1990) Science 250, 1560–1563 [DOI] [PubMed] [Google Scholar]
- 13.Scott D. L., Otwinowski Z., Gelb M. H., Sigler P. B. (1990) Science 250, 1563–1566 [DOI] [PubMed] [Google Scholar]
- 14.Dennis E. A. (1994) J. Biol. Chem. 269, 13057–13060 [PubMed] [Google Scholar]
- 15.Rogers J., Yu B. Z., Serves S. V., Tsivgoulis G. M., Sotiropoulos D. N., Ioannou P. V., Jain M. K. (1996) Biochemistry 35, 9375–9384 [DOI] [PubMed] [Google Scholar]
- 16.Yu B. Z., Rogers J., Nicol G. R., Theopold K. H., Seshadri K., Vishweshwara S., Jain M. K. (1998) Biochemistry 37, 12576–12587 [DOI] [PubMed] [Google Scholar]
- 17.Epstein T. M., Yu B.-Z., Pan Y. H., Tutton S. P., Maliwal B. P., Jain M. K., Bahnson B. J. (2001) Biochemistry 40, 11411–11422 [DOI] [PubMed] [Google Scholar]
- 18.Edwards S. H., Thompson D., Baker S. F., Wood S. P., Wilton D. C. (2002) Biochemistry 41, 15468–15476 [DOI] [PubMed] [Google Scholar]
- 19.Berg O. G., Gelb M. H., Tsai M. D., Jain M. K. (2001) Chem. Rev. 101, 2613–2654 [DOI] [PubMed] [Google Scholar]
- 20.Wery J. P., Schevitz R. W., Clawson D. K., Bobbitt J. L., Dow E. R., Gamboa G., Goodson T., Jr., Hermann R. B., Kramer R. M., McClure D. B., et al. (1991) Nature 352, 79–82 [DOI] [PubMed] [Google Scholar]
- 21.Pan Y. H., Yu B. Z., Singer A. G., Ghomashchi F., Lambeau G., Gelb M. H., Jain M. K., Bahnson B. J. (2002) J. Biol. Chem. 277, 29086–29093 [DOI] [PubMed] [Google Scholar]
- 22.Matoba Y., Katsube Y., Sugiyama M. (2002) J. Biol. Chem. 277, 20059–20069 [DOI] [PubMed] [Google Scholar]
- 23.Ståhl U., Ek B., Stymne S. (1998) Plant Physiol. 117, 197–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ståhl U., Lee M., Sjödahl S., Archer D., Cellini F., Ek B., Iannacone R., MacKenzie D., Semeraro L., Tramontano E., Stymme S. (1999) Plant Mol. Biol. 41, 481–490 [DOI] [PubMed] [Google Scholar]
- 25.Kim J. Y., Chung Y. S., Ok S. H., Lee S. G., Chung W. I., Kim I. Y., Shin J. S. (1999) Biochim. Biophys. Acta 1489, 389–392 [DOI] [PubMed] [Google Scholar]
- 26.Lee H. Y., Bahn S. C., Kang Y. M., Lee K. H., Kim H. J., Noh E. K., Palta J. P., Shin J. S., Ryu S. B. (2003) Plant Cell 15, 1990–2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bahn S. C., Lee H. Y., Kim H. J., Ryu S. B., Shin J. S. (2003) FEBS Lett. 553, 113–118 [DOI] [PubMed] [Google Scholar]
- 28.Mansfeld J., Ulbrich-Hofmann R. (2007) Chem. Phys. Lipids 150, 156–166 [DOI] [PubMed] [Google Scholar]
- 29.Valentin E., Singer A. G., Ghomashchi F., Lazdunski M., Gelb M. H., Lambeau G. (2000) Biochem. Biophys. Res. Commun. 279, 223–228 [DOI] [PubMed] [Google Scholar]
- 30.Thannhauser T. W., Konishi Y., Scheraga H. A. (1984) Anal. Biochem. 138, 181–188 [DOI] [PubMed] [Google Scholar]
- 31.Bligh E. G., Dyer W. J. (1959) J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 32.Otwinowski Z., Minor W. (1997) Methods Enzymol. 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 33.CCP4 (1994) Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 [DOI] [PubMed] [Google Scholar]
- 34.Leslie A. G. W. (1992) Joint CCP4 + ESF-EAMCB Newsletter on Protein Crystallography, No. 26 [Google Scholar]
- 35.Sheldrick G. M. (2008) Acta Crystallogr. A 64, 112–122 [DOI] [PubMed] [Google Scholar]
- 36.McCoy A. J., Grosse-Kunstleve R. W., Adams P. D., Winn M. D., Storoni L. C., Read R. J. (2007) J. Appl. Crystallogr. 40, 658–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cowtan K. (2000) Acta Crystallogr. D Biol. Crystallogr. 56, 1612–1621 [DOI] [PubMed] [Google Scholar]
- 38.Perrakis A., Morris R., Lamzin V. S. (1999) Nat. Struct. Biol. 6, 458–463 [DOI] [PubMed] [Google Scholar]
- 39.Emsley P., Cowtan K. (2004) Acta Crystallogr. D Biol. Crystallogr. 60, (Pt. 12 Pt. 1) 2126–2132 [DOI] [PubMed] [Google Scholar]
- 40.Murshudov G. N., Vagin A. A., Dodson E. J. (1997) Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 [DOI] [PubMed] [Google Scholar]
- 41.Cowtan K. (1994) Joint CCP4 and ESF-EACBM Newsletter on Protein Crystallography 31, 34–38 [Google Scholar]
- 42.Brünger A. T., Adams P. D., Clore G. M., DeLano W. L., Gros P., Grosse-Kunstleve R. W., Jiang J. S., Kuszewski J., Nilges M., Pannu N. S., Read R. J., Rice L. M., Simonson T., Warren G. L. (1998) Acta Crystallogr. D Biol. Crystallogr. 54, (Pt. 5) 905–921 [DOI] [PubMed] [Google Scholar]
- 43.Vagin A., Teplyakov A. (1997) J. Appl. Crystallogr. 30, 1022–1025 [Google Scholar]
- 44.Winn M. D., Isupov M. N., Murshudov G. N. (2001) Acta Crystallogr. D 57, 122–133 [DOI] [PubMed] [Google Scholar]
- 45.Laskowski R. A., MacArthur M. W., Moss D. S., Thornton J. M. (1993) J. Appl. Crystallogr. 26, 283–291 [Google Scholar]
- 46.Vaguine A. A., Richelle J., Wodak S. J. (1999) Acta Crystallogr. D Biol. Crystallogr. 55, 191–205 [DOI] [PubMed] [Google Scholar]
- 47.Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. (1991) Acta Crystallogr. A 47, 110–119 [DOI] [PubMed] [Google Scholar]
- 48.Sekar K., Kumar A., Liu X., Tsai M. D., Gelb M. H., Sundaralingam M. (1998) Acta Crystallogr. D Biol. Crystallogr. 54, 334–341 [DOI] [PubMed] [Google Scholar]
- 49.Kelley M. J., Crowl R. M., Dennis E. A. (1992) Biochim. Biophys. Acta 1118, 107–115 [DOI] [PubMed] [Google Scholar]
- 50.Dudler T., Chen W. Q., Wang S., Schneider T., Annand R. R., Dempcy R. O., Crameri R., Gmachl M., Suter M., Gelb M. H. (1992) Biochim. Biophys. Acta 1165, 201–210 [DOI] [PubMed] [Google Scholar]
- 51.Othman R., Baker S., Li Y., Worrall A. F., Wilton D. C. (1996) Biochim. Biophys. Acta 1303, 92–102 [DOI] [PubMed] [Google Scholar]
- 52.Han S. K., Lee B. I., Cho W. (1997) Biochim. Biophys. Acta 1346, 185–192 [DOI] [PubMed] [Google Scholar]
- 53.Han S. K., Yoon E. T., Cho W. (1998) Biochem. J. 331, 353–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holm L., Kääriäinen S., Rosenström P., Schenkel A. (2008) Bioinformatics 24, 2780–2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu S., Gu L., Wang Q., Shu Y., Song S., Lin Z. (2003) Acta Crystallogr. 59, 1574–1581 [DOI] [PubMed] [Google Scholar]
- 56.Pan Y. H., Epstein T. M., Jain M. K., Bahnson B. J. (2001) Biochemistry 40, 609–617 [DOI] [PubMed] [Google Scholar]
- 57.Jain M. K., Berg O. G. (2006) Curr. Opin. Chem. Biol. 10, 473–479 [DOI] [PubMed] [Google Scholar]
- 58.Mansfeld J., Gebauer S., Dathe K., Ulbrich-Hofmann R. (2006) Biochemistry 45, 5687–5694 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.