Abstract
Mast cell degranulation is a highly regulated, calcium-dependent process, which is important for the acute release of inflammatory mediators during the course of many pathological conditions. We previously found that Synaptotagmin-2, a calcium sensor in neuronal exocytosis, was expressed in a mast cell line. We postulated that this protein may be involved in the control of mast cell-regulated exocytosis, and we generated Synaptotagmin-2 knock-out mice to test our hypothesis. Mast cells from this mutant animal conferred an abnormally decreased passive cutaneous anaphylaxis reaction on mast cell-deficient mice that correlated with a specific defect in mast cell-regulated exocytosis, leaving constitutive exocytosis and nonexocytic mast cell effector responses intact. This defect was not secondary to abnormalities in the development, maturation, migration, morphology, synthesis, and storage of inflammatory mediators, or intracellular calcium transients of the mast cells. Unlike neurons, the lack of Synaptotagmin-2 in mast cells was not associated with increased spontaneous exocytosis.
Mast cells (MCs)2 participate in adaptive and innate immune responses. Their secreted products play important roles in immunoglobulin E (IgE)-dependent inflammatory reactions such as allergic asthma and anaphylaxis (1) and are also involved in other forms of inflammation such as immune arthritis (2, 3) and innate immune responses to bacterial infections (4, 5). Upon activation, MCs exhibit three main secretory responses: release of granule contents (i.e. degranulation), secretion of prostaglandins and leukotrienes, and secretion of cytokines and growth factors (6). The exocytic release of preformed mediators (e.g. histamine and proteases) stored in secretory granules is immediate and regulated at the step of fusion between the membrane of the granule and the plasma membrane. Thus, it is an example of regulated exocytosis, like neuronal synaptic neurotransmitter release and insulin secretion (7). Another early event is the release of metabolites of arachidonic acid (e.g. prostaglandin D2 (PGD2) and leukotriene C4 (LTC4)). These eicosanoids cross the plasma membrane using transmembrane transporters (8), and their production is regulated by the activation of their synthetic enzymes (9). A late response after MC activation is the secretion of cytokines and growth factors (e.g. tumor necrosis factor-α (TNF-α) and interleukin-4 (IL-4)). The gap in time of minutes to hours between stimulation and the secretion of these mediators is explained by the fact that regulation is at the transcriptional and post-transcriptional levels, with secretion occurring via constitutive exocytosis (10).
A common intracellular mediator linking the stimulation event to these three MC responses is calcium (Ca2+) that is released into the cytoplasm from intracellular stores and introduced from the extracellular environment via specialized channels. Increase in the cytoplasmic concentration of Ca2+ ([Ca2+]i) is required for the activation of phospholipase A2 and other enzymes in the synthetic pathway of prostaglandins and leukotrienes, for the expression of cytokines and growth factors, and for the regulated exocytosis of MC secretory granules (11).
MC degranulation is a highly regulated process, and, like other exocytic events, it requires the participation of soluble NSF (N-ethylmaleimide-sensitive fusion protein) adaptor protein receptor (SNARE) proteins. These are present both on the cytoplasmic surfaces of secretory vesicles (v-SNAREs such as vesicle-associated membrane protein, VAMP) and of the plasma membrane (target or t-SNAREs such as Syntaxin and synaptosome-associated protein of 25 kDa, SNAP-25). Cognate v- and t-SNAREs form a highly stable quadruple α-helix complex (core complex) apposing the vesicle and target membranes. According to the “zipper hypothesis” the coiling of this complex drives the fusion between these membranes, opening a fusion pore and releasing the vesicle contents into the extracellular space (12). This step is Ca2+-dependent and is under the control of multiple proteins such as Synaptotagmin (Syt) (13).
There are at least 15 Syts in mammals, and several of them mediate Ca2+-dependent exocytosis. All Syts have a short intravesicular N terminus, a transmembrane domain, a variable linker region, and two conserved C2 domains (C2A and C2B) at their C terminus. The binding of Ca2+ to the C2 domains alters their electrostatic surface charge and mediates most of the Ca2+-dependent functions of Syt (13). The C2A domains of Syt1, -2, and -9 bind Ca2+ with low affinity and high cooperativity. These Syts were first identified in neurons where they control fast, synchronous, Ca2+-dependent synaptic vesicle exocytosis. Syt1 mediates this form of exocytosis in forebrain neurons (14) and Syt9 in limbic and striatal neurons (15). Both of them also mediate rapid exocytosis of dense core granules in adrenal chromaffin cells. We showed that Syt2 functions as the Ca2+ sensor for fast synchronous synaptic vesicle release in hindbrain neurons and at the neuromuscular junction and that deletion of Syt2 produces a severe neurologic phenotype that results in death during the third week of postnatal life (16, 17). These low Ca2+ affinity Syts also inhibit spontaneous and slow asynchronous vesicle exocytosis in the neurons where they are expressed (14–17). We previously identified Syt2 as the major Syt isoform expressed in the RBL-2H3 MC line (18). Here we study the effects of Syt2 deficiency in MC secretion in vivo. We show that Syt2-null mice contain normal MCs in number and morphology in their peritoneal cavity and skin and that MCs can be generated from the bone marrow of these mutant mice. Although no defects were found in IgE-dependent generation and secretion of cytokines and eicosanoids, the MCs in our Syt2-null mice had a marked deficiency in the exocytosis of their preformed granule mediators.
EXPERIMENTAL PROCEDURES
Animals and Cells
MC-deficient and Syt2 knock-out mice have been described (17, 19). We produced all experimental animals and littermate controls by heterozygous crossings of a Syt2+/− line that we backcrossed 13 times into C57BL/6. We harvested and purified peritoneal MCs as described (4). We harvested bone marrow from femurs of postnatal day 16 (P16) mice to generate bone marrow-derived MCs (BMMCs). Cells were grown in RPMI 1640 (with 1% fetal calf serum, 100 μm Hepes buffer pH 7.3, 1 mm sodium pyruvate, 1× nonessential amino acids, and 0.1% β-mercaptoethanol) supplemented with mIL-3 5 ng/ml and stem cell factor (SCF) 100 ng/ml for 6 weeks. We followed the viability of BMMCs by Trypan Blue exclusion, and their maturation and differentiation by flow cytometry using labeled antibodies against the α-subunit of the high affinity receptor for IgE (FcϵRIα; eBioscience) and cKit/CD117 (BD Pharmingen).
Immunohistochemistry and Immunoblotting
Cytospins of peritoneal MCs were postfixed with cold acetone, blocked with 3% bovine serum albumin/0.4% Triton X-100, incubated first with rabbit anti-Syt2 antibody 1:500 (A320; Dr. T. C. Südhof, Stanford University School of Medicine, Stanford, CA) (17) for 16 h at 4 °C, then with peroxidase-conjugated antirabbit antibody (Jackson Immunoresearch) for 2 h at room temperature, and finally with 3,3′-diaminobenzidine (DAB; Vector). We used the same antibody for the immunoblotting of peritoneal MC lysates resolved by SDS-PAGE and transferred to nitrocellulose membranes. GAPDH (glyceraldehyde-3-phosphate dehydrogenase), detected with a specific antibody (Abcam), was used as a loading control.
Microscopy
We harvested and processed tissues, peritoneal lavage, and electron microscopy (EM) samples from P16 mice as described (4). In tissue sections, we stained MC secretory granules with fluorescein isothiocyanate (FITC)-avidin and nuclei with Hoechst (Invitrogen-Molecular Probes), and then counted nucleated MC profiles (FITC+/Hoechst+) in randomized sections. In ears, we used the autofluorescence of cartilage, epidermis, and muscle in the red channel to calculate the dermis area by subtracting the cartilage and muscle areas from the area between the two epidermal edges. We counted peritoneal MCs using a Neubauer chamber and cytospins (Wright-Giemsa). We obtained EM pictures of BMMCs and peritoneal MCs using an unbiased random sampling technique. We calculated the volume fraction and the surface density of the granules using the point counting method with a cycloid grid, and the profile area using a point grid (20).
Secretion Assays
For each experiment, we suspended 106 BMMCs in 1 ml of culture medium and sensitized them with anti-2,4-dinitrophenol (DNP) IgE 5 μg/ml for 3 h. After repeated washes, we stimulated them with 100 ng/ml of DNP-human serum albumin (DNP-HSA). We measured all products in supernatants before and after stimulation. We also measured β-hexosaminidase in cell lysates (106 BMMCs treated with 1 ml of 0.2% Triton X-100). After stimulation, we measured LTC4 and PGD2 at 30 min, β-hexosaminidase and histamine at 1 h, TNF-α at 6 h, and IL-4 at different time points. We used enzyme-linked immunosorbent assays (ELISA, Oxford Biomed Res) to measure all products except β-hexosaminidase activity, which was measured by colorimetry using as substrate 4-nitrophenyl N-acetyl-β-d-glucosaminide.
MC Reconstitution and Passive Cutaneous Anaphylaxis (PCA)
We used BMMCs derived from Syt2+/+ or Syt2−/− P16 mice for reconstitution of both ears of MC-deficient B6.Cg-KitW-sh/KitW-sh (Wsh/Wsh) mice. After 6 weeks in culture, 105 BMMCs were suspended in 25 μl of PBS and injected intradermally into each ear pinna. Six weeks later, we quantified the MCs in the ears of some animals by histology and then performed the PCA sensitization and challenge on the rest as described (4).
Intracellular Ca2+ Measurements
Peritoneal MCs from P16 mice were sensitized with 3 μg/ml of anti-DNP IgE for 2 h, plated onto coverslips and incubated in 5 μm Fura 2-AM. Intracellular calcium of each individual MC was ratiometrically calculated (21, 22). The fluorescence of an unstimulated MC was measured for 60 s prior to the addition of DNP-HSA to a final concentration of 0.5 μg/ml.
Electrophysiological Recordings
Whole cell capacitance measurements of individual peritoneal MCs were made at room temperature using 5 to 6 mΩ sylgard-coated patch pipettes (21). The intracellular recording solution both defined the [Ca2+]i and stimulated exocytosis (23–25). It contained (mm) 135 potassium gluconate, 7 MgCl2, 3 KOH, 2.5 K2EGTA, 7.5 Ca-EGTA, 0.2 Na-ATP, 0.005 Li4GTPγS, and 0.1 bis-Fura 2, 10 HEPES (pH 7.2, 305 mOsm) (23), with a calculated free [Ca2+] of ∼700 nm that was experimentally verified. The external recording solution contained (mm) 140 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 10 glucose, 10 HEPES (pH 7.2 and 310 mOsm).
RESULTS AND DISCUSSION
Syt2 Expression in MCs
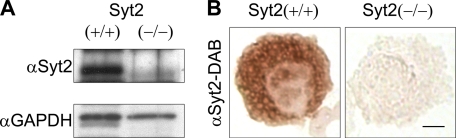
In previous studies, we showed that Syt2 is the main Syt isoform expressed in the RBL-2H3 MC line (18). Neurons from Syt2−/− mice have severe impairments in Ca2+-dependent-regulated exocytosis (16, 17), so we wondered if this low Ca2+ affinity exocytic Syt may play a similar role in a non-neuronal cell, such as the MC, in which activation does not depend on Ca2+ entry through voltage-gated channels in the plasma membrane. Our first step was to confirm expression of Syt2 in normal MCs using cells from our Syt2−/− mice as negative controls. Immunoblots of Syt2+/+ MC lysates showed intense staining at ∼80 kDa, whereas Syt2−/− MC lysates showed no immunoreactivity at this mobility (Fig. 1A), and we observed perigranular immunohistochemical staining for Syt2 only in Syt2+/+ peritoneal MCs (Fig. 1B). Together, these results provide evidence of the expression of Syt2 in mature endogenous MCs.
FIGURE 1.
Syt2 is expressed in mature MCs. Peritoneal MCs purified from P16 Syt2+/+ and Syt2−/− mice. A, immunoblot of cell lysates with anti-Syt2 antibody (αSyt2). GAPDH immunoreactivity was used as a loading control. B, immunohistochemistry of cytospins using the same antibody (bar, 4 μm). We demonstrated the presence of Syt2 in mature wild type MCs and the absence of Syt2 in Syt2−/− MCs.
Syt2 Deficiency Impairs an MC-dependent Allergic Reaction
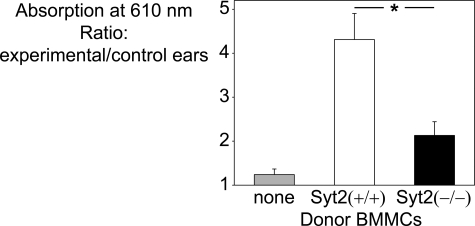
PCA models the acute phase of an allergic reaction. In PCA, we measure the localized vasodilation and increased endothelial permeability induced by products secreted by skin-resident MCs (26). All animals we used are on a C57BL/6 genetic background. We initially found a decreased PCA response in Syt2−/− mice compared with Syt2+/+ mice (not shown). A flaw in these pilot experiments was that altered responses of Syt2-deficient cells other than MCs (e.g. vascular smooth muscle relaxation and endothelial cell retraction) could also be responsible for the observed difference. To avoid this confounding factor and to guarantee that only the MCs in our model will be differentially deficient or sufficient in Syt2, we reconstituted Syt2-sufficient MC-deficient Wsh/Wsh mice with BMMCs from Syt2−/− and Syt2+/+ mice. In histological analysis performed 6 weeks after reconstitution, we identified 101 ± 11 and 98 ± 9 (mean ± S.E.; n = 4) MCs per mm2 of dermis at the site of injection in Wsh/Wsh mice that received Syt2+/+ and Syt2−/− BMMCs, respectively. As shown in Fig. 2, MC-deficient animals failed to mount a response to the antigen challenge. This reaction was rescued by intradermic reconstitution with Syt2+/+ BMMCs. Reconstitution with Syt2−/− BMMCs provided only a partial (about one-third) rescue compared with Syt2+/+ BMMCs. Pharmacologic interventions have determined that PCA is highly dependent on histamine. Given that this vasoactive amine is stored in MC secretory granules and released during degranulation, and that the presence of a Ca2+-binding exocytic Syt defines an exocytic event as regulated, we postulated that the lack of Syt2 was negatively affecting MC-regulated exocytosis.
FIGURE 2.
Syt2 deficiency impairs the response in passive cutaneous anaphylaxis. Ears of MC-deficient Wsh/Wsh mice reconstituted intradermally with Syt2+/+ or Syt2−/− BMMCs were sensitized with anti-DNP IgE (experimental ear) or polyclonal IgE (control ear). After intravenous challenge with DNP-HSA in Evans Blue, the amount of extravasated dye was measured as absorbance at 610 nm. Nonreconstituted MC-deficient animals failed to respond, and there was a marked difference between mice reconstituted with Syt2−/− and Syt2+/+ BMMCs. (Error bars represent S.E.; n = 9; *, p < 0.01.)
Syt2 Deficiency Does Not Affect MC Numbers, Differentiation, Structure, or Storage of Granule Contents
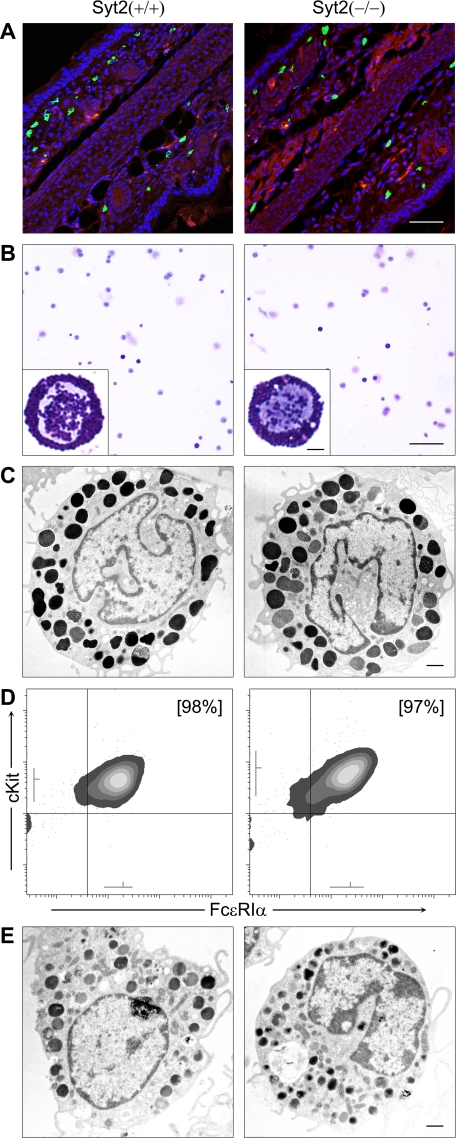
To rule out the possibility that the defective response to PCA was due to an unexpected effect of the absence of Syt2 on some aspect of MC development, we examined MC structure and differentiation in Syt2−/− mice. We found no difference in the MC density in skin (Fig. 3A and Table 1), peritoneal fluid (Fig. 3B and Table 1), tongue, trachea, stomach, or jejunum (not shown) between Syt2+/+ and Syt2−/− mice. EM images of peritoneal MCs from animals of both genotypes were indistinguishable (Fig. 3C) and unbiased stereological analysis to measure the size and surface complexity of the cells and the number, size, and density of their granules showed no difference (Table 1). We also did not find a difference in the rate of differentiation of bone marrow cells into BMMCs in SCF and IL-3-enriched medium (Table 1) or in the surface density of immunoreactivity against cKit/CD117 and FcϵRIα in BMMCs after 6 weeks in culture (Fig. 3D). Morphometric analysis of electron micrographs of Syt2+/+ and Syt2−/− BMMCs also failed to uncover a difference (Fig. 3E and Table 1). As part of the secretion assays described below we found almost identical amounts of total cellular histamine (Table 1) and β-hexosaminidase activity (not shown) in unstimulated Syt2+/+ and Syt2−/− BMMCs.
FIGURE 3.
Syt2 deficiency does not alter MC development, migration into tissues, or morphology. Representative images of samples obtained from P16 Syt2+/+ and Syt2−/− mice are shown. An in-depth morphometric analysis can be found in Table 1. A, fluorescent microscopy of ear sections (bar, 50 μm). MCs were labeled with FITC-avidin (green) and cell nuclei with Hoechst (blue). Background tissue was visualized as autofluorescence in the red channel. Syt2+/+ and Syt2−/− mice had similar densities of MCs in the dermis of their ears. B, cytospins of peritoneal lavages stained with Wright-Giemsa (bar, 100 μm). Insets depict higher power magnifications of representative MCs (bar, 5 μm). Syt2+/+ and Syt2−/− mice had similar numbers of MCs with similar gross morphology in their peritoneal cavities. C, transmission EM of peritoneal MCs (bar, 1 μm). MCs from Syt2+/+ and Syt2−/− mice had similar size and surface complexity, and similar number, size, and density of electron-dense granules. D, flow cytometry of BMMCs. After 6 weeks in medium supplemented with SCF and IL-3, BMMCs from Syt2+/+ and Syt2−/− mice had the same proportion of Kit+/FcϵRIα+ cells (% in brackets), and similar surface expression of both receptors (gray lines near both axes represent mean ± S.D. of fluorescence intensity). E, transmission EM of BMMCs (bar, 1 μm). Syt2+/+ and Syt2−/− BMMCs were indistinguishable.
TABLE 1.
Quantitation, morphometry, and cellular contents of studied MCs
Values obtained from four animals of each genotype are expressed as mean ± S.E. Vv denotes granule volume fraction, Sv, granule surface density, and A, cell profile area.
| Syt2+/+ | Syt2−/− | |
|---|---|---|
| Dermal MCsa | ||
| 106 cells/m2 | 183 ± 15 | 182 ± 12 |
| Peritoneal MCsb | ||
| % | 9.7 ± 1.7 | 9.3 ± 1.1 |
| 106 cells/liter | 180 ± 13 | 174 ± 16 |
| Peritoneal MCsc | ||
| Vv | 0.25 ± 0.02 | 0.26 ± 0.02 |
| Sv, 10−6 m−1 | 22.89 ± 1.71 | 21.82 ± 2.85 |
| A, 10−12 m2 | 33.82 ± 2.97 | 32.76 ± 0.78 |
| BMMCs in cultured | ||
| 2 weeks, % | 17.2 ± 3.1 | 19.1 ± 3.9 |
| 4 weeks, % | 89.1 ± 4.4 | 86.2 ± 5.4 |
| 6 weeks, % | 97.5 ± 1.2 | 97.1 ± 1.4 |
| BMMCs (6 w)c | ||
| Vv | 0.41 ± 0.04 | 0.39 ± 0.03 |
| Sv, 10−6 m−1 | 20.77 ± 2.01 | 20.46 ± 1.14 |
| A, 10−12 m2 | 43.11 ± 2.88 | 48.58 ± 2.78 |
| BMMCs histaminee | ||
| 10−3 g/liter | 0.93 ± 0.11 | 0.97 ± 0.09 |
a FITC-avidin+ cells with a Hoechst+ nuclear profile per area of dermis in random sections of ear (10 samples/animal).
b Calculated from Neubauer chamber counts and Wright-Giemsa staining of cytospins of peritoneal lavages (3 samples/animal).
c Stereological analysis of randomly acquired EM images from peritoneal MCs and BMMCs after 6 weeks in culture (10 cell profiles/sample, 4 samples/animal).
d Percent of Kit+/FcϵRIα+ double positive cells in bone marrow cultures in medium enriched with SCF and IL-3 assessed by flow cytometry (1 sample/animal/time point).
e Total histamine concentration in lysates of 106 BMMCs measured by ELISA (1 sample/animal).
Based on these results, we concluded that the lack of Syt2 did not affect the proliferation, migration, and maturation of MCs, the morphology of these cells and of their secretory granules, the expression of receptors important for their maturation and stimulation, and the storage of preformed inflammatory mediators in their secretory granules. Thus, none of these factors explained the defective acute allergic cutaneous response described in Fig. 2.
Syt2 Selectively Controls Regulated Exocytosis in MCs
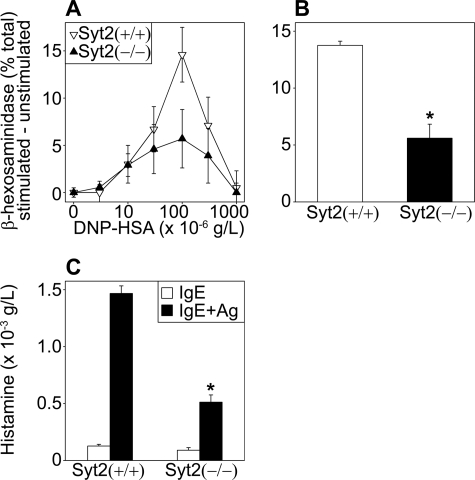
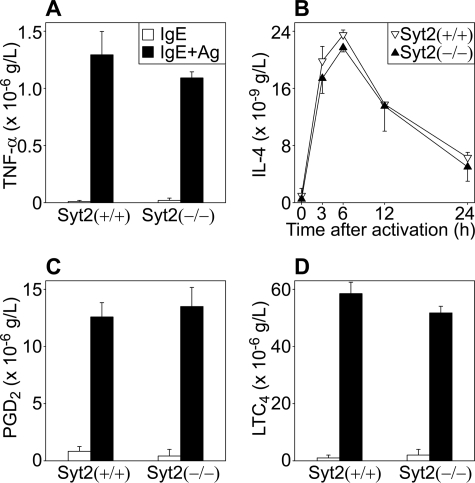
Other possibilities that we needed to test were that the deficient PCA response was part of a more extensive defect affecting all forms of MC secretion and/or the activation of MCs. We studied the functional consequences of the absence of Syt2 in the three main MC secretory effector responses: regulated exocytosis, constitutive exocytosis, and nonexocytic secretion (6). To simulate the conditions used in our PCA model, we used FcϵRI cross-linking to stimulate BMMCs from Syt2+/+ and Syt2−/− mice. After incubating the cells with anti-DNP IgE, we stimulated them by applying DNP-HSA. Because excess of polyvalent antigen can interfere with FcϵRI cross-linking, we used the antigen to cell ratio that induced the maximum secretory response in our system (106 BMMCs in 1 ml of DNP-HSA at 100 ng/ml, Fig. 4A). To assess exocytosed secretory granule contents, we measured the enzymatic activity of β-hexosaminidase (expressed as a fraction of total cellular content; Fig. 4, A and B) and the amount of histamine (expressed in absolute values, Fig. 4C) in the cell supernatants before and after stimulation. In both cases we found that Syt2−/− cells showed only about a third of the response found in Syt2+/+ cells. We next measured the release of cytokines under transcriptional control and secreted via constitutive exocytosis, and found no difference in the total amount released (TNF-α, Fig. 5A) or in the time course of this response (IL-4, Fig. 5B). We did not find any difference in the release of products that do not depend on exocytosis for their secretion such as PGD2 and LTC4 (Fig. 5, C and D).
FIGURE 4.
Syt2 deficiency affects MC-regulated exocytosis. BMMCs from Syt2+/+ and Syt2−/− mice were incubated with anti-DNP IgE and then exposed to DNP-HSA. Secreted products were measured in supernatants before and 1 h after stimulation. Total β-hexosaminidase enzymatic activity was also measured in cell lysates. A, secretion of β-hexosaminidase with different doses of DNP-HSA. Values are expressed as the difference in enzymatic activity in supernatants between stimulated and unstimulated cells in percent of total activity in cell lysates. The maximal response and greatest difference were found at 100 ng/ml DNP-HSA (n = 3). This concentration was used in all subsequent experiments. B, secretion of β-hexosaminidase at 100 ng/ml DNP-HSA (n = 8). C, histamine in supernatants before (open bars) and after (black bars) challenge (n = 5). Release of products stored in MC secretory granules and secreted via regulated exocytosis was significantly decreased in Syt2−/− BMMCs. (Error bars represent S.E.; *, p < 0.01.)
FIGURE 5.
Syt2 deficiency does not affect other MC secretory mechanisms. Secreted products were measured in supernatants of BMMCs from Syt2+/+ and Syt2−/− mice incubated with anti-DNP IgE before and at different time points after addition of DNP-HSA. A, TNF-α before (open bars) and after (6 h, black bars) challenge (n = 5; color legend applies also to C and D). B, time course of IL-4 secretion (n = 3 per time point). C, PGD2 and D, LTC4 30 min after challenge (n = 4 for both). Secretion dependent on constitutive exocytosis or independent of exocytosis was not affected in Syt2−/− BMMCs. (Error bars represent S.E.)
The normal secretion of cytokines and lipid mediators, together with the normal surface expression of FcϵRIα (Fig. 3D), point to an intact IgE-dependent stimulation mechanism in MCs lacking Syt2, and excludes a defect in this pathway as an explanation for our findings. The presence of an abnormal and selective defect in degranulation confirms that, like in neurons and neuroendocrine cells, the presence of a Ca2+-binding Syt defines an exocytic step as a regulated event (7). Our finding that Syt2 discriminates between the secretory compartment containing both β-hexosaminidase and histamine from that containing cytokines is very similar to the findings on VAMP-8 in MCs (27). Another study suggested that VAMP-8 may further discriminate between subpopulations of granules released by regulated exocytosis, as it may control the release of serotonin, cathepsin-D, and β-hexosaminidase but not of histamine (28). Because we found a similar defect in the secretion of β-hexosaminidase and histamine (Fig. 4, B and C), we postulate that there may be only a single secretory granule population that contains both mediators or that Syt2 controls the exocytosis of the two granule subpopulations.
In nonexcitable cells, another Syt that regulates exocytic events is the high affinity Ca2+-binding Syt7 (29), an ubiquitously expressed protein that associates with lysosomal compartments. Although its function in MCs is not known, it is a good candidate to explain the residual exocytic response seen in Syt2−/− MCs. Similar functional redundancy can explain why the inhibition of VAMP-7, VAMP-8, and Stx-4 with neutralizing antibodies does not entirely inhibit the release of histamine from MCs compared with the complete suppression obtained with anti-SNAP-23 antibodies (30). Finally, we found no structural or functional difference between Syt2+/+ and Syt2+/− MCs (not shown), so there is no phenotypic haploinsufficiency, similar to our findings in synapses and neuromuscular junctions (16, 17), but different to what we have observed in airway mucous cells (31).
Syt2 Deficiency Does Not Lead to Increased Spontaneous Degranulation in MCs
In neurons, Syt2 plays a double role: it is required for the fast synchronized component of the evoked exocytic release of synaptic vesicles, and it also suppresses the slow nonsynchronized component of this process and the spontaneous exocytic events that occur in the absence of stimuli (16, 17). As shown in Fig. 4C, we did not find a difference in the baseline concentration of histamine in the supernatants of Syt2+/+ and Syt2−/− BMMCs, even when these measurements were repeated over 24 h (results not shown). Also, as described in Table 1, the amount of histamine present in cells of both genotypes was almost identical, so there was no evidence of depletion of internal stores.
Thus, like in neurons, Syt2 in MCs is required for an effective regulated exocytic response, but unlike in neurons, its absence does not produce an increase in spontaneous fusion events resulting in depletion of stored products. An explanation for this difference may be the nature of the membrane fusion machinery in these different cells. Neurotransmission takes place within milliseconds after an action potential induces influx of Ca2+. In neurons, secretory vesicles are docked at active zones with VAMP-2, SNAP-25, and Syntaxin-1 forming a partially coiled SNARE complex. The cytoplasmic protein complexin “clamps” the complex in this state and prevents complete coiling and membrane fusion. At low [Ca2+]i Syt promotes this action of complexin, but as [Ca2+]i rises, Syt displaces complexin and actively promotes the “zippering” of the quadruple helix, driving membrane fusion (32). In MCs, regulated exocytosis takes place over minutes and depends on different complexins, v- and t-SNAREs. Perhaps because there is no need for an immediate coupling of Ca2+ entry with exocytosis, the “clamping” effect of Syt2 is not required in MCs, and that may be a consequence of a different interaction with complexin or with the SNARE complex. An alternative explanation is that another Syt isoform plays this negative role in MC exocytosis.
Syt2 Deficiency Does Not Alter the Stimulated Rise of [Ca2+]i in MCs
In neurons and muscle cells, some voltage-gated Ca2+ channels physically interact with the exocytic proteins Syntaxin, SNAP-25, and Syt. These interactions help localize synaptic vesicles to the active zone and alter the permeability to Ca2+ of these channels (33). Furthermore, some Syt channel complexes are the target of autoantibodies in Lambert-Eaton myastenic syndrome (34). Even though there is no reported physical or functional interaction between a Syt and the inositol trisphosphate receptor, STIM (stromal interaction molecule), or components of the Ca2+ release activated Ca2+ channel such as Orai1/CRACM1, it was important to confirm that the defective exocytic response we found in Syt2−/− MCs was not due to an impairment of store-operated Ca2+ entry in this nonexcitable cell (35).
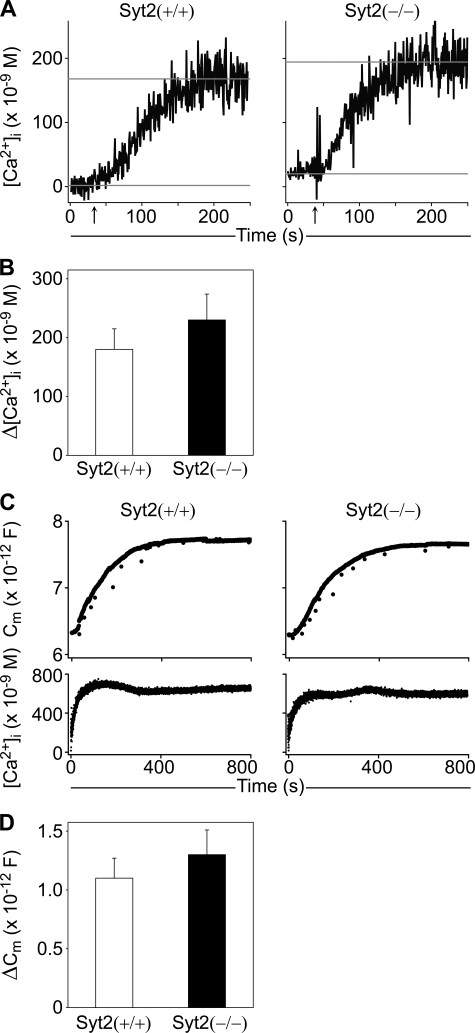
In purified peritoneal MCs passively sensitized with specific IgE, we found no difference in the increase of [Ca2+]i (Δ[Ca2+]i) after challenge by exposure to the cognate antigen (Fig. 6, A and B). Thus, it is a deficiency in a Ca2+ sensor, and not in upstream Ca2+ signaling that explains the degranulation defect in Syt2−/− MCs.
FIGURE 6.
Syt2 deficiency does not affect [Ca2+]i transients or MC exocytic competence. A, representative [Ca2+]i traces of isolated peritoneal MCs incubated with anti-DNP IgE and stimulated by addition of DNP-HSA (arrows). Gray lines represent the mean baseline and maximal [Ca2+]i used to calculate Δ[Ca2+]i. B, average Δ[Ca2+]i showed no difference between the two genotypes (n = 9). C, representative Cm traces (upper panels) measured in peritoneal MCs dialyzed with 5 μm GTPγS via the patch pipette. The [Ca2+]i in both cells was buffered to 700 nm (lower panels). D, average ΔCm demonstrated that degranulation can be induced in Syt2−/− MCs under these conditions (n = 8; error bars represent S.E.).
Syt2 Deficiency Does Not Impair Secretory Granule Exocytic Competence in MCs
Another possibility we explored was to determine whether the effects of lack of Syt2 in MCs were indirect, affecting exocytosis by altering the competence of the core exocytic machinery. For example, the deletion of VAMP-2 (36) or SNAP-25 (37) produces a defect in neurotransmitter release that cannot be overcome by any stimulus because a core component of the neuronal exocytic machinery is absent.
Single peritoneal MCs from Syt2+/+ and Syt2−/− mice were strongly stimulated by intracellular dialysis with GTPγS and a persistent high [Ca2+]i (25), while measuring plasma membrane capacitance (Cm). We found no difference in plasma membrane capacitance gain (ΔCm), demonstrating that the total amount of granule membrane incorporated into the plasma membrane via exocytosis was the same, and therefore that the membrane fusion machinery was intact (Fig. 6, C and D). Similar to our findings, application of an intervention that induces neuronal exocytosis via a mechanism independent of Syts (hypertonic sucrose) to neurons deficient in Syt1 (14), Syt2 (16), or Syt9 (15) elicited similar levels of vesicle fusion when compared with wild-type controls.
Summary
We found that Syt2 controls exclusively regulated exocytosis in MCs in vitro and in vivo and not constitutive exocytosis or nonexocytic secretion. We also found that this Ca2+ sensor has no “clamping” activity on spontaneous exocytosis in this nonexcitable cell. Although the defect in Syt2-deficient mice is profound, it is not absolute, and it would be interesting to explore if another Syt such as Syt7 could explain this residual exocytic activity. We also found that the PCA reaction is highly dependent on MC-regulated exocytosis, suggesting that it may be possible to control the acute phase of the allergic response by manipulating this exocytic step.
This work was supported by Grant 0655003Y from the American Heart Association (to R. A.).
- MC
- mast cell
- BMMC
- bone marrow-derived MC
- [Ca2+]i
- cytoplasmic Ca2+ concentration
- Cm
- plasma membrane capacitance
- Δ
- change
- DNP
- 2,4-dinitrophenol
- EM
- electron microscopy
- FcϵRIα
- α-subunit of the high affinity receptor for IgE
- FITC
- fluorescein isothiocyanate
- HSA
- human serum albumin
- IgE
- immunoglobulin E
- IL
- interleukin
- LTC4
- leukotriene C4
- NSF
- N-ethylmaleimide-sensitive fusion protein
- P16
- postnatal day 16
- PCA
- passive cutaneous anaphylaxis
- PGD2
- prostaglandin D2
- SCF
- stem cell factor
- SNAP-25
- synaptosome-associated protein of 25 kDa
- SNARE
- soluble NSF adaptor protein receptor
- Syt
- Synaptotagmin
- TNF-α
- tumor necrosis factor α
- VAMP
- vesicle-associated membrane protein
- Wsh/Wsh
- MC-deficient B6.Cg-KitW-sh/KitW-sh mouse
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate.
REFERENCES
- 1.Yu M., Tsai M., Tam S. Y., Jones C., Zehnder J., Galli S. J. (2006) J. Clin. Invest. 116, 1633–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McNeil H. P., Shin K., Campbell I. K., Wicks I. P., Adachi R., Lee D. M., Stevens R. L. (2008) Arthritis Rheum. 58, 2338–2346 [DOI] [PubMed] [Google Scholar]
- 3.Lee D. M., Friend D. S., Gurish M. F., Benoist C., Mathis D., Brenner M. B. (2002) Science 297, 1689–1692 [DOI] [PubMed] [Google Scholar]
- 4.Thakurdas S. M., Melicoff E., Sansores-Garcia L., Moreira D. C., Petrova Y., Stevens R. L., Adachi R. (2007) J. Biol. Chem. 282, 20809–20815 [DOI] [PubMed] [Google Scholar]
- 5.Malaviya R., Ikeda T., Ross E., Abraham S. N. (1996) Nature 381, 77–80 [DOI] [PubMed] [Google Scholar]
- 6.Adachi R., Nigam R., Dickey B. F. (2003) in Mast Cell Exocytosis in Airway Inflammation, Therapeutic Targets in Airway Inflammation (Eissa N. T., Huston D. P. eds) pp. 327–348, Marcel Dekker, New York [Google Scholar]
- 7.Verhage M., Toonen R. F. (2007) Curr. Opin. Cell Biol. 19, 402–408 [DOI] [PubMed] [Google Scholar]
- 8.Loe D. W., Almquist K. C., Deeley R. G., Cole S. P. (1996) J. Biol. Chem. 271, 9675–9682 [DOI] [PubMed] [Google Scholar]
- 9.Wong A., Cook M. N., Foley J. J., Sarau H. M., Marshall P., Hwang S. M. (1991) Biochemistry 30, 9346–9354 [DOI] [PubMed] [Google Scholar]
- 10.Theoharides T. C., Kempuraj D., Tagen M., Conti P., Kalogeromitros D. (2007) Immunol. Rev. 217, 65–78 [DOI] [PubMed] [Google Scholar]
- 11.Turner H., Kinet J. P. (1999) Nature 402, B24–B30 [DOI] [PubMed] [Google Scholar]
- 12.Jahn R., Lang T., Südhof T. C. (2003) Cell 112, 519–533 [DOI] [PubMed] [Google Scholar]
- 13.Chapman E. R. (2008) Annu. Rev. Biochem. 77, 615–641 [DOI] [PubMed] [Google Scholar]
- 14.Geppert M., Goda Y., Hammer R. E., Li C., Rosahl T. W., Stevens C. F., Südhof T. C. (1994) Cell 79, 717–727 [DOI] [PubMed] [Google Scholar]
- 15.Xu J., Mashimo T., Südhof T. C. (2007) Neuron 54, 567–581 [DOI] [PubMed] [Google Scholar]
- 16.Sun J., Pang Z. P., Qin D., Fahim A. T., Adachi R., Südhof T. C. (2007) Nature 450, 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pang Z. P., Melicoff E., Padgett D., Liu Y., Teich A. F., Dickey B. F., Lin W., Adachi R., Südhof T. C. (2006) J. Neurosci. 26, 13493–13504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baram D., Adachi R., Medalia O., Tuvim M., Dickey B. F., Mekori Y. A., Sagi-Eisenberg R. (1999) J. Exp. Med. 189, 1649–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolters P. J., Mallen-St Clair J., Lewis C. C., Villalta S. A., Baluk P., Erle D. J., Caughey G. H. (2005) Clin. Exp. Allergy 35, 82–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peterson D. A. (1999) Methods 18, 493–507 [DOI] [PubMed] [Google Scholar]
- 21.Zhou Z. Y., Wan Q. F., Thakur P., Heidelberger R. (2006) J. Neurophysiol. 96, 2539–2548 [DOI] [PubMed] [Google Scholar]
- 22.Heidelberger R., Matthews G. (1992) J. Physiol. 447, 235–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riedel D., Antonin W., Fernandez-Chacon R., Alvarez de, Toledo G., Jo T., Geppert M., Valentijn J. A., Valentijn K., Jamieson J. D., Südhof T. C., Jahn R. (2002) Mol. Cell. Biol. 22, 6487–6497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neher E. (1988) J. Physiol. 395, 193–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandez J. M., Neher E., Gomperts B. D. (1984) Nature 312, 453–455 [DOI] [PubMed] [Google Scholar]
- 26.Inagaki N., Goto S., Nagai H., Koda A. (1986) Int. Arch. Allergy Appl. Immunol. 81, 58–62 [DOI] [PubMed] [Google Scholar]
- 27.Tiwari N., Wang C. C., Brochetta C., Ke G., Vita F., Qi Z., Rivera J., Soranzo M. R., Zabucchi G., Hong W., Blank U. (2008) Blood 111, 3665–3674 [DOI] [PubMed] [Google Scholar]
- 28.Puri N., Roche P. A. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 2580–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chakrabarti S., Kobayashi K. S., Flavell R. A., Marks C. B., Miyake K., Liston D. R., Fowler K. T., Gorelick F. S., Andrews N. W. (2003) J. Cell Biol. 162, 543–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sander L. E., Frank S. P., Bolat S., Blank U., Galli T., Bigalke H., Bischoff S. C., Lorentz A. (2008) Eur. J. Immunol. 38, 855–863 [DOI] [PubMed] [Google Scholar]
- 31.Tuvim M. J., Mospan A. R., Burns K. A., Chua M., Mohler P. J., Melicoff E., Adachi R., Ammar-Aouchiche Z., Davis C. W., Dickey B. F. (2009) J. Biol. Chem. 284, 9781–9787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carr C. M., Munson M. (2007) EMBO Rep. 8, 834–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Catterall W. A. (1999) Ann. N. Y. Acad. Sci. 868, 144–159 [DOI] [PubMed] [Google Scholar]
- 34.Leveque C., Hoshino T., David P., Shoji-Kasai Y., Leys K., Omori A., Lang B., el Far O., Sato K., Martin-Moutot N. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 3625–3629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vig M., Kinet J. P. (2007) Cell Calcium 42, 157–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schoch S., Deák F., Königstorfer A., Mozhayeva M., Sara Y., Südhof T. C., Kavalali E. T. (2001) Science 294, 1117–1122 [DOI] [PubMed] [Google Scholar]
- 37.Washbourne P., Thompson P. M., Carta M., Costa E. T., Mathews J. R., Lopez-Benditó G., Molnár Z., Becher M. W., Valenzuela C. F., Partridge L. D., Wilson M. C. (2002) Nat. Neurosci. 5, 19–26 [DOI] [PubMed] [Google Scholar]