Abstract
Fewer surgical procedures have a history as fascinating and as terrifying as breast augmentation. Initial efforts at augmentation involved injection of substances such as paraffin or oil into the breast tissue, or the implantation of substances including ivory or glass balls, or rubber. More recent efforts have included the injection of liquid silicone or polyacrylamide hydrogel. The current paper reviews four distinct eras of breast augmentation, and provides the current status of these injection materials. A case report is presented on a woman whose breasts were injected with polyacrylamide hydrogel in Iran. The current status of this group of materials is also presented. During the past 110 years, history has repeated itself during each of the four eras of injection.
Keywords: Breast, Injectable, Liquid silicone, Paraffin, Polyacrylamide hydrogel
Abstract
Peu de techniques chirurgicales ont une histoire à la fois aussi fascinante et terrifiante que l’augmentation mammaire. Les premières tentatives ont porté sur l’injection de substances (paraffine, huile) ou sur l’implantation de matériaux (boules d’ivoire ou de verre, caoutchouc) dans les tissus mammaires. Plus récemment, on a procédé à des injections de silicone liquide ou d’hydrogel de polyacrylamide. Le présent article passe en revue les quatre époques de l’histoire de l’augmentation mammaire et nous dit où en est actuellement l’utilisation de ces injections. Il fait état d’un rapport de cas d’augmentation mammaire par injection d’hydrogel de polyacrylamide en Iran et fait également le point sur cette catégorie de substance. Au cours des 110 dernières années, l’histoire des injections s’est répétée, à chacune des quatre étapes.
Fewer surgical procedures have a history as fascinating and as terrifying as breast augmentation. There have been four main eras of injectable materials for breast augmentation. These include the following:
| • Paraffin | 1899 to 1914 |
| • A plethora of material | 1915 to 1943 |
| • Liquid silicone | 1944 to 1991 |
| • Polyacrylamide hydrogel | 1988 to 2009 |
The purpose of the present paper is to present a case report of the complications of polyacrylamide hydrogel (PAH) injections. History has repeated itself in each of the above four eras. Because of this, we undertook to review the history of injectable materials and their complications.
PARAFFIN, 1899 TO 1914
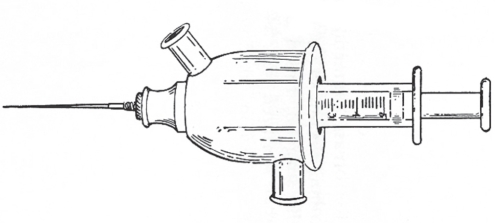
Paraffin is a group of hydrocarbons, which is saturated with carbon to hydrogen bonds, making them relatively inert. The basic repeating unit in the polymers is: –(CH2) n–. Paraffin exists as a hard form (wax) and a soft form (vaseline). Waxes are long-chain hydrocarbons, containing more than 20 repeating units. The softer form of paraffin, vaseline, is composed of shorter molecules. It looks and feels like the product that goes by that trade name today. Both the soft and hard forms of paraffin have a low melting point. Before injection, paraffin was heated inside a chamber surrounded by warm water (Figure 1), to form a semiliquid material, which allowed easier injection.
Figure 1).
Warming chamber for liquefaction of paraffin before injection (see reference 4)
The first published report of paraffin injections into a patient dates back to a report by Gersuny, of Vienna, in 1899 (1). This patient was a young man, who had undergone a bilateral orchiectomy for tuberculous disease. Gersuny injected paraffin into his scrotum, so that the patient could pass the physical examination necessary to join the army. Paraffin injections were subsequently used extensively from 1899 until 1914, primarily to augment the size of women’s breasts.
In 1903, Gersuny published a report about soft and hard paraffin injections (2). He chose to use a mixture of one part of vaseline (soft paraffin) and three parts of olive oil. His theory was that the olive oil would be absorbed by the body, leaving small particles of paraffin, which would become surrounded by the body’s own connective tissue. He surmised that the lasting effect of the method would come from the resulting permanent framework of connective tissue, rather than from the paraffin.
The early results of paraffin injections were often quite acceptable. The complications that followed these injections frequently did not show up until five or 10 years later. In 1912, Hollander provided an early report (3) on the complications of paraffin injections into a patient’s breasts. That patient had injections in 1904, and initially had an ‘acceptable’ result. However, five or six years later, she developed ugly knots and draining fistulae that required surgical intervention. Kolle’s 1911 textbook, Plastic and Cosmetic Surgery, contained a whole section on the complications of paraffin injections into breast tissue (4). These complications ranged from aesthetic failure to death. They included pulmonary embolism, migration, ulceration, fistulae, infection and necrosis. These complications would frequently lead to breast amputation.
During the early 1920s, a new term was coined – paraffinomas – to describe the delayed, chronic, inflammatory granulomatous reactions that developed after paraffin injections (5). This phenomenon involved both skin and underlying soft tissues. It was characterized by the development of indurated masses, pain, ulceration, fistulae and necrosis. When these complications were initially seen, plastic surgeons dismissed them as “cases of individual susceptibility”. Figure 2 shows the clinical status of a woman in our practice, who had received paraffin injections in the Far East 40 years earlier. She had undergone multiple debridement procedures and bilateral mastectomies over the years to treat multiple ulcers and fistulae. She continued to suffer from areas of induration, ulcers and fistulae.
Figure 2).
Clinical status of a woman who had received paraffin injections in the Far East 40 years previously. She has had many operations over the years, including bilateral mastectomies, to treat ulcers and fistulae. She continues to suffer from these problems
Radiologically, injected paraffin does not form a solid body in tissues. Rather, it consists of many droplets, widely dispersed in the tissues. On mammography, early paraffin injections appear as multiple, circumscribed, noncalcified masses in the retroglandular and subpectoral regions (6). By contrast, late-stage paraffin injections (paraffinomas) demonstrate numerous multiple dense nodules with arc and ring calcifications (7).
In the western medical world, injection of paraffin became progressively more popular from 1899 until approximately 1914, when its use began to taper. Ultimately, in his 1926 textbook, H Lyons Hunt called it an “inexcusable practice” and blamed “beauty doctors and other such imposters” for its continued use. However, in the Far East, the practice was continued into the 1950s and 1960s (8). Deaths continued to be sporadically reported after paraffin injections. In one such case in Hong Kong, in 1957, a 32-year-old mother of four died of a fat embolism, 48 h after she received 11 paraffin injections into her breasts by a “beauty clinic nurse”. At an inquest on this patient, the jury returned a unanimous verdict of manslaughter. The magistrate issued a warrant for the arrest of the nurse. In both Europe and the United States, a number of patients injected themselves with paraffin as a self-inflicted injury to escape the military service or deportation (8). Other patients injected themselves to enlarge the penis or breasts.
The disastrous experience with paraffin was to live on in the collective memory of the plastic surgery profession. Plastic surgery historians have blamed the paraffin saga for severely retarding the progress of the profession. Plastic surgeons became susceptible to the blandishments of quick results. As complications unfolded, physicians followed the pattern of first blaming the technique and the lay people doing the injections. Then, individual patient susceptibility, and finally the material itself, were blamed for the adverse effects that resulted. Poor outcomes made patients distrustful.
A PLETHORA OF MATERIALS, 1915 TO 1943
After the paraffin saga, there was a period of approximately 30 years during which time a huge plethora of materials was used for breast augmentation. The list of these materials was limited only by the extent of man’s imagination. During this time, the following materials were used: ivory balls, glass balls, vegetable oils, mineral oil, lanolin, beeswax, shellac, silk fabric, epoxy resin, ground rubber, ox cartilage, sponges, sacs, rubber, goat’s milk, Teflon, soybean and peanut oil, and glazier’s putty. The outcome with each of these materials was similar – chronic inflammation with foreign body granulomas. Many of the materials had severe tissue reactions. Infections were common. Ultimately, none of the materials proved to be useful for breast augmentation.
LIQUID SILICONE INJECTIONS, 1944 TO 1991
Surely the terrifying history of paraffin injections should have taught both physicians and patients to be wary of injectable materials for breast augmentation. However, in the 1940s and 1950s, many physicians and lay clinics turned to liquid silicone injections, for this purpose (9,10). Silicones are extensively cross-linked polymers of dimethyl siloxane, with a basic repeating unit of:
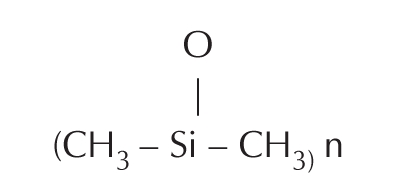
In 1943, the Dow Corning Corporation and Corning Glass formed a joint venture in the United States, to develop silicone products to be used for military purposes during World War II. Ultimately, these silicones were used for waterproofing, to prepare grease and oil products for aircraft, to insulate electrical transformers, and to prepare high-temperature-resistant rubbers. When the war came to an end, Dow Corning redirected their efforts to the formulation of medical-grade silicones. Medical grade refers to material that is pure in quality, sterile, and of constant viscosity. Medical-grade silicone was not available until 1960.
Near the end of World War II, prostitutes in Japan used industrial-grade liquid silicone extensively. United States servicemen preferred women with larger breasts than those of Asian women. Barrels of industrial-grade silicone began to mysteriously disappear from Japanese docks, destined for injection into the breasts of these enterprising ladies, to cater to their potential clients.
In Japan, liquid silicone was available under the names Elicon (a heat vulcanized silicone gel) and Zeflon (a fluid that was mixed with an organic salt of tin). Both of these preparations were industrial-grade silicone, not medical grade. A medical grade of silicone liquid was marketed by the Koken company in Japan, but not until many years later. Silicone injections continued to be used extensively in Japan and Asia after the war. Even today, they are used in certain areas of Asia.
Many of the complications of paraffin injections were repeated – a half-century later – with silicone injections. Some of them were even worse, because of impurities and additives in the silicone preparations. Medical-grade silicone was not available until 1960. Previously, only industrial-grade silicone was available. This material was never meant to be used for injection, because of its impurities. In addition, in many of the preparations, contaminants were purposely added to the injected material, as in the Sakurai formula (11). Their purpose was to cause a sclerosis reaction in the breasts, to contain the liquid silicone and to hopefully prevent it from migrating through the breast tissue to other sites. Common sclerosing agents included croton oil, cobra venom, olive oil and peanut oil. The adverse effects of injected liquid were very similar to those of paraffin. These included the following: migration of silicone to other parts of the body, inflammation, discolouration, and the formation of granulomas, ulceration and fistulae.
In 1960, Dow Corning developed its first medical-grade silicone (12), which was initially known as ‘Dow Corning 200’. This material was subsequently refined further and was then known as ‘Dow Corning 360’. It was marketed under the name of Dermagen. Its initial purpose was for waterproofing skin, primarily in burn victims. Although this material was not approved or intended for cosmetic injections in patients, it was used very extensively for this purpose, by certain physicians and by lay clinics. When it was used in breasts, huge volumes were often used. Large volumes of the silicone liquid were obtained under the false pretense that it was being used for burn mattresses, to treat injuries in racehorses and to prepare fertilizer products. All of these uses had been approved. Unscrupulous physicians in Las Vegas withdrew as much as a pint of the substance from five-gallon drums stored in their offices. This was injected into breasts under great pressure, using equipment resembling a caulking gun. In the entertainment business, these silicone injections were referred to as ‘Cleopatra’s Needle’. It has been estimated that in Las Vegas in the 1960s, two physicians, alone, used silicone to inject the breasts of over 10,000 women over a 10-year period (12,13). No records were kept on any of these patients.
By 1965, many complications began to surface from liquid silicone injections. Some injections had been done by plastic surgeons. However, many were done by laypersons, who were minimally qualified to undertake this type of procedure (9–12). Because of the problems caused by uncontrolled silicone injections by unqualified practitioners, in 1966, the Food and Drug Administration (FDA) designated silicone injections as a ‘new drug’ (14). This ruling stipulated that silicone must undergo certain laboratory investigations before it could be approved for use. To date, these studies have never been done.
In 1966, the FDA authorized nine plastic surgeons and one dermatologist to investigate the cosmetic use of Dow Corning’s highly purified medical-grade liquid silicone (Dow Corning 360) for certain problems in patients. The study was limited to only these particular physicians, who were treating certain facial deformities that were not treatable by other methods (9). However, it appears that Dow Corning also provided this medical-grade silicone to other physicians, although they were not part of the official study (15). Subsequently, Dow Corning noted that the original study “was not as tight as it could have been”. Therefore, they decided to discontinue efforts to gain FDA approval, because they could not “prevent misuse of the product” (10). In 1975, because of the horrendous complications from silicone injections into breasts in Las Vegas, the state of Nevada declared that it was a felony to inject silicone or to transport liquid silicone across the state line.
By 1990, over 100,000 patients had received facial silicone gel injections of known or unknown origin (13). In August 1991, the FDA issued guidelines (16) clearly forbidding the marketing or sale of injectable liquid silicone for aesthetic injection purposes, until appropriate studies had been completed. To date, these long-term clinical investigations have never been done. In 1991, silicone injections were labeled as ‘adulterated’ by the FDA, to indicate that they had not received FDA approval for marketing or scientific study. In 1992, the FDA issued press releases mandating that “Physicians will no longer be allowed to use injectable silicone for cosmetic treatment unless the product is approved by the FDA for marketing or investigational studies” (17). The FDA has never approved the use of injections of liquid silicone for the cosmetic treatment of patients, except in the 1966, 10-physician investigational study. In 1991/1992, the FDA officially banned the use of all silicone injection products by all physicians.
In spite of all of the warnings and cautions, liquid silicone injections were easily obtainable by any woman who wanted them. They were frequently injected in lay clinics in numerous regions of the United States. They were particularly popular in Mexico and even in San Francisco. Injectable silicone has also always been freely available from Asia.
In Canada, silicone has never been approved for injection purposes. In 1971, physicians and others obtained medical-grade liquid silicone freely from New York, in 1 lb jars! Physicians and lay people used these supplies of silicone through the 1990s. Subsequently, liquid silicone was available by mail order from Panama, Mexico and Asia. Thousands of patients likely received facial injections of liquid silicone in Toronto over the years.
Under Canada’s Medical Device Regulations, as amended in 1983, it is illegal to inject people with substances that have not been approved by federal authorities. This has always included liquid silicone. In 1992, the College of Physicians and Surgeons of Ontario declared that the use of silicone injections was “unlawful” and represented “professional misconduct”. In spite of these declarations, a number of Ontario physicians, and at least one lay clinic in Toronto, continued to regularly inject liquid silicone over the years. Their source of injectable silicone was likely the 1 lb jars that had been obtained from New York in 1971.
In 1994, the FDA approved a form of silicone oil for the treatment of a particularly devastating AIDS-related disorder – complicated retinal detachment secondary to cytomegalovirus retinitis (18). The purpose of the oil was to provide “prolonged retinal tamponade” so that the retina could reattach. In 1997, the FDA approved a commercial formulation of this silicone oil, Silikon 1000 (Alcon, USA), for the treatment of this disorder. In March 2001, the FDA cleared another commercial formulation, Adatosil 5000 (Bausch & Lomb, USA) for treatment of this form of detached retina. For the past several years, a number of practitioners in the United States have purchased these liquid silicone preparations, on the grounds that they were being used for the treatment of retinal detachment. However, they have been injecting the silicone to treat wrinkles and other cosmetic concerns (12–14). In the case of retinal detachment, the silicone is meant to be removed after reattachment of the retina. This removal is not possible when this material is injected into soft tissues.
The FDA has never approved liquid silicone for general cosmetic injection purposes.
However, the FDA has no jurisdiction over the practice of medicine. Therefore, physicians frequently use devices that are FDA-cleared for one indication, but they use it in a totally different application or off-label use. This off-label use is beyond the authority of the FDA. The FDA Modernization Act of 1997 permits FDA-cleared devices to be used off-label, for any condition within the doctor-patient relationship (18).
Currently, FDA-approved clinical trials are reported to be in progress for a liquid silicone product specifically for treatment of HIV-associated facial lipoatrophy and for use in cosmetic indications (14,18). Little is known about the study except that it involves the use of a microdroplet serial puncture technique, as described by Orentreich (19). This injection technique was reported in the late 1970s. It consists of depositing minute droplets of liquid silicone 0.01 mL or smaller into the subdermal tissues at 2 mm to 10 mm intervals. Treatments are spaced at least one month apart until the desired result is obtained. Injection of these microdroplets has been shown to produce a mild inflammatory reaction, resulting in a fibroblastic response. The resulting fibrosis is responsible for the apparent soft-tissue augmentation. By contrast, injections of larger doses (greater than 0.05 mL) has been shown to produce granulomas and foreign body reaction. In this study, patients will be evaluated for at least seven years. To date, no follow-up studies have been reported.
Mammography of breasts injected with silicone demonstrates one of two patterns: multiple cystic masses ranging from 0.2 cm to 2.0 cm in diameter, often with calcification (Figure 3A), or large areas of opacity if larger volumes have been injected into areas of the breast (Figure 3B).
Figure 3).
Mammography following silicone injections demonstrates two possible patterns: multiple cystic masses ranging from 0.2 cm to 2.0 cm in diameter, often with calcification (A); or large areas of opacity if large volumes have been injected (B)
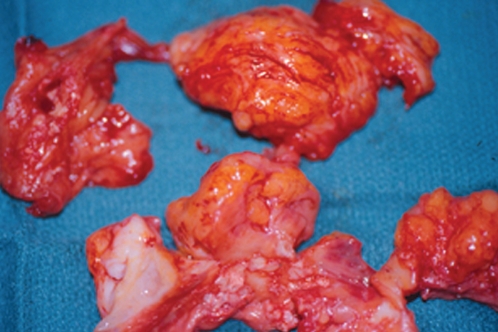
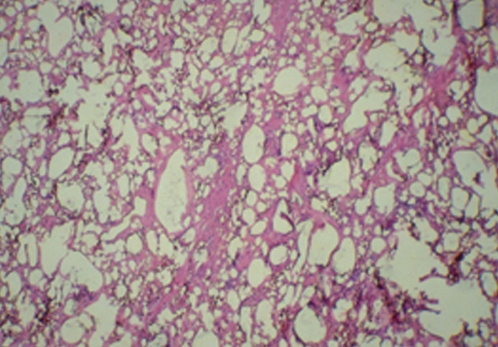
Figure 4 shows the clinical appearance of silicone granulomas, which have been excised from silicone-injected breast tissue. Histology of this tissue (Figure 5) shows extensive involvement of the breast tissue by silicone, which has been washed out during the tissue preparation and now appears as empty spaces or vacuoles. Histology also shows occasional giant cells, vascular obliteration, a chronic inflammatory response and destruction of the breast parenchyma.
Figure 4).
Silicone granulomas resected from breast tissue previously injected with liquid silicone
Figure 5).
Histology of hematoxylin and eosin staining of the tissue shown in Figure 4. There is extensive involvement of the breast tissue by silicone, which appears as empty spaces or vacuoles filled with silicone. There are occasional multinucleated giant cells, areas of vascular obliterans, chronic inflammation, and destruction of breast parenchyma (original magnification ×50)
The reactions of liquid silicone injected into breasts vary considerably. Not all patients appear to be equally susceptible to the deleterious effects of silicone. Some patients do not develop significant symptoms at all. In general, the average time from injection of silicone to the development of complications is nine years (13,15,20). In the study by Wilkie in Vancouver, British Columbia, only 13 of his 92 patients injected with silicone from 1966 to 1976 developed granulomas (15).
There are two main types of clinical presentations. The first type of patient usually presents with multiple and/or painful lumps in their breasts. In some patients, these may occur within two to three years of injection. In other patients, they may not occur until 10 or 15 years after injection (20–22). Many patients have also had multiple injections of cortisone, in an attempt to decrease the inflammatory reaction. This can complicate the clinical picture.
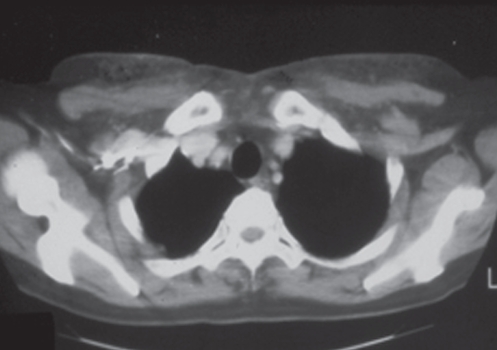
The second type of patient presents with skin inflammation and impending breakdown. As the silicone invades the dermis and epidermis of the overlying skin, the breast may show varying stages of skin circulatory difficulties, from fine telangiectasia to necrosis. Skin capillary filling time is increased. Migration of the silicone is common. Figure 6 shows a woman who received silicone injections into her breasts in 1972 in San Francisco. Over the next 30 years, much of this material migrated to her abdomen. Only a small amount of the silicone is seen on a computed tomography scan (Figure 7).
Figure 6).
This woman received silicone injections to her breasts in San Francisco in 1972. Over time, much of the silicone has migrated to her abdomen
Figure 7).
Computed tomography scan of the patient in Figure 6. Only a small amount of the silicone, which was initially injected into patient’s breasts, currently remains in that location
Smaller granulomas can be treated by localized resection. With more extensive involvement, there is skin destruction, ulceration, necrosis and fistula formation. Once fistulae have developed, treatment is much more complicated. Extensive surgery is usually necessary to fully excise these areas. There have been few reports of successful treatment using a tip-open 8 mm canula (22). The treatment of choice for advanced silicone granulomatosis with ulceration and fistulae is usually bilateral mastectomy. In many cases of major complications, their development is related to the use of industrial silicone, rather than medical-grade silicone. However, there are also well-documented cases of major complications, resulting in bilateral mastectomy, following the use of medical-grade silicone (23).
Injectable liquid silicone has many qualities that could make it a suitable material for long-term soft-tissue augmentation. At the same time, there are still many unanswered questions pertaining to potential complications that need to be addressed before it can be considered for this purpose. A well conducted, controlled, long-term study is needed to answer these questions.
PAH
PAH is an extensively cross-linked polymeric soft tissue filler substance (25) that has been used in Ukraine, Russia and China for the past 15 to 20 years (26–28). PAH was originally introduced to aesthetic surgery under the name of Royamid in Ukraine in the late 1980s. It has subsequently been marketed under many different names. PAH consists of 2.5% polyacrylamide and 97.5% nonpyrogenic water. The repeating unit for the polymers is polypropenoate:
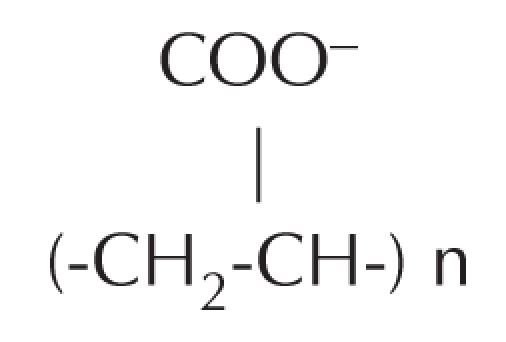
Water is bonded between cross-linked polymers, by hydrogen bonding at the COO− groups. Theoretically, 1.0 mL of 2.5% PAH can bind up to 1.25 mL of normal saline. The water is highly exchangeable with the water molecules of human tissue fluids. A 10% reduction in volume occurs during the first days after implantation, as a consequence of osmotic exchange (28), whereby sodium from tissue fluids displaces water bonded to the COO− groups. Preparations of PAH have been shown to be stable, nontoxic, nonallergenic, nonabsorbable and nonbiodegradable.
PAH is also used in the production of soft contact lenses and intraocular lenses (29). It is a major ingredient of microencapsulated gelosheres for drug delivery systems (30) and antibiotic release systems (31). It is also used in food packaging products (32), in the clarification of beet and cane sugar juices (27), as a filler in the cosmetic industry, and as a flocculating agent in sedimentation and water purification (32). It has been used as a tissue-bulking agent to treat female stress urinary incontinence.
CASE PRESENTATION
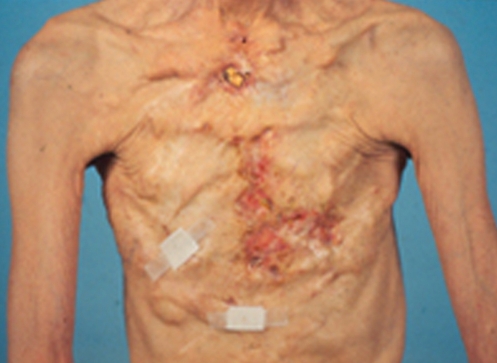
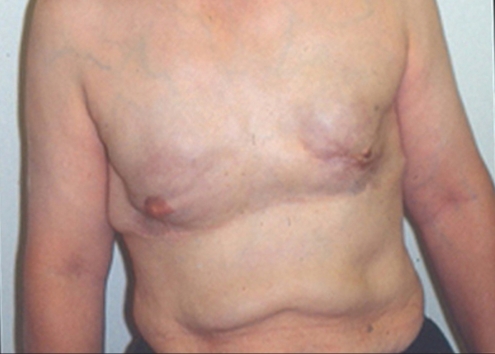
A 29-year-old computer programmer and mother of a two-year-old son presented at our clinic with a one-year history of multiple lumps in both breasts. This patient was originally from Iran. She moved to Toronto about 10 years previously. Following the birth of her son in 2003, her breasts underwent involution and became smaller. Therefore, in August 2004, while she was on a holiday in Iran, she attended a plastic surgery clinic and had injections of PAH into each breast. This was done under a general anaesthetic, through inframammary injection sites. The left side received 150 mL, the right side 190 mL. She had only minimal discomfort for the following two days.
She was initially very pleased with her result. However, approximately one year after the injections, she started to develop visible, palpable, tender lumps in both breasts. They had increased in size significantly over the past year. The lumps were most noticeable in the inframammary and inferior-lateral aspect of the left breast (Figure 8), particularly when the left shoulder was abducted (Figure 9). The right breast was larger than the left side, and it showed significantly more upper pole fullness than the left side (Figure 8). Clinically, the injection on the right side appeared to be submuscular, whereas on the left side, it appeared to be subglandular. The upper pole fullness on the right side became more apparent when she contracted her pectoralis major muscle.
Figure 8).
Breast appearance one year after polyacrylamide hydrogel injection into both breasts in Iran. There is a visible mass in the inframammary area of the left breast
Figure 9).
Visible and palpable masses are most apparent in the inframammary and inferior-lateral areas of the left breast, particularly when the left shoulder is abducted
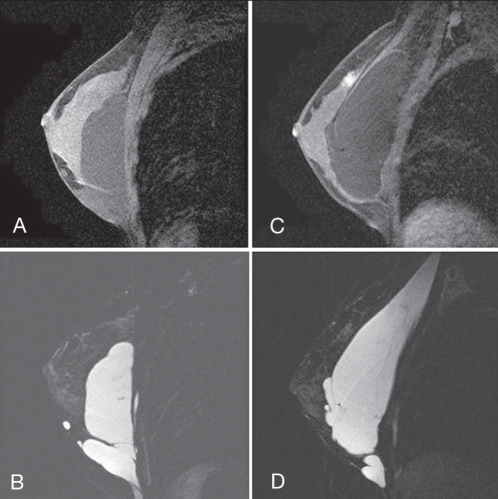
An magnetic resonance imaging study of sagittal gadolinium-enhanced images (mid breast through the nipple) is shown in Figures 10A, B, C and D. On the left side, the T1-weighted image shows low signal intensity material, mostly superficial to the pectoral muscles, in the subglandular plane (Figure 10A). The T2-weighted image shows high signal intensity material, again, mostly in the subglandular plane (Figure 10B). On the right side, the T1-weighted image shows low signal intensity material, mostly deep to the pectoral muscles (Figure 10C). The T2-weighted image shows high signal intensity material, also mostly deep to the pectoral muscles, with only a small amount in the subglandular plane, inferiorly (Figure 10D).
Figure 10).
A T1-weighted image of the left breast with low signal intensity material, mostly superficial to the pectoral muscles, in the subglandular plane. B T2-weighted image of left breast with high intensity material, mostly in the subglandular plane. C T1-weighted image of the right breast with low signal intensity material, mostly deep to the pectoral muscles. D T2-weighted image with high signal intensity material, mostly deep to the pectoral muscles, with only a small amount in the subglandular plane, inferiorly
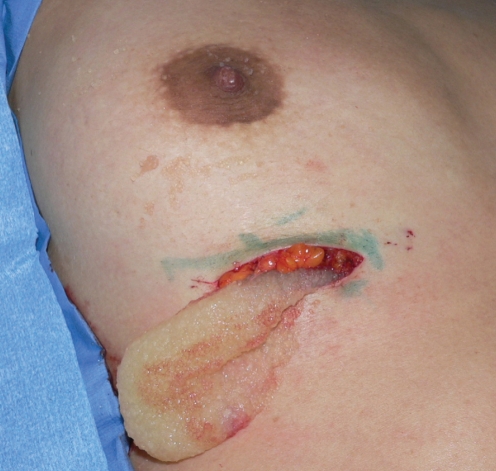
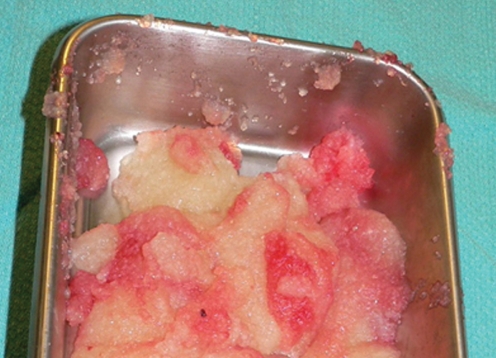
The patient was taken to the operating room and the PAH was removed from both breasts through inframammary incisions (Figure 11). It had the consistency of Cream of Wheat (Figure 12), and was easily ‘milked out’ through the inframammary incisions. The pockets were then thoroughly irrigated with a bacitracin/saline solution. She had an uneventful postoperative recovery.
Figure 11).
The polyacrylamide hydrogel was removed through bilateral inframammary incisions. It was easily expressed out of the pockets
Figure 12).
The polyacrylamide hydrogel had the consistency of Cream of Wheat
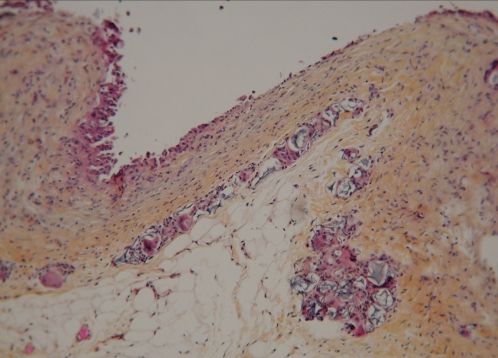
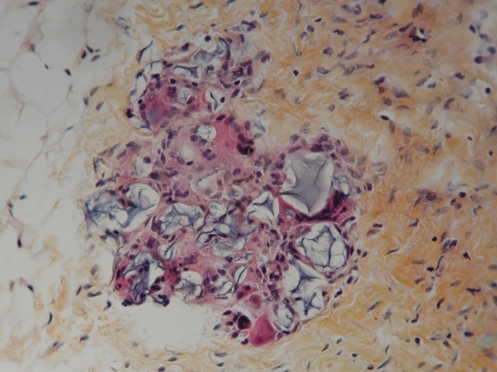
Figure 13 shows the histological appearance of capsular tissue surrounding the PAH material, stained with World Health Organization stain (hematoxylin, phloxin, saffron and alcian green). The interface surface of the capsule is covered by a row of mononuclear and multinucleated histiocytes. These cells are supported by multiple layers of collagenous fibrous tissue. On the surface of the capsule and within the capsule, there are pools of amorphous, nonbirefringent, granular, foreign material. These pools are lined by attenuated, stretched out, multinucleated giant cells (Figure 14).
Figure 13).
World Health Organization stain section of the capsule around the injected polyacrylamide hydrogel material. The interface surface of the capsule (at top of image) is covered by a row of mono-nuclear and multinucleated histiocytes. These cells are supported by multiple layers of collagenous fibrous tissue. On the surface of the capsule and within the capsule, there are pools of amorphous, nonbirefringent, granular, foreign material (original magnification × 12.5)
Figure 14).
World Health Organization stain of a pool of foreign material within the capsule. The pool within the capsule is lined by attenuated, stretched out, multinucleated giant cells (original magnification × 250)
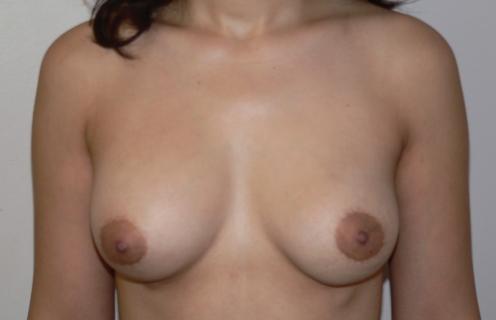
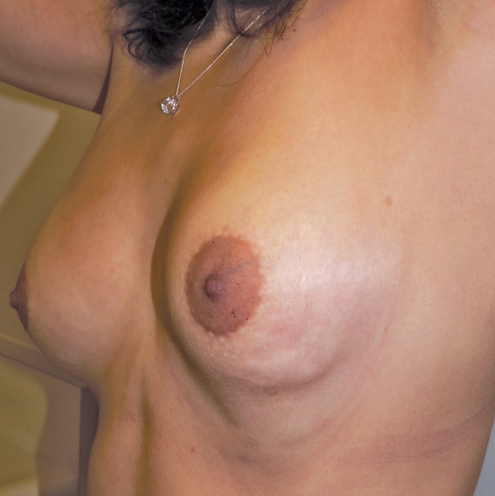
Six months later, the patient became pregnant and breast-fed her child for nine months. Three months later, she underwent a bilateral, inframammary, subglandular breast augmentation with 300 mL Mentor Moderate Plus Profile Elite Gel implants (Mentor Canada). Her final result is shown in Figure 15, which was taken six months months after insertion of her implants.
Figure 15).
Final appearance of breasts six months after the insertion of bilateral, 300 mL, subglandular Moderate Plus Profile Mentor Elite Gel implants (Mentor Canada)
DISCUSSION
During the past century, the history of breast augmentation has repeated itself on many occasions. The dreadful complications of paraffin injections (1899 to 1914) were repeated many times over the next 30 years, with the huge plethora of materials that were used for breast augmentation. During the 1940s and 1950s, a half-century after the paraffin saga, history repeated itself again with silicone injections. Some of the complications were even worse, because industrial grade silicone was injected and contaminants were purposely added to the silicone injections, to cause sclerosis of the silicone, to hopefully restrict its migration.
During the past 20 years, a newer class of injectable material has been used in Ukraine, Russia, and China: PAH. This material was originally introduced to aesthetic surgery under the name of Royamid in Ukraine in late 1980s (33,34). It was subsequently marketed under the name of Interfall (Contura International SA, Switzerland) in Ukraine, and Formacryl in Russia (25). In 1997, it was approved by the State Drug Administration in China (the Chinese equivalent of the FDA). Most preparations marketed in Russia, Ukraine and China today are manufactured by Contura International (Denmark), under the brand name Aquamid. Over the years, PAH has also been marketed as Outline, Bioformacryl, Argiform, Amazing Gel and Bio-Alkamid (35–38). These preparations have been used in over 30,000 cases in Europe and Russia (25). Although the most common site for PAH injections has been the breasts, they have also been used for soft tissue contouring in the face, lips and chest (25,37–39).
Initially, PAH appeared to be an ideal soft tissue filler material. However, several reports have subsequently appeared, demonstrating that numerous complications can occur after PAH injections. These can develop from several months to three years after injection. They include the following: migration, breast lumps, pain, infection, firmness and disfigurement (35–39). Migration of PAH appears to be due, in part, to a leak of the viscous injected material from the pocket, through the injection tract (40). To avoid this problem, injections have been done through very long 18-gauge needles, which are flushed out with saline, to push the PAH from the needle back into the pocket. When these problems have developed, attempts have been made to remove the PAH from the involved tissues. This has been done by both cannula suction and open techniques. Removal is much more effective if the PAH has been injected into large pockets, rather than into multiple small sites (39).
The current patient, who received PAH injections in Iran, showed only minimal complications with migration and surface lumps in her breasts. However, we have also seen other patients (eg, from Russia), who have presented with major recurrent infections and multiple sinuses, many years after PAH injection. It could well be that there are different chemical formulations and different purifications of PAH. These may be dependent on the source of the material. It is interesting that the Chinese State Food and Drug Administration (SFDA) has recently banned the production, sale and use of PAH (41). They received 183 reports of adverse effects from the use of PAH from 2002 to November 2005. Of those, 161 involved breast injection patients who suffered infections and disfigurement. The SFDA has stipulated that all existing supplies of PAH are to be recalled and destroyed under SFDA supervision. Failure to comply with this mandate will result in criminal prosecution. Once again, history has repeated itself!
REFERENCES
- 1.Gersuny R. Ueber eine subcutyane Prothese. Z Heilk. 1900;30:1–5. [Google Scholar]
- 2.Gersuny R. Harte and weiche Paraffinprothesen. Zentralbl Chir. 1903;30:1–3. [Google Scholar]
- 3.Hollander E. Abstract from Berliner Gesellschaft fur Chirurgie. Munch Med Wochenschr. 1912;59:2842. [Google Scholar]
- 4.Kolle FS. Plastic and Cosmetic Surgery. New York: D Appleton and Co; 1911. [Google Scholar]
- 5.Schmorl Paraffingranulome, Munch Med Wochenscr. 1922;69:215–20. [Google Scholar]
- 6.Erguvan-Dogan B, Yang WT. Direct injection of paraffin into the breast: Mammographic, sonographic, and MRI features of early complications. Am J Roentgenol. 2006;186:888–94. doi: 10.2214/AJR.05.0064. [DOI] [PubMed] [Google Scholar]
- 7.Sinclair DS, Freedy L, Spigos DG. Altered breast: Paraffin injection with development of paraffinomas. Am J Roentgenol. 2000;175:864–5. [PubMed] [Google Scholar]
- 8.Matton G. The history of injectable biomaterials and the biology of collagen. Aesthetic Plast Surg. 1985;9:133–40. doi: 10.1007/BF01570345. [DOI] [PubMed] [Google Scholar]
- 9.Mastrucerio DN, Pesqueira MJ. Severe granulomatous reaction and facial ulceration occurring after subcutaneous silicone injection. J Am Acad Dermatol. 1996;34:849–52. doi: 10.1016/s0190-9622(96)90043-2. [DOI] [PubMed] [Google Scholar]
- 10.Duffy DM. Injectable liquid silicone: New perspectives. In: Klein AW, editor. Tissue Augmentation in Clinical Practice. 1st edn. New York: Marcel Dekker Inc; 1998. pp. 237–27. [Google Scholar]
- 11.Milojevic B. Complications after silicone injection therapy in aesthetic plastic surgery. Aesthetic Plast Surg. 1982;20:267–276. doi: 10.1007/BF01570647. [DOI] [PubMed] [Google Scholar]
- 12.Romm S. The Changing Face of Beauty. Toronto: Mosby Year Book; 1992. pp. 188–90. [Google Scholar]
- 13.Christensen L, Breiting V, Jansen M, et al. Adverse reactions to injectable soft tissue permanent fillers. Aesthetic Plast Surg. 2005;29:34–48. doi: 10.1007/s00266-004-0113-6. [DOI] [PubMed] [Google Scholar]
- 14.Coleman SR. Injectable silicone returns to the United States. Aesthetic Surg J. 2001;21:576–8. doi: 10.1067/maj.2001.120705. [DOI] [PubMed] [Google Scholar]
- 15.Wilkie TF. Late development of granuloma after liquid silicone injections. Plast Reconstr Surg. 1977;60:179–88. doi: 10.1097/00006534-197708000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Food and Drug Administration. Current and Useful Information on Collagen and Liquid Silicone Injections. FDA Backgrounder, August 1991. BG91-2.0.
- 17.“Physicians to stop Injecting Silicone for Cosmetic Treatment of Wrinkles.” Food and Drug Administration. Press Release 11/07/1992;92–95.
- 18.Food and Drug Administration Press Release 11/07/1994;94–95.
- 19.Orentreich DS. Liquid injectable silicone: Techniques for soft tissue augmentation. Clin Plast Surg. 2000;27:595–612. [PubMed] [Google Scholar]
- 20.Vinnik CA. Silicone mastopathy. In: Owsley JQ, Peterson RA, editors. Symposium on Aesthetic Surgery of the Breast. St Louis: The C. V. Mosby Company; 1978. pp. 151–5. [Google Scholar]
- 21.Rohrich RJ, Potter JK. Liquid injectable silicone: Is there a role as cosmetic soft-tissue filler? Plast Reconstr Surg. 2004;113:1239–41. doi: 10.1097/01.prs.0000118022.71802.78. [DOI] [PubMed] [Google Scholar]
- 22.Lai CS, Lin TM, Lee SS, et al. Surgical treatment of facial siliconoma involving the temporal area. Plast Reconstr Surg. 2005;115:553–8. doi: 10.1097/01.prs.0000149483.70678.c9. [DOI] [PubMed] [Google Scholar]
- 23.Achauer BM. A serious complication following medical-grade silicone injections of the face. Plast Reconstr Surg. 1883;71:251–3. doi: 10.1097/00006534-198302000-00020. [DOI] [PubMed] [Google Scholar]
- 24.Bello G, Jackson I, Keskin M, et al. The use of polyacrylamide gel in soft-tissue augmentation; an experimental assessment. Plast Reconstr Surg. 2007;119:1326–36. doi: 10.1097/01.prs.0000254824.13065.3b. [DOI] [PubMed] [Google Scholar]
- 25.Stephens S. Final report on the safety assessment of polyacrylamide. J Am Coll Toxicol. 1991;10:193–8. [Google Scholar]
- 26.Christenen LH, Breiting VB, Aasted A, et al. Long-term effects of polyacrylamide hydrogel on human breast tissue. Plast Reconstr Surg. 2003;111:1883–90. doi: 10.1097/01.PRS.0000056873.87165.5A. [DOI] [PubMed] [Google Scholar]
- 27.de Cassia Novaes W, Berg A. Experiences with a non-biodegradable hydrogel (Aquamid): A pilot study. Aesthetic Plast Surg. 2003;27:376–81. doi: 10.1007/s00266-003-2119-x. [DOI] [PubMed] [Google Scholar]
- 28.Lloyd AW, Faragher RG, Denyer SP. Ocular biomaterials and implants. Biomaterials. 2001;22:769–72. doi: 10.1016/s0142-9612(00)00237-4. [DOI] [PubMed] [Google Scholar]
- 29.Vyas SP, Sood A, Venugopalan P, et al. Preparation and characterization of microencapsulated gelospheres for controlled oral theophylline delivery. J Microencapsul. 2000;17:767–70. doi: 10.1080/02652040050161756. [DOI] [PubMed] [Google Scholar]
- 30.Risbud MV, Bholde RR. Polyacrylamide-chitosan hydrogels: In vitro biocompatibiltiy and sustained antibiotic release studies. Drug Deliv. 2000;7:69–74. doi: 10.1080/107175400266623. [DOI] [PubMed] [Google Scholar]
- 31.McCollister DD, Hake CL, Sadek SE, et al. Toxicological investigations of polyacrylamides. Toxicol Appl Pharmacol. 1965;7:639–43. doi: 10.1016/0041-008x(65)90119-5. [DOI] [PubMed] [Google Scholar]
- 32.Puget FP, Halasz MR, Massarani G, et al. Influence of the flocculating agent in sedimentation and performance of a non flocculent strain of Zymomonas mobilis in the ethanol production process. Bioseparation. 2000;9:119–24. doi: 10.1023/a:1008140029482. [DOI] [PubMed] [Google Scholar]
- 33.Niechajev I. Lip enhancement: Surgical alternatives and histological aspects. Plast Reconstr Surg. 2000;105:375–80. doi: 10.1097/00006534-200003000-00055. [DOI] [PubMed] [Google Scholar]
- 34.Christensen L, Breiting V, Janssen M, et al. Adverse reactions to injectable soft issue permanent fillers. Aesthetic Plast Surg. 2005;29:34–48. doi: 10.1007/s00266-004-0113-6. [DOI] [PubMed] [Google Scholar]
- 35.Qiao Q, Wang X, Sun J, et al. Management for postoperative complications of breast augmentation by injected polyacrylamide hydrogel. Aesthetic Plast Surg. 2005;29:156–61. doi: 10.1007/s00266-004-0099-0. [DOI] [PubMed] [Google Scholar]
- 36.Cheng N, Wang Y, Wang J, et al. Complications of breast augmentation with injected hydrophilic polyacrylamide gel. Aesthetic Plast Surg. 2002;26:375–82. doi: 10.1007/s00266-002-2052-4. [DOI] [PubMed] [Google Scholar]
- 37.Niedzielska I, Pajak J, Drugacz J. Late complications after polyacrylamide hydrogel injection in to facial soft tissues. Aesthetic Plast Surg. 2006;l30:377–8. doi: 10.1007/s00266-005-0014-3. [DOI] [PubMed] [Google Scholar]
- 38.Cheng NX, Xu SL, Deng H, et al. Migration of implants: A problem with injectable polyacrylamide gel in aesthetic plastic surgery. Aesthetic Plast Surg. 2006;30:215–25. doi: 10.1007/s00266-005-0081-5. [DOI] [PubMed] [Google Scholar]
- 39.Christensen L, Breiting V. Management of postoperative complications of breast augmentation by injected polyacrylamide hydrogel. Aesthetic Plast Surg. 2006;30:132–3. doi: 10.1007/s00266-005-0156-3. [DOI] [PubMed] [Google Scholar]
- 40.Orenstein A, Bar-Meir E. Bio-Alkamid: Avoiding the leak. Plast Reconstr Surg. 2005;115:1789–90. doi: 10.1097/01.prs.0000162113.00488.22. [DOI] [PubMed] [Google Scholar]
- 41.China announces ban on polyacrylamide hydrogel breast implants. Press release: Chinese State Food and Drug Administration, May 2, 2006.