Short abstract
Resistance to β lactam antibiotics is an increasing problem worldwide. This review describes the classification and mechanism of action of β lactamases and the options available for detecting, treating, and controlling extended spectrum β lactamases
β lactam antimicrobial agents are the most common treatment for bacterial infections (table 1).1 Rates of bacterial resistance to antimicrobial agents are increasing worldwide, including in Lebanon.2 Production of β lactamases is the most common mechanism of bacterial resistance (table 2).1,3 These enzymes are numerous, and they mutate continuously in response to the heavy pressure of antibiotic use, leading to the development of extended spectrum β lactamases (ESBLs).4 Examples are the mutated TEM and SHV genes, mainly found in strains of Escherichia coli and Klebsiella pneumoniae respectively. Infections with ESBL producing bacterial strains are encountered singly or in outbreaks, especially in critical care units in hospitals, resulting in increasing costs of treatment and prolonged hospital stays. We aim to present a simplified review of this highly complex subject, in the hope that it will guide the practising physician in appropriate decisions relating to the use of β lactams in patient care.
Table 1.
Groups and examples of β lactam antimicrobial agents
| β lactam groups | Examples of antimicrobial agents |
|---|---|
| Penicillins | Penicillin G, penicillin
|
| Penicillinase resistant penicillins: methicillin, nafcillin, oxacillin, cloxacillin
|
|
| Aminopenicillins: ampicillin, amoxicillin
|
|
| Carboxypenicillins: carbenicillin, ticarcillin
|
|
| Ureidopenicillins: mezlocillin, piperacillin | |
| Cephalosporins | First generation: cefazolin, cephalothin, cephalexin
|
| Second generation: cefuroxime, cefaclor, cefamandole, cefamycins (cefotetan, cefoxitin)
|
|
| Third generation: cefotaxime, ceftriaxone, cefpodoxime, ceftizoxime, cefoperazone, ceftazidime
|
|
| Fourth generation: cefepime, cefpirome
|
|
| Carbapenems | Imipenem, meropenem, ertapenem |
| Monobactams | Aztreonam |
Table 2.
Antimicrobial agents, their modes of action, and the corresponding mechanisms of bacterial resistance
| Antimicrobial agents | Mode of action | Resistance mechanisms |
|---|---|---|
| β lactams | Cell wall synthesis, cell division | β lactamase, altered penicillin binding proteins |
| Glycopeptides (azoles, cycloserine) | Cell wall division | Blocking of drug access to pentapeptide |
| Aminoglycosides (spectinomycin) | Inhibit protein synthesis (bind to 30S ribosome) | Enzymatic inactivation, altered target, impermeability |
| Macrolides | Inhibit protein synthesis (bind to 50S ribosome) | Altered target, enzymatic inactivation |
| Tetracycline | Inhibit protein synthesis (affect t-RNA binding to 30S) | Efflux, altered target, impermeability, enzymatic inactivation |
| Chloramphenicol (lincosamides, streptogramin) | Inhibit protein synthesis (bind to 50S ribosome) | Enzymatic inactivation, impermeability |
| Quinolones | Replication: inhibit DNA gyrase | Altered target enzymes, impermeability |
| Rifampin | Transcription: inhibit DNA dependent RNA polymerase | Altered target enzymes, impermeability |
| Sulfonamides | Folic acid synthesis | Altered target |
| Trimethoprim | Folic acid synthesis | Altered target, impermeability |
| Polyenes (nystatin, amphotericin B) | Cell membrane permeability | Ergosterol deficient mutants |
Sources and selection criteria
We examined new information from the most recent relevant literature retrieved from PubMed and the internet.
Groups and mechanisms of action of β lactams
The β lactams are a family of antimicrobial agents consisting of four major groups: penicillins, cephalosporins, monobactams, and carbapenems (table 1). They all have a β lactam ring, which can be hydrolysed by β lactamases. The groups differ from each other by additional rings (thiazolidine ring for penicillins, cephem nucleus for cephalosporins, none for monobactams, double ring structure for carbapenems). The various antibiotics in each group differ by the nature of one or two side chains.
The β lactam antibiotics act on bacteria through two mechanisms targeting the inhibition of cell wall synthesis.5 Firstly, they are incorporated in the bacterial cell wall and inhibit the action of the transpeptidase enzyme responsible for completion of the cell wall. Secondly, they attach to the penicillin binding proteins that normally suppress cell wall hydrolases, thus freeing these hydrolases, which in turn act to lyse the bacterial cell wall. To bypass these antimicrobial mechanisms of action, bacteria resist by producing β lactam inactivating enzymes (β lactamases) or mutated types of penicillin binding proteins. Here, we will discuss only β lactamases.
Summary points
β lactamase producing bacteria are increasing in number and causing more severe infections, because of their continuous mutation
Extended mutation has led to the emergence of extended spectrum β lactamase enzymes, the incidence and types of which vary with geographical location and time
The functional and molecular classifications are complex for the practising physician who is facing problems in deciding how to treat infections caused by bacteria producing these enzymes
Awareness and detection of these enzymes are necessary for optimal patient care
β lactamases
Synthesis and mode of transfer
The synthesis of β lactamases is either chromosomal (constitutive), as in Pseudomonas aeruginosa, or plasmid mediated (inducible), as in Aeromonas hydrophila and Staphylococcus aureus. Plasmids are a major cause of bacterial resistance spreading, as they can be transferred between Gram negative bacteria by conjugation and between Gram positive bacteria by bacterial viruses called transducing phages. This transferability is responsible for many outbreaks of resistance, especially when appropriate infection control measures are breached in hospital settings.
Location
In the Gram positive bacteria β lactamases are secreted to the outside membrane environment as exoenzymes. In the Gram negative bacteria they remain in the periplasmic space, where they attack the antibiotic before it can reach its receptor site.3
Mechanisms of action
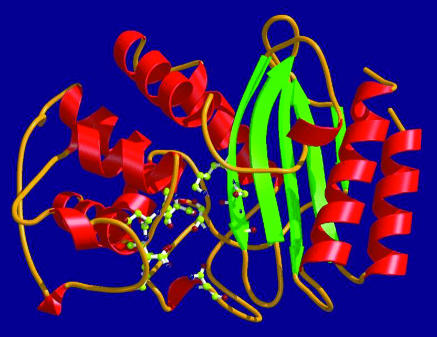
β lactamase enzymes destroy the β lactam ring by two major mechanisms of action. Firstly, most common β lactamases have a serine based mechanism of action. They are divided into three major classes (A, C, and D) on the basis of the amino acid sequences. They contain an active site consisting of a narrow longitudinal groove, with a cavity on its floor (the oxyanion pocket), which is loosely constructed in order to have conformational flexibility in terms of substrate binding (fig 1).1,3 Close to this lies the serine residue that irreversibly reacts with the carbonyl carbon of the β lactam ring, resulting in an open ring (inactive β lactam) and regenerating the β lactamase. These enzymes are active against many penicillins, cephalosporins, and monobactams. Secondly, a less commonly encountered group of β lactamases is the metallo β lactamases, or class B β lactamases. These use a divalent transition metal ion, most often zinc, linked to a histidine or cysteine residue or both, to react with the carbonyl group of the amide bond of most penicillins, cephalosporins, and carbapenems, but not monobactams.6
Fig 1.
Molecular structure of β lactamase. Adapted from http://biosafety.ihe.be/AR/betalactamase.html
Classification of β lactamases
Because of the diversity of enzymatic characteristics of the many β lactamases discovered so far, many attempts have been made to categorise and classify them since the late 1960s. These classifications involve two major approaches: the first and older one is based on the biochemical and functional characteristics of the enzyme; the second approach is based on the molecular structure of the enzyme.
Functional classification of β lactamases
Several criteria were used in the functional classification of the β lactamases, including the spectrum of antimicrobial substrate profile, enzyme inhibition profile, enzyme net charge (pI), hydrolysis rate (Vmax), binding affinity (Km), isoelectric focusing, protein molecular weight, and amino acid composition. Since the 1960s several functional classification schemes of β lactamases have evolved, as shown in table 3.7
Table 3.
Evolution of functional classification of β lactamases
| Year | Author | Basis of classification of β lactamases |
|---|---|---|
| 1968 | Sawai et al | Used cephalosporins versus penicillins as substrates |
| 1973 | Richmond and Sykes | Expanded substrate profile and suggested five major groups (la-d, II, III, IV, V) |
| 1976 | Sykes and Matthew | Differentiated the plasmid mediated β lactamases on the basis of isoelectric focusing |
| 1981 | Mitsuhachi and Inoue | Added the category “cefuroxime hydrolysing β lactamase” |
| 1989 | Bush | Expanded further the substrate profile, added the reaction with EDTA, correlated between functional and molecular classification |
| 1995 | Bush, Jacoby, and Medeiros | Expanded the Bush scheme and used biochemical properties, molecular structure, and nucleotide sequence. Suggested classification into four groups (1-4)* on the basis of the spectrum of activity and other functional characteristics |
Details in text and in table on bmj.com
Bush-Jacoby-Medeiros presented, in 1995, the latest classification of β lactamases based on four groups (1-4) and subgroups (a-f) as follows (see table on bmj.com).7
Group 1 are cephalosporinases not inhibited by clavulanic acid, belonging to the molecular class C
-
Group 2 are penicillinases, cephalosporinases, or both inhibited by clavulanic acid, corresponding to the molecular classes A and D reflecting the original TEM and SHV genes. However, because of the increasing number of TEM and SHV derived β lactamases, they were divided into two subclasses, 2a and 2b. The 2a subgroup contains just penicillinases, whereas 2b are broad spectrum β lactamases, meaning that they are capable of inactivating penicillins and cephalosporins at the same rate. Furthermore, new subgroups were segregated from subgroup 2b:
- Subgroup 2be, with the letter “e” for extended spectrum of activity, represents the ESBLs, which are capable of inactivating third generation cephalosporins (ceftazidime, cefotaxime, and cefpodoxime) as well as monobactams (aztreonam)
- The 2br enzymes, with the letter “r” denoting reduced binding to clavulanic acid and sulbactam, are also called inhibitor resistant TEM derivative enzymes; nevertheless, they are still susceptible to tazobactam
- Later, subgroup 2c was segregated from group 2 because these enzymes inactivate carbenicillin more than benzylpenicillin, with some effect on cloxacillin
- Subgroup 2d enzymes inactivate cloxacillin more than benzylpenicillin, with some activity against carbenicillin; these enzymes are poorly inhibited by clavulanic acid, and some of them are ESBLs
- Subgroup 2e enzymes are cephalosporinases that can also hydrolyse monobactams, and they are inhibited by clavulanic acid
- Subgroup 2f was added because these are serine based carbapenemases, in contrast to the zinc based carbapenemases included in group 3
Group 3 are the zinc based or metallo β lactamases, corresponding to the molecular class B, which are the only enzymes acting by the metal ion zinc as discussed above. They are able to hydrolyse penicillins, cephalosporins, and carbapenems. Thus, carbapenems are inhibited by both group 2f (serine based mechanism) and group 3 (zinc based mechanism)
Group 4 are penicillinases that are not inhibited by clavulanic acid, and they do not yet have a corresponding molecular class.
Molecular classification
The molecular classification of β lactamases is based on the nucleotide and amino acid sequences in these enzymes. To date, four classes are recognised (A-D), correlating with the functional classification (see table on bmj.com). Classes A, C, and D act by a serine based mechanism, whereas class B or metallo β lactamases need zinc for their action.8
Extended spectrum β lactamases
The persistent exposure of bacterial strains to a multitude of β lactams has induced a dynamic and continuous production and mutation of β lactamases in these bacteria, expanding their activity even against the third and fourth generation cephalosporins such as ceftazidime, cefotaxime, and cefepime and against aztreonam. Thus these new β lactamases are called extended spectrum β lactamases.9
The incidence of ESBLs varies with geographical location and time. In Lebanon, the incidence of ESBLs increased approximately twofold at a major tertiary hospital, the American University of Beirut Medical Center, between 1998 and 2002, for both E coli (3% v 5 %) and K pneumoniae (6.4% v 13%).2 In the USA the incidence in Enterobacteriacae ranges from zero to 25%, and in Europe the incidence is 23-25% for Klebsiella spp and 5.4% for E coli.4
These ESBLs enzymes are plasmid borne and have evolved from point mutations altering the configuration of the active site of the original and long known β lactamases designated TEM-1, TEM-2, and SHV-1. The activity of these enzymes is limited to ampicillin, penicillin, and carbenicillin. The original TEM was first discovered in E coli in a patient named Temoniera in Greece, but it spread rapidly to other bacteria. Although TEM-type β lactamases are most often found in E coli and K pneumoniae, they are also found in other genera of Enterobacteriacae and in other penicillin or ampicillin resistant Gram negative bacteria such as Haemophilus influenzae and Neisseria gonorrhoeae. The SHV enzymes, named after the “sulfhydryl variable” active site, are commonly associated with K pneumoniae. At first these bacteria contained a single ESBL gene, but now multiple ESBL genes are commonly present in a single strain, further complicating the process of detecting them and identifying an appropriate treatment regimen.10 To date, more than 90 TEM-type and more than 25 SHV-type β lactamases have been identified. Other recently recognised genes with similar activity include PER-1 β lactamases, first discovered in Pseudomonas aeruginosa in Turkey, and the VEB-1 and TLA-1 from single E coli isolates from Vietnam and Mexico respectively.4
The ESBL producing bacteria are typically associated with multidrug resistance, because genes with other mechanisms of resistance often reside on the same plasmid as the ESBL gene.10 Thus some ESBL producing strains also show resistance to quinolones, aminoglycosides, and trimethoprim-sulfamethoxazole.11 β lactamase inhibitors such as β lactam-β lactamase inhibitor combinations could show higher in vitro susceptibility results against bacterial strains with ESBL production than their original parent. However, their in vivo activity remains to be validated.12
Infections with ESBL producing bacteria can result in avoidable failure of treatment and increased cost in patients who have received inappropriate antibiotic treatment. Nosocomial outbreaks of this form of resistance are most often associated with intensive care units and oncology, burns, and neonatal wards. They can result in prolongation of hospital stay, as well as devastating or even fatal consequences.13
Methods of detecting ESBLs
The increasing prevalence of ESBL producing bacterial strains has caused many outbreaks. This has warranted the establishment of rapid and reliable laboratory methods for screening and confirmation (table 4, fig 2).14-18
Table 4.
Laboratory tests for detection of extended spectrum β lactamases
| Tests | Method and interpretation |
|---|---|
| Screening tests | |
| Double disk approximation or double disk synergy | Disk of third generation cephalosporin placed at 30 mm distance from amoxicillin-clavulanic acid. Enhanced inhibition indicates ESBL |
| Combination disk | Uses two discs of third generation cephalosporin alone and combined with clavulanic acid. An increase in the zone inhibition of >5 mm with the combination disk indicates ESBL |
| Microdilution test | Growth in a broth containing 1 μg/ml third generation cephalosporin indicates ESBL |
| Confirmatory tests | |
| MIC broth dilution | MIC of third generation cephalosporin alone or combined with clavulanic acid. A decrease in the MIC of the combination of ≥3 twofold dilutions indicates ESBL |
| E test (MIC ESBL strips) | Two sided strip containing ceftazidime on one side and ceftazidime-clavulanic acid on the other. The ratio of the MIC of the combination to that of ceftazidime alone of >8, or the presence of a phantom zone (or both) indicates ESBL |
| Automated instruments (for example, Vitek) | Measures MICs and compares growth of bacteria in presence of ceftazidime v ceftazidime-clavulanic acid |
| Molecular (DNA probes, PCR, RFLP) | Targets specific nucleotide sequences to detect different variants of TEM and SHV genes |
ESBL=extended spectrum β lactamases; MIC=minimum inhibitory concentration; PCR=polymerase chain reaction; RFLP=restriction fragment length polymorphism.
Fig 2.
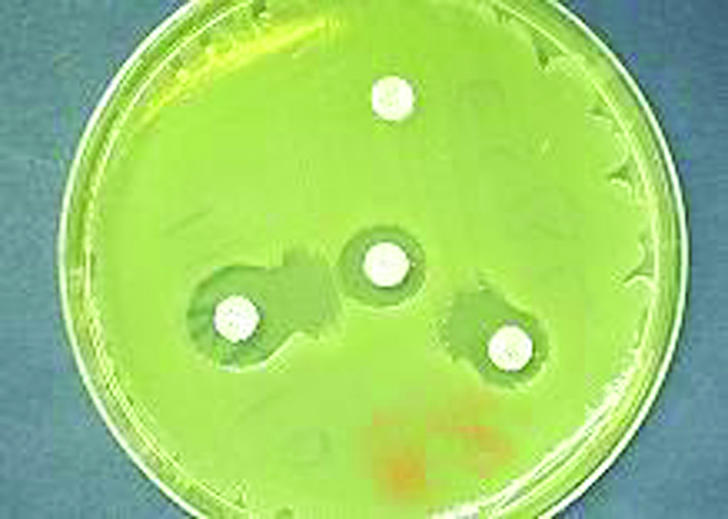
Double disk approximation, or double disk synergy, test to detect ESBL producing bacteria
Generally, an isolate is suspected to be an ESBL producer when it shows in vitro susceptibility to the second generation cephalosporins (cefoxitin, cefotetan) but resistance to the third generation cephalosporins and to aztreonam. Moreover, one should suspect these strains when treatment with these agents for Gram negative infections fails despite reported in vitro susceptibility. Once an ESBL producing strain is detected, the laboratory should report it as “resistant” to all penicillins, cephalosporins, and aztreonam, even if they test as susceptible.19,20 Other antimicrobial agents can be reported as they are tested.
Additional educational resources
www.cdc.gov/ncidod/hip/Lab/FactSheet/esbl.htm—information on laboratory detection of extended spectrum β lactamases (ESBLs)
www.phppo.cdc.gov/dls/master/qa-arc02.asp—answers the question of why only Klebsiella pneumoniae, Klebsiella oxytoca, and Escherichia coli, and not other Enterobacteriaceae, are screened for ESBL production
biosafety.ihe.be/AR/betalactamases.html—Belgian biosafety server: antibioresistance archive on β lactam resistance
www.ncbi.nlm.nih.gov—national center for biotechnology information
www.cdc.gov/ncidod/eid/vol7no2/pdfs/thomson.pdf—Emerging Infectious Diseases: special issue on ESBLs
Treatment of ESBLs
Essentially, the choice of drug for treating ESBL producing bacteria is limited to carbapenems—for example, imipenem. Alternatively, fluoroquinolones and aminoglycosides may be used if they show in vitro activity. Although clinical data for their use are absent, a β lactam-β lactamase inhibitor combination such as amoxicillin-clavulanate or piperacillin-tazobactam may also be a further option to consider. All these agents should be used with caution, however, as their susceptibility varies among ESBL producers. Cefamycins, such as cefoxitin and cefotetan, although active in vitro, are not recommended for treating such infections, because of the relative ease with which these strains decrease the expression of outer membrane proteins, rendering them resistant.21
Control measures
Proper infection control practices and barriers are essential to prevent spreading and outbreaks of ESBL producing bacteria. The reservoir for these bacteria seems to be the gastrointestinal tract of patients.22 Alternative reservoirs could be the oropharynx, colonised wounds, and urine. The contaminated hands and stethoscopes of healthcare providers are important factors in spreading infection between patients.23 Essential infection control practices should include hand washing by hospital personnel, increased barrier precautions, and isolation of patients colonised or infected with ESBL producers. Other practices that have minimised the spread of such organisms include clinical and bacteriological surveillance of patients admitted to intensive care units and antibiotic cycling, as well as policies of restriction, especially on the empirical use of broad spectrum antimicrobial agents such as the third and fourth generation cephalosporins and imipenem.24
Supplementary Material
 An extra table appears on bmj.com
An extra table appears on bmj.com
Contributors: GFA initiated the concept and plan of the review. JNS-K did the literature search, wrote the first draft, and was involved in the evolution of the review.
Competing interests: None declared.
References
- 1.Kotra LP, Samama J, Mobashery S. Beta-lactamases and resistance to beta-lactam antibiotics. In: Lewis K, Salyers AA, Taber HW, Wax RG, eds. Bacterial resistance to antimicrobials. New York: Marcel Decker, 2002: 123-60.
- 2.Araj GF, Kanj SA. Current status and changing trends of antimicrobial resistance in Lebanon. Leb Med J 2000;48: 221-6. [PubMed] [Google Scholar]
- 3.Stratton CW. Mechanisms of bacterial resistance to antimicrobial agents. Leb Med J 2000;48: 186-98. [PubMed] [Google Scholar]
- 4.Bradford PA. Extended-spectrum beta-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev 2001;14: 933-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dbaibo GS. Old and new targets of antibacterial therapy. Leb Med J 2000;48: 177-81. [PubMed] [Google Scholar]
- 6.Page MI. Understanding metallo-β-lactamases. ASM News 2002;68: 217-21. [Google Scholar]
- 7.Bush K, Jacoby GA, Medeiros AA. A functional classification scheme for beta-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother 1995;39: 1211-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ambler RP. The structure of beta-lactamases. Philos Trans R Soc Lond B Biol Sci 1980;289: 321-31. [DOI] [PubMed] [Google Scholar]
- 9.Bush K. New beta-lactamases in gram-negative bacteria: diversity and impact on the selection of antimicrobial therapy. Clin Infect Dis 2001;32: 1085-9. [DOI] [PubMed] [Google Scholar]
- 10.Bradford P, Cherubin CE, Idemyor V, Rasmussen BA, Bush K. Multiply resistant Klebsiella pneumoniae from two Chicago hospitals: identification of the extended spectrum TEM-12 and TEM-10 ceftazidime hydrolyzing beta-lactamases in a single isolate. Antimicrob Agents Chemother 1994;38: 761-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paterson DL, Mulazimoglu L, Casellas JM, Ko WC, Goossens H, Von Gottberg A, et al. Epidemiology of ciprofloxacin resistance and its relationship to extended-spectrum beta-lactamase production in Klebsiella pneumoniae isolates causing bacteremia. Clin Infect Dis 2000;30: 473-8. [DOI] [PubMed] [Google Scholar]
- 12.Ferrara A, Dos Santos C, Cimbro M. Effect of different beta-lactams in combination with beta-lactamase inhibitors in the presence or absence of tobramycin against some enterobacteriaceae producing extended-spectrum beta-lactamases. Chemotherapy 1998;44: 313-7. [DOI] [PubMed] [Google Scholar]
- 13.Bisson G, Fishman NO, Patel JB, Edelstein PH, Lautenbach E. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella species: risk factors for colonization and impact of antimicrobial formulary interventions on colonization prevalence. Infect Control Hosp Epidemiol 2002;23: 254-60. [DOI] [PubMed] [Google Scholar]
- 14.Thomson KS, Sanders CC, Moland ES. Use of microdilution panels with and without beta-lactamase inhibitors as phenotypic test for beta-lactamase production among Escherichia coli, Klebsiella spp., Enterobacter spp., Citrobacter freundii, and Serratia marcescens. Antimicrob Agents Chemother 1999;43: 1393-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cormican MG, Marshall SA, Jones RN. Detection of extended-spectrum beta-lactamase (ESBL)-producing strains by the Etest ESBL screen. J Clin Microbiol 1996;34: 1880-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leverstein-van Hall MA, Fluit AC, Paauw A, Box AT, Brisse S, Verhoef J. Evaluation of the Etest ESBL and the BD Phoenix, VITEK 1, and VITEK 2 automated instruments for detection of extended-spectrum beta-lactamases in multiresistant Escherichia coli and Klebsiella spp. J Clin Microbiol 2002;40: 3703-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arlet G, Philippon A. Construction by polymerase chain reaction and intragenic DNA probes for three main types of transferable beta-lactamases (TEM, SHV, CARB). FEMS Microbiol Lett 1991;66: 19-25. [DOI] [PubMed] [Google Scholar]
- 18.Nüesch-Inderbinen MT, Hächler FH. Detection of genes coding for extended-spectrum SHV beta-lactamases in clinical isolates by a molecular genetic method, and comparison with the E test. Eur J Clin Microbiol Infect Dis 1996;15: 398-402. [DOI] [PubMed] [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing: twelfth informational supplement (M100-S12). Wayne, PA: National Committee for Clinical Laboratory Standards, 2002.
- 20.Paterson DL, Ko WC, Gottberg AV, Casellas JM, Mulazimoglu L, Klugman KP, et al. Outcome of cephalosporin treatment for serious infections due to apparently susceptible organisms producing extended-spectrum beta-lactamases: implications for the clinical microbiology laboratory. J Clin Microbiol 2001;39: 2206-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez-Martinez L, Hernandez-Alles S, Alberti S, Jacoby GA. In vivo selection of porin-deficient mutants of Klebsiella pneumoniae with increased resistance to cefoxitin and expanded-spectrum cephalosporins. Antimicrob Agents Chemother 1996;40: 342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Champs C, Sauvant MP, Chanal C, Sirot D, Gazuy N, Malhuret R, et al. Prospective survey of colonization and infections caused by expanded-spectrum beta-lactamase-producing members of the family Enterobacteriaceae in an intensive care unit. J Clin Microbiol 1989;12: 2887-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casewell MW, Phillips I. Hands as a route of transmission for Klebsiella species. BMJ 1977;2: 1315-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lautenbach E, Patel JB, Bilker WB, Edelstain PH, Fishman NO. Extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for infection and impact on resistance of outcomes. Clin Infect Dis 2001;32: 1162-71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.