Abstract
Although outnumbered more than 20:1 by rod photoreceptors, cone cells in the human eye mediate daylight vision and are critical for visual acuity and color discrimination. A variety of human retinal diseases, e.g. age-related macular degeneration (AMD), are characterized by a progressive loss of cone photoreceptors, but the low abundance of cones and the absence of a macula in non-primate mammalian retinas have made it difficult to investigate them directly owing to lack of suitable experimental models. Conventional rodents (laboratory mice and rats) are nocturnal rod dominated species with few cones in the retina, while investigating other animals with cone-rich retinas presents various logistic and technical difficulties. Past work, originating in the early 1900s, has begun to provide insights into cone ultrastructure, but has yet to afford a unified model of cone cell organization. This review summarizes this past work and focuses on the recent introduction of special mammalian models (transgenic mice and diurnal rats rich in cones) that promise to reveal a more unified model of cone photoreceptor organization and its role in retinal diseases. These new mammalian models should allow new investigative techniques such as atomic force microscopy and cryo-electron tomography to advance our understanding of cone photoreceptors, much as has been done with rod photoreceptors.
Keywords: cone photoreceptors, rod photoreceptors, retinoids, retinoid cycle, chromophore, opsins, retina, vision, rhodopsin, cone pigments, enhanced S-cone syndrome, retinitis pigmentosa, age-related macular degeneration
1. Introduction
1.1 The Eye Across Species
Vertebrate eyes are based on a common structural plan. Despite differences in embryological development and optical layouts among invertebrates, visual pigment genes are all descended from the same remote ancestor. Furthermore, genes involved in formation of the vertebrate eye have proved to be homologous with those of Drosophila eye (Cook and Zelhof, 2008). Remarkably, all jawed vertebrates possess eyes so closely similar to our own that it is virtually indisputable that the last common ancestor of jawed vertebrates (that lived around 430 million years ago) was equipped with an eye fundamentally like ours (Lamb, et al., 2008). This strongly suggests that, despite certain differences, eyes across vertebrates have a common origin, with perhaps PAX6 as the universal master control gene for eye morphogenesis (Gehring, 2002; Gehring, 2005). Differences among vertebrates, therefore, must reflect adaptation to particular environments. Visual capabilities of animals have evolved to match aspects of their photic environment, and it is likely that the primary adaptive selective pressure is the spectral range and intensity of daylight needed to optimize color vision. Other structural features like size and packing density of photoreceptors are important for low light settings. Furthermore, structural differences of cone photoreceptors across species (Table 1) reflect adaptations of these species to their photic habitat and the demands of visual acuity. At the most basic level, nocturnal animals have the most rod-dominated retinas, whereas diurnal species have more cone-rich retinas. One of the most striking modifications of the ancestral pattern of four spectral classes of cone opsins is found in mammals, where only the two spectrally extreme classes are present. One explanation for this intermediate class loss relates to the evolution of mammals when reptilian ancestors went through a prolonged nocturnal phase. It is thought that because genes have no long-term storage mechanism, a gene cannot be retained unless it continuously remains functional, although there are exceptions to this idea, as supported by the blind cavefish (Parry, et al., 2003). Among mammals, only primates have evolved trichromatic color vision. The primary mechanism for trichromacy in New World primates is through allelic diversity of the L/M cone opsin gene on the X-chromosome. This single visual pigment gene has multiple alleles. Heterozygous females segregate expression of the alleles into separate populations of cones that are trichromatic. Old World primates, including humans, have evolved trichromatic vision through gene duplication and divergence of the cone opsin gene on the X-chromosome. Primates are trichromatic with three cone pigments (Bowmaker and Hunt, 2006). The three cone types, termed L, M, and S, are distinguished mainly by the portion of the visible spectrum to which each is maximally sensitive. L cones are most sensitive to low-frequency photons (λmax~555–565 nm), M cones to middle-frequency photons (λmax~530–537 nm), and S cones to supra-frequency photons (λmax~415–430 nm). Compared to L and M cones, inner segments (IS) of S cones are slightly extended. On average, there are over twice as many L-cones than M-cones in humans, but Old World primates do exist that exhibit more variation with some actually exhibiting M-cones that outnumber L-cones (Marc and Sperling, 1977); otherwise these two cone types show similar spatial distributions and appear to be randomly intermixed. L and M cones are most concentrated in the fovea where they are densely packed in a hexagonal pattern that accounts for the high visual acuity capability of the fovea. However, the spatial distribution of S cones across the retina differs from that of L and M cones in several respects. S cones constitute only about 5% of the total cone population (Roorda, et al., 2001), they are more peripherally located in the retina and are absent from the center of the human fovea. Indeed, pigments in the lens and macula selectively reduce the fraction of higher-frequency photons that reach the retina and, hence, the photoreceptors, thereby reducing the need for S-cones. This ‘filtration’ process improves vision in two respects. First, removal of higher-frequency photons serves to sharpen the image (due to the refractive properties of water in the interior of the eye). Second, such ‘filtration’ reduces damage to the retina and photoreceptors produced by high frequency photons. Because most high frequency photons are absorbed by the lens, images are produced mainly by L and M cones with less of a contribution from S-cones, which are nonetheless important because they contribute to color in image formation.
Table 1. Comparison of cone photoreceptor dimensions across species.
| Feature | Mousea | Nile ratb | Ground squirrelc | Lizard (Sceloporus-Occidentalis)d |
Pige | Emuf | Humang |
|---|---|---|---|---|---|---|---|
| Length | 13.4 ± 0.7 μm | 10.84 ± 1.17 μm | 7.4 μm | 12.5 ± 0.50 μm | 4–5 μm | 10 μm | 41–50 μm |
| Width | 1.2 ± 0.03 μm | ND | 2.0 μm | ND | ND | 1–3 μm | 1–1.2 μm |
ND: Not determined
(Yuodelis and Hendrickson, 1986), survey of fovea from a 37 year old patient
1.2 Roles of Rods and Cones in the Retina
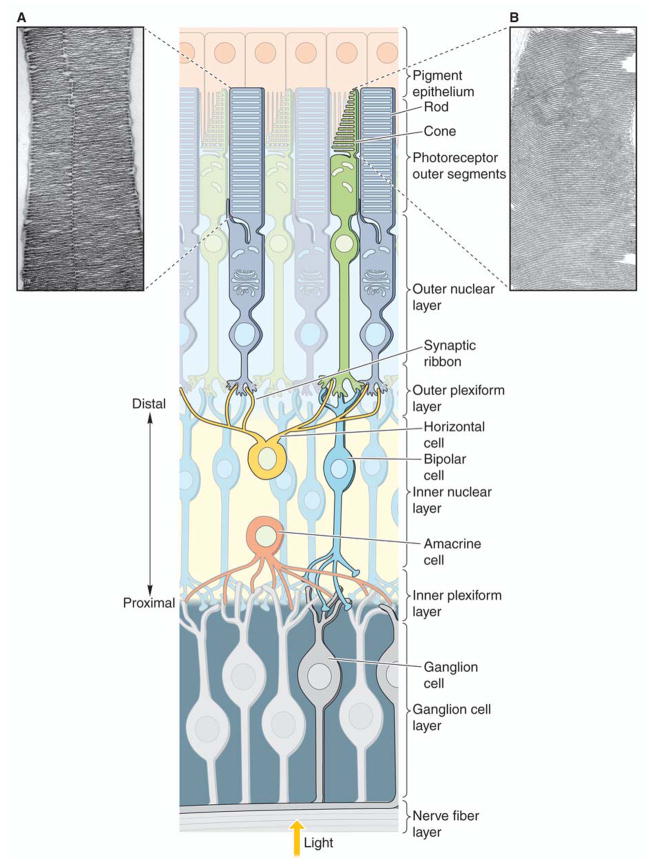
The retina is the eye tissue layer that converts light into visual signals transmitted to the brain. This process is carried out by two major types of photoreceptors, rods and cones that are distinguished by their shape, type of photopigment, retinal distribution, and pattern of synaptic connections (Figure 1). These properties reflect the fact that rod and cone systems are specialized for different aspects of vision. The rod system has low spatial resolution but is extremely sensitive to light so it is specialized for sensitivity at the expense of resolution. Conversely, the cone system has high spatial resolution but is relatively insensitive to light. Thus, it is specialized for visual acuity at the expense of sensitivity.
Figure 1.
Differences in photoreceptors and their arrangement in the retina. Rod and cone photoreceptors are displayed in a cross-sectional depiction of the retina also showing connections of these photoreceptors to retinal pigment epithelium distally and relaying cells (bipolar, horizontal, amacrine, ganglion) proximally. Electron microscopic images are shown of a ROS (A) and a COS (image provided by Dr. Steven K. Fisher) (B). The rod structure has a longer outer segment with discs packed without connections to the ciliary membrane, in stark contrast to the COS discs that are continuously connected by the ciliary membrane.
Basically, the different architectures of their outer segments (OS) represent a major distinctive feature of these two cell types. Rods with their longer OS composed of individualized discs unconnected to the ciliary plasma membrane contrast starkly with cones. The latter features shorter OS that arise initially as evaginations with subsequent formation of a series of discs (or invaginations), which are continuously connected to the membrane of the cilium that extends over the length of the OS. Lack of rim formation is the reason for this open formation of cone discs (Arikawa, et al., 1992).
Arrangement of the circuits that transmit rod and cone information to retinal ganglion cells contributes to the different characteristics of scotopic (rod) and photopic (cone) vision. Pathways linking rods and cones to ganglion cells are largely independent at early stages. A striking difference between rod and cone circuitry is the degree of their convergence. Each rod bipolar cell is contacted by a number of rods, and many rod bipolar cells contact a given amacrine cell. In contrast, the cone system is much less convergent. Thus, each retinal ganglion cell that dominates central vision receives input from only one cone bipolar cell, which in turn, is contacted by only a single cone. More convergence makes the rod system a better detector of light, because small signals from many rods are pooled to generate a large response in the bipolar cell. However, such convergence also reduces the spatial resolution of the rod system. The one-to-one relationship of cones to bipolar and ganglion cells is just what is required to maximize visual acuity.
Differences in transduction mechanisms utilized by these two receptor types largely account for the ability of rods and cones to respond to different ranges of light intensity. For example, a rod produces a reliable response to a single photon of light, whereas more than 100 photons are required to produce a comparable response in a cone. This difference does not reflect cone failure to capture photons effectively. Rather, the change in current produced by single photon capture in cones is comparatively small and difficult to distinguish from background noise. Another difference is that the response of an individual cone does not saturate at high levels of steady illumination, as does the rod response. Finally, compared to cones, rods show little, if any, directional sensitivity. Molecular mechanisms of phototransduction related to architecture and directional sensitivity are discussed in the subsequent section.
2. Structural Basis of Cone Phototransduction
2.1 Cone Response to Photons
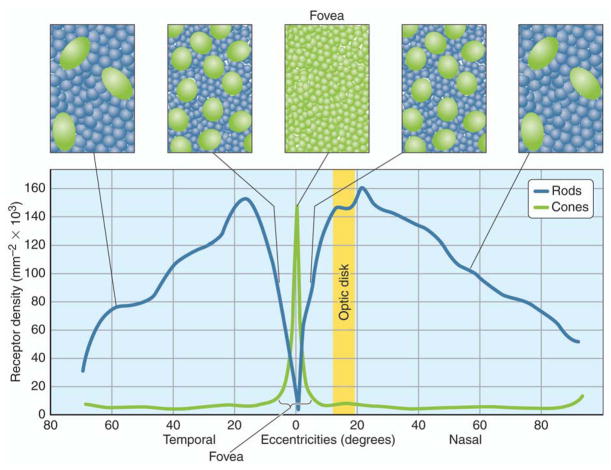
Of light incident on the eye, about 75% reaches the fovea, the region that triggers the greatest degree of visual acuity, responds to higher light intensities, and where there is the greatest cone photoreceptor density (Figure 2),. The image that falls on the retina is sufficiently sharp that a single cone can encounter significantly more photons than an adjacent one. Of the photons reaching the fovea, about one-quarter of the photons fall within an inner region that contains about 30 cones (Rodieck, 1998). Cones in outer regions of the fovea capture the rest of the photons. Outside the fovea, the rest of the surrounding eye is predominantly populated by rods.
Figure 2.
Distribution of photoreceptors in the eye. Overall, rods outnumber cones by a ratio of 20:1 or greater in the retina. However, in the fovea, the cone density is the highest and is correlated with visual acuity.
The probability that a photon will be absorbed by a cone is based on three factors in temporal order:
The direction of arrival of the photon (most efficient when along the long axis of cone).
The frequency of the photon (only ~67% are of the correct frequency to photoisomerize visual pigment molecules due to the spectral content of the signal).
The type of cone (L, M, or S-type).
Cone phototransduction is a complex process that has been elucidated mechanistically (Cideciyan and Jacobson, 1996; Ebrey and Koutalos, 2001; Hattar, et al., 2003; Hood and Birch, 1995; Kawamura and Tachibanaki, 2008; Rebrik and Korenbrot, 1998). Depolarization of cones increases the rate of neurotransmitter release whereas hyperpolarization decreases this rate. These parameters are also modulated by light. Light causes hyperpolarization whereas darkness causes depolarization, so that maximum release of the glutamate neurotransmitter occurs in the dark and the action of light is to reduce this rate of release. The only actions of a photoreceptor that directly affect horizontal and bipolar cells are the release of glutamate by the invaginated nerve terminal and the removal of this transmitter via reuptake mechanisms.
Whereas a rod cell has a single synaptic ribbon and contacts no more than seven or so processes of horizontal or bipolar cells (Figure 1), each cone cell contains many synaptic ribbons and contacts hundreds of such processes. Processes of horizontal cells make invaginating contacts and form the lateral elements in each triad. Processes of bipolar cells are of three types: invaginating processes, which lie directly below the synaptic ribbon; triad-associated processes that are aligned on each side of the overlying synaptic ribbon; and non-triad-associated processes, which are at the base of the cone pedicle.
The process of phototransduction can be broken down into several general steps. The first is photoactivation where 11-cis-retinal, the chromophore for both rods and cones, is photoisomerized to the all-trans-retinal isomer, thereby inducing a conformational change in the structure of the opsin protein molecule (Palczewski, 2006; Ridge and Palczewski, 2007). This conformational change allows the catalytically active opsin to bind to transducin, a G-protein, to initiate phototransduction. Binding of opsin to transducin replaces the GDP with GTP activating the α-subunit of transducin which then dissociates to activate the membrane-associated phosphodiesterase (PDE) by removing the two regulatory (γ) subunits. In the dark, the OS cation channels are gated by cyclic guanosine monophosphate (cGMP), controlling the influx of ions across the photoreceptor plasma membrane. The hydrolysis of cGMP by PDE results in channel closure, thus decreasing the conductance of the plasma membrane to cations, which hyperpolarizes the plasma membrane, inhibits neurotransmitter release, and signals the adjacent neurons of the light stimulus (Polans, et al., 1996). The disc is an important structural component in this step, and in the cone, the disc shape and composition is a critical determinant of its extended activity in light, as contrasted to rods. In cones, the protein molecules of the biochemical cascade are similar to those of rods, but they are located for the most part on open discs that are continuous with the plasma membrane, rather than on discrete disc membranes (Figure 3). This allows membrane proteins to diffuse among different cone discs. The structure of cone discs and their development is addressed in detail in later sections. In the second step, there is a decreased release of the excitatory neurotransmitter, glutamate. In the third and final step, cone photoreceptors cells recover from the photoresponse through a series of quenching/termination reactions of all activated phototransduction proteins to bring these cells back to their dark-adapted state.
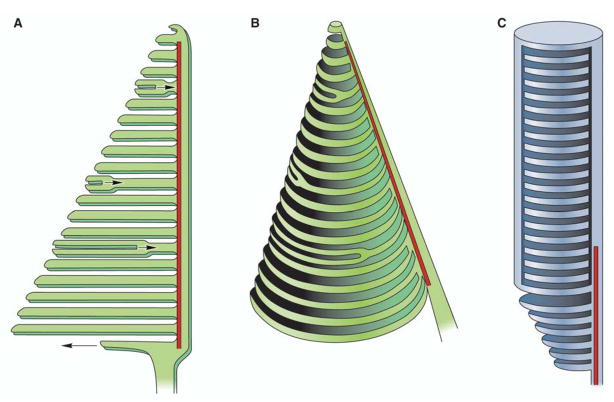
Figure 3.
Structure and renewal of rod and cone discs. Discs in the cone photoreceptor (A) are not unique evaginations completely separated from the cilium membrane, but instead retain connection to the cilium that extends the entire length of the outer segment. Early EM data indicated that discs of the COS feature partial folds composed of BE and DI. The arrow indicating growth away from the ciliary stalk represents a BE showing a developing COS surrounded by new membrane whereas arrows pointing toward the ciliary stalk represent more DI forming new but shorter COS. More recent work has shown that this representation is not a unifying model and the disc morphogeneis is more dependent on incomplete rim protein (perpherin/rds) formation. Regardless of the mechanism, the continuous membrane structure of cone discs permits an increased surface area that could explain a phototransduction cascade distinct from rod cells. (B) The cone axoneme (shown in red) extends the length of the cone ciliary stalk, indicating its importance in disc morphogenesis and turnover. The rod photoreceptor (C) features individualized discs that do not maintain any connection to the rod cilium. Furthermore, the ciliary axoneme (shown in red) does not extend the entire length of the ROS.
2.2 Structural Dynamics of Cone Opsins during Phototransduction
There is a pressing need to elicit more precise measurements of cone photoreceptor responses to permit correlations of spatial dynamics with the time scale of phototransduction. Mouse photoreceptors are quite similar to primate photoreceptors with respect to their physical dimensions and therefore present an appropriate model for study. The OS in mice is about 1.4 μm in diameter and 24 μm in length for rods, but about 1.2 μm and 13 μm, respectively, for cones (Baylor, et al., 1984; Carter-Dawson and LaVail, 1979; Pugh and Lamb, 2000). Rods and cones have four primary structural/functional regions: OS, IS, cell bodies, and synaptic terminals. Similar to humans, murine rod discs are completely internalized and therefore physically separated from the cellular plasma membrane, whereas cone discs are delineated by foldings of the plasma membrane itself (Figure 3). Thus, open cone discs offer a much larger surface area for rapid exchange of substances between the cell exterior and interior, such as chromophore transfer for pigment regeneration and fast calcium dynamics during light adaptation (Fu and Yau, 2007). COS possess open discs, which are continuous with the plasma membrane of the connecting cilium whereas OS of rods are separated from the plasma membrane. This correlates with the observations that deletion of peripherin/rds, a protein present in both cone and rod OS required for normal OS disc morphogenesis (Arikawa, et al., 1992; Molday, et al., 1987; Portier, et al., 1984), produced nonfunctional rod precursors that undergo apoptosis, whereas cones remain viable, despite the development of atypical OS with reduced phototransduction efficiency (Farjo, et al., 2007; Farjo and Naash, 2006; Farjo, et al., 2006).
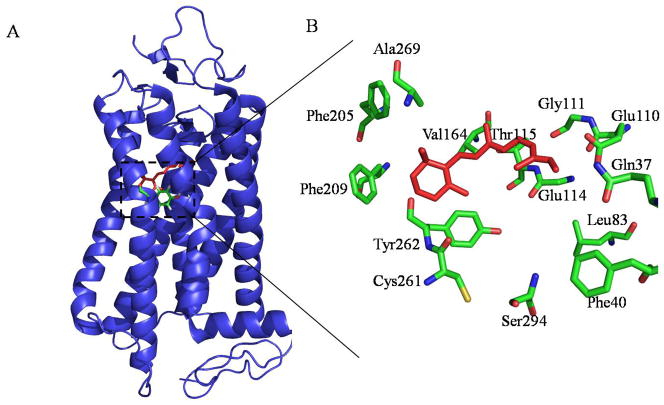
Opsins, as a subclass of GPCRs (Palczewski, 2006), are defined by their ability to bind a retinal-based chromophore in order to form a light-sensitive photopigment (Nickle and Robinson, 2007). Properties that differ among the various opsin classes suggest biochemical and structural differences among opsin classes (Stenkamp, et al., 2002). Hydroxylamine bleaching susceptibility of cone opsins as contrasted to rhodopsin in the dark state (Kawamura and Yokoyama, 1997; Okano, et al., 1992) implies that cone opsin classes may have a relatively open conformation in the dark that allows hydroxylamine to compete with opsin for binding to 11-cis-retinal. Also, site-directed mutagenesis studies have shown that each opsin class may have different residues that affect its overall stability (Nickle, et al., 2006). Despite these differences, rhodopsin and transgenic rod/cone pigments employ identical downstream signaling mechanisms when compared side-by-side in Xenopus rods and cones (Kefalov, et al., 2003). The same was reported for rhodopsin and transgenic red/cone pigments in mouse rods (Fu, et al., 2002). Thus, not only do rod and cone pigments interact with a given transducin identically, but the shutoff mediated by a given protein kinase and arrestin is also similar (Fu and Yau, 2007). Despite these observations the three-dimensional structure of COS needs to be elucidated by more advanced techniques such as cryo-electron tomography (cryo-ET) as has been done for ROS to reveal an accurate structural framework for the space within which phototransduction occurs (Nickell, et al., 2007). Even as rhodopsin comprises ~90% of the protein in rod disc membranes, the composition and organization of opsins in cone cells have yet to be determined. Such information would be broadly applicable to other signal transduction cascades because GPCRs represent the largest known class of drug, hormone and neuropeptide receptors. Homology modeling of cone opsins with the X-ray defined structure of rhodopsin (Stenkamp, et al., 2002) reveals that the three cone opsins are similar. The S-cone opsin homology model is shown to illustrate the structural elements. Key amino acids in the retinal binding pocket shown for the blue cone opsin indicate that the central residue Tyr262, is much different than the central residue of Trp281 (analogous to Trp262 in bovine rhodopsin) in red and green cone opsins (Figure 4). Moreover, the binding pocket of the blue cone opsin lacks a glutamate residue to act as a counter ion to the chromophore Schiff base, resulting in the blue shift of this opsin.
Figure 4.
Homology model of S-cone (blue) opsin. (A) The S-cone opsin (pdb id: 1kpn) is shown in blue with the retinal chromophore shown as red sticks and Tyr262 side chain colored by atom. The shown opsin model is a homology based structure from bovine rhodopsin generated with the program Modeler in the Insight II package. The only obvious differences between the two lie in the N- and C-terminal regions, with differences between the blue opsin and rhodopsin being minimal. Homology modeling of the other cone pigment opsins also revealed very similar differences. (B) The modeled retinal binding site is shown with the retina depicted as red sticks and blue opsin residues as sticks colored by atom. The major difference seen between the three pigments is that, unlike the red and green opsins where the central residue forming the retinal cavity is Trp281 (analogous to Trp265 in bovine rhodopsin), the central residue forming the cavity in blue cone pigment is Tyr262 and there is no Glu residue to act as a counter ion to the chromophore Schiff base, resulting in the blue shift of this opsin.
2.3 Evolution of Retinal Circuitry
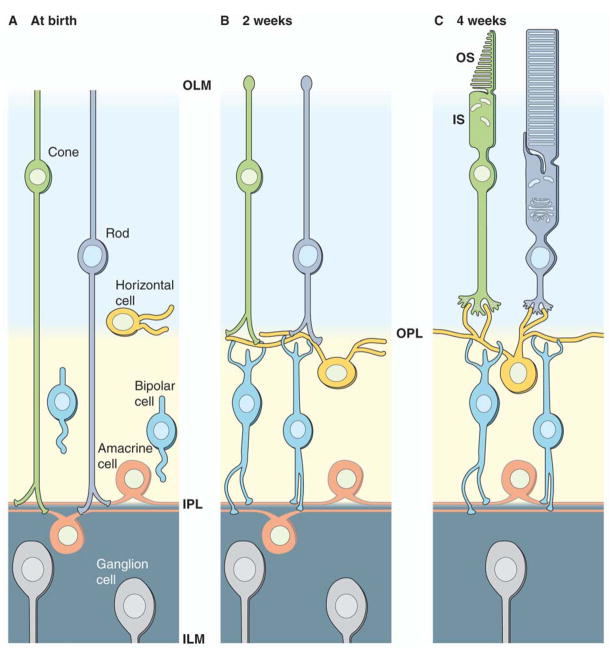
To understand cone cell architecture and its role in cone function, an understanding of retinal and ultimately cone cell evolution is also required. Early photoreceptor cells that evolved into the current rod and cone structures were the ciliary and rhadomeric cells. Until recently the evolutionary relevance of rhadomeric photoreceptors to rods and cones was unclear. This has been elucidated in invertebrates (Gomez and Nasi, 2005), and recently clarified in vertebrates by the proposal that vertebrate retinal ganglion cells actually are daughter cells of rhadomeric photoreceptors, found mainly in the compound eyes of arthropods. Unlike ciliary cells where modification of the cilium increases the membrane surface area, rhadomeric cells lacking cilia increase their surface area through microvilli. This view is based primarily on the close homology of transcription factors used by the two classes of cells. Secondly, melanopsin, an opsin integral to circadian control found in special retinal ganglion cells that are depolarized rather than hyperpolarized by light, is a member of the rhadomeric class of opsins (Arendt, 2003; Arendt, et al., 2004; Arendt, et al., 2002). Therefore, a reasonable assumption is that modern mammalian retinal ganglion cells actually are rhadomeric photoreceptors that have lost their original membrane structure, but have retained their axons, their ancestral responses to neurogenic factors and, in some cases, their rhadomeric opsin and G protein-signaling cascade. Modern ganglion cells also have acquired the ability to receive synaptic input from ciliary photoreceptors, which have evolved into present day cone and rod cells. Additionally, evolution has provided a gradual transition towards a highly organized laminar structure of the OS and the appearance of ribbons in the synaptic terminals (Collin, et al., 1999; Govardovskii and Lychakov, 1984; Imai, et al., 2005; Pu and Dowling, 1981; Samejima, et al., 1989). Much about the development of the circuitry in the mammalian retina is known from studying ferrets, which are convenient to work with because their young are born at a very immature developmental stage with eyes that do not open until 2 weeks after birth (Johnson, et al., 1999) (Figure 5).
Figure 5.
Evolution of photoreceptors. This retinal model is based on the ferret, a mammal with a developmentally immature retina at birth. (A) Cones and rods have indistinct morphologies at birth with both photoreceptors extending processes to the inner plexiform layer (IPL). (B) At 2 weeks of age, morphologies are still indistinct but their processes retract and contact horizontal and bipolar cells. (C) Finally at 4 weeks or age, cones and rods are morphologically distinct with discernable inner and outer segments.
2.4 Cone Cell Evolution
Both autoradiographic and kinetic data of COS renewal (Eckmiller, 1993) predict that many opsin molecules in COS will become much older than the oldest opsin molecules in ROS. The presence of a significant number of older molecules within COS membranes is not detrimental to cone function, because cones function in daylight and signal the absorption of thousands to millions of photons despite a high level of noise (Lamb and Pugh, 1990). But even so, the efficiency of COS renewal mechanisms may limit the sensitivity of a photoreceptor to light (Eckmiller, 1993). Although the actual turnover rate of cones compared to rods is unknown, it is thought that rods exhibit more rapid decay than cones.
The slow turnover of COS actually is typical of renewal rates for most intracellular components in a majority of cells. The highly efficient OS renewal mechanism in rods is thus unusual, suggesting a specialized evolution from cones, rather than the opposite. This fundamental difference between ROS and COS strongly suggesting that rods are modified cones is supported by the following observations:
Most vertebrates develop COS prior to ROS and the initial topology of developing photoreceptors is cone-like (Dorn, et al., 1995; Raymond, 1985).
Vertebrate retinas have many cone types, but fewer rod types (Rodieck, 1973).
Close amino acid homology between photoreceptor opsins indicates that genes encoding the three human cone pigments and rhodopsin are all derived from a common ancestral gene (Nathans, 1987).
These past insights into cone evolution have been recently substantiated by studies of the transcriptional network that regulates photoreceptor development. One of these transcription factors central to our understanding of cone cell development, is named the neural retina leucine zipper (Nrl). The Nrl gene was identified by subtraction cloning and detected only in the neural retina, including the photoreceptor cells and inner nuclear layers (Swaroop, et al., 1992). Subsequent papers by the Swaroop group and others implicated the Nrl gene in rod photoreceptor development because Nrl knockout mice were functionally ‘rodless’ with photoreceptors that functioned exclusively as cones (Mears, et al., 2001; Mitton, et al., 2003; Rehemtulla, et al., 1996; Strettoi, et al., 2004; Yoshida, et al., 2004).
By using the Nrl-promoter to express green fluorescent protein (GFP) in transgenic mice, researchers have shown that Nrl is indeed the earliest rod lineage-specific marker. To evaluate the origin of enhanced S-cones in the Nrl−/− retina directly, Swaroop and colleagues crossed wild type-GFP mice with Nrl−/− mice. GFP+ photoreceptors were isolated by fluorescence activated cell sorting (FACS) from wild type retinas and spatial and temporal expression of GFP was found to correlate completely with the timing of rod genesis and the central to peripheral gradient which drives rod genesis. Furthermore, GFP+ cells (rod precursors in wild type retina) were also co-labeled with S-opsin in Nrl−/− GFP retinas. Because Nrl−/− mice produce only S-cones, representing the “default fate” for photoreceptors in mice, Swaroop and colleagues proposed that Nrl determines the rod fate of “bipotent” photoreceptor precursors by modulating gene networks that simultaneously activate rod- and suppress cone-specific genes. In the cone development pathway, a second transcription factor, thyroid hormone receptor β2 (TR-β2) regulates the developmental “choice” between S-opsin and M/L-opsin expression; mice without a functional TR-β2 have no M-opsin expressing cones (Ng, et al., 2001; Roberts, et al., 2006). A percentage of early cones are thus directed to an M-opsin expressing fate by TR-β2; unliganded TR-β2 acts to repress S-opsin expression in cones, whereas liganded TR-β2 activates M-opsin expression (Roberts, et al., 2006), with a contribution from the retinoid X receptor (Roberts, et al., 2005). Recent evidence suggests that there still may be more unidentified factors involved in cone evolution and development. Class III myosin 3B (Myo3B), in addition to having motor function, has a kinase domain thought to participate in signaling. Myo3B is specific to rhadomeric photoreceptors (Battelle, et al., 1998; Edwards and Battelle, 1987). Recent work shows that Myo3B is expressed at birth, before most rod photoreceptors differentiate, and that it is highly concentrated in the OS of mouse cones expressing S opsin, but not in the OS of other cones (Katti, et al., 2009). These, and possibly other factors yet to be discovered, dictate a gradient of cone types that is set up in mice. Once set up during development, the cones’ identities appear to be fixed.
More proof for this hypothesis is provided by retinal disease profiles. Immunohistochemical and physiological studies (Oh, et al., 2008) suggest that Nrl modulates the development of S-cones, and that its gain or loss of function primarily results in alterations of the S-cone pathway. One possible explanation is that S-cones represent the “default fate” for early-born photoreceptors in mice (Szel, et al., 2000) and that the expression of Nrl controls an important node in this process. These results are consistent with evolutionary data suggesting that rods are derived from an ancestral cone (Akimoto, et al., 2006). There is even further evidence from the branching pattern of vertebrate retinal opsins that classes of cone pigments existed before evolution of the rod pigment, rhodopsin (Okano, et al., 1992). The order in which different classes of retinal cells are generated is generally conserved across vertebrate species. During the first wave of cell genesis, retinal ganglion cells, horizontal cells, amacrine cells, and cone cells are born. A subsequent wave produces the majority of rods, the remaining amacrine cells, the bipolar cells and the Müller cells as well (Lamb, et al., 2007).
3. Cone Structure
The evolving evolutionary perspective of cone photoreceptor function has been further corroborated by structural investigations. Elucidation of the spatial organization of the cone photoreceptor and its components has been of great interest and our understanding of it began to develop when electron microscopy (EM) was used to study the ultrastructure of the retina. Very early on, it was demonstrated that the retinal pigment epithelium (RPE) extends long processes that reach cone tips. Tubular processes protrude from the apical surface of the RPE to ensheath the COS (Walls, 1934). This early description was later confirmed by EM data (Hogan and Wood, 1973) and subsequently by ultrastructural studies of this relationship in human extrafoveal cones (Steinberg, et al., 1977).
Because ROS are cylindrically shaped and each rod disc becomes independent after its initial formation at the OS base, continuous displacement of discs toward the OS tip is easy to visualize in three dimensions. If one presumes that cone discs are also displaced, the process must be more complex because many, and perhaps all, discs retain some connection with not only the adjacent discs, but also the outer plasma membrane (Anderson, et al., 1978; Fetter and Corless, 1987) (Figure 3).
3.1 Cone Disc Renewal
The availability of rod dominated retinal samples from mouse models has allowed us to understand not only rod cell structure, but also the steps inherent for renewal of its discs. ROS are renewed in an orderly fashion, as first revealed by autoradiographic studies in which radioactive protein molecules became trapped in new membranous discs generated at the OS base, producing autoradiographically labeled bands. Unchanged bands were displaced sclerally as additional discs formed below and finally were discarded from the OS tip and phagocytized by the RPE (Young, 1967). This was further supported for the first time in 1969 with biochemical work (Hall, et al., 1969). These and other studies (Bok, 1985; Steinberg, et al., 1980; Young, 1976), indicate that new membrane is incorporated into ROS via the connecting cilium by distributing into successive new membrane folds that evaginate from the cilium at the OS base. These evaginations expand to the full OS width and are displaced away from the base. They then lose their connections and become isolated into separate discs, all surrounded by the plasma membrane. An alternative mechanism was recently proposed in which vesicles fuse to form nascent discs that are assembled entirely within the cell’s membrane (Chuang, et al., 2007). This fusion model, however, assumes that nascent discs are closed and do not differ from the mature discs except in size. This membrane fusion idea possibly stemmed from a preservation-artifact as previously described (Townes-Anderson, 1995).
COS differ from ROS in structural organization, autoradiographic labeling pattern, and three-dimensional shape. COS consist of numerous parallel membrane foldings oriented at right angles to the connecting cilium that apparently retain continuity with each other and with the plasma membrane, forming a single topologically continuous membrane system in some species. Early studies revealed the tapered conical shape of COS and presented circumstantial evidence that their membranes are not renewed due to their mode of development (Young, 1969). Since then, it has been generally accepted that the tips of mature COS are regularly shed, and therefore their membranes must be shed as well. Presumably, membranes in COS are renewed in a manner unlike ROS due to these structural differences, but which is not completely understood. Although cones are more difficult to study than rods, it is especially important to clarify how COS are renewed because humans rely much more on cone-based day vision than on rod-based night vision. Instead of distinct discs, COS in all vertebrate retinas share the structural feature of numerous parallel lamellae connected by a single longitudinal ciliary stalk (Anderson, et al., 1978; Bok, 1985; Carter-Dawson and LaVail, 1979). Most lamellae in a COS extend laterally across the full COS width, but a few at the base are incomplete (Figure 3).
In non-mammalian cones, the OS are composed of a stack of discs, all of which are continuous with each other and with the outer membrane adjacent to the connecting cilium (Cohen, 1968) (Figure 3). In mammalian cones, however, only the basal part of the OS seems to retain this organization in single thin sections (Anderson, et al., 1978). It was initially thought that displacement of cone discs from the base to the OS tip must be accompanied by a similar displacement of the cilium and outer membrane, because otherwise connections between the discs and outer membrane would have to continually be formed and broken to permit disc displacement, an unlikely situation. Coordinated displacement, in turn, indicates that the outer membrane must also be continually replaced in conjunction with the discs. That this actually might occur for both rods and cones steamed from evidence that shed disc packets from both photoreceptor types are surrounded by shed outer plasma membrane (Anderson and Fisher, 1976; Steinberg, et al., 1977). This understanding of disc displacement in cones has been revised with more current work that embraces the idea that because the autoradiographic data suggested that protein is randomly distributed, protein and lipid components must freely flow throughout the system and that there is terminal loss of discs from the OS and phagocytosis by RPE cells as demonstrated by ground squirrel, monkey, and human cone turnover (Anderson, et al., 1978).
The entire COS appears to be continually remodeled or reshaped as a unit, from the time of its initial outgrowth until its mature dimensions and shape are achieved, a process supported by the open disc structure of COS. It is thought that mature COS remain tapered despite shedding because their distal lamellae shrink by losing membrane (Steinberg, et al., 1980). The entire COS is thought to expand in all three dimensions during morphogenesis. The COS shape changes because the length increases more than the width. Rather than requiring shrinkage of lamellae at the tip, the change in taper of developing COS can be interpreted as reflecting differential growth (Eckmiller, 1997). Actin is thought to be involved in the formation of evaginations at the base of the OS and this has been substantiated by examining how the microfilament-destabilizing drug, cytochalasin D, affects photoreceptor OS morphology. No new discs/lamellae seem to form at the OS base in the presence of this inhibitor (Vaughan and Fisher, 1989; Williams, et al., 1988). However, these findings must be interpreted with caution because the basic mechanism is unknown and the main drug effect may be disruption of actin filaments at the COS base that indirectly interferes with processes that occur more distally.
3.2 Cone Disc Morphogenesis
Disc morphogenesis in ROS is quite different than in COS, giving rise to the more open cone discs that permit continuous protein flow. ROS lamellae are formed successively and discs become isolated from the plasma membrane near the ROS base in young rods. In contrast, the first membrane foldings during OS development in cones arise as evaginations of the ciliary membrane because the cilium is the only structure initially present (Kinney and Fisher, 1978; Steinberg, et al., 1980). The prevailing theory of cone morphogenesis has evolved over time leading to the present day theory that cone disc structure is related to incomplete rim development.
Earlier it was thought that as cones develop, lamellae can expand simultaneously at many levels of the COS (Ditto, 1975). In 1987, studies of Xenopus COS revealed unique structures termed distal invaginations (DI). In these early EM studies, it was shown that some of the distal folds of the disc margins are incomplete in that they extend from the non-ciliary side of the COS only part way across its width (Figure 3). These structures were termed DI because they are invaginations of the plasma membrane that occur throughout all basal COS levels. The presence of DI causes minimal interruption of the regular spacing between COS lamellae, but complete lamellae above and below a large group of DI are oblique, rather than parallel to one other (Eckmiller, 1987). DI were not observed within the distal membranes of developing ROS. This was consistent with previous research indicating that all new membranes in the developing ROS are assembled in the basal evagination (BE) at the ROS base, so that additional membrane cannot flow into ROS discs that have been separated from the plasma membrane (Steinberg, et al., 1980).
The variable amount of taper of different COS can result from variation in the relative amounts of new membrane that flow into the BE versus the DI. This idea was based on COS that are highly tapered (as in amphibian retinas) such that they form few BE and many DI. However, COS can be slightly tapered (as in mammalian retinas) if they form many BE and few DI and in some cases OS can be cylindrical (as are ROS) if they form only BE (Eckmiller, 1990). Such variable degrees of taper indicate that the idea of DI may not be a correct unifying theory for cone disc formation and it was actually hinted to be a possible mechanism for cone disc resorption and recycling (Corless, et al., 1989). More recent work on the localization of peripherin/rds, specifically in the disc rim region of cone disc membranes (Arikawa, et al., 1992; Farjo and Naash, 2006; Farjo, et al., 2006), explains previous data from mammalian cones where only the basal part of the cone OS seems to retain this organization in single thin sections (Anderson, et al., 1978). This also indicates that the more gradual development of the disc rim in cones (Steinberg, et al., 1980) gives rise to the open structure, permitting the nascent disc zone to extend further distally. Studies of peripherin/rds are consistent with the new proposed theory that the rim development is a slow, incomplete process arising from the cilium.
The cilium is clearly important for normal photoreceptor function, especially for cones, because the OS develops as an elaboration of this structure and cone discs are more intimately connected to it than rod discs. The cilium is the major cytoskeletal element of the OS in mature photoreceptors and also is the only cytoplasmic connection between the IS and OS. Thus, the cilia constitute the major route through which materials, such as opsins (Liu, et al., 1999), synthesized in the IS are delivered to the OS. So it is hardly surprising that human diseases with ciliary defects may result in retinal degeneration and night blindness (Arden and Fox, 1979; Barrong, et al., 1992; Berson and Adamian, 1992; Brown, et al., 1963; Cohen, 1965). The importance of OS assembly and maintenance in its function is supported by the findings of intraflagellar transport proteins (Insinna and Besharse, 2008) and the detrimental effects seen when these protein complexes are mutated (Krock and Perkins, 2008; Pazour, et al., 2002). More importance of the cilium to cones may be explained by the fact that the relative length of the ciliary axoneme is different in ROS than COS (Figure 3). In COS, the axoneme is thought to extend the entire length of the OS (Eckmiller, 1996), whereas in the ROS, the axoneme extends for most, but not the entire length (Luby-Phelps, et al., 2008). However, there is evidence that, aside from the axoneme in the ROS, there are distinct microtubule-like structures distally that extend the over the remaining length of the OS. These structures seem to be modulated by light-induced interactions between the RPE and photoreceptors, as occurs with disc shedding (Roof, et al., 1991).
Past work centering on EM analysis and more current molecular work have shed light on the disc arrangements and different components of cone photoreceptors important for their function, but shortcomings arise from the lack of overlap between some studies and their applicability to mammalian models. Much work has been done on species that do not approximate models for human disease or mammalian disease in general. So it is hard to say if the data obtained are unique to the species of study or if they are more broadly applicable to understanding mammalian cone structure and function. Subsequent sections emphasize the importance of cone photoreceptors in retinal diseases and new mammalian models that have been developed to address this issue.
4. Importance of Cones in Retinal Diseases
Photoreceptor degeneration results in vision loss in diseases like retinitis pigmentosa and age-related macular degeneration. In these diseases, the main cause of clinically significant vision loss is cone cell degeneration rather than rod cell death. Although most mutations responsible for retinitis pigmentosa in humans and animal models affect rod-photoreceptor-specific genes, rod apoptosis is often followed by secondary cone degeneration (Shelley, et al., 2009). Nevertheless, people with a night blindness disease can lead a normal life, especially in industrialized countries, because they can still see satisfactorily despite the loss of rods. Thus, prevention of cone cell loss is a major goal of therapeutic strategies (Travis, 1998).
Leber congenital amaurosis (LCA) is an inherited retinal disease that leads to blindness in humans. Studies of LCA has revealed insight into cone cell degeneration and the importance of RPE specific protein 65 kDa (RPE65)-based visual chromophore production for cone survival (Jacobson, et al., 2007). It has been demonstrated that cone photoreceptors degenerate quicker than their rod counterparts in RPE65−/− mice (Znoiko, et al., 2005), highlighting the need for the chromophore, 11-cis-retinal, in cone function and survival (Samardzija, et al., 2009). Further studies of exogenous administration of 11-cis-retinal to RPE65−/− mice indicated 11-cis-retinal bound to cone opsins is essential for retinal protein sorting, transport, and targeting and, ultimately, cell survival (Zhang, et al., 2008). Also, administration of the artificial pro-drug, 9-cis-retinyl acetate, has shown protective effects on cone cell degeneration (Maeda, et al., 2009). These results suggest a possible mechanism of cone degeneration as the underlying cause of AMD.
Identification of potential targets for treating retinal degenerative diseases has improved upon investigation of the relevant transcription factors, and more recently, the regulatory mechanisms that control photoreceptor-specific gene expression (Figure 6). Crx, a homeobox gene expressed in both rods and cones, is required for the expression of a variety of photoreceptor specific genes (Chen, et al., 1997). Due to its importance in differentiation and maturation for both rods and cones, Crx mutations lead to general nonselective photoreceptor degeneration in both mice and humans (Freund, et al., 1997; Freund, et al., 1998; Swain, et al., 1997). Nr2e3/photoreceptor cell-specific nuclear receptor, a transcription factor of the ligand-dependent family of nuclear hormone receptors crucial for the development and function of rod and cone photoreceptors, is thought to suppress cone-specific genes in rods (Chen, et al., 2005). Nr2e3 directly interacts with Nrl and Crx to regulate expression of rod photoreceptor genes during differentiation while simultaneously suppressing the expression of cone-specific genes. The physiological function of Nr2e3 was demonstrated in transgenic mice ectopically expressing Nr2e3 under the control of the Crx promoter in photoreceptor precursor cells where suppression of cone-specific gene expression generating nonfunctional rod-like photoreceptors was evident (Cheng, et al., 2006). Recently, it also was shown that Nr2e3 becomes a potent repressor of cone-specific gene expression after SUMOylation by Pias3, a transcriptional co-regulator (Onishi, et al., 2009). The role of Nr2e3 was further elucidated by experiments in rd7−/− mice, a spontaneous loss of function model of Nr2e3 (Akhmedov, et al., 2000). In rd7−/− retinas, there was overexpression of S-type cones (Haider, et al., 2006; Haider, et al., 2000). The resulting cone overpopulation not only damaged photoreceptor cells, but also undermined the integrity of their postsynaptic partners, that in turn caused laminar disorganization and fragmentation of the retina with progressive loss of cone and rod cell function. The enhanced S-cone syndrome (ESCS) phenotype in human patients with mutations in Nr2e3 may therefore be due to misregulation of both L and M cone progenitors, leading to an excess of S-cones expressing blue opsin, and to atypical differentiation of rods and cones (Haider, et al., 2006).
Figure 6.
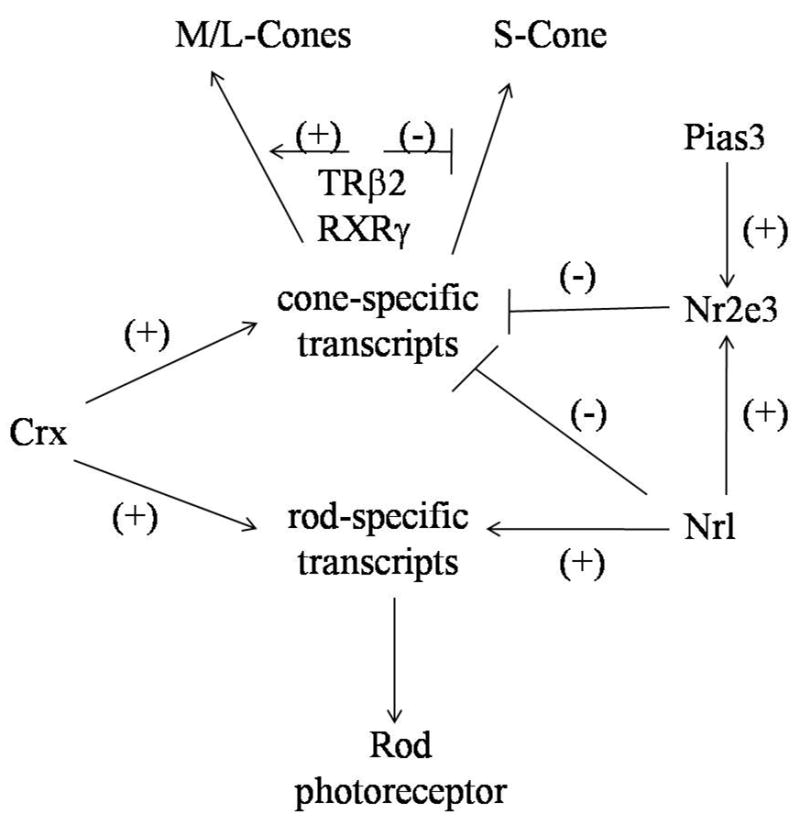
Model for transcriptional control of photoreceptor development. Different transcriptional factors that control rod and cone cell differentiation are indicated. Crx activation is required for both rod and cone development, but is not essential for photoreceptor differentiation. Both Nrl and Nr2e3 are essential. Nrl activates rod specific transcripts and inhibits cone specific transcripts and also activates Nr2e3, which in turn amplifies the effect of Nrl by inhibiting cone specific transcripts. Nr2e3 is also a physiological target of the transcriptional co-regulator Pias3. SUMOylation by Pias3 directs the action of Nr2e3 as a promoter of rod genesis and suppression of cone pathways. TR-β2 and RXRγ are downstream of these transcription factors in the cone pathway that take committed cone progenitors and modulate S versus M/L cone opsin maturation.
Loss of visual function in hereditary human retinal degenerative diseases usually reduces photoreceptor cells by apoptosis (Rattner, et al., 1999), but the one exception is ESCS, manifested as a gain in function in photoreceptor development (Haider, et al., 2000; Hood, et al., 1995; Jacobson, et al., 1991; Milam, et al., 2002; Stone, et al., 2000). ESCS patients suffer visual loss, with or without night blindness occurring early in life. Abnormal overexpression of S-type cones is accompanied by varying degrees of L and M cone depletion and retinal degeneration (Escher, et al., 2008). The final transcription factor of note, Nrl described previously, not only provides insights into photoreceptor development, but Nrl−/− animal species serve as excellent models for study of ESCS. Functional switching of photoreceptors from a rod to an S-cone phenotype in the Nrl−/− retina is evident from a change both in the expression of components of the phototransduction cascade, and in electroretinogram (ERG) recordings, which measure the electrical response in different cell types of the retina, namely rods and cones (Mears, et al., 2001). Of the transcription factors studied, Nrl appears to have the most widespread effect in cone cell morphogenesis because of its diverse interactions with other factors. Nrl interacts with Crx, Nr2e3, and other transcription factors (Figure 6) and activates the expression of many rod-specific genes, such as that for rhodopsin (Friedman, et al., 2004). Nrl also seems to act as a molecular switch during photoreceptor differentiation by promoting the rod differentiation program while simultaneously repressing cones. Suppression of cone fate is achieved, at least in part, through direct or indirect regulation of the transcription factor, Nr2e3, whose expression is undetectable in Nrl−/− retina (Yoshida, et al., 2004).
All published dominant Nrl mutations are missense changes affecting one of three residues (S50, P51, and G122). Researchers have identified patients with recessive, loss-of-function mutations in the Nrl genes P51S and L160P, who exhibited a phenotype similar to Nrl−/− mice. These patients have preserved S-cone function as deduced from visual field tests and also show intraretinal pigmentation in their fundi (Nishiguchi, et al., 2004).
5. New Mammalian Models to Study Cone Structure
Lack of a suitable experimental mammalian model constitutes the major impediment to understanding cone pathophysiology. Nocturnal species like laboratory rats and mice, which are useful for a variety of studies, have little place in cone photoreceptor research due to the dearth of cone cells in these species (Peichl, 2005; Peichl and Gonzalezsoriano, 1994). However, animals with cone dominance such as ground squirrels (~85% cones) (Blakeslee, et al., 1988; Kryger, et al., 1998; Long and Fisher, 1983), chickens (~65% cones) (Blanks and Johnson, 1984), and pigs (~20% cones) (Hendrickson and Hicks, 2002) not only are hard to breed in captivity, but also cannot be studied with pre-existing antibodies generated for rodent cone targets. Therefore, despite the cone-dominated retina in these species, they do not serve well for scientific research purposes. A cone-rich transgenic mouse model and a similar cone-rich natural diurnal rat should provide scientists the ability to work with cone dominant animals that are not only easy to breed, but also will allow preexisting well developed technologies to be exploited to their fullest extent.
In addition to elucidating cone cell evolution, the Nrl mouse model provides an ideal opportunity to study cone cell structure and function. The Nrl−/− mice should enable a simplified isolation of COS for structure-function studies by high resolution AFM and cryo-ET previously adopted for ROS analyses (Fotiadis, et al., 2003; Nickell, et al., 2007). That these photoreceptors resemble cones was confirmed through EM, immunohistochemistry, protein analyses, and electroretinographic recordings (Nikonov, et al., 2005). The Pugh group did a series of structural, ultrastructural, histochemical, molecular, and electrophysiological studies to establish that Nrl−/− photoreceptors indeed possess cone-like characteristics (Table 2) (Daniele, et al., 2005). However, it is clear that these cells are hybrid between rods and cones.
Table 2. Comparison of photoreceptor properties of wild type mice.
Key molecules of the cone phototransduction cascade such as mouse cone ultraviolet (MUV) pigment, cone transducin (Gtα2), mouse cone arrestin (mCarr), and cone sheath stainable peanut agglutinin (PNA) were identified to confirm the isolated photoreceptors as cone cells as opposed to rod cells that lacked staining of these molecular markers. Wild type cones displayed a low level of staining for “rod” arrestin.
| Feature | Wild type S-Cones1 | Wild type Rods1 |
|---|---|---|
| Ultrastructure | ||
| OS length (μm) | 13.4 ± 0.7 | 23.6 ± 0.4 |
| OS width (μm) | 1.2 ± 0.03 | 1.4 ± 0.1 |
| OS volume (μm3) | 14 | 36 |
| Open discs | >15 | 5–7 |
| Mitochondrial length (μm) | 1.31 ± 0.7 | 2.20 ± 0.7 |
| Histology | ||
| Chromatin clumping | Yes | No |
| PNA-stained OS sheath | Yes | No |
| Molecular | ||
| MUV | Yes | No |
| Gtα2 | Yes | No |
| mCarr | Yes | No |
| Arrestin | Yes, low level | Yes |
| ERG a-wave | ||
| λmax (nm) | 360 | 500 |
| lpeak (ms) | Unknown | 140 |
Key molecules of the cone phototransduction cascade, i.e., mouse cone ultraviolet (MUV) pigment, cone transducin, and cone arrestin—are present at cellular concentrations comparable to those of homologous transduction proteins measured in rods and expected to be present in wild type cones. Also, proteins of the cone phototransduction cascade in Nrl−/− photoreceptors drive photoresponses with high efficiency and cone-like recovery kinetics. Nonetheless, Nrl−/− photoreceptors are not a perfect model of normal wild type mouse cones as proofed by apparently disordered and deteriorating cones with OS shorter than wild type cones that express “rod” arrestin. Photoreceptor function is healthy and stable in the Nrl−/− retina during the 4 to 6 week period after birth but it deteriorates subsequently, as displayed by a decline in the maximal amplitude of the a-wave (Daniele, et al., 2005), which is the initial negative deflection in response to a bright flash. Despite this decline, each Nrl−/− photoreceptor, like wild type mouse cones, has an associated peanut agglutinin (PNA)-stainable sheath. This observation supports the intriguing hypothesis that the sheath is secreted by cones themselves because Nrl−/− photoreceptors are far removed from the RPE cell apical surface. These limitations should not detract from the contribution of this species to the understanding cone ultrastructure. The Nrl−/− species promises to facilitate cone purification protocols for three-dimensional analyses that can not only start to shed light on normal cone architecture but also lead to a better understanding of ESCS by examining older animals undergoing retinal degeneration.
Another promising species is the Nile rat (Arvicanthis niloticus). This newly studied rodent has a diurnal behavior pattern similar to humans and it also uniquely possesses a large percentage of easily identifiable cones (~33%) (Bobu, et al., 2006; Galliard, et al., 2008). At the ultrastructural level, the RPE-OS interface exhibits an orderly arrangement of ROS and COS apposed to the RPE apical surface. The ROS appear as cylindrical structures with clearly visible stacked discs. The COS are narrower and tapered, with areas of clear cytoplasm and a surrounding cone matrix sheath (Bobu, et al., 2006). More importantly, the cones can be conveniently stained with antibodies raised against murine peptide sequences and other proteins involved in phototransduction, namely arrestin, recoverin and cGMP-gated channels (Bobu, et al., 2008). Studies with the Nile rat should build on research with Nrl−/− species to establish the first cone photoreceptor structure-function relationships in naturally occurring mammalian species and further allow parallel analyses of rods and cones from the same species.
6. Conclusions and Future Outlook
The future of cone photoreceptor structure/function discovery is bright with emerging models consisting of the cone-rich Nrl−/− mouse line and Nile rat species. Current EM data will be augmented by application of advanced structural methods such as AFM (Fotiadis, et al., 2006) and cryo-ET (Nickell, et al., 2007) that have been successfully used to elucidate the fine architecture of rod photoreceptor organization. Use of these procedures has substantially contributed to a greater understanding of the rod phototransduction cascade and the roles of individual components such as rhodopsin and other supporting proteins. Some may ask why understanding cone photoreceptor structure is of such pressing concern. Other than feeding scientific curiosity, its importance lies in the myriad of visual problems associated with cone photoreceptor degeneration that, not only are presented here, but are yet to be discovered.
Acknowledgments
We thank Drs. L.T. Webster and J. von Lintig, (Case Western Reserve Univ.), Thomas Reh and Maureen Neitz (Univ. of Washington), Arthur S. Polans (Univ. of Wisconsin), Robert Eugene Anderson (University of Oklahoma Health Sciences Center), David Williams (UCLA School of Medicine), and Joseph Besharse (Medical College of Wisconsin) for their valuable comments on the manuscript, and members of Palczewski laboratory for their help in preparation of the manuscript. We also thank Dr. Steven K. Fisher (Univ. of California-Santa Barbara) for providing us with an EM image of the COS.
Financial Support: This research was supported by NIH grants EY09339, GM 079191, and by an unrestricted grant from Amgen Inc. D.M. was supported in part by the CWRU Medical Scientist Training Program (MSTP) and National Institutes of Health grant T32-GM007250.
Abbreviations used
- AFM
atomic force microscopy
- BE
basal evagination
- cGMP
cyclic guanosine monophosphate
- COS
cone outer segment(s)
- Crx
cone-rod homeobox
- cryo-ET
cryo-electron tomography
- DI
distal invagination
- EM
electron microscopy
- ERG
electronretinography
- ESCS
enhanced S-cone syndrome
- GDP
guanosine diphosphate
- GFP
green fluorescent protein
- GPCR
G protein-coupled receptor
- GTP
guanosine triphosphate
- IS
inner segment(s)
- LCA
Leber congenital amaurosis
- Myo3B
Myosin 3B
- Nr2e3
photoreceptor nuclear receptor transcription factor
- Nrl
neural retina leucine zipper
- OS
outer segment(s)
- PDE
phosphodiesterase
- PNA
peanut agglutinin
- ROS
rod outer segment(s)
- RPE
retinal pigment epithelium
- RPE65
RPE specific protein 65 kDa
- TR-β2
thyroid hormone receptor-β2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akhmedov NB, Piriev NI, Chang B, Rapoport AL, Hawes NL, Nishina PM, Nusinowitz S, Heckenlively JR, Roderick TH, Kozak CA, Danciger M, Davisson MT, Farber DB. A deletion in a photoreceptor-specific nuclear receptor mRNA causes retinal degeneration in the rd7 mouse. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:5551–5556. doi: 10.1073/pnas.97.10.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akimoto M, Cheng H, Zhu DX, Brzezinski JA, Khanna R, Filippova E, Oh ECT, Jing YZ, Linares JL, Brooks M, Zareparsi S, Mears AJ, Hero A, Glaser T, Swaroop A. Targeting of GFP to newborn rods by Nrl promoter and temporal expression profiling of flow-sorted photoreceptors. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3890–3895. doi: 10.1073/pnas.0508214103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DH, Fisher SK. The photoreceptors of diurnal squirrels: outer segment structure, disc shedding and protein renewal. Journal of Ultrastructure Research. 1976;55:119–141. doi: 10.1016/s0022-5320(76)80087-1. [DOI] [PubMed] [Google Scholar]
- Anderson DH, Fisher SK, Steinberg RH. Mammalian cones: Disc shedding, phagocytosis, and renewal. Investigative Ophthalmology & Visual Science. 1978;17:117–133. [PubMed] [Google Scholar]
- Arden GB, Fox B. Increased incidence of abnormal nasal cilia in patients with retinitis pigmentosa. Nature. 1979;279:534–536. doi: 10.1038/279534a0. [DOI] [PubMed] [Google Scholar]
- Arendt D. Evolution of eyes and photoreceptor cell types. International Journal of Developmental Biology. 2003;47:563–571. [PubMed] [Google Scholar]
- Arendt D, Tessmar-Raible K, Snyman H, Dorresteijn AW, Wittbrodt J. Ciliary photoreceptors with a vertebrate-type opsin in an invertebrate brain. Science. 2004;306:869–871. doi: 10.1126/science.1099955. [DOI] [PubMed] [Google Scholar]
- Arendt D, Tessmar K, de Campos-Baptista MIM, Dorresteijn A, Wittbrodt J. Development of pigment-cup eyes in the polychaete Platynereis dumerilii and evolutionary conservation of larval eyes in Bilateria. Development. 2002;129:1143–1154. doi: 10.1242/dev.129.5.1143. [DOI] [PubMed] [Google Scholar]
- Arikawa K, Molday LL, Molday RS, Williams DS. Localization of Peripherin/Rds in the Disk Membranes of Cone and Rod Photoreceptors - Relationship to Disk Membrane Morphogenesis and Retinal Degeneration. J Cell Biol. 1992;116:659–667. doi: 10.1083/jcb.116.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrong SD, Chaitin MH, Fliesler SJ, Possin DE, Jacobson SG, Milam AH. Ultrastructure of Connecting Cilia in Different Forms of Retinitis-Pigmentosa. Archives of Ophthalmology. 1992;110:706–710. doi: 10.1001/archopht.1992.01080170128040. [DOI] [PubMed] [Google Scholar]
- Battelle BA, Andrews AW, Calman BG, Sellers JR, Greenberg RM, Smith WC. A myosin III from Limulus eyes is a clock-regulated phosphoprotein. Journal of Neuroscience. 1998;18:4548–4559. doi: 10.1523/JNEUROSCI.18-12-04548.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, Nunn BJ, Schnapf JL. The Photocurrent, Noise and Spectral Sensitivity of Rods of the Monkey Macaca-Fascicularis. Journal of Physiology-London. 1984;357:575–607. doi: 10.1113/jphysiol.1984.sp015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein SA, Breding DJ, Fisher SK. The Influence of Light on Cone Disk Shedding in the Lizard, Sceloporus-Occidentalis. J Cell Biol. 1984;99:379–389. doi: 10.1083/jcb.99.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson EL, Adamian M. Ultrastructural Findings in an Autopsy Eye from a Patient with Ushers Syndrome Type-Ii. American Journal of Ophthalmology. 1992;114:748–757. doi: 10.1016/s0002-9394(14)74055-3. [DOI] [PubMed] [Google Scholar]
- Blakeslee B, Jacobs GH, Neitz J. Spectral Mechanisms in the Tree Squirrel Retina. J Comp Physiol A-Sens Neural Behav Physiol. 1988;162:773–780. doi: 10.1007/BF00610966. [DOI] [PubMed] [Google Scholar]
- Blanks JC, Johnson LV. Specific Binding of Peanut Lectin to a Class of Retinal Photoreceptor Cells -a Species Comparison. Investigative Ophthalmology & Visual Science. 1984;25:546–557. [PubMed] [Google Scholar]
- Bobu C, Craft CM, Masson-Pevet M, Hicks D. Photoreceptor organization and rhythmic phagocytosis in the nile rat Arvicanthis ansorgei: A novel diurnal rodent model for the study of cone pathophysiology. Investigative Ophthalmology & Visual Science. 2006;47:3109–3118. doi: 10.1167/iovs.05-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobu C, Lahmam M, Vuillez P, Ouarour A, Hicks D. Photoreceptor organisation and phenotypic characterization in retinas of two diurnal rodent species: Potential use as experimental animal models for human vision research. Vision Res. 2008;48:424–432. doi: 10.1016/j.visres.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Bok D. Retinal Photoreceptor-Pigment Epithelium Interactions - Friedenwald Lecture. Investigative Ophthalmology & Visual Science. 1985;26:1659–1694. [PubMed] [Google Scholar]
- Bowmaker JK, Hunt DM. Evolution of vertebrate visual pigments. Current Biology. 2006;16:R484–R489. doi: 10.1016/j.cub.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Braekevelt CR. Fine-Structure of the Retinal Rods and Cones in the Domestic Pig. Graefes Archive for Clinical and Experimental Ophthalmology. 1983;220:273–278. doi: 10.1007/BF00231355. [DOI] [PubMed] [Google Scholar]
- Braekevelt CR. Fine structure of the retinal photoreceptors of the emu (Dromaius novaehollandiae) Tissue Cell. 1998;30:137–148. doi: 10.1016/s0040-8166(98)80062-1. [DOI] [PubMed] [Google Scholar]
- Brown PK, Gibbons IR, Wald G. The visual cells and visual pigment of the mudpuppy. Necturus Journal of Cell Biology. 1963;19:79–106. doi: 10.1083/jcb.19.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Dawson LD, LaVail MM. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J Comp Neurol. 1979;188:245–262. doi: 10.1002/cne.901880204. [DOI] [PubMed] [Google Scholar]
- Chen JC, Rattner A, Nathans J. The rod photoreceptor-specific nuclear receptor Nr2e3 represses transcription of multiple cone-specific genes. Journal of Neuroscience. 2005;25:118–129. doi: 10.1523/JNEUROSCI.3571-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SM, Wang QL, Nie ZQ, Sun H, Lennon G, Copeland NG, Gilbert DJ, Jenkins NA, Zack DJ. Crx, a novel Otx-like paired-homeodomain protein, binds to and transactivates photoreceptor cell-specific genes. Neuron. 1997;19:1017–1030. doi: 10.1016/s0896-6273(00)80394-3. [DOI] [PubMed] [Google Scholar]
- Cheng H, Aleman TS, Cideciyan AV, Khanna R, Jacobson SG, Swaroop A. In vivo function of the orphan nuclear receptor NR2E3 in establishing photoreceptor identity during mammalian retinal development. Human Molecular Genetics. 2006;15:2588–2602. doi: 10.1093/hmg/ddl185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang JZ, Zhao Y, Sung CH. SARA-regulated vesicular targeting underlies formation of the Light-Sensing organelle in mammalian rods. Cell. 2007;130:535–547. doi: 10.1016/j.cell.2007.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, Jacobson SG. An alternative phototransduction model for human rod and cone ERG a-waves: Normal parameters and variation with age. Vision Res. 1996;36:2609–2621. doi: 10.1016/0042-6989(95)00327-4. [DOI] [PubMed] [Google Scholar]
- Cohen AI. New details of the ultrastructure of the outer segment and ciliary connectives of the rods of human and macaque retinas. Anatomical Record. 1965;152:63–80. doi: 10.1002/ar.1091520108. [DOI] [PubMed] [Google Scholar]
- Cohen AI. New evidence supporting the linkage to extracellular space of outer segment saccules of frog cones but not rods. JCB. 1968;37:424–444. doi: 10.1083/jcb.37.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin SP, Potter IC, Braekevelt CR. The ocular morphology of the southern hemisphere lamprey Geotria australis gray with special reference to optical specialisations and the characterisation and phylogeny of photoreceptor types. Brain Behavior and Evolution. 1999;54:96–118. doi: 10.1159/000006616. [DOI] [PubMed] [Google Scholar]
- Cook B, Zelhof AC. Photoreceptors in evolution and disease. Nature Genetics. 2008;40:1275–1276. doi: 10.1038/ng1108-1275. [DOI] [PubMed] [Google Scholar]
- Corless JM, Worniallo E, Fetter RD. Modulation of Disk Margin Structure During Renewal of Cone Outer Segments in the Vertebrate Retina. J Comp Neurol. 1989;287:531–544. doi: 10.1002/cne.902870410. [DOI] [PubMed] [Google Scholar]
- Daniele LL, Lillo C, Lyubarsky AL, Nikonov SS, Philp N, Mears AJ, Swaroop A, Williams DS, Pugh EN. Cone-like morphological, molecular, and electrophysiological features of the photoreceptors of the Nrl knockout mouse. Investigative Ophthalmology & Visual Science. 2005;46:2156–2167. doi: 10.1167/iovs.04-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditto M. A difference between developing rods and cones in the formation of outer segment membranes. Vision Res. 1975;15:535–537. doi: 10.1016/0042-6989(75)90031-0. [DOI] [PubMed] [Google Scholar]
- Dorn EM, Hendrickson L, Hendrickson AE. The Appearance of Rod Opsin During Monkey Retinal Development. Investigative Ophthalmology & Visual Science. 1995;36:2634–2651. [PubMed] [Google Scholar]
- Ebrey T, Koutalos Y. Vertebrate photoreceptors. Progress in Retinal and Eye Research. 2001;20:49–94. doi: 10.1016/s1350-9462(00)00014-8. [DOI] [PubMed] [Google Scholar]
- Eckmiller MS. Cone Outer Segment Morphogenesis - Taper Change and Distal Invaginations. J Cell Biol. 1987;105:2267–2277. doi: 10.1083/jcb.105.5.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckmiller MS. Distal invaginations and the renewal of cone outer segments in anuran and money retinas. Cell Tissue Res. 1990;260:19–28. doi: 10.1007/BF00297486. [DOI] [PubMed] [Google Scholar]
- Eckmiller MS. Shifting distribution of autoradiographic label in cone outer segments and its implications for renewal. J Hirnforsch. 1993;34:179–191. [PubMed] [Google Scholar]
- Eckmiller MS. Renewal of the ciliary axoneme in cone outer segments of the retina of Xenopus laevis. Cell Tissue Res. 1996;285:165–169. doi: 10.1007/s004410050632. [DOI] [PubMed] [Google Scholar]
- Eckmiller MS. Morphogenesis and renewal of cone outer segments. Progress in Retinal and Eye Research. 1997;16:401–441. [Google Scholar]
- Edwards SC, Battelle BA. Octopamine-Stimulated and Cyclic Amp-Stimulated Phosphorylation of a Protein in Limulus Ventral and Lateral Eyes. Journal of Neuroscience. 1987;7:2811–2820. doi: 10.1523/JNEUROSCI.07-09-02811.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escher P, Gouras P, Roduit R, Tiab L, Bolay S, Delarive T, Chen S, Tsai C, Hayashi M, Zernant J, Meriam JE, Mermod N, Allikmets R, Munier FL, Schorderet DF. Mutations in NR2E3 Can Cause Dominant or Recessive Retinal Degenerations in the Same Family. Human Mutation. 2008;0:1–13. doi: 10.1002/humu.20858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farjo R, Fliesler SJ, Naash MI. Effect of Rds abundance on cone outer segment morphogenesis, photoreceptor gene expression, and outer limiting membrane integrity. J Comp Neurol. 2007;504:619–630. doi: 10.1002/cne.21476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farjo R, Naash MI. The role of Rds in outer segment morphogenesis and human retinal disease. Ophthalmic Genet. 2006;27:117–122. doi: 10.1080/13816810600976806. [DOI] [PubMed] [Google Scholar]
- Farjo R, Skaggs JS, Nagel BA, Quiambao AB, Nash ZA, Fliesler SJ, Naash MI. Retention of function without normal disc morphogenesis occurs in cone but not rod photoreceptors. J Cell Biol. 2006;173:59–68. doi: 10.1083/jcb.200509036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetter RD, Corless JM. Morphological components associated with frog cone outer segment disc margins. Investigative Ophthalmology & Visual Science. 1987;28:646–657. [PubMed] [Google Scholar]
- Fotiadis D, Jastrzebska B, Philippsen A, Muller DJ, Palczewski K, Engel A. Structure of the rhodopsin dimer: a working model for G-protein-coupled receptors. Current Opinion in Structural Biology. 2006;16:252–259. doi: 10.1016/j.sbi.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Fotiadis D, Liang Y, Filipek S, Saperstein DA, Engel A, Palczewski K. Atomic-force microscopy: Rhodopsin dimers in native disc membranes. Nature. 2003;421:127–128. doi: 10.1038/421127a. [DOI] [PubMed] [Google Scholar]
- Freund CL, GregoryEvans CY, Furukawa T, Papaioannou M, Looser J, Ploder L, Bellingham J, Ng D, Herbrick JAS, Duncan A, Scherer SW, Tsui LC, LoutradisAnagnostou A, Jacobson SG, Cepko CL, Bhattacharya SS, McInnes RR. Cone-rod dystrophy due to mutations in a novel photoreceptor-specific homeobox gene (CRX) essential for maintenance of the photoreceptor. Cell. 1997;91:543–553. doi: 10.1016/s0092-8674(00)80440-7. [DOI] [PubMed] [Google Scholar]
- Freund CL, Wang QL, Chen SM, Muskat BL, Wiles CD, Sheffield VC, Jacobson SG, McInnes RR, Zack DJ, Stone EM. De novo mutations in the CRX homeobox gene associated with Leber congenital amaurosis. Nature Genetics. 1998;18:311–312. doi: 10.1038/ng0498-311. [DOI] [PubMed] [Google Scholar]
- Friedman JS, Khanna H, Swain PK, DeNicola R, Cheng H, Mitton KP, Weber CH, Hicks D, Swaroop A. The minimal transactivation domain of the basic motif-leucine zipper transcription factor NRL interacts with TATA-binding protein. Journal of Biological Chemistry. 2004;279:47233–47241. doi: 10.1074/jbc.M408298200. [DOI] [PubMed] [Google Scholar]
- Fu Y, Kefalov VJ, Lai J, Yau KW. Study of cone pigment function with a transgenic mouse model. Investigative Ophthalmology & Visual Science. 2002;43 E-Abstract 1962. [Google Scholar]
- Fu YB, Yau KW. Phototransduction in mouse rods and cones. Pflugers Archiv-European Journal of Physiology. 2007;454:805–819. doi: 10.1007/s00424-006-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliard F, Bonfield S, Gimour GS, Kuny S, Mema SC, Martin BT, Smale L, Crowder N, Stell WK, Sauve Y. Retinal anatomy and visual performance in a diurnal cone-rich laboratory rodent, the Nile grass rat (Arvicanthis niloticus) J Comp Neurol. 2008;510:525–538. doi: 10.1002/cne.21798. [DOI] [PubMed] [Google Scholar]
- Gehring WJ. The genetic control of eye development and its implications for the evolution of the various eye-types. International Journal of Developmental Biology. 2002;46:65–73. [PubMed] [Google Scholar]
- Gehring WJ. New perspectives on eye development and the evolution of eyes and photoreceptors. J Hered. 2005;96:171–184. doi: 10.1093/jhered/esi027. [DOI] [PubMed] [Google Scholar]
- Gomez MD, Nasi E. Calcium-independent, cGMP-mediated light adaptation in invertebrate ciliary photoreceptors. Journal of Neuroscience. 2005;25:2042–2049. doi: 10.1523/JNEUROSCI.5129-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govardovskii VI, Lychakov DV. Visual Cells and Visual Pigments of the Lamprey, Lampetra-Fluviatilis. Journal of Comparative Physiology. 1984;154:279–286. [Google Scholar]
- Haider NB, Demarco P, Nystuen AM, Huang XN, Smith RS, McCall MA, Naggert JK, Nishina PM. The transcription factor Nr2e3 functions in retinal progenitors to suppress cone cell generation. Visual Neurosci. 2006;23:917–929. doi: 10.1017/S095252380623027X. [DOI] [PubMed] [Google Scholar]
- Haider NB, Jacobson SG, Cideciyan AV, Swiderski R, Streb LM, Searby C, Beck G, Hockey R, Hanna DB, Gorman S, Duhl D, Carmi R, Bennett J, Weleber RG, Fishman GA, Wright AF, Stone EM, Sheffield VC. Mutation of a nuclear receptor gene, NR2E3, causes enhanced S cone syndrome, a disorder of retinal cell fate. Nature Genetics. 2000;24:127–131. doi: 10.1038/72777. [DOI] [PubMed] [Google Scholar]
- Hall MO, Bok D, Bacharach ADE. Biosynthesis and assembly of the rod outer segment membrane system. Formation and fate of visual pigment in the frog retina. Journal of Molecular Biology. 1969;45:397–402. doi: 10.1016/0022-2836(69)90114-4. [DOI] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau KW. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson A, Hicks D. Distribution and density of medium- and short-wavelength selective cones in the domestic pig retina. Experimental Eye Research. 2002;74:435–444. doi: 10.1006/exer.2002.1181. [DOI] [PubMed] [Google Scholar]
- Hogan JH, Wood I. The retinal pigment epithelium. Trans Pac Coast Otoophthalmol Soc. 1973;54:11. [Google Scholar]
- Hood DC, Birch DG. Phototransduction in Human Cones Measured Using the Alpha-Wave of the Erg. Vision Res. 1995;35:2801–2810. doi: 10.1016/0042-6989(95)00034-w. [DOI] [PubMed] [Google Scholar]
- Hood DC, Cideciyan AV, Roman AJ, Jacobson SG. Enhanced S-Cone-Syndrome - Evidence for an Abnormally Large Number of S-Cones. Vision Res. 1995;35:1473–1481. doi: 10.1016/0042-6989(95)98727-q. [DOI] [PubMed] [Google Scholar]
- Imai H, Kuwayama S, Onishi A, Morizumi T, Chisaka O, Shichida Y. Molecular properties of rod and cone visual pigments from purified chicken cone pigments to mouse rhodopsin in situ. Photochemical & Photobiological Sciences. 2005;4:667–674. doi: 10.1039/b416731g. [DOI] [PubMed] [Google Scholar]
- Insinna C, Besharse JC. Intraflagellar transport and the sensory outer segment of vertebrate photoreceptors. Dev Dyn. 2008;237:1982–1992. doi: 10.1002/dvdy.21554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SG, Aleman TS, Cideciyan AV, Heon E, Golczak M, Beltran WA, Sumaroka A, Schwartz SB, Roman AJ, Windsor EAM, Wilson JM, Aguirre GD, Stone EM, Palczewski K. Human cone photoreceptor dependence on RPE65 isomerase. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15123–15128. doi: 10.1073/pnas.0706367104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SG, Roman AJ, Roman MI, Gass JDM, Parker JA. Relatively Enhanced S-Cone Function in the Goldmann-Favre Syndrome. American Journal of Ophthalmology. 1991;111:446–453. doi: 10.1016/s0002-9394(14)72379-7. [DOI] [PubMed] [Google Scholar]
- Johnson PT, Williams RR, Cusato K, Reese BE. Rods and cones project to the inner plexiform layer during development. J Comp Neurol. 1999;414:1–12. [PubMed] [Google Scholar]
- Katti C, Dalal JS, Dose AC, Burnside B, Battelle BA. Cloning and distribution of Myosin 3B in the mouse retina: Differential distribution in cone outer segments. Experimental Eye Research. 2009 doi: 10.1016/j.exer.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura S, Tachibanaki S. Rod and cone photoreceptors: Molecular basis of the difference in their physiology. Comparative Biochemistry and Physiology a-Molecular & Integrative Physiology. 2008;150:369–377. doi: 10.1016/j.cbpa.2008.04.600. [DOI] [PubMed] [Google Scholar]
- Kawamura S, Yokoyama S. Expression of visual and nonvisual opsins in American chameleon. Vision Res. 1997;37:1867–1871. doi: 10.1016/s0042-6989(96)00309-4. [DOI] [PubMed] [Google Scholar]
- Kefalov V, Fu YB, Marsh-Armstrong N, Yau KW. Role of visual pigment properties in rod and cone phototransduction. Nature. 2003;425:526–531. doi: 10.1038/nature01992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney MS, Fisher SK. The photoreceptors and pigment epithelium of the larval Xenopus retina: morphogenesis and outer segment renewal. Proceedings of the Royal Society of London B. 1978;201:149–167. doi: 10.1098/rspb.1978.0037. [DOI] [PubMed] [Google Scholar]
- Krock BL, Perkins BD. The intraflagellar transport protein IFT57 is required for cilia maintenance and regulates IFT-particle-kinesin-II dissociation in vertebrate photoreceptors. J Cell Sci. 2008;121:1907–1915. doi: 10.1242/jcs.029397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryger Z, Galli-Resta L, Jacobs GH, Reese BE. The topography of rod and cone photoreceptors in the retina of the ground squirrel. Visual Neurosci. 1998;15:685–691. doi: 10.1017/s0952523898154081. [DOI] [PubMed] [Google Scholar]
- Lamb TD, Collin SP, Pugh EN. Evolution of the vertebrate eye: opsins, photoreceptors, retina and eye cup. Nature Reviews Neuroscience. 2007;8:960–975. doi: 10.1038/nrn2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb TD, Pugh EN. Physiology of transduction and adaptation in rod and cone photoreceptors. Seminars in Neuroscience. 1990;2:3–13. [Google Scholar]
- Lamb TD, Pugh EN, Collin SP. The Origin of the Vertebrate Eye. Evolution: Education and Outreach. 2008;1:415–426. [Google Scholar]
- Liu XR, Udovichenko IP, Brown SDM, Steel KP, Williams DS. Myosin VIIa participates in opsin transport through the photoreceptor cilium. Journal of Neuroscience. 1999;19:6267–6274. doi: 10.1523/JNEUROSCI.19-15-06267.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long KO, Fisher SK. The Distributions of Photoreceptors and Ganglion-Cells in the California Ground-Squirrel, Spermophilus-Beecheyi. J Comp Neurol. 1983;221:329–340. doi: 10.1002/cne.902210308. [DOI] [PubMed] [Google Scholar]
- Luby-Phelps K, Fogerty J, Baker SA, Pazour GJ, Besharse JC. Spatial distribution of intraflagellar transport proteins in vertebrate photoreceptors. Vision Res. 2008;48:413–423. doi: 10.1016/j.visres.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Cideciyan AV, Maeda A, Golczak M, Aleman TS, Jacobson SG, Palczewski K. Loss of cone photoreceptors caused by chromophore depletion is partially prevented by the artificial chromophore pro-drug, 9-cis-retinyl acetate. Human Molecular Genetics. 2009;18 doi: 10.1093/hmg/ddp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE, Sperling HG. Chromatic organization of primate cones. Science. 1977;196:454–456. doi: 10.1126/science.403607. [DOI] [PubMed] [Google Scholar]
- Mears AJ, Kondo M, Swain PK, Takada Y, Bush RA, Saunders TL, Sieving PA, Swaroop A. Nrl is required for rod photoreceptor development. Nature Genetics. 2001;29:447–452. doi: 10.1038/ng774. [DOI] [PubMed] [Google Scholar]
- Milam AH, Rose L, Cideciyan AV, Barakat MR, Tang WX, Gupta N, Aleman TS, Wright AF, Stone EM, Sheffield VC, Jacobson SG. The nuclear receptor NR2E3 plays a role in human retinal photoreceptor differentiation and degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:473–478. doi: 10.1073/pnas.022533099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitton KP, Swain PK, Khanna H, Dowd M, Apel IJ, Swaroop A. Interaction of retinal bZIP transcription factor NRL with Flt3-interacting zinc-finger protein Fiz1: possible role of Fiz1 as a transcriptional repressor. Human Molecular Genetics. 2003;12:365–373. doi: 10.1093/hmg/ddg035. [DOI] [PubMed] [Google Scholar]
- Molday RS, Hicks D, Molday L. Peripherin - a Rim-Specific Membrane-Protein of Rod Outer Segment Disks. Investigative Ophthalmology & Visual Science. 1987;28:50–61. [PubMed] [Google Scholar]
- Nathans J. Molecular-Biology of Visual Pigments. Annual Review of Neuroscience. 1987;10:163–194. doi: 10.1146/annurev.ne.10.030187.001115. [DOI] [PubMed] [Google Scholar]
- Ng L, Hurley LB, Dierks B, Srinivas M, Salto C, Vennstrom B, Reh TA, Forrest D. A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nature Genetics. 2001;27:94–98. doi: 10.1038/83829. [DOI] [PubMed] [Google Scholar]
- Nickell S, Park PSH, Baumeister W, Palczewski K. Three-dimensional architecture of murine rod outer segments determined by cryoelectron tomography. J Cell Biol. 2007;177:917–925. doi: 10.1083/jcb.200612010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickle B, Robinson PR. The opsins of the vertebrate retina: insights from structural, biochemical, and evolutionary studies. Cellular and Molecular Life Sciences. 2007;64:2917–2932. doi: 10.1007/s00018-007-7253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickle B, Wilkie SE, Cowing JA, Hunt DM, Robinson PR. Vertebrate opsins belonging to different classes vary in constitutively active properties resulting from salt-bridge mutations. Biochemistry. 2006;45:7307–7313. doi: 10.1021/bi060234g. [DOI] [PubMed] [Google Scholar]
- Nikonov SS, Daniele LL, Zhu XM, Craft CM, Swaroop A, Pugh EN. Photoreceptors of Nrl(−/−) mice coexpress functional S- and M-cone opsins having distinct inactivation mechanisms. Journal of General Physiology. 2005;125:287–304. doi: 10.1085/jgp.200409208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiguchi KM, Friedman JS, Sandberg MA, Swaroop A, Berson EL, Dryja TP. Recessive NRL mutations in patients with clumped pigmentary retinal degeneration and relative preservation of blue cone function. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17819–17824. doi: 10.1073/pnas.0408183101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh ECT, Cheng H, Hao H, Jia L, Khan NW, Swaroop A. Rod differentiation factor NRL activates the expression of nuclear receptor NR2E3 to suppress the development of cone photoreceptors. Brain Res. 2008;1236:16–29. doi: 10.1016/j.brainres.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano T, Kojima D, Fukada Y, Shichida Y, Yoshizawa T. Primary Structures of Chicken Cone Visual Pigments - Vertebrate Rhodopsins Have Evolved out of Cone Visual Pigments. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:5932–5936. doi: 10.1073/pnas.89.13.5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi A, Peng GH, Hsu C, Alexis U, Chen S, Blackshaw S. Pias3-Dependent SUMOylation Directs Rod Photoreceptor Development. Neuron. 2009;61:234–246. doi: 10.1016/j.neuron.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K. G protein-coupled receptor rhodopsin. Annu Rev Biochem. 2006;75:743–767. doi: 10.1146/annurev.biochem.75.103004.142743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry JWL, Peirson SN, Wilkens H, Bowmaker JK. Multiple photopigments from the Mexican blind cavefish, Astyanax fasciatus: a microspectrophotometric study. Vision Res. 2003;43:31–41. doi: 10.1016/s0042-6989(02)00404-2. [DOI] [PubMed] [Google Scholar]
- Pazour GJ, Baker SA, Deane JA, Cole DG, Dickert BL, Rosenbaum JL, Witman GB, Besharse JC. The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J Cell Biol. 2002;157:103–113. doi: 10.1083/jcb.200107108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peichl L. Diversity of mammalian photoreceptor properties: Adaptations to habitat and lifestyle? Anatomical Record Part a-Discoveries in Molecular Cellular and Evolutionary Biology. 2005;287A:1001–1012. doi: 10.1002/ar.a.20262. [DOI] [PubMed] [Google Scholar]
- Peichl L, Gonzalezsoriano J. Morphological Types of Horizontal Cell in Rodent Retinae - a Comparison of Rat, Mouse, Gerbil, and Guinea-Pig. Visual Neurosci. 1994;11:501–517. doi: 10.1017/s095252380000242x. [DOI] [PubMed] [Google Scholar]
- Polans A, Baehr W, Palczewski K. Turned on by Ca2+! The physiology and pathology of Ca2+-binding proteins in the retina. Trends in Neurosciences. 1996;19:547–554. doi: 10.1016/s0166-2236(96)10059-x. [DOI] [PubMed] [Google Scholar]
- Portier MM, Denechaud B, Gros F. Peripherin, a New Member of the Intermediate Filament Protein Family. Dev Neurosci. 1984;6:335–344. doi: 10.1159/000112360. [DOI] [PubMed] [Google Scholar]
- Pu GA, Dowling JE. Anatomical and Physiological-Characteristics of Pineal Photoreceptor Cell in the Larval Lamprey, Petromyzon-Marinus. Journal of Neurophysiology. 1981;46:1018–1038. doi: 10.1152/jn.1981.46.5.1018. [DOI] [PubMed] [Google Scholar]
- Pugh ENJ, Lamb TD. Phototransduction in vertebrate rods and cones: molecular mechanisms of amplification, recovery and light adaptation. Handbook of biological physics Molecular mechanisms of visual transduction. 2000;3:183–255. [Google Scholar]
- Rattner A, Sun H, Nathans J. Molecular genetics of human retinal disease. Annual Review of Genetics. 1999;33:89–131. doi: 10.1146/annurev.genet.33.1.89. [DOI] [PubMed] [Google Scholar]
- Raymond PA. Cytodifferentiation of Photoreceptors in Larval Goldfish - Delayed Maturation of Rods. J Comp Neurol. 1985;236:90–105. doi: 10.1002/cne.902360108. [DOI] [PubMed] [Google Scholar]
- Rebrik TI, Korenbrot JI. In intact cone photoreceptors, a Ca2+-dependent, diffusible factor modulates the cGMP-gated ion channels differently than in rods. Journal of General Physiology. 1998;112:537–548. doi: 10.1085/jgp.112.5.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehemtulla A, Warwar R, Kumar R, Ji XD, Zack DJ, Swaroop A. The basic motif leucine zipper transcription factor Nrl can positively regulate rhodopsin gene expression. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:191–195. doi: 10.1073/pnas.93.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reme EC, Young RC. The effects of hibernation on cone visual cells in the ground squirrel. Investigative Ophthalmology & Visual Science. 1977;16:815–840. [PubMed] [Google Scholar]
- Ridge KD, Palczewski K. Visual rhodopsin sees the light: Structure and mechanism of G protein signaling. Journal of Biological Chemistry. 2007;282:9297–9301. doi: 10.1074/jbc.R600032200. [DOI] [PubMed] [Google Scholar]
- Roberts MR, Hendrickson A, McGuire CR, Reh TA. Retinoid X receptor gamma is necessary to establish the S-opsin gradient in cone photoreceptors of the developing mouse retina. Investigative Ophthalmology & Visual Science. 2005;46:2897–2904. doi: 10.1167/iovs.05-0093. [DOI] [PubMed] [Google Scholar]
- Roberts MR, Srinivas M, Forrest D, de Escobar GM, Reh TA. Making the gradient: Thyroid hormone regulates cone opsin expression in the developing mouse retina. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:6218–6223. doi: 10.1073/pnas.0509981103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodieck RW. The Vertebrate Retina. W. H. Freeman; San Francisco: 1973. [Google Scholar]
- Rodieck RW. The first steps in seeing. Sinauer Associates; Sunderland, Mass: 1998. [Google Scholar]
- Roof D, Adamian M, Jacobs D, Hayes A. Cytoskeletal Specializations at the Rod Photoreceptor Distal Tip. J Comp Neurol. 1991;305:289–303. doi: 10.1002/cne.903050210. [DOI] [PubMed] [Google Scholar]
- Roorda A, Metha AB, Lennie P, Williams DR. Packing arrangement of the three cone classes in primate retina. Vision Res. 2001;41:1291–1306. doi: 10.1016/s0042-6989(01)00043-8. [DOI] [PubMed] [Google Scholar]
- Samardzija M, Tanimoto N, Kostic C, Beck S, Oberhauser V, Joly S, Thiersch M, Fahl E, Arsenijevic Y, von Lintig J, Wenzel A, Seeliger MW, Grimm C. In conditions of limited chromophore supply rods entrap 11-cis-retinal leading to loss of cone function and cell death. Human Molecular Genetics. 2009;18:1266–1275. doi: 10.1093/hmg/ddp026. [DOI] [PubMed] [Google Scholar]
- Samejima M, Tamotsu S, Watanabe K, Morita Y. Photoreceptor Cells and Neural Elements with Long Axonal Processes in the Pineal Organ of the Lamprey, Lampetra-Japonica, Identified by Use of the Horseradish-Peroxidase Method. Cell Tissue Res. 1989;258:219–224. [Google Scholar]
- Shelley EJ, Madigan MC, Natoli R, Penfold PL, Provis JM. Cone degeneration in aging and age-related macular degeneration. Arch Ophthalmol. 2009;127:483–492. doi: 10.1001/archophthalmol.2008.622. [DOI] [PubMed] [Google Scholar]
- Steinberg RH, Fisher SK, Anderson DH. Disk Morphogenesis in Vertebrate Photoreceptors. J Comp Neurol. 1980;190:501–518. doi: 10.1002/cne.901900307. [DOI] [PubMed] [Google Scholar]
- Steinberg RH, Wood I, Hogan MH. Pigment epithelial ensheathment and phagocytosis of extrafoveal cones in human retina. Philosophical Transactions of the Royal Society of London. 1977;277:459–471. doi: 10.1098/rstb.1977.0028. [DOI] [PubMed] [Google Scholar]
- Stenkamp RE, Filipek S, Driessen C, Teller DC, Palczewski K. Crystal structure of rhodopsin: a template for cone visual pigments and other G protein-coupled receptors. Biochimica Et Biophysica Acta-Biomembranes. 2002;1565:168–182. doi: 10.1016/s0005-2736(02)00567-9. [DOI] [PubMed] [Google Scholar]
- Stone EM, Haider NB, Jacobson SG, Cideciyan AV, Swiderski RE, Bennett J, Weleber RG, Fishman GA, Wright AF, Sheffield VC. Mutations in a photoreceptor-specific nuclear receptor (NR2E3) cause the enhanced S-cone syndrome. Investigative Ophthalmology & Visual Science. 2000;41:S94–S94. [Google Scholar]
- Strettoi E, Mears AJ, Swaroop A. Recruitment of the rod pathway by cones in the absence of rods. Journal of Neuroscience. 2004;24:7576–7582. doi: 10.1523/JNEUROSCI.2245-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain PK, Chen SM, Wang QL, Affatigato LM, Coats CL, Brady KD, Fishman GA, Jacobson SG, Swaroop A, Stone E, Sieving PA, Zack DJ. Mutations in the cone-rod homeobox gene are associated with the cone-rod dystrophy photoreceptor degeneration. Neuron. 1997;19:1329–1336. doi: 10.1016/s0896-6273(00)80423-7. [DOI] [PubMed] [Google Scholar]
- Swaroop A, Xu JZ, Pawar H, Jackson A, Skolnick C, Agarwal N. A Conserved Retina-Specific Gene Encodes a Basic Motif Leucine Zipper Domain. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:266–270. doi: 10.1073/pnas.89.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szel A, Lukats A, Fekete T, Szepessy Z, Rohlich P. Photoreceptor distribution in the retinas of subprimate mammals. J Opt Soc Am A-Opt Image Sci Vis. 2000;17:568–579. doi: 10.1364/josaa.17.000568. [DOI] [PubMed] [Google Scholar]
- Townes-Anderson E. Intersegmental Fusion in Vertebrate Rod Photoreceptors - Rod Cell Structure Revisited. Investigative Ophthalmology & Visual Science. 1995;36:1918–1933. [PubMed] [Google Scholar]
- Travis GH. Mechanisms of cell death in the inherited retinal degenerations. American Journal of Human Genetics. 1998;62:503–508. doi: 10.1086/301772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan DK, Fisher SK. Cytochalasin-D Disrupts Outer Segment Disk Morphogenesis Insitu in Rabbit Retina. Investigative Ophthalmology & Visual Science. 1989;30:339–342. [PubMed] [Google Scholar]
- Walls GL. Human rods and cones. Archives of Ophthalmology. 1934;12:914–930. [Google Scholar]
- Williams DS, Linberg KA, Vaughan DK, Fariss RN, Fisher SK. Disruption of Microfilament Organization and Deregulation of Disk Membrane Morphogenesis by Cytochalasin-D in Rod and Cone Photoreceptors. J Comp Neurol. 1988;272:161–176. doi: 10.1002/cne.902720202. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Mears AJ, Friedman JS, Carter T, He S, Oh E, Jing YZ, Farjo R, Fleury G, Barlow C, Hero AO, Swaroop A. Expression profiling of the developing and mature Nrl(−/−) mouse retina: identification of retinal disease candidates and transcriptional regulatory targets of Nrl. Human Molecular Genetics. 2004;13:1487–1503. doi: 10.1093/hmg/ddh160. [DOI] [PubMed] [Google Scholar]
- Young RW. The renewal of photoreceptor cell outer segments. J Cell Biol. 1967;33:61–72. doi: 10.1083/jcb.33.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW. The difference between rods and cones in the renewal of outer segment protein. Investigative Ophthalmology & Visual Science. 1969;8:222–231. [PubMed] [Google Scholar]
- Young RW. Visual cells and the concept of renewal. Investigative Ophthalmology & Visual Science. 1976;15:700–725. [PubMed] [Google Scholar]
- Yuodelis C, Hendrickson A. A Qualitative and Quantitative-Analysis of the Human Fovea During Development. Vision Res. 1986;26:847–855. doi: 10.1016/0042-6989(86)90143-4. [DOI] [PubMed] [Google Scholar]
- Zhang HB, Fan J, Li S, Karan S, Rohrer B, Palczewski K, Frederick JM, Crouch RK, Baehr W. Trafficking of membrane-associated proteins to cone photoreceptor outer segments requires the chromophore 11-cis-retinal. Journal of Neuroscience. 2008;28:4008–4014. doi: 10.1523/JNEUROSCI.0317-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Znoiko SL, Rohrer B, Lu KN, Lohr HR, Crouch RK, Ma JX. Downregulation of cone-specific gene expression and degeneration of cone Photoreceptors in the Rpe65(−/−) mouse at early ages. Investigative Ophthalmology & Visual Science. 2005;46:1473–1479. doi: 10.1167/iovs.04-0653. [DOI] [PubMed] [Google Scholar]