Abstract
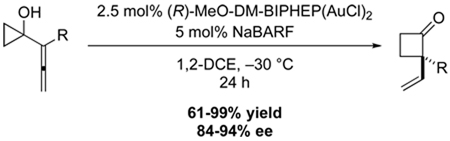
The asymmetric gold(I)-catalyzed ring expansion of 1-allenylcyclopropanols is described. The method provides synthetically valuable cyclobutanones with a vinyl-substituted quaternary stereogenic center in high enantioselectivities and yields. The method shows a broad substrate scope, tolerating protected alcohols and amines, alkenes, unsaturated esters and acetals. The reaction is easily adjustable to large scale synthesis, leading to product formation without significant loss of selectivity or yield with only 0.5 mol% catalyst loading.
Substituted cyclobutanes constitute valuable building blocks for organic synthesis due to their rich chemistry and are additionally found as motifs in numerous natural products.1 Among the various approaches to cyclobutanes, the ring expansion of cyclopropanols by a Wagner-Meerwein shift to the corresponding cyclobutanones is considered one of the most powerful and versatile methods.2 While ring expansion of small ring systems can generally be induced by coordination of π-acidic transition metal catalysts to alkenyl-, alkynyl-, and allenylcycloalkanols,3 only few reports have described asymmetric catalytic Wagner-Meerwein shifts based on this strategy for the synthesis of cycloalkanones.4
In the last decade, cationic gold(I) complexes have evolved as mild Lewis acid catalysts for transformations requiring the activation of π-bonds.5 In 2005, we reported the ring expansion of 1-alkynylcyclopropanols catalyzed by [(p-CF3C6H4)3P]AuSbF6, which provides efficient access to a range of alkylidenecyclobutanols.6,7 We hypothesized that an analogous rearrangement of 1-allenylcyclopropanols using chiral gold-phosphine complexes might allow for the catalytic construction of cyclobutanones possessing a vinyl-substituted quaternary stereogenic center.8
During initial experiments to establish standard reaction conditions, (R)-xylyl-BINAP was identified as promising ligand. When allenylcyclopropanol 1 was treated with the (R)-xylyl-BINAP derived cationic gold(I) complex at 0 °C, cyclobutanone 2 was isolated in 75% ee (Table 1, entry 1). When the temperature for the reaction was decreased, the enantioselectivity increased to 84% at −30 °C (entry 4). Notably, the enantioselectivity showed a pronounced leveling behavior, with only a small increase in selectivity between −10 °C and −30 °C (entries 2–4). Decreasing the steric encumbrance around the phosphine led to a drop in the enantioselectivity (entries 5 and 6).9 A slight improvement in selectivity was obtained with MeO-DM-BIPHEP, giving 2 in 86% ee at −30 °C (Table 1, entry 7). The replacement of AgNTf2 with sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]-borate (NaBARF) further increased the enantioselectivity, providing 2 in 89% and 91% ee for xylyl-BINAP and MeO-DM-BIPHEP, respectively (entries 8 and 9).10 Control experiments indicated that the improved selectivity in the case of NaBARF presumably results from suppression of an inherent background reaction by trace amounts of HNTf2 formed under the reaction conditions.11
Table 1.
Optimization of Reaction Conditions
 | ||||
|---|---|---|---|---|
| entry | ligand (L) | MX | T (°C) | ee (%) |
| 1 | (R)-xylyl-BINAP | AgNTf2 | 0 | 75 |
| 2 | (R)-xylyl-BINAP | AgNTf2 | −10 | 81 |
| 3 | (R)-xylyl-BINAP | AgNTf2 | −20 | 83 |
| 4 | (R)-xylyl-BINAP | AgNTf2 | −30 | 84 |
| 5 | (R)-tolyl-BINAP | AgNTf2 | −30 | 48 |
| 6 | (R)-BINAP | AgNTf2 | −30 | 32 |
| 7 | (R)-MeO-DM- BIPHEP |
AgNTf2 | −30 | 86 |
| 8 | (R)-xylyl-BINAP | NaBARFa | −30 | 89 |
| 9 | (R)-MeO-DM- BIPHEP |
NaBARFa | −30 | 91 |
NaBARF: sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate.
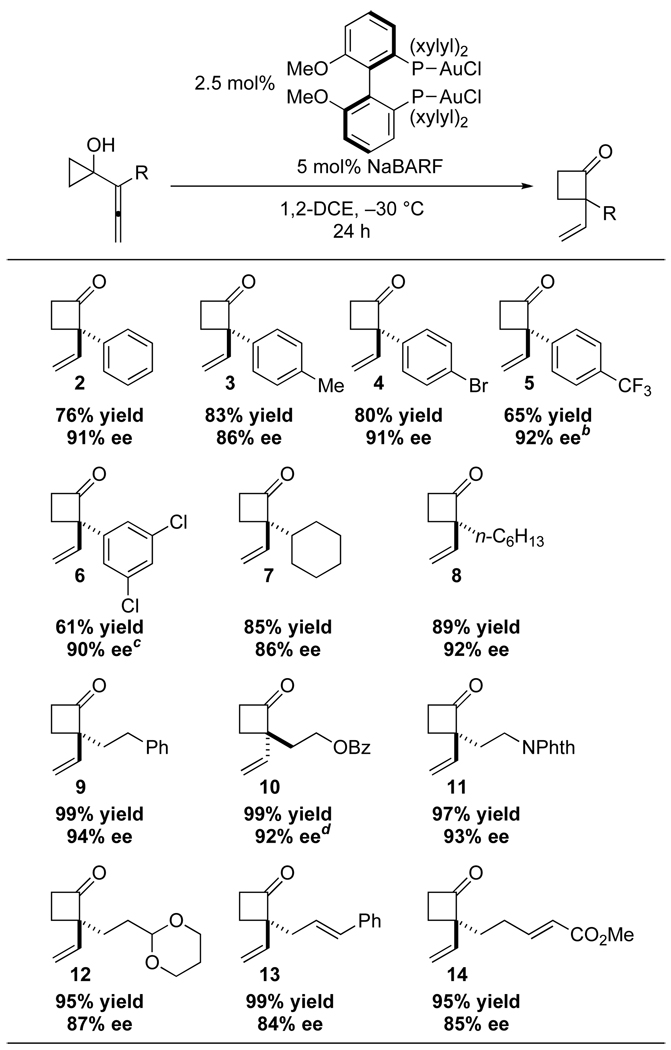
With these results in hand, we examined the scope of the gold(I)-catalyzed enantioselective ring expansion of 1-allenylcyclopropanols (Scheme 1). Straightforward access to all substrates was achieved using three different strategies: (1) direct allenylation of cyclopropanone generated in situ from 1-ethoxycyclopropanol; (2) Johnson-Claisen rearrangement of propargyl alcohols; and (3) SN2' displacement of propargyl mesylates with cuprate reagents (see Supporting Information for details). A wide range of cyclobutanones are accessible in synthetically useful yields12 and good to excellent enantio-selectivities.13 Substrates possessing aromatic substituents are generally well tolerated (2–6), with the more electron-deficient 1-allenylcyclopropanols 5 and 6 requiring longer reaction times. Alkyl-substituted compounds are excellent substrates for this method, giving cyclobutanones 7 to 9 in high enantioselectivities. The mildness of the method allows for the incorporation of various functional groups in the side chain, e. g. protected alcohols (10) or amines (11), acetals (12), alkenes (13), and α,β-unsaturated esters (14).
Scheme 1.
Substrate Scope a
aConditions: 2.5 mol% (R)-MeO-DM-BIPHEP(AuCl)2, 5 mol% NaBARF, 0.100 mmol substrate, 0.12 M in 1,2-DCE, −30 °C, 24 h; yields refer to isolated material; ee’s determined by chiral HPLC, see Supporting Information for details. bReaction run at 0.25 M for 48 h. cReaction run with 5 mol% catalyst and 10 mol% NaBARF at 0.08 M for 48 h. dReaction run with (S)-xylyl-BINAP(AuCl)2.
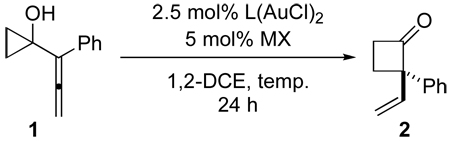
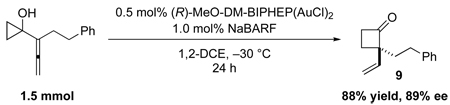
The amount of catalyst can be reduced without significant loss of enantioselectivity or yield. Cyclobutanone 9 was obtained in 88% yield and 89% ee at a 1.5 mmol scale with only 0.5 mol% of the chiral gold(I) catalyst (equation 1). In combination with the air and moisture tolerance of the reaction, this method should be suitable for the synthesis of chiral cyclobutanones on large scale.
 |
(1) |
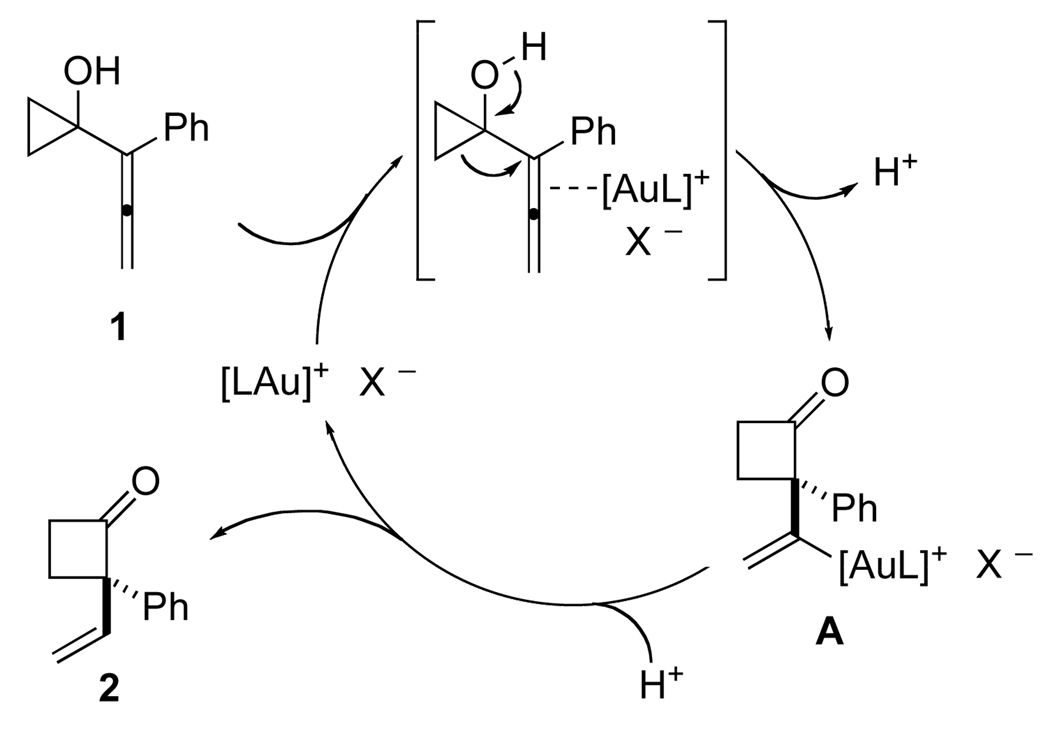
A proposed mechanism for the gold(I)-catalyzed enantioselective ring expansion of 1-allenylcyclopropanols is outlined in Scheme 2. Coordination of the cationic gold(I) catalyst to the internal double bond of the allene moiety in 1 triggers a ring expansion by a Wagner-Meerwein shift, generating a vinyl gold-intermediate A. A subsequent protodemetallation liberates the catalyst and releases the product 2. This mechanistic proposal is in agreement with results from computational studies carried out for the related rearrangement of 1-alkynylcyclopropanols.14
Scheme 2.
Proposed Reaction Mechanism
In summary, we have developed an asymmetric ring expansion reaction of 1-allenylcyclopropanols catalyzed by chiral gold(I)-phosphine complexes. The method provides access to a wide range of cyclobutanones with a vinyl-substituted quaternary stereogenic center. Moreover, this method constitutes the first report of an enantioselective gold-catalyzed 1,2-alkyl migration,7 and thereby expands the class of reactions amenable to asymmetric catalysis by gold.15 Further studies on the application of this methodology to the synthesis of natural products are ongoing in our laboratories.
Supplementary Material
Experimental procedures, compound characterization data, and crystallographic data (CIF). This material is available free of charge via the internet at http://pubs.acs.org.
Acknowledgment
We thank Dr. David J. Buchanan and Daniel L. Gray for initial studies. We gratefully acknowledge NIHGMS (RO1 GM073932), Bristol-Myers Squibb, and Novartis for financial support. F.K. thanks the German Academic Exchange Service (DAAD) for a postdoctoral fellowship. We acknowledge Solvias and Takasago for the generous donation of phosphine ligands and Johnson Matthey for a gift of AuCl3.
References
- 1.For general reviews on the chemistry of cyclobutanes, see: Belluš D, Ernst B. Angew. Chem. 1988;100:820. Lee-Ruff E, Mladenova G. Chem. Rev. 2003;103:1449. doi: 10.1021/cr010013a. Namyslo JC, Kaufmann DE. Chem. Rev. 2003;103:1485. doi: 10.1021/cr010010y.
- 2.Lee-Ruff E. In: The Chemistry of Cyclobutanes. Rappoport Z, Liebman JF, editors. Chichester: John Wiley & Sons; 2005. [Google Scholar]
- 3.(a) Hudlicky T, Reed JW. In: Comprehensive Organic Synthesis. Trost BM, Fleming I, editors. Oxford: Pergamon Press; 1991. [Google Scholar]; (b) Hudlicky T, Kutchan TM, Naqvi SM. Org. React. 1985;33:247. [Google Scholar]; (c) Iwasawa N. Synlett. 1999:13. [Google Scholar]; (d) Iwasawa N, Narasaka K. Top. Curr. Chem. 2000;207:69. [Google Scholar]; (e) Muzart J. Tetrahedron. 2005;61:9423. [Google Scholar]; (f) Muzart J. Tetrahedron. 2008;64:5815. [Google Scholar]
- 4.Cyclobutanones: Trost BM, Yasukata T. J. Am. Chem. Soc. 2001;123:7162. doi: 10.1021/ja010504c. Cyclopentanones: Trost BM, Xie J. J. Am. Chem. Soc. 2006;128:6044. doi: 10.1021/ja0602501. Trost BM, Xie J. J. Am. Chem. Soc. 2008;130:6231. doi: 10.1021/ja7111299. For an alternative entry to chiral cyclobutanones based on a Wagner-Meerwein shift induced by a Sharpless asymmetric epoxidation, see: Nemoto H, Ishibashi H, Nagamochi M, Fukumoto K. J. Org. Chem. 1992;57:1707.
- 5.For general recent views on gold catalysis, see: Jiménez-Núñez E, Echavarren AM. Chem. Comm. 2007:333. doi: 10.1039/b612008c. Gorin DJ, Toste FD. Nature. 2007;446:395. doi: 10.1038/nature05592. Fürstner A, Davies PW. Angew. Chem. Int. Ed. 2007;46:3410. doi: 10.1002/anie.200604335. Hashmi ASK. Chem. Rev. 2007;107:3180. doi: 10.1021/cr000436x. Gorin DJ, Sherry BD, Toste FD. Chem. Rev. 2008;108:3351. doi: 10.1021/cr068430g. Shen HC. Tetrahedron. 2008;64:3885. Shen HC. Tetrahedron. 2008;64:7847.
- 6.Markham JP, Staben ST, Toste FD. J. Am. Chem. Soc. 2005;127:9708. doi: 10.1021/ja052831g. [DOI] [PubMed] [Google Scholar]; For a ruthenium-catalyzed version of the same ring expansion reaction, see: Trost BM, Xie J, Maulide N. J. Am. Chem. Soc. 2008;130:17258. doi: 10.1021/ja807894t.
- 7.For a review on 1,2-alkyl migrations catalyzed by π-acids, see: Crone B, Kirsch SF. Chem. Eur. J. 2008;14:3514. doi: 10.1002/chem.200701985. and references therein.
- 8.For reviews on catalytic enantioselective formation of quaternary stereocenters, see: Corey EJ, Guzman-Perez A. Angew. Chem. Int. Ed. 1998;37:388. doi: 10.1002/(SICI)1521-3773(19980302)37:4<388::AID-ANIE388>3.0.CO;2-V. Douglas CJ, Overman LE. Proc. Nat. Acad. Sci. 2004;101:5363. doi: 10.1073/pnas.0307113101. Trost BM, Jiang C. Synthesis. 2006:369.
- 9.For all three major bisphosphine ligand families (BINAP, BIPHEP, and Segphos), the same trend was observed, with the xylyl-substituted representatives providing the highest selectivity within each family.
- 10.While AgNTf2, AgPF6, and ASbF6 all provided cyclobutanone 2 in 83% ee with (R)-xylyl-BINAP as ligand, AgBF4, AgOTf, and AgOTs led to formation of 2 in only 67% ee, 34% ee and 15% ee, respectively.
- 11.AgNTf2 was found to slowly catalyze the ring expansion of 1. While AgOAc was not a competent catalyst, almost instantaneous reaction was observed with HNTf2. These observations suggest that the achiral catalyst causing the background reaction is not the silver cation.
- 12.As opposed to 1,1-disubstituted allenes, for which intramolecular addition of the alcohol onto the allene was never observed, tri- and tetrasubstituted allenes generated substantial amounts of dihydrofuran byproducts. For an example of formation of dihydrofurans from alkoxyallenes, see: Yeom H-S, Yoon S-J, Shin S. Tetrahedron Lett. 2007;48:4817.
- 13.The absolute configuration of cyclobutanone 4 was determined by crystal structure analysis of the corresponding tosylhydrazone. The absolute configurations of compounds 2 and 9 was assigned by comparison of the optical rotation with reported values (see ref. 4a). The absolute configuration of all other compounds was assigned by analogy.
- 14.Sordo TL, Ardura D. Eur. J. Org. Chem. 2008:3004. [Google Scholar]
- 15.For examples of gold-catalyzed enantioselective reactions of allenes, see: Luzung MR, Mauleón P, Toste FD. J. Am. Chem. Soc. 2007;129:12402. doi: 10.1021/ja075412n. Tarselli MA, Chianese AR, Lee SJ, Gagné MR. Angew. Chem., Int. Ed. 2007;46:6670. doi: 10.1002/anie.200701959. Hamilton GL, Kang EJ, Mba M, Toste FD. Science. 2007;317:496. doi: 10.1126/science.1145229. Liu C, Widenhoefer RA. Org. Lett. 2007;9:1935. doi: 10.1021/ol070483c. LaLonde RL, Sherry BD, Kang EJ, Toste FD. J. Am. Chem. Soc. 2007;129:2452. doi: 10.1021/ja068819l. Zhang Z, Widenhoefer RA. Angew. Chem. Int. Ed. 2007;46:283. doi: 10.1002/anie.200603260.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures, compound characterization data, and crystallographic data (CIF). This material is available free of charge via the internet at http://pubs.acs.org.