Abstract
Children with autism often have difficulty performing skilled movements. Praxis performance requires basic motor skill, knowledge of representations of the movement (mediated by parietal regions), and transcoding of these representations into movement plans (mediated by premotor circuits). The goals of this study were: (a) to determine whether dyspraxia in autism is associated with impaired representational (“postural”) knowledge, and (b) to examine the contributions of postural knowledge and basic motor skill to dyspraxia in autism. Thirty-seven children with autism spectrum disorder (ASD) and 50 typically developing (TD) children, ages 8–13, completed: (a) an examination of basic motor skills, (b) a postural knowledge test assessing praxis discrimination, and (c) a praxis examination. Children with ASD showed worse basic motor skill and postural knowledge than controls. The ASD group continued to show significantly poorer praxis than controls after accounting for age, IQ, basic motor skill, and postural knowledge. Dyspraxia in autism appears to be associated with impaired formation of spatial representations, as well as transcoding and execution. Distributed abnormality across parietal, premotor, and motor circuitry, as well as anomalous connectivity may be implicated.
Keywords: developmental dyspraxia, premotor cortex, autism spectrum disorder, movement representation, motor learning
Autism is a developmental disorder that is characterized by three key features: deficits in communication and language, social difficulties, and the presence of repetitive/stereotyped behaviors and interests (American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision). In addition to these hallmark features, children with autism consistently display motor deficits; parents and clinicians frequently observe children with autism displaying clumsy gait, poor muscle tone, imbalance, as well as poor manual dexterity and coordination (see Gidley Larson & Mostofsky, 2006, for a review). Considering the consistent clinical reports of impaired motor functioning in autism, motor examination may provide a window into the underlying neurobiological substrate of the disorder. Motor signs may serve as markers for deficits in parallel or neighboring brain systems important for control of socialization and communication. Measures of motor function tend to be more overtly observable than measures of more complex social and behavioral systems (e.g., one can count the number of finger taps a child performs, but cannot as easily quantify executive functioning). Furthermore, examination of praxis and more basic motor skills is advantageous in that the neuroanatomic and physiologic basis of motor control is well understood in contrast to that for complex social behavior, so that one knows where in the brain to look when examining for anatomic correlates of functional impairment. Additionally, motor functioning is easier to assess than social and emotional functioning at pre-verbal ages. However, research on the efficacy of motor examinations in diagnostic assessments of suspected ASD patients is limited and is an area that warrants further scrutiny by the scientific community.
Motor deficits in autism include impairment in basic motor control (such as gait, tone, posture, coordination, and balance). In addition, children with autism have been found to have difficulties with praxis performance and as such are often labeled with “developmental dyspraxia”. While the term developmental dyspraxia encompasses various symptoms in the motor systems literature, here it is defined as a developmental impairment in the performance of learned skilled limb movements that does not stem from a basic motor or perceptual deficit (in the adult literature, the term apraxia has been used to describe these deficits when acquired later in life as the result of traumatic brain injury). Skilled movements can be categorized as transitive movements, which involve demonstration of tool use (e.g., using a hammer or a toothbrush) or intransitive movements, which are symbolic, communicative gestures (e.g., waving goodbye or giving the thumbs up sign; for reviews see Gibbs, Appleton, & Appleton, 2007; Heilman & Rothi, 1993; Wheaton & Hallett, 2007). Performance of transitive verses intransitive gestures is likely to be distinct, given the differences in complexity between the two (transitive gestures adding the requirement to orient the hand properly in relation to the tool, in addition to moving it correctly according to its function) and meaning (transitive gestures being specific to tool use, intransitive gestures specific to communication), each likely recruiting differing cognitive resources.
Effective development of praxis involves connections between multiple brain regions. The angular and supramarginal gyri are thought to be the site of storage of learned time-space movement representations, or “action sequences” (Buxbaum, Kyle, Grossman, & Coslett, 2007; Frey, 2007; Halsband et al., 2001; Heilman, Rothi, & Valenstein, 1982; Sirigu et al., 1996; Weiss, Rahbari, Hesse, & Fink, 2008). It is thought that these movement representations help to program the premotor cortex, which is involved in transcoding them into motor programs that in turn activate the motor cortex for execution (Heilman & Rothi, 1993). A breakdown at any stage in this process (i.e., in recruitment of correct movement representations - henceforth referred to as “postural knowledge”, Mozaz, Rothi, Anderson, Crucian, & Heilman, 2002 - in transcoding, or in execution) can manifest as dyspraxia.
Although much is known about dyspraxia from a clinical perspective, there has been little research on the neurological basis of dyspraxia in children with autism. Given what is known about the neurology underlying the performance of skilled gestures (Heilman & Rothi, 1993), there appear to be three potential contributors to dyspraxia in autism: (a) impairments in the storage of learned time-space movement representations, mediated by parietal regions; (b) impairments in transcoding of these movement representations in the premotor cortex; and, (c) impairments in execution/basic motor skills (mediated by the motor cortex). Jansiewicz et al. (2006) found that children with ASD showed significant impairments in basic motor control. Dziuk et al. (2007) found that children with ASD have been shown to demonstrate poorer praxis performance, after accounting for the effects of basic motor skills. These findings are consistent with Gibbs’ definition of dyspraxia, which he and others have described as a developmental deficit in performance of motor functioning, beyond what can be explained by simple clumsiness (Gibbs, Appleton, & Appleton, 2007).
Deficits in imitation have also been emphasized in the autism literature (Hobson & Lee, 1999; Stieglitz Ham, Corley, Rajendran, Carletta, & Swanson, 2008; Vanvuchelen, Roeyers, & de Weerdt, 2007; Williams, Whiten, & Singh, 2004). Abnormalities in “self-other mapping” (Rogers & Pennington, 1991), thought to be associated with dysfunction within mirror neuron systems (Cattaneo et al., 2007; Martineau, Cochin, Magne, & Barthelemy, 2008; Rizzolatti & Fabbri-Destro, 2008; Rizzolatti, Fadiga, Gallese, & Fogassi, 1996; Williams, Whiten, Suddendorf, & Perrett, 2001, for a review), have been hypothesized as contributing to impaired development of empathy, joint attention, and theory of mind. For example, Mostofsky et al. (2006) explored the relationship between autism and developmental dyspraxia. In this study, children with ASD and TD controls were administered a version of the Florida Apraxia Battery (Rothi et al. 2003), modified for children, in which participants performed gestures learned during early childhood (such as waving goodbye) as well as skills which developed later (such as cutting with scissors). Children performed gestures to command (in which verbal instructions were kept simple to minimize any significant language component), gestures to imitation (of a live model), and gestures with actual tool use. Gestures included novel (“nonsense”) movements as well as learned movements (e.g., using a toothbrush), and the complexity of the movements ranged from single movements (e.g., making a “stop” signal with one hand) to more complex sequenced movements (e.g., opening a door with a key). The authors found that while children with autism showed significant impairments in skilled motor gestures, these deficits do not appear to be specific to imitation. Rather, children with autism showed significant impairments in performance of gestures to command and with tool use, as well as to imitation (Mostofsky et al., 2006). Furthermore, follow-up analyses utilizing the same praxis assessment methods revealed that performance of gestures to command, to imitation, and with tool use each predict the defining social, communication, and behavioral deficits in autism (Dziuk et al., 2007).
Taking into account the basic motor skills deficits in children with ASD, it remains unclear whether the observed dyspraxia can otherwise be accounted for by deficits in the storage of the movement representations (mediated by parietal regions), or alternatively, by abnormal connectivity between these areas and those parts of the brain responsible for translation of these representations into performed movements (mediated by premotor circuits). If the problem is primarily in the area of storage of these motor representations, then presumably children with dyspraxia would have difficulty not only with performance of skilled gestures, but also with the recognition of these gestures (in other words, their postural knowledge would be deficient), in the absence of any motor execution requirement. Consistent with this notion, children with autism performed significantly worse on a task in which they were required to perceive biological motion, which the authors postulated may be related to the hallmark social deficits in autism (Blake, Turner, Smoski, Pozdol, & Stone, 2003). Other studies have shown that children with autism demonstrate poorer performance on complex visual motion tasks, but not on non-motion visual tasks (Milne et al., 2002; Pellicano, Gibson, Maybery, Durkin, & Badcock, 2005; Spencer et al., 2000). However, given that more complex phenotypes of ASD are more likely (for example, Takarae, Luna, Minshew, & Sweeney, 2008, showed that performance on a visual motion discrimination task varied with language development in an ASD sample), the role of visual perception in postural knowledge is important to take into account.
As such, the current study sought to examine whether autism is associated with impaired representational knowledge of skilled movements (i.e., “postural knowledge”; Mozaz, Rothi, Anderson, Crucian, & Heilman, 2002) as well as to examine the additive contributions of postural knowledge and basic motor skill to dyspraxia in autism. It was hypothesized that children with autism would show impaired recognition of skilled movements (with poorer recognition of transitive than intransitive gestures), but that these deficits (as well as basic motor skill deficits) would still not entirely account for their deficits in praxis performance. In addition, we predicted that praxis performance would continue to predict social, communicative, and behavioral features of autism, after accounting for postural knowledge and basic motor skill deficits.
Method
Participants
Eighty-seven children ages 8 years, 1 month to 13 years, 1 month participated in the study. Of these, 37 children (5 females; M age = 10.26, SD = 1.7) were diagnosed with an autism spectrum disorder (ASD) - either high functioning autism (HFA) or Asperger's syndrome (AS). Children with HFA and AS were combined into one ASD group based on recent research that supports the notion that children from both groups demonstrate comparable performance on assessments of praxis and basic motor skills (Jansiewicz et al., 2006; Mostofsky et al., 2006). Additionally, preliminary analyses from the current dataset showed that children with HFA and AS did not differ in performance on the assessment of postural knowledge (see Results). Fifty typically developing (TD) children (9 females; M age = 10.55, SD = 1.3) served as a control group.
Children in both groups were recruited from advertisements posted in the local communities, local magazines, pediatricians’ offices, schools, and word of mouth. Additionally, children in the ASD group were recruited from local Autism Society of America chapters, and outpatient clinics at the Kennedy Krieger Institute.
ASD diagnoses were established using the Autism Diagnostic Interview - Revised (ADI-R; Lord, Rutter, & Le Couteur, 1994), the Autism Diagnostic Observation Schedule - G, Module 3 (ADOS-G; Lord et al., 2000), and the clinical judgment of the examiners. Participants were required to meet criteria for ASD based on the clinical judgment of the examiner and either the ADOS-G, the ADI-R, or both. All participants met criteria for ASD based on the ADOS-G and clinical impression. One participant was not administered the ADI-R; in this case, a diagnosis of ASD was based on clinical impression as well as ADOS-G results. Three participants did not meet criteria for ASD on the ADI-R; however, a diagnosis was confirmed based on clinical judgment and ADOS-G results.
Children were excluded from the both groups if there was a prior documented history of a definitive neurological disorder (including seizures, tumors, traumatic brain injury, stroke, or lesions), presence of a severe chronic medical disorder, visual impairment, history of substance abuse or dependence, or presence of childhood schizophrenia or psychosis. Children were excluded from the ASD group if there was a history of known etiology for autism (e.g., Fragile X, Tuberous Sclerosis, PKU, congenital rubella), or history of documented prenatal or perinatal insult.
Children were excluded from the control group if they had a history of a developmental disorder or a psychiatric disorder based on responses from a standardized parent interview, the Diagnostic Interview for Children and Adolescents (DICA-IV; Reich, Welner, & Herjanic, 1997). They were also excluded if they had an immediate family member (sibling or parent) with autism or another pervasive developmental disorder.
Children in the control group were not taking any psychotropic medications. Eight children in the ASD group were taking stimulants, six were taking selective serotonin reuptake inhibitors, four were taking neuroleptics, three were taking atomoxetine, one was taking clonidine, and one child was taking bupropion. Children were asked to discontinue taking stimulant medications the day prior to and the day of each study visit; all other medications were taken as prescribed.
Of note, of the children included in the current sample, 70% are distinct from those included in the sample analyzed in our most recent prior praxis study (Dziuk et al., 2007). The Johns Hopkins Medicine Institutional Review Board approved this study. Written consent was obtained from a parent or legal guardian of each child; all participants gave their verbal and written assent.
Procedures
Each participant was administered the Wechsler Intelligence Scale for Children - 4th Edition (WISC-IV; Wechsler, 2003) to assess intellectual functioning. Recent research supports the notion that using a task-specific measure of intelligence is a more appropriate assessment of intellectual functioning in children with ASD than a more general measure (Mottron, 2004). Therefore, the present study used the Perceptual Reasoning Index (PRI) from the WISC-IV as the primary measure of intellectual functioning, rather than the Full Scale IQ (FSIQ), taking into account that the three tasks performed by the participants were nonverbal, perceptually based motor tasks.
Throughout each motor examination, verbal instructions were simple and standardized in order to minimize any confounding elements of language and comprehension. All participants appeared to understand the directions and any questions were answered before beginning a task.
Praxis examination
Praxis performance was assessed using a version of the Florida Apraxia Battery (Rothi et al., 2003), modified for children (Mostofsky et al., 2006). Children were asked to perform a variety of skilled gestures in three ways: by responding to a verbal command (gesture to command, or GTC), by imitating the gestures of the examiner (gesture to imitation, or GTI), and when applicable, by demonstrating the gesture using an actual tool (gesture with tool use, or GTU). The examination was videotaped and later scored independently by two raters who were blind to diagnosis. Each gesture was examined for the presence of errors according to criteria described in Mostofsky et al. (2006). Average Total Errors was the primary dependent measure of praxis performance. At least 80% concurrence between raters was achieved for each assessment to ensure reliability of scoring. Detailed descriptions of the praxis battery, scoring methodology, and reliability data are provided in Mostofsky et al. (2006) and Dziuk et al. (2007). A summary of the types of errors is included as Table 1.
Table 1.
Types of Errors Committed on Praxis Examination
| Error | Type | Description |
|---|---|---|
| Spatial | Amplitude | Irregularity of height or width of motion |
| Internal Configuration | Incorrect position of hand to hold tool | |
| External Configuration | Movements do not direct tool toward object | |
| Movement | Movement at incorrect joints | |
| Content | Concretization | Pantomimes on real object |
| Perseverative | Responds with previously enacted movement | |
| Related | Associated to target, but different | |
| Non-related | Accurate but not associated to target | |
| Hand | Performs action with hand, not tool | |
| Temporal | Sequence | Any change in correct sequence of movements |
| Timing | Alteration of correct timing | |
| Occurrence | Incorrect number of actions performed | |
| Body Part for Tool | Body used as tool | |
| Other | No Response | No response |
| Unrecognizable | Not a recognizable response | |
Basic motor skill examination
Basic motor skills were assessed using the Physical and Neurological Examination of Subtle Signs (PANESS; Denckla, 1985). The PANESS is a standardized childhood assessment that tests basic motor skills such as gait, balance, coordination, and aim, and assesses for the presence of subtle neurological signs such as overflow movements, abnormal posturing, and dysrhythmia. Relevant to the current study, one section of the PANESS measures the child's ability to perform repetitive movements of the hands (patting the front of the hand on the lap while keeping the heel of the hand stationary), fingers (tapping the thumb and pointer finger together), and feet (tapping the front of the foot while keeping the heel on the floor). The time taken to perform each movement 20 times (on both the left and right sides) was recorded and summed to provide a “total timed repetitive movements” score. Because limb movements are integral to praxis performance, this score was selected to examine the contribution of basic motor skills used in executing skilled movements during the praxis examination; the frontal and frontal-subcortical contributions to these basic motor skills are integrated into the more complex praxis movements.
Examination of postural knowledge
A postural knowledge test (PKT), adapted for children from Mozaz et al. (2002), was used to assess recognition of skilled gestures. The assessment was comprised of three sections. In the first section, the child was presented with pictures of a person with a missing hand performing a transitive gesture (one involving the use of a tool, such as hammering or painting), and then asked to indicate by pointing (to one of three options) the hand that best depicted how the tool should be held. In the second section, the child was presented with a picture of someone with a missing hand performing an intransitive gesture (one not involving tool use, such as waving goodbye or clapping) and asked to point to (from three options) the hand that best demonstrated how the gesture should be performed. In the third section, the child was presented with three pictures of hands holding a tool and asked to identify (again, by pointing) which of the three pictures best depicted how the tool should be held. The numbers of correct responses from the first and third sections were added together, resulting in a “transitive gestures score”; the number of correct responses from the second section yielded an “intransitive gestures score.” The sum of these two scores was used as an overall measure of postural, or representational, knowledge.
Statistical Analyses
Group differences in age, socio-economic status (SES; measured using the Hollingshead four-factor index of social status; Hollingshead, 1975), PRI, Total Timed Repetitive Movements score from the PANESS, Average Total Errors on the praxis examination, and Total PKT scores were first individually examined using univariate analyses of variance. Chi square analysis was used to examine group differences in gender and racial distribution. Hierarchical linear regression analysis was used to evaluate the contribution of five variables in predicting praxis performance (i.e., Average Total Errors). The predictors were entered in the following order: (a) age, (b) intelligence (measured by the PRI), (c) basic motor skill (measured by Total Timed Repetitive Movements from the PANESS), (d) postural knowledge (measured by the total number of correct responses on the PKT), and (e) diagnosis. Although the control and ASD groups did not differ significantly on age or PRI performance, both were included as independent variables in the regression analysis to control for any residual effects of age and intelligence. A two-tailed significance level of .05 was used as the criterion for significance in all analyses.
Results
Preliminary Analyses
Results of preliminary analyses are listed in Table 2. The control and ASD groups did not differ significantly in age, F(1, 85) = .751, p = .389. They were also matched by PRI, F(1, 85) = .007, p =.932, as well as gender ratio, χ2(1, N = 87) = .317, p = .573, SES, F(1, 85) = 3.3235, p = .072, and racial distribution, χ2(3, N = 87) = 4.933, p = .177. Children with ASD demonstrated significantly slower timed repetitive movements (i.e., basic motor skill) from the PANESS than controls, F(1, 85) = 12.545, p = .001, and significantly poorer performance on the PKT (total correct responses), F(1, 85) = 4.626, p = .034. The two groups showed similar performance on the transitive gestures score of the PKT as TD controls, F(1, 85) = 2.52, p = .116; however, the ASD group showed significantly worse performance on the intransitive gestures score of the PKT, F(1, 85) = 4.228, p = .0428. Consistent with past findings (Dziuk et al., 2007; Mostofsky et al., 2006), children in the ASD group committed a significantly higher number of errors on the praxis examination, F(1, 85) = 58.343, p < .0001, than the children in the TD group. Further analyses revealed that children in the ASD group performed significantly worse than controls on all three sections of the praxis examination: GTC (total errors), F(1, 85) = 43.129, p <.0001; GTI (total errors), F(1, 85) = 32.659, p <.0001; and GTU (total errors), F(1, 85) = 48.571, p < .0001. Children diagnosed with AS and HFA demonstrated similar performance on the PKT, F(1, 35) = 0.066, p = .799, supporting the decision to collapse the AS and HFA groups into one ASD group.
Table 2.
Analysis of Variance of Group Performance on Motor Assessments
| Assessment | Autism | TD | df | F | p | η2 | ||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||||
| PANESS (Total Timed Repetitive Movements) | 37.7 | 8.07 | 32.4 | 5.80 | 1 | 12.545** | 0.001 | 0.129 |
| PKT (Total Correct) | 28.3 | 2.73 | 29.5 | 2.49 | 1 | 4.626* | 0.034 | 0.052 |
| Praxis (Average Total Errors) | 26.2 | 11.9 | 12.1 | 4.68 | 1 | 58.343** | <.001 | 0.407 |
Note: p<.05
p<.01; TD = Typically developing group; PANESS = Physical and Neurological Assessment of Subtle Signs; PKT = Postural Knowledge Test
Prediction of Praxis Scores
Results of the hierarchical regression analyses predicting praxis scores are listed in Table 3. Age was a significant predictor of praxis performance (ΔR2 = .058, p = .024); however, PRI was not (ΔR2 = .022, p = .164). After accounting for age and PRI, basic motor skill (ΔR2 = .138, p <.0001), postural knowledge (ΔR2 = .119, p < .0001), and diagnosis (ΔR2 = .214, p < .0001) all contributed significant proportions of unique variance to prediction of praxis performance. Correlation statistics among the five variables in the regression are listed in Table 4.
Table 3.
Hierarchical Regression Examining Contribution to Praxis Performance
| Predictor | B | SE B | β | ΔR2 | ΔF |
|---|---|---|---|---|---|
| Age | −1.755 | .766 | −0.241* | 0.058 | 5.246 |
| PRI | −.121 | .087 | −0.147 | 0.022 | 1.968 |
| PANESS (Total Timed Repetitive Movements) | .666 | .174 | 0.444** | 0.138 | 14.629 |
| PKT (Total Correct) | −1.572 | .409 | −0.38** | 0.119 | 14.759 |
| Diagnosis | 11.238 | 1.812 | 0.51** | 0.214 | 38.469 |
Note: p<.05
p<.01; PRI = WISC-IV Perceptual Reasoning Index; PANESS = Physical and Neurological Assessment of Subtle Signs; PKT = Postural Knowledge Test
Table 4.
Intercorrelations Between Factors Contributing to Praxis Performance
| 1. | 2. | 3. | 4. | 5. | |
|---|---|---|---|---|---|
| 1. Age | – | ||||
| 2. PRI | −.021 | – | |||
| 3. Total Timed Rep. | −.490** | −.232* | – | ||
| 4. PKT Total Correct | .373** | .138 | −.308** | – | |
| 5. Diagnosis | −.094 | .009 | .359** | −.227* | – |
Note. p<.05
p<.01; PRI = WISC-IV Perceptual Reasoning Index; Total Timed Rep. = Total Timed Repetitive Movements from PANESS; PKT = Postural Knowledge Test.
Relationship between ADOS and Praxis Scores
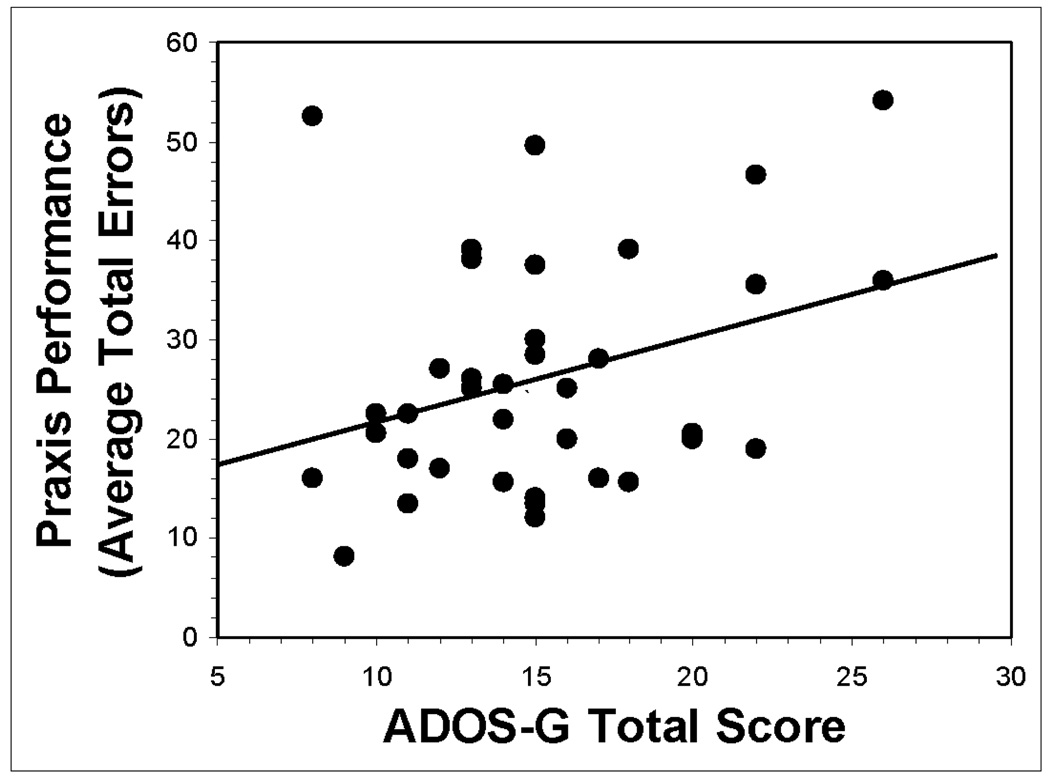
Within the ASD group, results of a linear regression revealed that praxis performance was significantly associated with the ADOS-G Total Score, which is the sum of three scores in areas of Communication, Reciprocal Social Interaction, and Stereotyped and Restricted Behaviors, R = .327, p = .049. Follow-up hierarchical regression reveals that this association remains significant after accounting for basic motor skill and postural knowledge, ΔR2 = 0.184, p = 0.01 (see Figure 1).
Figure 1.
Plot showing significant correlation (R = .327, p = .049) between praxis performance (average total errors) and total scores from the Autism Diagnostic Observation Schedule – Generic (ADOS-G) in 37 children with ASD. This correlation remains significant (ΔR2 = .184, p = .01) after accounting for PANESS (total timed repetitive movements) and Postural Knowledge Test (total correct) performance.
Discussion
Consistent with prior studies (Dziuk et al., 2007), children with ASD show substantial impairments on praxis examination, with deficits observed not only in the performance of gestures to imitation, but also gestures to command and gestures with actual tool use. In addition, while results indicate that children with ASD demonstrate deficits in postural knowledge (as well as basic motor execution), these deficits do not entirely account for the observed dyspraxia in ASD. Both measures of basic motor skill and postural knowledge were significant predictors of praxis performance. Nevertheless, the HFA group continued to show significantly poorer praxis than controls after accounting for these abilities. Furthermore, consistent with prior findings (Dziuk et al., 2007), performance on the praxis examination was correlated with the behavioral characteristics of autism measured by the ADOS-G. Additionally, this correlation remained significant after accounting for the effects of basic motor functioning and postural knowledge.
In children with ASD, the associations among dyspraxia, basic motor skills, and postural knowledge suggest dysfunction within motor circuits important for execution as well as parietal regions important for representational knowledge of gestures. However, there remains a robust statistical effect of diagnosis even after accounting for basic motor skill and postural knowledge, which suggests additional contributions from circuits important for selection and transcoding of spatial representations into motor actions necessary to execute the skilled gesture. The premotor cortex (PMC) serves as a relay point that houses the relationship between sensory information (both spatial and sequential) and the motor command for a particular movement (see Halsband & Lange, 2006, for review). Dysfunction due to abnormalities of the structure or function of the PMC (particularly the dorsal PMC, which appears to be responsible for selection of movements based on external visual cues; Schluter, Rushworth, Passingham, & Mills, 1998) may indeed contribute to impaired praxis performance in ASD. Further studies, perhaps utilizing functional imaging, should be helpful in clarifying the role of the premotor cortex in dyspraxia in autism. For example, given the role of the PMC (particularly the supplementary motor area) in motor planning and sequencing (see review in Nachev, Kennard, & Husain, 2008), simple sequencing tasks (such as those administered during the PANESS that require alternating movement of the fingers, hands, and feet) would provide an elementary assessment of one role of the premotor cortex. In addition, given the association between intra-individual variability and supplementary motor circuits (Suskauer et al., 2008), a task involving response selection and inhibition (such as a simple go-no-go paradigm) may provide a method of assessing this critical role of the PMC.
Alternatively, or perhaps additionally, the combined contributions of parietal, premotor, and motor systems to dyspraxia in autism suggest that abnormalities in connectivity between these regions may contribute to difficulties with acquisition and performance of skilled gestures. There is accumulating evidence that autism is associated with anomalous white matter connectivity (see Minshew & Williams, 2007 for a review). Examination of postmortem tissue from individuals with autism reveals an abundance of short relative to long connective fibers in frontal and temporal regions (Casanova, Buxhoeveden, Switala, & Roy, 2002). Similarly, neuroimaging studies of autism have revealed increased volume of radiate (immediately subcortical) white matter, which is principally comprised of short U-fibers (Herbert et al., 2004) and decreased size in the corpus callosum (see Stanfield et al., 2008, for a review) comprising distant connections. Furthermore, these differences in white matter volume appear to have functional relevance; in children with autism, increased radiate white matter volume in the primary motor cortex was found to be a robust predictor of deficits in basic motor skill (Mostofsky, Burgess, & Gidley Larson, 2007). Future studies should aim to explore whether impairments in performance of skilled motor gestures (impairments in imitation and dyspraxia) are associated with abnormalities in parietal-frontal connectivity; techniques assessing anatomic connectivity (anatomic MRI and diffusion tensor imaging) and functional connectivity (fMRI) would help to address this question.
It is acknowledged that the above formulations are based on adult lesion-based models of deficient praxis performance (in which the individual loses skills after a focal lesion). These models may not be as appropriate as a developmental perspective. Rather, it may be more suitable to conceptualize dyspraxia in autism as anomalous development resulting in impaired acquisition (learning) of motor skills, which also depend on long-range cortical and subcortical connectivity. Impaired motor skill learning, which has been shown in autism (Gidley Larson & Mostofsky, 2008; Mostofsky, Goldberg, Landa, & Denckla, 2000), may lead to anomalous formation of spatial and motor representations of skilled movements. Abnormalities in the lateral PMC, which have been shown to be activated during motor learning (Deiber et al., 1997; Inoue et al., 1997), would be consistent with this model. Future studies should address the associations between impairments in motor skill learning and dyspraxia in autism. For instance, investigating visually guided motor learning (assessed using a rotary pursuit or serial reaction time task), as well as somatosensory guided motor learning (using a maze tracing paradigm) may contribute to our understanding of the cognitive processes and related neural networks underlying dyspraxia in autism. In addition, it will be important to ascertain whether abnormal connectivity (with overgrowth of localized connections and dysfunctional distant connections) contributes to impaired procedural learning, particularly in autism. The authors acknowledge that while various processes involved of the completion of motor tasks (i.e., accessing postural knowledge, transcoding it into motor programs, motor execution, and motor learning) are often studied behaviorally in isolation, we recognize that in a developmental context, the neural systems underlying these functions overlap and may be difficult to disentangle.
The current findings, in combination with recent literature, lend further support to the contention that impairments in motor functioning are key players in a large constellation of associated features of ASD. Although there is some inconsistency in the literature regarding the reliability of motor examinations in the early identification of autism (Ozonoff et al., 2008), there is evidence that motor abnormalities may be detectable at an early age, particularly in the high-risk population and may be important to include in a comprehensive assessment (Brian et al., 2008). For instance, abnormal general movements (GMs) in infancy have been shown to be predictive of developmental disorders (Hadders-Algra, 2004) and there is evidence that GM assessments may be useful in the early identification of autism (Phagava et al, 2008). As motor skills are integral to imitation (Vanvuchelen, Roeyers, & de Weerdt, 2007), communication (such as the oromotor skills involved in speech and fine motor skills involved in sign language and the use of assistive communication devices; Gernsbacher, Sauer, Geye, Schweigert, & Goldsmith, 2008), and other skills with which children with ASD struggle, interventions targeted at motor impairments may be key in addressing core areas of impairment in autism. Further research into the efficacy of physical and occupational therapeutic interventions aimed at improving motor skills is warranted.
There are several limitations of this study that warrant mentioning. The Postural Knowledge Test was developed using adult participants (Mozaz et al., 2002); thus, there is a need for an instrument that assesses representational knowledge using more child-appropriate gestures. In addition, expanding the number of test items may improve the instrument’s utility in distinguishing postural knowledge skills between groups. In the present study, surprisingly, children with autism did not show impaired performance on the transitive gestures score of the PKT (although there appears to be a statistical trend toward children with autism demonstrating worse recognition of transitive gestures); a lack of statistical power may be a contributing factor to this finding. Additionally, although there was no evidence of visual perception impairments in this sample, they were not specifically assessed and should be considered in interpretation of the findings. Also, as the children in the current ASD sample were all high functioning (i.e., of at least average intelligence), the current findings and interpretations may not be applicable across the entire autism spectrum; future studies examining dyspraxia across a wider section of the spectrum are necessary. Furthermore, it may be more revealing in future studies to examine the ability of children with autism to interpret video representations of movement; in addition to eliminating the possible confound of limited perspectives in the illustrations of the PKT (some are frontal views, some peripheral), analysis of postural knowledge using video may reveal a contribution of temporal sequencing in postural knowledge, in addition to spatial configuration.
Acknowledgements
This research was funded by grants from the National Alliance for Autism Research/Autism Speaks and from NIH: R01 NS048527 (SHM), K02 NS044850 (SHM), HD-24061 (Developmental Disabilities Research Center) and the Johns Hopkins University School of Medicine Institute for Clinical and Translational Research, an NIH/NCRR CTSA Program, UL1-RR025005.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/neu.
Contributor Information
Lauren R. Dowell, Laboratory for Neurocognitive and Imaging Research, Kennedy Krieger Institute
E. Mark Mahone, Department of Neuropsychology, Kennedy Krieger Institute, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine
Stewart H. Mostofsky, Laboratory for Neurocognitive and Imaging Research, Kennedy Krieger Institute Departments of Neurology, Psychiatry, and Pediatrics, Johns Hopkins University School of Medicine
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed., text revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Blake R, Turner LM, Smoski MJ, Pozdol SL, Stone WL. Visual recognition of biological motion is impaired in children with autism. Psychological Science. 2003;14(2):151–157. doi: 10.1111/1467-9280.01434. [DOI] [PubMed] [Google Scholar]
- Brian J, Bryson SE, Garon N, Roberts W, Smith IM, Szatmari P, et al. Clinical assessment of autism in high-risk 18-month-olds. Autism. 2008;12(5):433–456. doi: 10.1177/1362361308094500. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Kyle K, Grossman M, Coslett HB. Left inferior parietal representations for skilled hand-object interactions: Evidence from stroke and corticobasal degeneration. Cortex. 2007;43(3):411–423. doi: 10.1016/s0010-9452(08)70466-0. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism. Neurology. 2002;58:428–432. doi: 10.1212/wnl.58.3.428. [DOI] [PubMed] [Google Scholar]
- Cattaneo L, Fabbri-Destro M, Boria S, Pieraccini C, Monti A, Cossu G, et al. Impairment of actions chains in autism and its possible role in intention understanding. Proceedings of the National Academy of Sciences. 2007;104(45):17825–17830. doi: 10.1073/pnas.0706273104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiber M-P, Wise SP, Honda M, Catalan MJ, Grafman J, Hallett M. Frontal and parietal networks for conditional motor learning: A positron emission tomography study. Journal of Neurophysiology. 1997;78:977–991. doi: 10.1152/jn.1997.78.2.977. [DOI] [PubMed] [Google Scholar]
- Denckla MB. Revised neurological examination for subtle signs. Psychopharmacology Bulletin. 1985;21(4):773–800. [PubMed] [Google Scholar]
- Dziuk MA, Gidley Larson JC, Apostu A, Mahone EM, Denckla MB, Mostofsky SH. Dyspraxia in autism: association with motor, social, and communicative deficits. Developmental Medicine & Child Neurology. 2007;49:734–739. doi: 10.1111/j.1469-8749.2007.00734.x. [DOI] [PubMed] [Google Scholar]
- Frey SH. What puts the how in where? Tool use and the divided visual streams hypothesis. Cortex. 2007;43(3):368–375. doi: 10.1016/s0010-9452(08)70462-3. [DOI] [PubMed] [Google Scholar]
- Gernsbacher MA, Sauer EA, Geye HM, Schweigert EK, Goldsmith HH. Infant and toddler oral-and manual-motor skills predict later speech fluency in autism. The Journal of Child Psychology and Psychiatry. 2008;49(1):43–50. doi: 10.1111/j.1469-7610.2007.01820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J, Appleton J, Appleton R. Dyspraxia or developmental coordination disorder? Unraveling the enigma. Archives of Disease in Childhood. 2007;92:534–539. doi: 10.1136/adc.2005.088054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidley Larson JC, Mostofsky SH. Motor deficits in autism. In: Tuchman R, Rapin I, editors. Autism: A Neurological Disorder of Early Brain Development. London: MacKeith Press; 2006. pp. 231–247. [Google Scholar]
- Gidley Larson JC, Mostofsky SH. Evidence that the pattern of visuomotor sequence learning is altered in children with autism. 2008 doi: 10.1002/aur.54. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadders-Algra M. General movements: A window for early identification of children at high risk for developmental disorders. The Journal of Pediatrics. 2004;145(2):S12–S18. doi: 10.1016/j.jpeds.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Halsband U, Lange RK. Motor learning in man: A review of functional and clinical studies. Journal of Physiology. 2006;99:414–424. doi: 10.1016/j.jphysparis.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Halsband U, Schmitt J, Weyers M, Binkofski F, Grützner G, Freund H-J. Recognition and imitation of pantomimed motor acts after unilateral parietal and premotor lesions: A perspective on apraxia. Neuropsychologia. 2001;39:200–216. doi: 10.1016/s0028-3932(00)00088-9. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Rothi LJG. Apraxia. In: Heilman K, Valenstein E, editors. Clinical Neuropsychology. 3rd Edition. New York: Oxford University Press; 1993. pp. 141–163. [Google Scholar]
- Heilman KM, Rothi LJ, Valenstein E. Two forms of ideomotor apraxia. Neurology. 1982;32(4):342–346. doi: 10.1212/wnl.32.4.342. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Makris N, Filipek PA, Kemper TL, Normandin JJ, et al. Localization of white matter volume increase in autism and developmental language disorder. Annals of Neurology. 2004;55(4):530–540. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- Hobson RP, Lee A. Imitation and identification in autism. Journal of Child Psychology and Psychiatry. 1999;40(4):649–659. [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. New Haven, Connecticut: Yale University; 1975. Unpublished manuscript, Department of Sociology. [Google Scholar]
- Inoue K, Kawashima R, Satoh K, Kinomura S, Goto R, Sugiura M, et al. Activity in the parietal area during visuomotor learning with optical rotation. NeuroReport. 1997;8:3979–3983. doi: 10.1097/00001756-199712220-00026. [DOI] [PubMed] [Google Scholar]
- Jansiewicz EM, Goldberg MC, Newschaffer CJ, Denckla MB, Landa R, Mostofsky SH. Motor signs distinguish children with high functioning autism and Asperger's syndrome from controls. Journal of Autism and Developmental Disorders. 2006;36:613–621. doi: 10.1007/s10803-006-0109-y. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Martineau J, Cochin S, Magne R, Barthelemy C. Impaired cortical activation in autistic children: Is the mirror neuron system involved? International Journal of Psychophysiology. 2008;68:35–40. doi: 10.1016/j.ijpsycho.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Milne E, Swettenham J, Hansen P, Campbell R, Jeffries H, Plaisted K. High motion coherence thresholds in children with autism. Journal of Child Psychology and Psychiatry. 2002;43(2):255–263. doi: 10.1111/1469-7610.00018. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Williams DL. The new neurobiology of autism. Archives of Neurology. 2007;64(7):945–950. doi: 10.1001/archneur.64.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Burgess MP, Gidley Larson JC. Increased motor cortex white matter volume predicts motor impairment in autism. Brain. 2007;130:2117–2122. doi: 10.1093/brain/awm129. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Dubey P, Jerath VK, Jansiewicz EM, Goldberg MC, Denckla MB. Developmental dyspraxia is not limited to imitation in children with autism spectrum disorders. Journal of the International Neuropsychological Society. 2006;12:314–326. doi: 10.1017/s1355617706060437. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Goldberg MC, Landa RJ, Denckla MB. Evidence for a deficit in procedural learning in children and adolescents with autism: Implications for cerebellar contribution. Journal of the International Neuropsychological Society. 2000;6:752–759. doi: 10.1017/s1355617700677020. [DOI] [PubMed] [Google Scholar]
- Mottron L. Matching strategies in cognitive research with individuals with high-functioning autism: Current practices, instrument biases, and recommendations. Journal of Autism and Developmental Disorders. 2004;34(1):19–27. doi: 10.1023/b:jadd.0000018070.88380.83. [DOI] [PubMed] [Google Scholar]
- Mozaz M, Rothi LJG, Anderson JM, Crucian GP, Heilman KM. Postural knowledge of transitive pantomimes and intransitive gestures. Journal of the International Neuropsychological Society. 2002;8:958–962. doi: 10.1017/s1355617702870114. [DOI] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nature Reviews Neuroscience. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Goldring S, Greiss-Hess L, Herrera AM, Steele J, et al. Gross motor development, movement abnormalities, and early identification of autism. Journal of Autism and Developmental Disorders. 2008;38:644–656. doi: 10.1007/s10803-007-0430-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicano E, Gibson L, Maybery M, Durkin K, Badcock DR. Abnormal global processing along the dorsal visual pathway in autism: A possible mechanism for weak visuospatial coherence? Neuropsychologia. 2005;43:1044–1053. doi: 10.1016/j.neuropsychologia.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Phagava H, Muratori F, Einspieler C, Maestro S, Apicella F, Guzzetta A, et al. General movements in infants with autism spectrum disorders. Georgian Medical News. 2008;156:100–105. [PubMed] [Google Scholar]
- Reich W, Welner Z, Herjanic B. North Tonawanda: Multi-Health Systems; 1997. The Diagnostic Interview for Children and Adolescents-IV. [Google Scholar]
- Rizzolatti G, Fabbri-Destro M. The mirror system and its role in social cognition. Current Opinion in Neurobiology. 2008;18:1–6. doi: 10.1016/j.conb.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Cognitive Brain Research. 1996;3:131–141. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Pennington BF. A theoretical approach to the deficits in infantile autism. Development and Psychopathology. 1991;3:137–162. [Google Scholar]
- Rothi GLJ, Raymer AM, Ochipa C, Maher LM, Greenwald MI, Heilman KM. Florida Apraxia Battery-Revised. 2003 [Google Scholar]
- Schluter ND, Rushworth MFS, Passingham RE, Mills KR. Temporary interference in human lateral premotor cortex suggests dominance for the selection of movements. Brain. 1998;121:785–799. doi: 10.1093/brain/121.5.785. [DOI] [PubMed] [Google Scholar]
- Sirigu A, Duhamel J-R, Cohen L, Pillon B, Dubois B, Agid Y. The mental representation of hand movements after parietal cortex damage. Science. 1996;273(5281):1564–1568. doi: 10.1126/science.273.5281.1564. [DOI] [PubMed] [Google Scholar]
- Spencer J, O′Brien J, Riggs K, Braddick O, Atkinson J, Wattam-Bell J. Motion processing in autism: Evidence for a dorsal stream deficiency. NeuroReport. 2000;11(12):2765–2767. doi: 10.1097/00001756-200008210-00031. [DOI] [PubMed] [Google Scholar]
- Stanfield AC, McIntosh AM, Spencer MD, Philip R, Gaur S, Lawrie SM. Towards a neuroanatomy of autism: A systematic review and meta-analysis of structural magnetic resonance imaging studies. European Psychiatry. 2008;23:289–299. doi: 10.1016/j.eurpsy.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Stieglitz Ham H, Corley M, Rajendran G, Carletta J, Swanson S. Brief report: Imitation of meaningless gestures in individuals with Asperger syndrome and high-functioning autism. Journal of Autism and Developmental Disorders. 2008;38:569–573. doi: 10.1007/s10803-007-0417-x. [DOI] [PubMed] [Google Scholar]
- Suskauer SJ, Simmonds DJ, Caffo BS, Denckla MB, Pekar JJ, Mostofsky SH. fMRI of intrasubject variability in ADHD: Anomalous premotor activity with prefrontal compensation. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(10):1141–1150. doi: 10.1097/CHI.0b013e3181825b1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takarae Y, Luna B, Minshew N, Sweeney JA. Patterns of visual sensory and sensorimotor abnormalities in autism vary in relation to history of early language delay. Journal of the International Neuropsychological Society. 2008;14:980–989. doi: 10.1017/S1355617708081277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanvuchelen M, Roeyers H, de Weerdt W. Nature of motor imitation problems in school-aged boys with autism. Autism. 2007;11(3):225–240. doi: 10.1177/1362361307076846. [DOI] [PubMed] [Google Scholar]
- Wechsler DL. Fourth Edition. San Antonio, TX: The Psychological Corporation; 2003. Wechsler Intelligence Scale for Children. [Google Scholar]
- Weiss PH, Rahbari NN, Hesse MD, Fink GR. Deficient sequencing of pantomimes in apraxia. Neurology. 2008;70:834–840. doi: 10.1212/01.wnl.0000297513.78593.dc. [DOI] [PubMed] [Google Scholar]
- Wheaton LA, Hallett M. Ideomotor apraxia: A review. Journal of the Neurological Sciences. 2007;260:1–10. doi: 10.1016/j.jns.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Williams JHG, Whiten A, Singh T. A systematic review of action imitation in autistic spectrum disorder. Journal of Autism and Developmental Disorders. 2004;34(3):285–299. doi: 10.1023/b:jadd.0000029551.56735.3a. [DOI] [PubMed] [Google Scholar]
- Williams JHG, Whiten A, Suddendorf T, Perrett DI. Imitation, mirror neurons and autism. Neuroscience and Biobehavioral Reviews. 2001;25:287–295. doi: 10.1016/s0149-7634(01)00014-8. [DOI] [PubMed] [Google Scholar]