Abstract
Objectives
To determine the metabolism of favipiravir (T-705, 6-fluoro-3-hydroxy-2-pyrazinecarboxamide) to its ribosylated, triphosphorylated form (T-705 RTP) in uninfected and influenza A/Duck/MN/1525/81 (H5N1) virus-infected cells. Effects of treatment on intracellular guanosine triphosphate (GTP) pools and influenza virus-inhibitory activity were also assessed.
Methods
A strong anion exchange HPLC separation method with UV detection was used to quantify T-705 RTP and GTP levels in Madin–Darby canine kidney cells. Antiviral activity was determined by virus yield reduction assay.
Results
Accumulation of T-705 RTP in uninfected cells increased linearly from 3 to 320 pmol/106 cells in cells exposed to 1–1000 µM extracellular T-705 for 24 h, approaching maximum levels by 9 h. Virus infection did not result in greater T-705 RTP accumulation compared with uninfected cells. Catabolism of T-705 RTP occurred after removal of T-705 from the extracellular medium, with a half-life of decay of 5.6 ± 0.6 h. Based upon these results, short-term incubation of T-705 with H5N1 virus-infected cells was predicted to provide an antiviral benefit. Indeed, 4–8 h 10–100 µM T-705 treatment of cells resulted in virus yield reductions, but less than continuous exposure. A 100-fold higher extracellular concentration of T-705 was required to inhibit intracellular GTP levels compared with ribavirin, which helps explain ribavirin's greater toxicity.
Conclusions
The favourable intracellular metabolic properties of T-705 combined with its reduced cell-inhibitory properties make this compound an attractive candidate for treating human influenza virus infections.
Keywords: antiviral, phosphorylation, guanosine triphosphate, RNA polymerase, ribavirin
Introduction
Favipiravir (T-705, 6-fluoro-3-hydroxy-2-pyrazinecarboxamide) is a broad-spectrum antiviral agent inhibiting diverse kinds of viruses, such as arenaviruses, bunyaviruses, flaviviruses, foot-and-mouth disease virus and influenza viruses, in cell culture and in animal model systems.1 The mode of action of the compound has been studied in influenza virus infections but not against other viruses. T-705 undergoes conversion into a nucleotide analogue, with monophosphate (T-705 RMP) and triphosphate (T-705 RTP) derivatives detected in cell lysates when using radioactive T-705 as a probe.2 The diphosphate form (T-705 RDP) is below the limit of detection, however. Conversion of T-705 into T-705 RMP occurs in one step via hypoxanthine-guanine phosphoribosyltransferase (H. Egawa, K. Takahashi, T. Kadota and Y. Furuta, unpublished results), whereas enzymes responsible for conversion of T-705 RMP into di- and triphosphate forms have not been identified. T-705 RTP is inhibitory to influenza virus RNA polymerase.2 Overall, its activity is similar to that of ribavirin, since ribavirin also undergoes conversion into a triphosphorylated form3–5 that inhibits influenza virus RNA polymererase.2,6 Ribavirin monophosphate inhibits the cellular enzyme inosine monophosphate dehydrogenase (IMPDH),2,3 resulting in reductions in intracellular guanosine triphosphate (GTP) pools.3,5,7 This effect leads to cell cytostasis8 and other manifestations of cytotoxicity,7 and likely contributes to the toxic effects of ribavirin in animals and humans. In contrast, T-705 RMP is only weakly inhibitory to IMPDH in enzymatic assays;2 thus, may prove to be less toxic to humans. The effects of T-705 on intracellular GTP pools have not been reported. Both compounds possess a carboxamide moiety that makes them appear like purines to cellular enzymes, even though their single-ring structures are more similar to pyrimidines. A reversal (antagonism) of antiviral activity occurs in cell culture when T-705 or ribavirin is co-incubated with certain purines,2,3 confirming their purine-like nature. T-705 RTP and ribavirin triphosphate are competitive with GTP (a purine ribonucleotide) in influenza virus RNA polymerase assays.2,6
The metabolism of ribavirin to mono-, di- and triphosphate forms has been investigated in uninfected cells and in cells infected with respiratory syncytial virus.4 Preliminary studies of T-705 phosphorylation in uninfected cells have been reported,2 but they are not quantitative in nature. In the present report, the metabolism and catabolism of T-705 in uninfected and influenza A (H5N1) virus-infected cells was investigated. A strong anion exchange (SAX) HPLC method coupled with UV spectrophotometry was used to separate and detect T-705 RTP in crude cell extracts. T-705 RTP co-migrated with ATP on the SAX column. However, because the UV absorbance spectrum of T-705 differs from that of ATP (360 versus 254 nm) and the other natural nucleoside triphosphates, this allowed detection of T-705 RTP without interference from ATP. Because of this, radioactive T-705 was not required to conduct these studies. The results indicate that T-705 underwent efficient concentration-dependent conversion into T-705 RTP, which then catabolized slowly after T-705 was removed from the extracellular medium.
Materials and methods
Antiviral compounds
T-705 and T-705 RTP were prepared by Toyama Chemical Company (Toyama, Japan). Ribavirin was obtained from ICN Pharmaceuticals (Costa Mesa, CA, USA). T-705 and ribavirin were dissolved in cell culture medium for testing. T-705 RTP was used only as a UV standard for equating integrated peak areas from a UV tracing to calculate T-705 RTP levels.
Virus and cells
The low pathogenicity North American influenza A/Duck/MN/1525/81 (H5N1) virus was provided by Dr Robert Webster (St Jude Children's Research Hospital, Memphis, TN, USA). The virus was replicated in Madin–Darby canine kidney (MDCK) cells. The cells were obtained from the American Type Culture Collection (Manassas, VA, USA), and were propagated in Eagle's medium supplemented with 10% fetal bovine serum and 0.22% sodium bicarbonate.
T-705 phosphorylation and HPLC analysis
T-705 metabolism and T-705 RTP catabolism experiments were performed with confluent monolayers of MDCK cells in T-25 flasks. One flask was used for each concentration of compound and contained between 6 × 106 and 8 × 106 cells, depending upon the experiment. T-705, at various half-log10 concentrations in Eagle's medium (containing 50 mg/L gentamicin and 0.22% sodium bicarbonate, but devoid of serum) was incubated with the cells for various lengths of time. At the end of the incubation period, the medium was aspirated from the flasks. Each flask was treated with 540 µL of 3.5% perchloric acid at 4°C, and incubated for 5 min under refrigeration. Next, 270 µL of a solution of 1 N KOH/1 M imidazole was added to neutralize the sample. Finally, the liquid contents of each flask were collected and frozen at –80°C until analysis for T-705 RTP concentration.
HPLC analysis of the samples was conducted as follows. Chromatographic separations were performed using an HPLC apparatus (Waters Corp., Milford, MA, USA) fitted with a 10 × 250 mm column packed with Whatman partisil SAX resin (Phenomenex Inc., Torrance, CA, USA). A linear gradient from 10 mM to 1 M ammonium phosphate (Sigma-Aldrich, St Louis, MO, USA) at pH 3.5 was run over 30 min at 1 mL/min. The 1 M buffer was run for an additional 10 min before re-equilibrating the column in low-salt buffer. We have previously published similar separation methods using SAX HPLC for studying nucleoside triphosphate accumulation in cells.4,5,9,10 Detection and quantification of T-705 RTP was made at 360 nm with a Waters LC Spectrophotometer. Sensitivity of the UV detector was set for full scale of 0.01 absorbance units. Baseline drift was too great below this level of sensitivity. In general, 500 µL samples from each flask were analysed, except from cells treated with 1000 µM T-705 that contained high levels of T-705 RTP (200 µL samples were analysed). Using this method, T-705 RTP eluted at ∼26 min in a region of no interfering peaks.
Estimation of T-705 RTP intracellular concentration was made based upon the absorbance of comparable standard micromolar solutions of T-705 and T-705 RTP at 360 nm. This gave a T-705:T-705 RTP optical density ratio of 1:3.8. This factor was used to convert T-705 RTP peak areas obtained from integrated HPLC tracings into picomoles (pmol) of compound. The number of cells in an untreated flask was determined by trypsinization and haemocytometer counting at the end of experiments in order to calculate pmol of T-705 RTP per 106 cells. The lower limit of detection, based upon baseline drift, was about 2 pmol/106 cells. In these studies this meant treatment of cells with 1 µM T-705. Greater accuracy at this level of detection can be achieved by assaying crude cell extracts from a larger number of treated cells, such as from a T-75 flask.
The half-life of T-705 RTP in cells following removal of compound from the extracellular medium was calculated using cells that were treated with T-705 for 24 h in order to accumulate T-705 RTP. Half-life calculations were made for each concentration of extracellular treatment using data starting at the 2 h timepoint. Calculations were performed by plotting T-705 RTP concentrations on a log10 scale versus time plotted on linear scale.
T-705 phosphorylation in infected cells
Certain studies were performed with influenza A/Duck/MN/1525/81 (H5N1) virus-infected MDCK cells compared with uninfected cells. For these studies, the virus infectious dose was approximately one infectious particle per cell [or a multiplicity of infection (moi) of 1]. After adsorbing virus for 1 h, the infecting medium was replaced with medium containing T-705 at a concentration of 32 µM. The medium used for these studies was the same as for uninfected cells, with the addition of 10 units/mL trypsin and 1 µg/mL EDTA to promote virus replication. Uninfected flasks of cells for these comparative studies contained the same trypsin/EDTA medium. Cell lysate samples were processed for HPLC analysis as described above.
T-705 antiviral experiment
Antiviral experiments were conducted to determine the effects of short-term incubation of T-705 on influenza A/Duck/MN/1525/81 (H5N1) virus production in cells. Confluent monolayers of MDCK cells in 24-well microplates were treated with compound in half-log10 dilutions (two microwells per dilution at each timepoint), followed 5 min later by infection with the virus at ∼1 moi. Studies were performed using the virus replication medium described above. At various times after infection, certain wells had the medium removed. The cells were rinsed once with fresh medium, and then medium devoid of T-705 was applied. At 24 h, the medium from duplicate wells was pooled, and the samples were frozen at −80°C. At a later time, the virus concentration in each sample was determined by endpoint dilution in newly prepared 96-well microplates of MDCK cells. Titrations were made in 10-fold dilution increments, using four microwells per dilution. Calculations of virus titre in 50% cell culture infectious dose (CCID50)/0.1 mL units were made using the method of Reed and Muench.11 Concentrations of compound required to reduce virus titre by 90% (1 log10) were estimated by plotting extracellular concentration versus log of virus titre on semi-log10 paper.
Methods for studying effects of compounds on intracellular GTP pools
Uninfected MDCK cells in T-25 flasks were treated with each compound at several doses for 24 h. The cell lysates were processed for HPLC analysis as described above, but at 254 nm UV wavelength to detect GTP. GTP eluted from the SAX column at ∼30 min. GTP peak areas from treated cultures were converted into percentages of the untreated control GTP levels (set at 100%).
Results
T-705 RTP detection in cells by the UV method
In previous studies, the use of radioactive compound was required for detection because the phosphorylated metabolites were either too low in concentration and/or co-migrated with natural nucleotides present in cells,4,5,9,10 thus making detection by UV spectroscopy coupled with the HPLC method impossible. However, T-705 differs from natural nucleosides and nucleoside analogues because it absorbs at 360 rather than 254 nm. At 360 nm, ATP and GTP were not detectable. In cells treated with T-705 for 24 h, a peak corresponding to T-705 RTP eluted at 26 min. T-705 RTP eluted in a region where there were no interfering peaks (at 360 nm), although ATP (detectable at 254 nm) co-migrated with T-705 RTP. T-705 RMP could not be distinguished by this method due to interfering peaks in the region where it would elute, and T-705 RDP was too low in concentration to be detected, which is in agreement with other reported findings.2
Concentration-dependent accumulation of T-705 RTP in cells
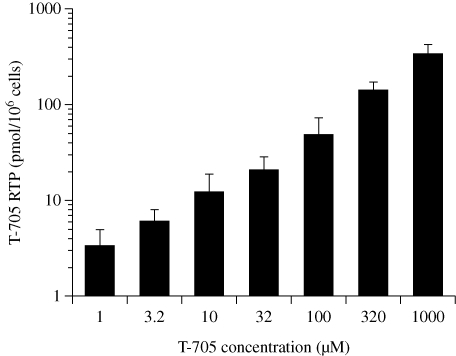
An experiment was conducted to determine levels of T-705 RTP in uninfected MDCK cells treated with extracellular concentrations of 1–1000 µM T-705 for 24 h (Figure 1). A linear relationship between extracellular T-705 concentration and intracellular levels of T-705 RTP was obtained when the data were plotted on a log10 scale. The 1 µM concentration of T-705 resulted in ∼3 pmol of T-705 RTP/106 cells, and the 1000 µM concentration resulted in ∼320 pmol/106 cells. UV background fluctuations at baseline prevented lower concentrations of T-705 RTP from being accurately measured.
Figure 1.
Dose-dependent effects of extracellular T-705 concentration on T-705 RTP accumulation in uninfected MDCK cells. Cells were incubated with compound for 24 h; then cell lysate samples were obtained for HPLC analysis of T-705 RTP levels. Data points are mean values ± SD (n = three independent experiments).
Time course of T-705 RTP formation
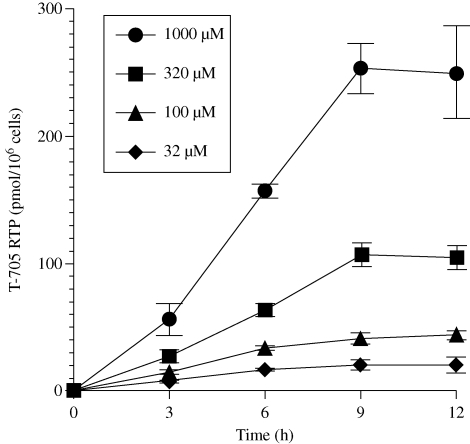
Uninfected MDCK cells were exposed to T-705, and the accumulation of T-705 RTP was assessed at different times for 12 h (Figure 2). Rapid accumulation of T-705 RTP that depended upon extracellular T-705 concentration was observed. At each concentration, T-705 RTP concentration approached a maximum level by 9 h. At that time, the levels of triphosphate were similar to those measured at 24 h (Figure 1).
Figure 2.
Time course of T-705 RTP production during treatment of uninfected cells with 32-1000 µM extracellular concentrations of T-705 for up to 12 h. Cells were incubated with compound for various times, and cell lysate samples were obtained for HPLC analysis of T-705 RTP levels. Data points are mean values ± SD (n = two independent experiments).
Effect of virus infection on phosphorylation
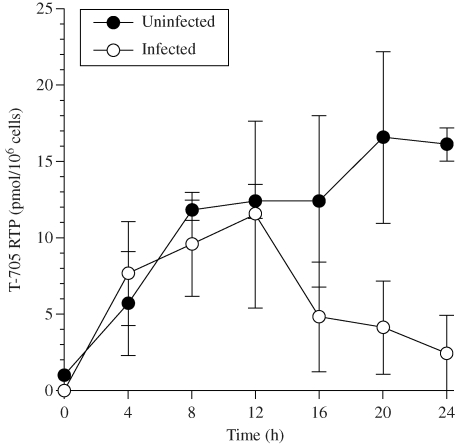
The anabolism of T-705 RTP in uninfected cells and in cells infected with influenza A/Duck/MN/1525/81 (H5N1) virus is shown in Figure 3. Similar levels of triphosphate were formed in uninfected and infected cells during the first 12 h. From 16 to 24 h, there were declines in T-705 RTP levels in infected cells, whereas small increases in triphosphate were observed between 16 and 24 h in uninfected cells. The steady decline in T-705 RTP in infected cells was associated with increasing virus-induced cytopathology. At the high moi, the compound at 32 µM was not able to combat the deleterious effects of virus replication that resulted in cell lysis.
Figure 3.
Formation of T-705 RTP over 24 h in uninfected cells or in cells infected with influenza A/Duck/MN/1525/81 (H5N1) virus. Cells were treated with 32 µM T-705 starting after 1 h of virus adsorption (time 0 on the figure). The infecting virus dose was approximately 1 CCID50/cell. Cell lysate samples were obtained for HPLC analysis of T-705 RTP levels. Data points are mean values ± SD (n = three independent experiments). Calculations (pmol/106 cells) were based upon the starting number of live cells in the flasks, and were not adjusted to account for cell degradation and death later on as a result of the virus infection.
Catabolism of T-705 RTP following removal of extracellular T-705
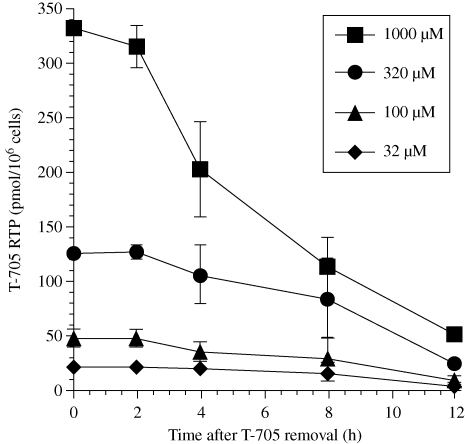
Cells were exposed to T-705 for 24 h to accumulate T-705 RTP. The catabolism of T-705 RTP was monitored for 12 h following removal of T-705 from the medium (Figure 4). At time 0 (corresponding to maximum accumulation of triphosphate at 24 h), a concentration-dependent amount of T-705 RTP ranging from 20 to 330 pmol/106 cells for treatments of 32–1000 µM was detected in cells. The amount of triphosphate in cells remained relatively constant between 0 and 2 h after removal of T-705 from the medium, with minimal losses in T-705 RTP detected. Decline in triphosphate levels was progressive after 2 h. The time to a 50% decrease in intracellular T-705 RTP (the half-life of the compound) was calculated for each curve of extracellular T-705 concentration. Half-life values of T-705 RTP catabolism [calculated from when the decline in concentration began (at 2 h)] from cells initially treated with T-705 at 32, 100, 320 and 1000 µM were 5.7, 6.0, 6.0 and 4.8 h, respectively, giving a mean value and standard deviation of 5.6 ± 0.6 h. The overall half-life of T-705 RTP in cells is longer, based upon the lag time between 0 and 2 h before the decline in concentration occurred.
Figure 4.
Catabolism of T-705 RTP following removal of T-705 from the extracellular medium. Cells were incubated with T-705 at 32–1000 µM for 24 h (time 0 on the figure). Then the medium was removed and replaced with medium free of T-705. Cell lysate samples were taken at different timepoints for HPLC analysis of T-705 RTP levels. Data points are mean values ± SD (n = two independent experiments).
Effect of short-term treatment of cells on antiviral activity
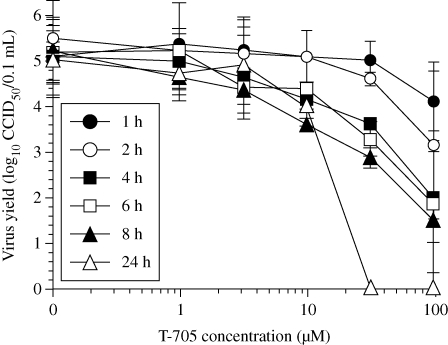
Based upon the persistence of T-705 RTP in cells for nearly 6 h following removal of compound from the extracellular medium, it was anticipated that short-term treatment of cells with T-705 would result in antiviral activity. To test this hypothesis, infected MDCK cells were treated with compound for 1 to 8 h compared with a 24 h (continuous) incubation. Virus titres were assessed from samples harvested at 24 h. The results indicate that virus incubation times as short as 4 h with 10–100 µM concentrations caused reductions in influenza virus titres (Figure 5). The highest (100 µM) concentration caused the greatest degree of inhibition, as was expected. At this concentration, even the 2 h incubation with T-705 resulted in a moderate suppression of virus titre. Interestingly, the 10 µM treatments for 4, 6 and 8 h were as inhibitory as the same concentration applied for 24 h. The calculated concentrations required to inhibit virus production by 90% (90% effective concentration or EC90 value) for treatments ranging from 4 to 24 h in duration were 8–10 µM. In contrast, 90% inhibition of virus production for 1 and 2 h incubation times required 45 and 95 µM concentrations of the inhibitor, respectively. The 32 and 100 µM treatments were much more effective at reducing virus titre when sustained for 24 h, compared with shorter incubation times.
Figure 5.
Effects of short-term incubation of cells with T-705 on influenza A/Duck/MN/1525/81 (H5N1) virus production. After infection of cells with a virus challenge dose of ∼1 CCID50/cell for 1 h, the cells were incubated with compound for various lengths of time ranging from 1 to 24 h. Fresh medium devoid of T-705 was applied to cells exposed to compound only for short periods of time [1–8 h, compared with continuous (24 h) treatment]. Extracellular virus yields were determined from all samples collected at 24 h. Data points are mean values ± SD (n = two independent experiments).
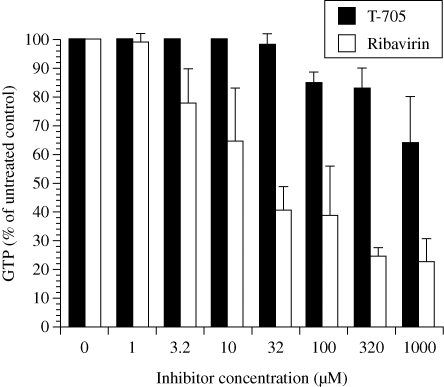
T-705 and ribavirin effects on intracellular GTP pools
The effects of T-705 and ribavirin on GTP pool levels were determined in treated cells (Figure 6). Ribavirin was more suppressive to GTP pools than T-705 at equimolar concentrations. The degree of GTP inhibition caused by 10 µM ribavirin was the same as that exhibited by 1000 µM T-705, indicating that ribavirin was 100-fold more potent than T-705 at suppressing GTP levels. The negative consequences of GTP pool suppression on cell viability have previously been discussed for ribavirin.7
Figure 6.
Effects of treatment with T-705 and ribavirin on intracellular GTP pool levels in uninfected MDCK cells. Treatments were for 24 h, followed by HPLC analysis of GTP levels. Data points are mean values ± SD (n = two independent experiments).
Discussion
A SAX HPLC method was developed for studying T-705 RTP formation in uninfected and influenza virus-infected cells, made possible by the favourable UV absorbance profile of the molecule. Studies were performed without the use of radioactive T-705, resulting in considerably reduced costs, rapid analysis of samples and a greater number of replicate samples that could be tested. Using this methodology, it may be possible to determine T-705 RTP levels in tissues obtained from animals.
T-705 RTP co-migrated with ATP on the SAX column, but ATP was invisible at 360 nm. Thus, it did not interfere with detection of T-705 RTP. The SAX HPLC method does not resolve T-705 RMP or T-705 RDP, but these molecules are of lesser significance since it is T-705 RTP that inhibits the viral RNA polymerase.2
The results of phosphorylation studies demonstrate that T-705 is efficiently converted into T-705 RTP in cell culture in a concentration-dependent manner. Ribavirin is also converted in a similar manner into its triphosphate form in various cell lines.3–5 A comparison of those studies with the present one indicates that ribavirin phosphorylates to higher levels in cells than T-705. Since T-705 is a more potent inhibitor of influenza viruses than ribavirin in cell culture,12,13 the data imply that T-705 RTP is more active than ribavirin triphosphate at inhibiting the influenza virus RNA polymerase. This was confirmed in polymerase assays performed by Furuta et al.2
T-705 RTP was shown to accumulate in MDCK cells until it approached a maximum level by 9 h (Figure 2), with some additional accumulation occurring up to 24 h in other studies (Figure 3). Similarly, ribavirin triphosphate levels in uninfected monkey kidney cells peaked at 8 h.4 T-705 RTP levels were not increased by infection with influenza virus. This indicates that the virus encodes no enzymes and does not stimulate cellular enzymes to increase intracellular T-705 RTP levels. To date, studies of ribavirin phosphorylation in MDCK cells have not been reported. Ribavirin triphosphate levels were elevated in respiratory syncytial virus-infected cells compared with uninfected cells.4
Following removal of T-705 from the cell culture medium the catabolism of T-705 RTP was slow (with a half-life of 5.6 ± 0.6 h). In contrast, degradation of ribavirin triphosphate in monkey kidney cells was rapid, with a half-life of 70 min.4 Because of the slow degradation of T-705 RTP in MDCK cells, we predicted that short exposure times of T-705 with cells would produce an antiviral effect. This indeed was demonstrated (Figure 5), although the potency of inhibition was not as great as occurred with continuous exposure. The effect seen may not be due purely to persistence of T-705 RTP for a period of time, but could also be related to the impact of treatment on the early phase of virus replication that reduces virus titre at 24 h. Nevertheless, the slow degradation of T-705 RTP is favourable for treatment of animals wherein rapid clearance of T-705 from the blood will not mean rapid clearance of T-705 RTP from the cells. Studies using the A549 human cell line indicate that T-705's catabolic half-life is more rapid (∼2 h) (H. Egawa, K. Takahashi, T. Kadota and Y. Furuta, ubpublished results). Thus, catabolism may vary with animal species and/or cell type.
T-705 RMP was shown to be less inhibitory to cellular IMPDH than ribavirin monophosphate.2 This effect translated into less potent suppression of intracellular GTP pools than occurred with ribavirin (Figure 6). This may result in better tolerance of T-705 compared with ribavirin in animals. In support of this idea, we were able to safely treat mice with high doses of T-705 (up to 600 mg/kg/day)13 compared with using ribavirin up to 75 mg/kg/day to avoid toxicity.
The metabolism of T-705 to T-705 RTP and the persistence of the triphosphate in cells for a period of time after extracellular clearance of T-705 appear favourable for treatment of infected animals. Additionally, T-705 is a more potent inhibitor of influenza virus replication than ribavirin and is less cell inhibitory. These beneficial properties make T-705 an attractive candidate for treating human influenza virus infections.
Funding
This work was supported by contract NO1-AI-30048 from the Virology Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA.
Transparency declarations
None to declare.
Disclaimer
The contents of this article do not necessarily reflect the position or policy of the government and no official endorsement should be inferred.
References
- 1.Furuta Y, Takahashi K, Shiraki K, et al. T-705 (favipiravir) and related compounds: novel broad-spectrum inhibitors of RNA viral infections. Antiviral Res. 2009;82:95–102. doi: 10.1016/j.antiviral.2009.02.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furuta Y, Takahashi K, Kuno-Maekawa M, et al. Mechanism of action of T-705 against influenza virus. Antimicrob Agents Chemother. 2005;49:981–6. doi: 10.1128/AAC.49.3.981-986.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Streeter DG, Witkowski JT, Khare GP, et al. Mechanism of action of 1-β-D-ribofuranosyl-1,2,4-triazole-3-carboxamide (Virazole), a new broad spectrum antiviral agent. Proc Natl Acad Sci USA. 1973;70:1174–8. doi: 10.1073/pnas.70.4.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smee DF, Matthews TR. Metabolism of ribavirin in respiratory syncytial virus-infected and uninfected cells. Antimicrob Agents Chemother. 1986;30:117–21. doi: 10.1128/aac.30.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smee DF, Bray M, Huggins JW. Antiviral activity and mode of action studies of ribavirin and mycophenolic acid against orthopoxviruses in vitro. Antivir Chem Chemother. 2001;12:327–35. doi: 10.1177/095632020101200602. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson B, Helgstrand E, Johannson NG, et al. Inhibition of influenza virus ribonucleic acid polymerase by ribavirin triphosphate. Antimicrob Agents Chemother. 1977;11:946–51. doi: 10.1128/aac.11.6.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lowe JK, Brox L, Henderson JF. Consequences of inhibition of guanine nucleotide synthesis by mycophenolic acid and Virazole. Cancer Res. 1977;37:736–43. [PubMed] [Google Scholar]
- 8.Müller WEG, Maidhof A, Taschner H, et al. Virazole (1-β-D-ribofuranosyl-1,2,4-triazole-3-carboxamide), a cytostatic agent. Biochem Pharmacol. 1977;26:1071–5. doi: 10.1016/0006-2952(77)90246-5. [DOI] [PubMed] [Google Scholar]
- 9.Smee DF, Bray M, Huggins JW. Intracellular phosphorylation of carbocyclic 3-deazaadenosine, an anti-Ebola virus agent. Antivir Chem Chemother. 2001;12:251–8. doi: 10.1177/095632020101200406. [DOI] [PubMed] [Google Scholar]
- 10.Smee DF, Humphreys DE, Hurst BL, et al. Antiviral activities and phosphorylation of 5-halo-2′-deoxyuridines and N-methanocarbathymidine in cells infected with vaccinia virus. Antivir Chem Chemother. 2008;19:15–24. doi: 10.1177/095632020801900103. [DOI] [PubMed] [Google Scholar]
- 11.Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–7. [Google Scholar]
- 12.Furuta Y, Takahashi K, Fukuda Y, et al. In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob Agents Chemother. 2002;46:977–81. doi: 10.1128/AAC.46.4.977-981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sidwell RW, Barnard DL, Day CW, et al. Efficacy of orally administered T-705 on lethal avian influenza A (H5N1) virus infections in mice. Antimicrob Agents Chemother. 2007;51:845–51. doi: 10.1128/AAC.01051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]