Abstract
Objectives
To compare the in vitro and in vivo activities of a 4:1 (w/w) fosfomycin/tobramycin combination (FTI) with those of fosfomycin and tobramycin alone against cystic fibrosis (CF) and non-CF bronchiectasis pathogens.
Methods
Clinical isolates of CF Pseudomonas aeruginosa, Staphylococcus aureus, Haemophilus influenzae, Stenotrophomonas maltophilia, Burkholderia cepacia complex, Escherichia coli and Klebsiellia spp. were evaluated by MIC, MBC, post-antibiotic effect (PAE), synergy, time–kill, a rat pneumonia model and spontaneous mutation frequency (SMF).
Results
FTI showed high activity against E. coli, H. influenzae, S. aureus and Klebsiella spp. For the S. aureus strains, 75% of which were methicillin resistant (MRSA), FTI had a lower MIC90 than tobramycin. For P. aeruginosa, FTI had a lower MIC90 than fosfomycin, but tobramycin was more active than either. Synergy studies showed no antagonism between fosfomycin and tobramycin, and 93% of the isolates demonstrated no interaction. FTI was rapidly bactericidal and exhibited concentration-dependent killing in time–kill studies. In the rat pneumonia model, FTI and tobramycin demonstrated bactericidal killing of P. aeruginosa; both were more active than fosfomycin alone. The SMF for S. aureus resistance to FTI was 2–4 log10 lower than that for tobramycin and 2–7 log10 lower than that for fosfomycin. For P. aeruginosa, the FTI SMF was 2–3 log10 lower than that for fosfomycin and 1–2 log10 lower than that for tobramycin.
Conclusions
FTI is a broad-spectrum antibiotic combination with high activity in vitro and in vivo. These data suggest FTI could be a potential treatment for respiratory infections caused by Gram-positive and Gram-negative aerobic bacteria.
Keywords: P. aeruginosa, S. aureus, respiratory infections
Introduction
Cystic fibrosis (CF) and non-CF bronchiectasis patients are predisposed to chronic respiratory infections caused by a variety of bacteria including Pseudomonas aeruginosa, Staphylococcus aureus and non-typeable Haemophilus influenzae. Stenotrophomonas maltophilia, Achromobacter xylosoxidans and Burkholderia cepacia complex are also frequent pathogens in CF,1,2 and Moraxella catarrhalis, Streptococcus pneumoniae and enteric Gram-negative bacilli are seen in non-CF bronchiectasis.3 Extensive use of intravenous, oral and inhaled antibiotics has improved the survival of CF patients,4,5 but has also led to the development of bacterial resistance.6,7 There is a clear need for new antibiotics, and novel approaches including combination drugs should be explored.
An ideal therapy would be delivered directly to the lungs, kill a broad spectrum of bacteria, have a favourable safety profile and reduce the development of resistance. A combination antibiotic consisting of fosfomycin and tobramycin may be an appropriate addition to the current treatments for the management of respiratory infections. Fosfomycin is a phosphonic acid antibiotic8 with little reported human toxicity when administered parenterally.9 It is active against both Gram-positive10,11 and Gram-negative bacteria,12 and inhibits the first step of peptidoglycan biosynthesis in the bacterial cell wall.8,9 Both oral (fosfomycin calcium and fosfomycin trometamol) and intravenous (fosfomycin disodium) formulations are available, but only oral fosfomycin trometamol is approved in the USA for treating uncomplicated urinary tract infections.9,13 Parenteral administration of fosfomycin is sporadically used in the UK to treat CF bacterial respiratory infections,6,14 but an aerosol formulation deliverable directly to the lungs has not yet been developed.
Tobramycin is an aminoglycoside and is highly potent against Gram-negative bacteria, in particular P. aeruginosa.15 Tobramycin is rapidly bactericidal and acts by inhibiting bacterial protein synthesis.16 Aerosolized tobramycin (TOBI®) is used for the management of P. aeruginosa respiratory infections in CF patients.5 Nephrotoxicity and ototoxicity are adverse reactions associated with tobramycin therapy.17 The greatest risk factor for development of toxicity is cumulative exposure to large doses of tobramycin. Bronchiectasis patients may be at increased risk of developing tobramycin toxicity because they receive prolonged and repeated antibiotic therapies over their lifetime.18
The purpose of this study was to evaluate the in vitro and in vivo activities of a 4:1 (w/w) fosfomycin/tobramycin combination (FTI) against bacterial respiratory pathogens seen in the CF and non-CF bronchiectasis populations, and to compare them with those of fosfomycin and tobramycin, individually. S. aureus and P. aeruginosa were the major focus of this work, both because of their frequency and virulence in bronchiectasis infections and because, as resistance increases in these two organisms, treatment becomes problematic.
Materials and methods
Bacterial strains
P. aeruginosa strains from patients with CF (n = 100) were obtained from the Therapeutics Development Network Center for CF Microbiology at the Children's Hospital and Regional Medical Center (Seattle, WA, USA). B. cepacia (n = 20) complex strains were obtained from the University of British Columbia (Vancouver, BC, Canada). Non-CF (respiratory, bloodstream, skin/soft tissue) P. aeruginosa (n = 60), Enterococcus faecalis (n = 5), E. coli (n = 22), H. influenzae (n = 16), Klebsiella spp. (n = 22), M. catarrhalis (n = 5), S. maltophilia (n = 17), S. aureus (n = 16), S. pneumoniae (n = 8) and Streptococcus pyogenes (n = 5) strains were obtained from The Jones Group Laboratories (North Liberty, IA, USA) and The Clinical Microbiology Institute (Wilsonville, OR, USA). P. aeruginosa ATCC 27853, E. coli ATCC 25922, S. aureus ATCC 29213, S. pneumoniae ATCC 49619, E. faecalis ATCC 29212 and H. influenzae ATCC 49247 served as quality control and reference strains.19 An animal-passaged derivative of P. aeruginosa ATCC 27853 (C177) was used in the rat pneumonia studies.
Antibiotics
Fosfomycin disodium, tobramycin sulphate and vancomycin hydrochloride were obtained from Sigma-Aldrich (St Louis, MO, USA). Ciprofloxacin hydrochloride was obtained from Cellgro (Herndon, VA, USA). FTI consisted of a 4:1 ratio (w/w basis) of fosfomycin and tobramycin. Glucose-6-phosphate (Sigma-Aldrich) was added to the media at a final concentration of 25 mg/L for all in vitro evaluations of fosfomycin and FTI.19,20
MICs and MBCs
MICs were determined by agar plate dilution and broth microdilution methods.19,20 The MIC was defined as the lowest concentration of antibiotic that prevented visible growth after incubation at 35°C for 18–24 h. FTI MIC values were expressed as the concentration of both drugs (example, FTI MIC of 8 mg/L = 6.4 mg/L fosfomycin + 1.6 mg/L tobramycin). Vancomycin and ciprofloxacin MICs were determined only for the S. aureus isolates. MBCs were determined according to CLSI (formerly NCCLS) guidelines.21 The MBC was defined by a ≥3 log10 decrease in cfu/mL of the original inoculum.
Chequerboard synergy
Interactions between fosfomycin and tobramycin were determined by the broth microdilution chequerboard method.22 Two-fold serial dilutions bracketing the expected MIC value of both antibiotics were evaluated. The fractional inhibitory concentration (FIC) was calculated as the MIC of compound 1 in combination with a second compound, divided by the MIC of compound 1 alone. An FIC index (FICI) was calculated for each drug combination as the sum of the individual FICs of compounds 1 and 2. Synergy was defined as an FICI of ≤0.5, no interaction as an FICI >0.5 and ≤4, and antagonism as an FICI >4. The lowest FICI was used for final interpretation of drug interactions.
Post-antibiotic effect (PAE)
PAE values were determined by the viable plate count method.23 Bacteria were incubated with antibiotic at 2× the MIC for 1–2 h in a shaking 35°C water bath. Growth controls were included in each experiment. Following exposure, the cultures were diluted 1:1000 and the cfu/mL determined hourly. The PAE was defined as T–C, where T is the time required for the viable counts of an antibiotic-exposed culture to increase 1 log10 cfu/mL above the counts determined immediately after dilution and C is the corresponding time for the growth control.
Time–kill studies
Time–kill experiments were performed according to CLSI standards.21 Antibiotics were evaluated at multiples of the MIC in cation-adjusted Mueller–Hinton broth (CAMHB) (Remel, Lenexa, KS, USA). A no-drug control was run in each assay. Bacterial cultures and antibiotic were incubated at 35°C in a shaking water bath and killing activity assessed at 1, 2, 4, 6 and 24 h. Antibiotic concentrations that reduced the original inoculum by ≥3 log10 cfu/mL were considered bactericidal.
Animal efficacy studies
Animals were handled according to the Guidelines for the Care and Use of Laboratory Animals.24 All animal protocols were approved by an IRB/Ethics Committee. Male Sprague–Dawley rats (180–200 g) were obtained from Charles River Laboratories (Hollister, CA, USA) and acclimatized for 5 days prior to use. Animals were housed individually in ventilated cages, fed Purina Lab Diet ad libitum and allowed free access to water.
Antibiotic efficacy was determined using a rat bacterial pneumonia model.25 Rats were anaesthetized with isoflurane, and ∼103 cfu of P. aeruginosa C177 in a 2% agar solution were instilled into the lungs with an oral gavage needle. The inoculum was deposited at the first bifurcation and distributed throughout the lungs by inspiration. Animals were allowed to recover for 18 h post-infection. Each experiment consisted of pre-treatment (n = 5–7), saline control (n = 5–7) and antibiotic (n = 5–7) groups. Rats were anaesthetized with isoflurane, and 100 µL of antibiotic solution or saline was instilled into the trachea using a Microsprayer™ (Penn-Century Inc., Philadelphia, PA, USA). Antibiotics were administered intratracheally twice daily for 3 days.
Animals were euthanized by intraperitoneal administration of sodium pentobarbital. The pre-treatment control group was harvested 18 h post-infection, and the saline and treatment groups 18 h after the last antibiotic exposure. Lungs were removed aseptically, homogenized in sterile normal saline and viable bacteria determined by the colony count method. Statistical differences between the saline control group and treatment groups were evaluated by the Mann–Whitney Rank Sum Test using GraphPad Prism® software package version 3.03 (GraphPad Software, Inc., San Diego, CA, USA).
Single-step resistance
Development of resistance after a single exposure to antibiotic was determined using four clinical and one reference strain of S. aureus and P. aeruginosa. Late log-phase cultures (109–1010 cfu) were spread onto Mueller–Hinton agar (BBL, Sparks, MD, USA) plates containing 4× the MIC of each antibiotic. The culture plates were incubated at 35°C for 48 h and the number of colonies on each plate was enumerated manually. The frequency of resistance was calculated by dividing the number of bacteria growing at the defined antibiotic concentration by the number of bacteria in the inoculum.26 MIC values were calculated for three representative spontaneous mutants and compared with those for the parental strain.
Results
MICs
Table 1 summarizes the MICs at which 50% (MIC50) and 90% (MIC90) of the clinical isolates were inhibited. FTI had high activity against the 16 random S. aureus strains, and moderate activity against S. pneumoniae, S. pyogenes and E. faecalis. Twelve of the 16 S. aureus strains were categorized as methicillin-resistant (MRSA). The FTI MIC50 value (2 mg/L) was nearly identical to that of vancomycin (1 mg/L) and was superior to that of ciprofloxacin (>4 mg/L) for S. aureus. FTI was also active against single linezolid-resistant (C059) and glycopeptide-intermediate S. aureus (GISA) (C060) isolates, with MICs of 2 and 1 mg/L, respectively.
Table 1.
MICs of FTI, tobramycin and fosfomycin for Gram-positive and Gram-negative bacteria
| MIC (mg/L) |
||||||
|---|---|---|---|---|---|---|
| FTI |
tobramycin |
fosfomycin |
||||
| Organism (no. of strains) | range | MIC50 (MIC90) | range | MIC50 (MIC90) | range | MIC50 (MIC90) |
| S. aureusa (16) | 0.5–16 | 2 (8) | 0.125–512 | 0.5 (256) | 0.125–16 | 2 (4) |
| S. pneumoniaea (8) | 4–32 | ND | 16–64 | ND | 8–32 | ND |
| S. pyogenesb (5) | 16–32 | ND | 16–64 | ND | 16–64 | ND |
| E. faecalisa (5) | 32 | ND | 8–512 | ND | 32 | ND |
| E. colia (22) | 0.125–1 | 0.5 (1) | 0.5–1 | 1 (1) | 0.25–4 | 0.5 (2) |
| H. influenzaea (16) | ≤0.13–4 | 0.5 (2) | 0.5–1 | 1 (1) | 0.25–4 | 0.5 (2) |
| Klebsiella spp.b (22) | 0.5–16 | 1 (8) | 0.13–>512 | 0.13 (16) | 0.5–16 | 4 (16) |
| M. catarrhalisa (5) | 0.5–1 | ND | 0.5–1 | ND | 4–16 | ND |
| P. aeruginosaa, non-CF (60) | 1–256 | 4 (128) | 0.13–>512 | 1 (128) | 1–>512 | 32 (128) |
| P. aeruginosaa, CF (100) | 1–128 | 8 (64) | 0.25–>512 | 2 (16) | 4–>512 | 64 (512) |
| S. maltophiliaa (17) | 8–256 | 64 (128) | 2–>512 | 64 (256) | 32–512 | 64 (128) |
| B. cepacia complexa (20) | 0.5–>512 | 512 (>512) | 1–>512 | 64 (512) | 512–>512 | >512 (>512) |
ND, not determined due to the small number of isolates examined.
aMICs were determined by the agar dilution method.
bMICs were determined by the broth microdilution method.
Among the Gram-negative organisms examined FTI had the lowest MIC50 for E. coli (0.5 mg/L), H. influenzae (0.5 mg/L), Klebsiella spp. (1 mg/L) and P. aeruginosa (non-CF, 4 mg/L; and CF, 8 mg/L) strains. FTI also had high activity against M. catarrhalis strains, but poor activity against S. maltophilia and B. cepacia complex. Against tobramycin-resistant and high fosfomycin MIC (≥128 mg/L) strains, FTI had MICs comparable to that of the most active single antibiotic component. Tobramycin had the lowest MIC50 and MIC90 values for the CF (2 and 16 mg/L) and non-CF P. aeruginosa (1 and 128 mg/L) strains. Fosfomycin had potent activity against S. aureus, H. influenzae, E. coli and Klebsiella spp. It showed moderate activity against P. aeruginosa and S. maltophilia, and poor activity against B. cepacia complex.
MBCs
FTI and tobramycin were bactericidal against the S. aureus (100%), S. pneumoniae (100%), P. aeruginosa (100%), E. coli (100%), Klebsiella spp. (100%) and H. influenzae (83% and 100%, respectively) strains (Table 2). Fosfomycin was bactericidal against S. aureus (80%), S. pneumoniae (86%), P. aeruginosa (78%), E. coli (90%), Klebsiella spp. (100%) and H. influenzae (83%) strains. FTI and tobramycin had MBC/MIC ratios ≤8, suggesting that both antibiotics work by killing bacteria rather than by inhibiting bacterial growth.
Table 2.
MBC/MIC ranges of clinical strains
| Range of MBC/MICa |
|||
|---|---|---|---|
| Organism (no. of strains) | FTI | tobramycin | fosfomycin |
| S. aureus (10) | 1–2 | 1–2 | 1–8 |
| S. pneumoniae (7) | 1–4 | 1–2 | 1–16 |
| P. aeruginosa, non-CF (10) | 1–4 | 1–2 | 2–8 |
| P. aeruginosa, CF (8) | 1–4 | 1–4 | 2–>16 |
| E. coli (10) | 1–4 | 1–4 | 1–8 |
| Klebsiella spp. (5) | 1 | 1–4 | 1–4 |
| H. influenzae (6) | 1–8 | 1–2 | 1–8 |
aThe MBC/MIC ratio was calculated by dividing the MBC (mg/L) by the MIC (mg/L).
Chequerboard synergy
No antagonism was seen between fosfomycin and tobramycin by the chequerboard method for any of the 27 strains tested: S. aureus, n = 4; P. aeruginosa, n = 17; E. coli, n = 5; and H. influenzae, n = 1. The combination was categorized as no interaction for 25 of the 27 strains (93%), and synergistic for 1 P. aeruginosa strain and 1 E. coli strain.
PAE
FTI had the longest PAE for the three type strains, followed by tobramycin and fosfomycin (Table 3). FTI had the longest PAE with S. aureus (3.8 h) followed by P. aeruginosa (3 h) and E. coli (2.8 h).
Table 3.
PAE of FTI, tobramycin and fosfomycin for S. aureus P. aeruginosa and E. coli
| Organism | Antibiotic | MIC (mg/L) | PAE (h) |
|---|---|---|---|
| S. aureusa ATCC 29213 | FTI | 2.0 | 3.8 |
| tobramycin | 0.5 | 2.8 | |
| fosfomycin | 2.0 | 1.3 | |
| P. aeruginosab ATCC 27853 | FTI | 4.0 | 3.0 |
| tobramycin | 0.5 | 2.0 | |
| fosfomycin | 4.0 | 1.0 | |
| E. colib ATCC 25922 | FTI | 1.0 | 2.8 |
| tobramycin | 0.5 | 2.5 | |
| fosfomycin | 2.0 | 1.0 |
aPAE was determined after a 2 h exposure to 2 × the MIC of antibiotic.
bPAE was determined after a 1 h exposure to 2 × the MIC of antibiotic.
Time–kill studies
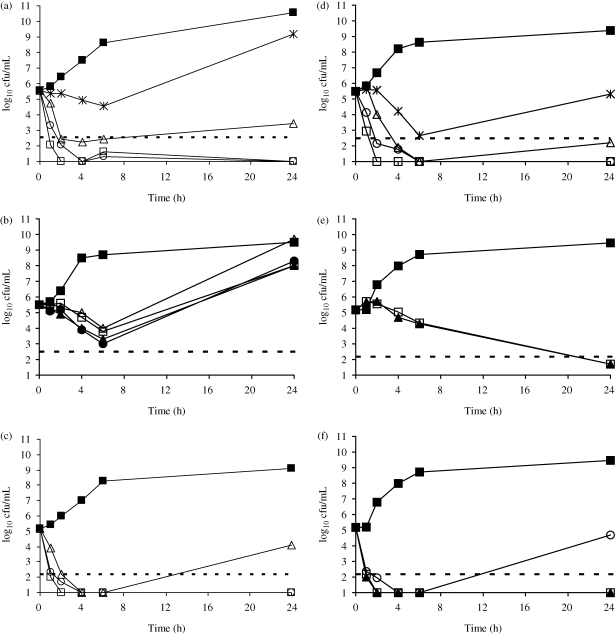
FTI and tobramycin killed in a concentration-dependent fashion while fosfomycin killed in a time-dependent fashion against S. aureus and P. aeruginosa (Figure 1). Against a methicillin-susceptible S. aureus (MSSA) strain (ATCC 29213), FTI and tobramycin were bactericidal at 2 mg/L (2× MIC) and 1 mg/L (2× MIC), respectively. FTI was bactericidal against the MRSA strain (C354) at 1 mg/L (data not shown). FTI and tobramycin were rapidly (1–2 h) bactericidal against P. aeruginosa ATCC 27853 at 4 mg/L (1× MIC) and 0.5 mg/L (1× MIC), respectively. FTI and tobramycin maintained bactericidal killing at 24 h, while cultures exposed to fosfomycin experienced regrowth.
Figure 1.
Time–kill curves for P. aeruginosa ATCC 27853 and (a) FTI, (b) fosfomycin and (c) tobramycin, and for S. aureus ATCC 29213 and (d) FTI, (e) fosfomycin and (f) tobramycin. Antibiotics were tested at concentrations of 0.5 × MIC (asterisks), 1 × MIC (open triangles), 2 × MIC (open circles), 4 × MIC (open squares), 8 × MIC (filled triangles) and 16 × MIC (filled circles). Closed squares, no drug; broken line, bactericidal line.
Animal efficacy studies
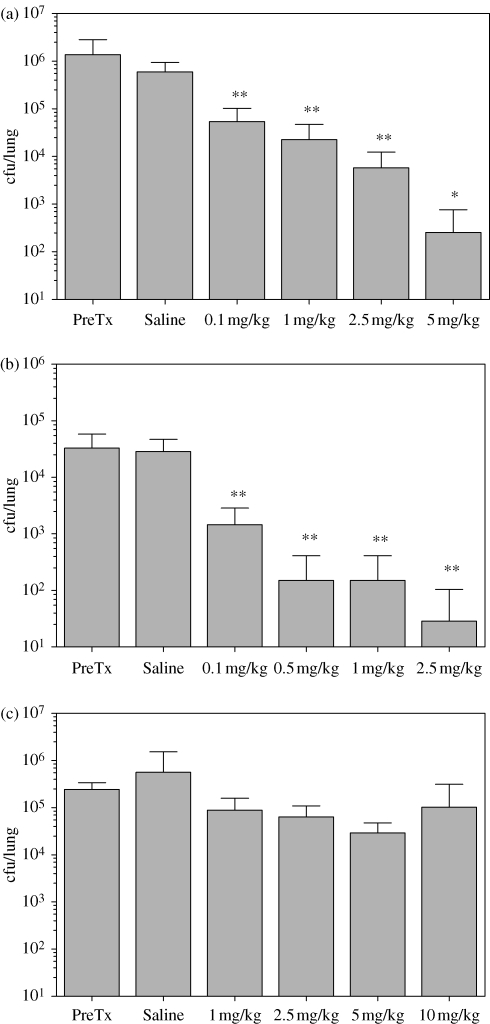
In the absence of antibiotic treatment, cfu/lung decreased <1 log10 at days 4 and 7 post-infection. Intratracheal administration of FTI showed progressively greater killing of P. aeruginosa with increasing dose (Figure 2). In subsequent experiments, complete eradication of the C177 infection was seen with 5 and 12.5 mg/kg FTI. Tobramycin showed 3 log10 bacterial killing at 2.5 mg/kg. Administration of tobramycin doses higher than 3 mg/kg resulted in complete eradication of the P. aeruginosa infection, while doses ≤0.5 mg/kg did not result in bacterial killing. A reduction in cfu/lung was not observed after administration of ≤10 mg/kg fosfomycin.
Figure 2.
Reduction of P. aeruginosa (strain C177) cfu in the rat lung after intratracheal administration of antibiotic twice daily for 3 days. (a) FTI. (b) Tobramycin. (c) Fosfomycin. Data are expressed as means ± SD. *P < 0.05; **P < 0.01. PreTx, pre-treatment.
Single-step resistance
Table 4 shows the frequencies of spontaneous single-step mutations leading to antibiotic resistance. Against the five S. aureus strains, FTI had a mutation frequency 2–4 log10 less than tobramycin and 2–7 log10 less than fosfomycin. Against P. aeruginosa, FTI was superior to tobramycin, but the differences were ≤2 log10. Relative to the parent strains, MIC values of the spontaneous mutants increased 4-, 16- and 32- to 128-fold for FTI, tobramycin and fosfomycin, respectively.
Table 4.
Spontaneous mutation frequency resulting in development of antibiotic resistance
| Frequency |
|||
|---|---|---|---|
| Organism (strain) | FTI | fosfomycin | tobramycin |
| S. aureus | |||
| C051 | <1.8 × 10–10 | 3.0 × 10–5 | 3.5 × 10–6 |
| C053 | <1.8 × 10–10 | 7.7 × 10–3 | 2.0 × 10–7 |
| C055 | <4.3 × 10–9 | 2.5 × 10–6 | 3.8 × 10–7 |
| C057 | 1.0 × 10–9 | 2.1 × 10–5 | 1.1 × 10–6 |
| ATCC 29213 | <3.1 × 10–10 | 2.6 × 10–8 | 1.6 × 10–7 |
| P. aeruginosa | |||
| C002 | 5.0 × 10–6 | 6.5 × 10–3 | 1.1 × 10–5 |
| C003 | 1.1 × 10–6 | 1.1 × 10–6 | 4.2 × 10–5 |
| C013 | 1.2 × 10–7 | 9.2 × 10–3 | 1.4 × 10–6 |
| C014 | 3.4 × 10–6 | 1.4 × 10–4 | 1.3 × 10–6 |
| ATCC 27853 | 4.6 × 10–7 | 7.2 × 10–4 | 3.0 × 10–5 |
Discussion
This study investigated the in vitro and in vivo antibacterial activities of FTI, a novel inhaled antibiotic combination. Fosfomycin was selected as the major component because it is active against both Gram-positive10,11 and Gram-negative bacteria,12 is bactericidal10 and has a good safety profile.8 However, fosfomycin kills in a time-dependent fashion,10 is only moderately active against P. aeruginosa and some of the more resistant Gram-negative organisms,12 and has a high mutation frequency resulting in bacterial resistance in vitro.26,27 Tobramycin is rapidly bactericidal, exhibits concentration-dependent killing activity,16 is highly active against many resistant Gram-negatives15 and has a low prevalence of bacterial resistance. Tobramycin constitutes the minor component because of the benefits of reducing the lifetime accumulation of aminoglycoside toxicity,18 which can be minimized by lowering the dose of tobramycin.
Polymicrobial respiratory infections are a significant cause of morbidity and mortality in CF and non-CF bronchiectasis patients2,3 as well as other diseases characterized by chronic airway infection. Important pathogens common to both populations include S. aureus, P. aeruginosa and non-typeable H. influenzae. FTI demonstrated excellent activity against both S. aureus and H. influenzae. S. aureus may be particularly pathogenic and the prevalence of MRSA is increasing.1 We demonstrated that FTI had high activity against ciprofloxacin- and methicillin-resistant S. aureus, suggesting that it might be a good therapeutic agent for these infections. Neither CF nor non-CF P. aeruginosa was found to be as susceptible to FTI as it was to tobramycin when susceptibility testing was conducted by CLSI standards.19 However, in vitro antibiotic activity does not always correlate with in vivo activity, particularly in CF because CF sputum has been shown to inhibit the activity of aminoglycosides.28,29 FTI demonstrated relatively poor activity against other CF pathogens, particularly S. maltophilia and B. cepacia complex. However, it was active against other non-CF bronchiectasis pathogens including M. catarrhalis, E. coli, Klebsiella and S. pneumoniae.
Examination of synergy by the chequerboard method demonstrated no interaction between fosfomycin and tobramycin for the majority of clinical P. aeruginosa, E. coli and S. aureus strains. Antagonism was not observed, while synergy was detected in 7% of the strains. To our knowledge this is the first report describing the interactions between fosfomycin and tobramycin. Our results were consistent with those reported between fosfomycin and other aminoglycosides.30,31 The PAE of FTI was superior to that of fosfomycin and tobramycin for all three bacterial species. These data were consistent with time–kill kinetics, which also demonstrated slower bacterial regrowth with FTI compared with tobramycin or fosfomycin.
Time–kill experiments were conducted to evaluate the rate and degree of bacterial killing. FTI and tobramycin were rapidly bactericidal against P. aeruginosa and S. aureus. FTI killed in a concentration-dependent fashion, which is somewhat surprising because fosfomycin, the major component of the combination, killed in a time-dependent fashion.10 Tobramycin killed in a concentration-dependent fashion like other aminoglycoside antibiotics.16 We also demonstrated that fosfomycin was bactericidal against S. aureus, which is consistent with previous studies.10 Fosfomycin's mechanism of killing against P. aeruginosa is not well characterized. This study demonstrates that bactericidal killing is not reached at concentrations ≤16× the fosfomycin MIC for P. aeruginosa ATCC 27853. MBC experiments also confirmed that FTI reached bactericidal killing against clinical P. aeruginosa, S. aureus and E. coli strains.
The in vitro studies with FTI were supported by animal efficacy experiments in rats. Lung cfus were stable over the treatment period (3 days) and demonstrated that bacterial killing was due to the activity of the antibiotics (data not shown). P. aeruginosa colony counts in rat lung dropped at least 3 log10 with treatment and the organisms were eradicated with higher doses of FTI. While the in vivo activity of FTI is slightly less than that of tobramycin on a weight basis, tobramycin accounts for only 20% of FTI. The tobramycin MIC for C177 (0.5 mg/L) is 8-fold less than the FTI MIC (4 mg/L) and may explain the slight difference in activity. Fosfomycin alone has very little activity against P. aeruginosa in vivo and confirms the in vitro data presented in this study.
Development of antibiotic resistance is of particular concern in bronchiectasis patients. Antibiotic options are limited and bacterial isolates, particularly those from CF individuals, are resistant to many of the currently approved antibiotics.2 Combinations of antibiotics administered independently (parenteral or parenteral + oral) are commonly used to treat multidrug-resistant P. aeruginosa during exacerbations,31,32 and to slow the development of resistance.2 Therefore, it would also seem promising to combine fosfomycin and tobramycin in the same aerosol formulation if the combination shows a clinically relevant benefit such as slowing the development of resistance. The spontaneous mutation frequency resulting in resistance after a single exposure was dramatically less than the frequencies of fosfomyin or tobramycin for S. aureus and P. aeruginosa. Fosfomycin resistance occurred very rapidly after a single in vitro exposure, in our studies. This finding is consistent with previous reports.26 However, evidence for the in vivo development of fosfomycin resistance is lacking. Fosfomycin has been extensively used for >20 years in Japan and Europe for the treatment of urinary tract infections. Despite this, the reported fosfomycin resistance in urinary E. coli isolates remains <2%.13
Antibiotic options for CF or non-CF bronchiectasis patients are limited. FTI, a novel antibiotic combination, has many desirable properties. Pre-clinical research demonstrates that FTI is active against important CF and non-CF respiratory pathogens including S. aureus, H. influenzae, M. catarrhalis, coliforms and multidrug-resistant P. aeruginosa. FTI was rapidly bactericidal and had activity comparable to that of tobramycin. Moreover, FTI reduced the development of antibiotic resistance. Fosfomycin, the major component of FTI, has a very favourable safety profile when administered parenterally.9 Additionally, several studies have shown that fosfomycin reduces aminoglycoside-induced nephrotoxicity.33–35 Since tobramycin constitutes 20% of FTI on a weight basis, the cumulative toxic effects due to tobramycin could also be reduced. The comparative safety of FTI should ultimately be evaluated in future studies in the affected populations. These data suggest that FTI should be investigated further for the treatment of CF and non-CF bronchiectasis infections.
Funding
This study was supported by Gilead Sciences, Inc.
Transparency declarations
D. L. M., T. F. K and W. R. B. are currently employed by Gilead Sciences, Inc. D. L. M., W. R. B. and T. F. K. own stock and options in Gilead Sciences, Inc. J. L. B. serves as a consultant for Gilead Sciences, Inc.
Acknowledgements
We thank Chad Nicely and Chris Hardwick for developing the microbiology database as well as Lisa Moorehead and Benjamin Duncan for their assistance with statistical analysis of the animal efficacy data. We also thank Geri Orta, Jolene Kidney and Lisa Corey for assisting with the animal efficacy work. Subsets of the MIC, resistance and animal efficacy data were presented at the Twenty-first Annual North American Cystic Fibrosis Conference, Anaheim, CA, USA, 2007 (Abstract 328).
References
- 1.Anonymous. Cystic Fibrosis Foundation Patient Registry Annual Report. Bethesda, MD, USA: Cystic Fibrosis Foundation; 2003. [Google Scholar]
- 2.Conway SP, Brownlee KG, Denton M, et al. Antibiotic treatment of multidrug-resistant organisms in cystic fibrosis. Am J Respir Med. 2003;2:321–32. doi: 10.1007/BF03256660. [DOI] [PubMed] [Google Scholar]
- 3.Sykes A, Mallia P, Johnston SL. Diagnosis of pathogens in exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2007;4:642–6. doi: 10.1513/pats.200707-101TH. [DOI] [PubMed] [Google Scholar]
- 4.Gibson RL, Emerson J, McNamara S, et al. Significant microbiological effect of inhaled tobramycin in young children with cystic fibrosis. Am J Respir Crit Care Med. 2003;167:841–9. doi: 10.1164/rccm.200208-855OC. [DOI] [PubMed] [Google Scholar]
- 5.Ramsey BW, Pepe MS, Quan JM, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. N Engl J Med. 1999;340:23–30. doi: 10.1056/NEJM199901073400104. [DOI] [PubMed] [Google Scholar]
- 6.Mirakhur A, Gallagher MJ, Ledson MJ, et al. Fosfomycin therapy for multiresistant Pseudomonas aeruginosa in cystic fibrosis. J Cyst Fibros. 2003;2:19–24. doi: 10.1016/S1569-1993(02)00143-1. [DOI] [PubMed] [Google Scholar]
- 7.Saiman L, Chen Y, Gabriel PS, et al. Synergistic activities of macrolide antibiotics against Pseudomonas aeruginosa, Burkholderia cepacia, Stenotrophomonas maltophilia, and Alcaligenes xylosoxidans isolated from patients with cystic fibrosis. Antimicrob Agents Chemother. 2002;46:1105–7. doi: 10.1128/AAC.46.4.1105-1107.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahan FM, Kahan JS, Cassidy PJ, et al. The mechanism of action of fosfomycin (phoshonomycin) Ann NY Acad Sci. 1974;235:364–86. doi: 10.1111/j.1749-6632.1974.tb43277.x. [DOI] [PubMed] [Google Scholar]
- 9.Woodruff HB, Mata JM, Hernandez S, et al. Fosfomycin: laboratory studies. Chemotherapy. 1977;23(Suppl 1):1–22. doi: 10.1159/000222020. [DOI] [PubMed] [Google Scholar]
- 10.Grif K, Dierich MP, Pfaller K, et al. In vitro activity of fosfomycin in combination with various antistaphylococcal substances. J Antimicrob Chemother. 2001;48:209–17. doi: 10.1093/jac/48.2.209. [DOI] [PubMed] [Google Scholar]
- 11.Menendez A, Tutor A, Sousa AS. Treatment of respiratory infections with fosfomycin. Chemotherapy. 1977;23(Suppl 1):348–57. doi: 10.1159/000222075. [DOI] [PubMed] [Google Scholar]
- 12.Schulin T. In vitro activity of the aerosolized agents colistin and tobramycin and five intravenous agents against Pseudomonas aeruginosa isolated from cystic fibrosis patients in southwestern Germany. J Antimicrob Chemother. 2002;49:403–6. doi: 10.1093/jac/49.2.403. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu M, Shigeobu F, Miyakozawa I, et al. Novel fosfomycin resistance of Pseudomonas aeruginosa clinical isolates recovered in Japan in 1996. Antimicrob Agents Chemother. 2000;44:2007–8. doi: 10.1128/aac.44.7.2007-2008.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katznelson D, Yahav Y, Rubinstein E. Fosfomycin in the treatment of cystic fibrosis. Eur J Clin Microbiol. 1984;3:213. doi: 10.1007/BF02014882. [DOI] [PubMed] [Google Scholar]
- 15.Shawar RM, MacLeod DL, Garber RL, et al. Activities of tobramycin and six other antibiotics against Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Antimicrob Agents Chemother. 1999;43:2877–80. doi: 10.1128/aac.43.12.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vakulenko SB, Mobashery S. Versatility of aminoglycosides and prospects for their future. Clin Microbiol Rev. 2003;16:430–50. doi: 10.1128/CMR.16.3.430-450.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammett-Stabler CA, Johns T. Laboratory guidelines for monitoring of antimicrobial drugs. Clin Chem. 1998;44:1129–40. [PubMed] [Google Scholar]
- 18.Al-Aloul M, Miller H, Alapati S, et al. Renal impairment in cystic fibrosis patients due to repeated intravenous aminoglycoside use. Pediatr Pulmonol. 2005;39:15–20. doi: 10.1002/ppul.20138. [DOI] [PubMed] [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically—Sixth Edition: Approved Standard M7-A6. Wayne, PA, USA: NCCLS; 2003. [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Susceptibility Testing—Fourteenth Edition: Approved Standard M100-S13. Wayne, PA, USA: NCCLS; 2003. [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards. Methods for Determining Bactericidal Activity of Antimicrobial Agents—Approved Standard M26-A. Wayne, PA, USA: NCCLS; 1999. [Google Scholar]
- 22.Eliopoulos GM, Moellering RC., Jr . Antimicrobial combinations. In: Lorian V, editor. Antibiotics in Laboratory Medicine. 4th edn. Baltimore: Williams & Wilkins Co.; 1996. pp. 330–96. [Google Scholar]
- 23.Craig WA, Gudmundsson S. Postantibiotic effect. In: Lorian V, editor. Antibiotics in Laboratory Medicine. 4th edn. Baltimore: Williams & Wilkins Co.; 1996. pp. 296–329. [Google Scholar]
- 24.NRC (National Research Council) Guide for the Care and Use of Laboratory Animals. Washington, DC, USA: National Academy Press; 1996. [Google Scholar]
- 25.Cash HA, Woods DE, McCullough B, et al. A rat model of chronic respiratory infection with Pseudomonas aeruginosa. Am Rev Respir Dis. 1979;119:453–9. doi: 10.1164/arrd.1979.119.3.453. [DOI] [PubMed] [Google Scholar]
- 26.Martinez JL, Baquero F. Mutation frequencies and antibiotic resistance. Antimicrob Agent Chemother. 2000;44:1771–7. doi: 10.1128/aac.44.7.1771-1777.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nilsson AI, Berg OG, Aspevall O, et al. Biological costs and mechanisms of fosfomycin resistance in Escherichia coli. Antimicrob Agents Chemother. 2003;47:2850–8. doi: 10.1128/AAC.47.9.2850-2858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunt BE, Weber A, Berger A, et al. Macromolecular mechanisms of sputum inhibition of tobramycin activity. Antimicrob Agents Chemother. 1995;39:34–9. doi: 10.1128/aac.39.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendelman PM, Smith AL, Levy J, et al. Aminoglycoside penetration, inactivation, and efficacy in cystic fibrosis sputum. Am Rev Respir Dis. 1985;132:761–5. doi: 10.1164/arrd.1985.132.4.761. [DOI] [PubMed] [Google Scholar]
- 30.Hayami H, Goto T, Kawahara M, et al. Activities of β-lactams, fluoroquinolones, amikacin, and fosfomycin alone and in combination against Pseudomonas aeruginosa isolated from complicated urinary tract infections. J Infect Chemother. 1999;5:130–8. doi: 10.1007/s101560050022. [DOI] [PubMed] [Google Scholar]
- 31.Tessier F, Quentin C. In vitro activity of fosfomycin combined with ceftazidime, imipenem, amikacin, and ciprofloxacin against Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis. 1997;16:159–62. doi: 10.1007/BF01709477. [DOI] [PubMed] [Google Scholar]
- 32.Smith AL, Doershuk C, Goldmann D, et al. Comparison of a β-lactam alone versus β-lactam and an aminoglycoside for pulmonary exacerbation in cystic fibrosis. J Pediatr. 1999;134:413–21. doi: 10.1016/s0022-3476(99)70197-6. [DOI] [PubMed] [Google Scholar]
- 33.Inouye S, Niizato T, Takeda U, et al. Protective effect of fosfomycin on the experimental nephrotoxicity induced by dibekacin. J Pharmacobiodyn. 1982;5:659–69. doi: 10.1248/bpb1978.5.659. [DOI] [PubMed] [Google Scholar]
- 34.Inouye S, Niizato T, Komiya I, et al. Mode of protective action of fosfomycin against dibekacin-induced nephrotoxicity in the dehydrated rats. J Pharmacobiodyn. 1982;5:941–50. doi: 10.1248/bpb1978.5.941. [DOI] [PubMed] [Google Scholar]
- 35.Yanagida C, Ito K, Komiya I, et al. Protective effect of fosfomycin on gentamicin-induced lipid peroxidation of rat renal tissue. Chem Biol Interact. 2004;148:139–47. doi: 10.1016/j.cbi.2004.05.005. [DOI] [PubMed] [Google Scholar]