Abstract
Cyanovirin-N (CV-N), a protein derived from Nostoc ellipsosporum, neutralizes influenza virus infectivity by binding to specific high-mannose oligosaccharides (oligomannose-8 and -9) at glycosylation sites on the viral hemagglutinin HA1 subunit. Mouse-adapted viruses lose sensitivity to CV-N due to HA1 mutations that eliminate these glycosylation sites. Recently we created a hybrid (reassortant) influenza A/WSN/33 (H1N1) virus containing the HA gene of A/New Caledonia/20/99 (H1N1) with an Asp225Gly mutation in the HA1, that was lethal to mice yet retained sensitivity to CV-N. We then utilized this model system to test the efficacy of CV-N against influenza. CV-N efficacy was dose-responsive from 0.0625 to 1 mg/kg/day when administered intranasally twice daily for 4 days starting 4 h prior to virus exposure. In a second study, survival benefit was seen with CV-N treatments (0.5 mg/kg/day for 4 days) beginning at −4 or +6 hours, but was significantly reduced at +12 hours. The early treatment resulted in up to 100% survival and 1,000-fold reduction in lung virus titer on day 3 of the infection. In contrast, ribavirin (a positive control − 75 mg/kg/day) treatment resulted in 30% survival and 30-fold decrease in lung virus titers. Lung consolidation scores and lung weights were significantly reduced by CV-N and ribavirin treatment on day 6 of the infection. Ferrets infected with a non-animal adapted influenza A/Charlottesville/31/95 (H1N1) virus were treated intranasally with CV-N (50 μg twice daily for 5 days starting 24 h before virus challenge). They exhibited 100-fold lower viral titers in nasal washes than placebos 1 day after treatment, but virus titers were equivalent on days 2–7. CV-N has the potential for prophylaxis and early initiation of treatment of influenza virus infections.
1. Introduction
Cyanovirin-N (CV-N) is a 101 amino acid protein derived from the cyanobacterium Nostoc ellipsosporum. The compound was originally discovered as an inhibitor of HIV, but was later found to inhibit other enveloped viruses such as influenza and Ebola (Boyd et al., 1997; Shenoy et al., 2001; Barrientos et al., 2003; O’Keefe et al., 2003). CV-N binds to envelope glycoproteins on the surface of each of these viruses. Binding to influenza occurs through high mannose oligosaccharides (oligomannose-8 and oligomannose-9) on glycosylation sites of the viral hemagglutinin HA1 molecule (O’Keefe et al., 2003). Loss of these glycosylation sites due to mutations leads to decreases in CV-N binding and antiviral activity (O’Keefe et al., 2003; Smee et al., 2008).
CV-N is a representative member of a class of proteins, termed carbohydrate-binding agents (CBAs) that inhibit a variety of enveloped viruses (Balzarini, 2007). CV-N has been investigated as a microbicidal agent against SIV and HIV in monkeys (Tsai et al., 2003, 2004). It also demonstrated systemic activity against Ebola virus infections following subcutaneous injection in mice by delaying the time to death, but not preventing mortality (Barrientos et al., 2003). Until this present investigation, treatment of influenza virus infections in mammals with CV-N has not been reported. Most investigators use mouse models for assaying anti-influenza virus activities of substances in vivo. However, adaptation of viruses to become lethal to mice results in mutations that render the viruses resistant to CV-N (Smee et al., 2008). These mutations are in the viral hemagglutinin HA gene, and cause the elimination of certain glycosylation sites important for CV-N binding and virus neutralization. One key mutation is at position 94A (in universal H3 numbering, corresponds to position 87 in H1 subtype) of the HA1 molecule that by itself confers CV-N resistance. Another mutation that arises during mouse adaptation is at position 225. We reported a 94A mutation and an Asp225Gly mutation that arose during adaptation of influenza A/New Caledonia/20/99 (H1N1) virus to mice (Smee et al., 2008).. The Asp225Gly substitution is a mutation that effects the receptor binding site for the HA1 that appears to aid in virus adaptation to a new host as previously reported in the context of the 1918 pandemic influenza virus (Tumpey et al., 2007).
For several years we were faced with the lack of a mouse model for evaluating the efficacy of CV-N or other carbohydrate-binding agents in vivo because the mouse-adapted viruses in our possession were all resistant to such compounds (O’Keefe et al., 2003; Smee et al., 2008). The idea was presented to create a hybrid (recombinant) virus using a previously mouse-adapted virus strain with an engineered HA gene from another virus. The resulting recombinant virus contained all but one gene segment of the mouse-adapted lethal influenza A/WSN/33 (H1N1) virus. The HA gene of A/WSN/33 virus was replaced with the HA gene from A/New Caledonia/20/99 (H1N1). In this case the HA gene of A/New Caledonia/20/99 was genetically altered to contain an Asp225Gly mutation in the HA1 subunit. The newly created recombinant virus was weakened in its virulence compared to wild-type A/WSN/33. It required further passages in mice to reestablish virulence, suggesting further mutations in regions of the virus that we did not analyze. Surprisingly, the mouse-adapted recombinant virus retained its sensitivity to inhibition by CV-N. The HA gene of the mouse adapted recombinant virus was sequenced and found to contain no additional mutations other than the introduced Asp225Gly. Other strains of A (H1N1) influenza viruses that we adapted to kill mice acquired the 94A mutation, which abolished a glycosylation site but this recombinant virus did not. This new virus became the tool we needed to evaluate CV-N in mice.
Mice are not the only species that can be used as a small animal model for testing compounds against influenza viruses in vivo. Ferrets are another animal species that is used (Fenton et al., 1977; Mendel et al., 1998; Sidwell and Smee, 2000; Mishin et al., 2005a). Ferrets can be infected with non-animal adapted influenza viruses. Human influenza viruses will not kill ferrets, and the modern human A (H1N1) viruses produce only mild disease in these animals. Antiviral and vaccine studies with them generally use virus quantitation from nasal washes to assess antiviral activity. Other disadvantages with the use of ferrets are cost, their limited availability, expense in housing, and amount of compound needed for testing. Thus, the number of published studies with ferrets is small. Cotton rats have also been used in influenza infection studies (Eichelberger, 2007). Cotton rats can be infected with non-animal adapted virus strains, thus, could be useful for evaluating CBAs.
In this report we examined the utility of CV-N to treat influenza A virus infections in mice and, to a more limited extent, in ferrets. The results are the first indication that this compound may have potential application for the treatment of influenza virus infections in humans.
2. Materials and Methods
2.1. Compounds and viruses
Recombinant CV-N was provided by the Center for Cancer Research, National Cancer Institute (NCI), Frederick, MD. It was purified at NCI by applying the E. coli (strain BL21 - DE3, containing the cyanovirin-N gene from Nostoc ellipsosporum) lysate to a column of wide-pore C4 packing (Bakerbond prep LC packing) and successively eluting with 100% H2O, H2O-MeOH (2:1), H2O-MeOH (1:2), and 100% MeOH. The material that eluted with H2O-MeOH (2:1) was further purified by C18 HPLC using a linear gradient from 80:20 H2O-CH3CN to 60:40 H2O-CH3CN to provide > 95% pure CV-N. Purity was assessed by SDS-PAGE, analytical HPLC and LC-MS analysis. Production methods were similar to those of Mori et al., 1998. After production the material was stored at −80°C. CV-N is stable at room temperature for months. However, since bacterial contamination might occur when used in mouse studies, the protein in solution was kept at 4°C between treatments. Verification of biological activity of recombinant CV-N was performed prior to use in the influenza virus experiments. Briefly, the anti-HIV activity and cytotoxicity of the the protein were assessed by a screen measuring the ability of CV-N to prevent the cytotoxicity of HIV against T-lymphoblastic (CEM-SS) cells challenged by HIV-1 (RF strain). The recombinant CV-N was also tested for HIV-1 gp120 binding using a direct protein binding assay. This same batch of CV-N was evaluated later in influenza cell culture studies (Smee et al., 2007) prior to being used in the present experiments in mice.
Ribavirin, a positive control, was obtained from ICN Pharmaceuticals (Costa Mesa, CA). Although not clinically approved for the treatment of influenza, ribavirin has often been used as a positive control in influenza studies in mice. We use it because it is consistently active against a broad number of influenza viruses (Sidwell et al., 2005). Ribavirin and CV-N were dissolved in sterile saline for treatment of mice, and sterile saline served as the placebo control.
The hybrid (recombinant) influenza A/WSN/33 (H1N1) virus containing the HA gene of influenza A/New Caledonia/20/99 (H1N1) was generated by reverse genetics as described previously (Mishin et al., 2005b; Hoffmann et al., 2000). The Asp225Gly mutation was introduced in the HA gene encoding the HA1 subunit by PCR based site-directed mutagenesis. The recombinant virus was designated influenza A/WSN/33 HAnc-Asp225Gly (H1N1). The virus became lethal after 7 serial passages in the lungs of the animals. An aliquot of the virus pool was titrated for lethality in mice to obtain the proper dose for antiviral studies. A stock of non-adapted influenza A/Charlottesville/31/95 (H1N1) virus (A/Bayern/7/95-like) was prepared in Madin-Darby canine kidney (MDCK) cells, aliquoted, and stored at −80°C. The 50% cell culture infectious virus titers (CCID50 per ml) in the viral stocks were determined in MDCK cells. The virus infectious dose 50% (ID50) was assessed in a ferret model as described (Mishin et al., 2005a).
2.2. Mouse infection studies
Female BALB/c mice (11–13 g, from Charles River Labs, Wilmington, MA) were anesthetized with ketamine (100 mg/kg) by intraperitoneal (i.p.) injection. They were infected intranasally (i.n.) with approximately 1 × 103 cell culture infectious doses (CCID50) of influenza A/WSN/33 HAnc-Asp225Gly (H1N1) virus per mouse in a 50-μl inoculum volume. Treatments with CV-N and ribavirin were given twice a day for 4 days by subcutaneous (s.c.) or i.n. route. The s.c. dose of CV-N was 5 mg/kg/day based upon the dose given to combat Ebola virus infections in mice (Barrientos et al., 2003), whereas the i.n. dose varied from 0.0625 to 2 mg/kg/day to cover the non-toxic range. Ribavirin was administered at 75 mg/kg/day, which is an active dose in mice by oral route against various strains of influenza viruses (Sidwell et al., 2005). The i.n. treatments in a 50-μl volume were given while the mice were under ketamine anesthesia similar to what was done for the infection. We found that the i.n. treatments could not continue too long into the infection due to the weakening condition of the animals, making recovery from anesthesia difficult. Ten treated animals per group and 20 placebo control mice were held 21 days to observe for death, and were weighed as a group every other day. An additional 10 animals per group were sacrificed to assess lung infection parameters. Uninfected mice treated in parallel were weighed daily and served as toxicity controls.
Lung infection parameters were determined on days 3 and 6 of the infection, using groups of 5 mice at each time point. Dissected lungs were assigned a consolidation score ranging from 0 (normal appearing) to 4 (100% of lung exhibiting a plum color), and then the lungs were weighed and frozen at −80°C until titrated for virus. Homogenization of each lung was done in 1 ml of cell culture medium using a stomacher. Samples were serially diluted in 10-fold increments in 96-well plates of MDCK cells. Four wells were used per virus dilution. Cytopathic effect induced by the virus was determined at 6 days, with virus titers calculated by the method of Reed and Muench (1938). Fifty percent cell culture infectious doses were converted to CCID50 per gram of lung tissue.
2.3. Ferret model
Young female ferrets (0.5 kg–0.8 kg) seronegative for the influenza A (H1N1) virus (HI titers <1:10) were obtained from Marshall Farms, North Rose, NY. The ferrets were placed two per cage. Food, water and plastic toys were provided. Following a 2-day acclimation period, the animals were lightly anesthetized by intramuscular injection of ketamine (25mg/kg) and inoculated i.n. with 1 ml of MDCK-grown, A/Charlottesville/31/95 (H1N1) virus diluted in phosphate buffered saline (PBS). The challenge dose was approximately 105 CCID50 per ferret (equal to 104 ferret infectious dose 50%) (Mishin et al., 2005a).
To collect nasal washes, the animals were lightly anesthetized and 0.5 ml of sterile PBS was inoculated into each nostril. The expelled liquid was collected into a centrifuge tube and placed on ice. The nasal washes were centrifuged and the supernatants were used to assess viral titers in MDCK cells. Nasal washes were collected daily for 7 days. CV-N was given at dose 50 μg twice daily (100 μg/day) for 5 days starting 1 day before virus challenge. The placebo-treated group received PBS instead of CV-N.
2.4. Statistical analysis
Differences in numbers of survivors were determined using the two-tailed Fisher exact test. Differences in the mean day to death, lung consolidation scores, lung weights, and lung virus titers were made using the two-tailed Mann-Whitney U-test. Comparisons were made between drug-treated and placebo groups.
3. Results
3.1. Dose-responsive CV-N treatment of mice
An experiment was conducted to determine safe and effective concentrations of CV-N for uninfected mice (Table 1). I.n. treatment of mice with CV-N at 2 mg/kg/day was overtly toxic, killing all animals. The 1 mg/kg/day dose caused weight loss exceeding that of the placebo group, as did the ribavirin group, but no animals died from these treatments. CV-N doses of 0.0625 to 0.5 mg/kg/day were considered to be well tolerated. I.n. treatments were stressful since they have to be given under anesthesia, thus, even the placebo group experienced some weight loss.
Table 1.
Dose-responsive effect of i.n. treatment with CV-N compared to one dose of ribavirin on a lethal influenza A/WSN/33 HAnc-Asp225Gly (H1N1) virus infection in mice.
| Toxicity Controls
|
Infected, Treated Mice
|
|||||
|---|---|---|---|---|---|---|
| Treatmenta | Dose (mg/kg/day) | Survivors/Total | MDDb ± SD | Mean Host Wt Changec (g) | Survivors/Total | MDDb ± SD |
| CV-N | 2 | 0/5 | 3.8 ± 1.0 | −2.0 | 2/10 | 3.4 ± 0.5*** |
| 1 | 5/5 | >21 | −1.6 | 8/10*** | 10.0 ± 0.0** | |
| 0.5 | 4/4 | >21 | −0.2 | 10/10*** | >21 | |
| 0.25 | 5/5 | >21 | 0.6 | 9/9*** | >21 | |
| 0.125 | 4/4 | >21 | 1.0 | 4/10** | 7.2 ± 3.8 | |
| 0.0625 | 4/4 | >21 | 0.4 | 6/10** | 7.0 ± 3.4 | |
| Ribavirin | 75 | 4/4 | >21 | −1.4 | 7/10*** | 10.7 ± 0.6** |
| Placebo | - | 4/4 | >21 | −0.8 | 0/20 | 7.9 ± 0.8 |
|
| ||||||
| Normal | - | 10/10 | >21 | 1.0 | - | - |
| Controlsd | ||||||
I.n. treatments were given twice a day for 4 days starting 4 h prior to virus exposure.
Mean day of death of mice that died prior to day 21.
Difference between initial weight and weight after final treatment.
Uninfected, untreated.
P<0.01,
P<0.001 compared to placebo-treated controls.
Mice infected with the influenza A/WSN/33 HAnc-Asp225Gly (H1N1) virus were treated with doses of CV-N starting 4 h prior to virus exposure (Table 1). The 2 mg/kg/day dose provided no significant protection due to toxicity. Doses of 0.0625 to 1 mg/kg/day provided statistically significant protection from death, with doses of 0.25 to 1 mg/kg/day being the most beneficial. The 1 mg/kg/day dose also caused delays in death of animals that died. Ribavirin treatment was 70% protective, and significantly delayed death in mice that died.
3.2. Prophylaxis and delayed treatment with CV-N on infections in mice
Infected mice were treated at varying times with CV-N (0.5 mg/kg/day) by i.n. route (Table 2). Treatments were effective when initiated either 4 h pre- or 6 h post-virus exposure (80% and 60% survival, respectively), but not when begun at +12 h. Ribavirin (75 mg/kg/day, initiated at −4 h) was weakly active (30% survival, significant [P<0.05] by one-tailed but by not two-tailed analysis), but greatly extended the time to death in mice that died. In the −4 h groups, CV-N treatment reduced lung virus titers 1000-fold on day 3, and reduced lung consolidation scores and lung weights on day 6. Virus titer in the CV-N treated mice rose 40-fold between days 3 and 6. Ribavirin treatment reduced lung virus titers 30-fold on day 3, and caused reductions in lung scores and weights on day 6. Virus titers on day 6 were 4-fold less than in the placebo group, but the results were not statistically significant. Lung consolidation was reduced by CV-N treatment started at +6 h after infection, but no other parameters were significantly reduced. Minimal positive benefit was seen with CV-N treatments started at +12 h. The increase in lung virus titer in the CV-N group compared to placebo was statistically significant, but not necessarily indicative that this treatment enhanced virus infection, due to the positive benefits seen with the other treatments.
Table 2.
Effects of i.n. treatment with CV-N and ribavirin on a lethal influenza A/WSN/33 HAnc-Asp225Gly (H1N1) virus infection in mice.
| Mean Lung Parameters (Day 3)
|
Mean Lung Parameters (Day 6)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compounda (mg/kg/day) | Start Timea (h) | Survivors/Total | MDDb ± SD | Score | Weight (mg) | Virus Titerc | Score | Weight (mg) | Virus Titerc |
| CV-N (0.5) | −4 | 8/10*** | 7.5 ± 0.7 | 0.4 ± 0.2 | 124 ± 13 | 4.3 ± 2.2** | 0.4 ± 0.2** | 134± 21* | 5.9 ± 1.4 |
| Ribavirin (75) | −4 | 3/10 | 13.4 ± 2.0*** | 0.2 ± 0.3 | 102 ± 13* | 6.0 ± 0.4** | 0.1 ± 0.2** | 140 ± 12* | 5.8 ± 0.6 |
| Placebo | −4 | 1/20 | 6.8 ± 1.2 | 0.0 ± 0.0 | 136 ± 11 | 7.5 ± 0.4 | 3.4 ± 0.8 | 210 ± 46 | 6.3 ± 0.2 |
| CV-N (0.5) | +6 | 6/10** | 6.8 ± 0.5 | 0.5 ± 0.0 | 116 ± 15 | 6.7 ± 1.1 | 1.7 ± 1.7* | 190 ± 59 | 6.3 ± 0.6 |
| Placebo | +6 | 0/20 | 6.3 ± 1.7 | 0.2 ± 0.3 | 116 ± 5 | 7.3 ± 0.3 | 3.7 ± 0.7 | 266 ± 31 | 5.8 ± 0.3 |
| CV-N (0.5) | +12 | 2/10 | 7.5 ± 0.9 | 0.4 ± 0.2 | 112 ± 11** | 6.5 ± 0.9 | 2.5 ± 0.9 | 178 ± 49 | 6.7 ± 0.2** |
| Placebo | +12 | 0/20 | 6.4 ± 0.7 | 0.3 ± 0.3 | 136 ± 9 | 7.1 ± 0.3 | 3.7 ± 0.4 | 236 ± 52 | 5.9 ± 0.3 |
I.n. treatments were given twice a day for 4 days starting at the time indicated relative to virus exposure.
Mean day of death of mice that died prior to day 21 of the infection.
Log10 CCID50/g.
P<0.05,
P<0.01,
P<0.001, compared to placebo-treated controls.
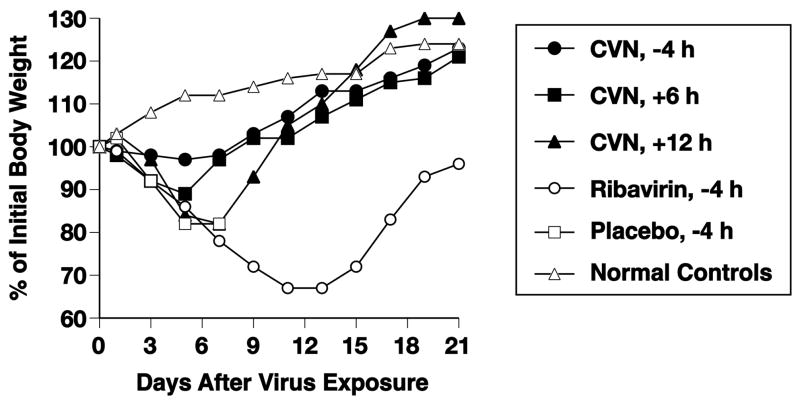
Mean body weights during the infections are presented in Figure 1. CV-N treatments starting at −4 or +6 h resulted in minimal weight loss. CV-N treatments starting 12 h after infection resulted in more substantial weight loss prior to the recovery phase of the two animals that survived. Mice treated with ribavirin lost considerable weight before the survivors rebounded starting after day 13.
Figure 1.
Effects of i.n. treatment with CV-N and ribavirin on mean body weights during a lethal influenza A/WSN/33 HAnc-Asp225Gly (H1N1) virus infection in mice. I.n. treatments (CV-N, 0.5 mg/kg/day; ribavirin, 75 mg/kg/day) were given twice daily for 4 days starting at the indicated times relative to virus exposure. Initial body weights of treated mice were 12.1 ± 0.2 g. Note: normal controls are uninfected, untreated mice.
3.3. Treatment of infections in mice by s.c. route
Infected mice were treated s.c. with CV-N at a non-toxic dose of 5 mg/kg/day or with ribavirin at 75 mg/kg/day, twice a day for 7 days starting 4 h prior to virus exposure. Survival rates were as follows: CV-N – 10%, ribavirin – 100%, and placebo –30% (data not shown). The lack of efficacy of CV-N by s.c. route suggests failure to reach the lungs in sufficient concentration to be active.
3.4. Treatment of ferrets
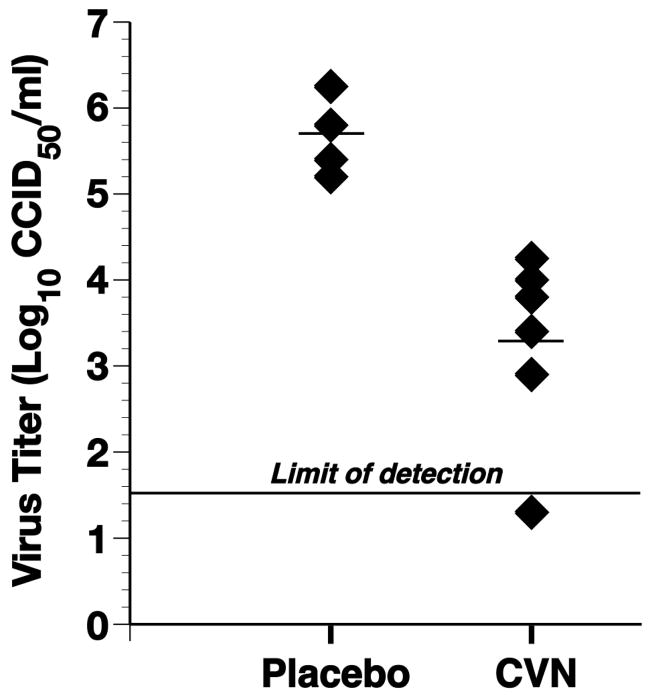
Ten ferrets were challenged with the influenza A/Charlottesville (H1N1) virus at a high dose of 105 CCID50. CV-N was administered to six ferrets i.n. starting 24 h before virus exposure. On the day of virus challenge, the compound was administered i.n. 4 h before and 4 h after virus challenge followed by twice daily treatment for 3 additional days. The remaining four ferrets were used as a control and received PBS only. In the placebo-treated control group, the nasal wash virus titers peaked on day 1 post infection and ranged from 5.5 log10CCID50/ml to 6.2 log10 CCID50/ml (Figure 2). On day 1 post infection, the viral titers were lower (ranged from 2.9 to 4.2 log10 CCID50/ml) in the six CV-N-treated ferrets compared to the placebo-treated animals (P<0.01). No virus was recovered from one of the CV-N-treated ferrets on day 1 post infection. Thereafter (days 2–7), there was no statistically significant difference in the viral titers between the placebo-and the CV-N-treated groups (data not shown). CV-N was well tolerated and no signs of apparent toxicity were observed at the dose administered. Proof-of-principal was obtained from this experiment, although the antiviral effect observed was moderate.
Figure 2.
Effects of i.n. treatment with CV-N on nasal virus titers recovered from ferrets on day 1 of a non-lethal influenza A/Charlottesville/31/95 (H1N1) virus infection. I.n. treatments with CV-N (100 μg/animal) or placebo were given twice daily for 7 days starting 24 h prior to virus exposure. On the day of infection CV-N was administered 4 h prior to and 4 h after virus exposure. Mean virus titers are shown by the horizontal bars. The difference between CV-N-treated and placebo is statistically significant (P<0.01).
3.5. CV-N neutralization of virus recovered from ferrets
Replication in a new host (ferret) may result in the loss of CV-N’s ability to neutralize virus infectivity, as described for mice (Smee et al., 2008). To investigate this possibility, we tested the virus in nasal washes collected from the infected ferrets that did not receive CV-N-treatment. The infectivity of this virus was completely neutralized after 30 min pre-incubation with CV-N (4.5 log10 CCID50/ml before pre-incubation with CV-N vs. <1.4 log10 CCID50/ml after pre-incubation). These data indicate that the A/Charlottesville/95 virus retains susceptibility to CV-N following a single passage in ferrets.
4. Discussion
In this report we demonstrated that a hybrid (recombinant) influenza A/WSN × A/New Caledonia HA virus was susceptible to inhibition by CV-N in an mouse model. Inhibition occurred with prophylaxis and early treatment after infection, but not when treatment initiation was delayed to 12 h post-infection. The hybrid (recombinant) virus should be useful for evaluating in vivo other carbohydrate binding agents the have a similar binding mode of action for the influenza virus HA1 molecule.
Activity of CV-N in the mouse model was associated with reduction in lung virus titers and pneumonitis, as well as with improved body weight during the infection. The 1000-fold reduction in virus titer (compared to placebo) on day 3 of the infection seen with the -4 h start of treatment was clearly superior to that of ribavirin (30-fold reduction), and exceeded the degree of inhibition generally reported for other antiviral compounds in the scientific literature (e.g., Sidwell et al., 2001; Smee et al., 2004). The higher virus titers observed on day 6 are likely attributed to the ability of virus to replicate to high titers after cessation of treatment, since CV-N treatments were only given through day 3 of the infection. CV-N treatment may simply have altered the dynamics of influenza replication in mice by shifting the timing of the peak viral load from day 3 to a later day. In this respect, the antiviral activity of CVN is not different from that of the neuraminidase inhibitor oseltamivir (10 mg/kg) used to treat mice lethally infected with A/NWS/33 (H1N1) virus (Mendel et al., 1998). Noteworthy, the major outcome of both studies (the present study and Mendel et al., 1998) is the clear protective effect seen in the CVN or oseltamivir-treated animals. The reduction of the lung viral titers during the early days of infection (Day 3) seems to play a key role in the animals’ survival.
Antiviral activity of CV-N in the mouse model was demonstrated by i.n. but not s.c. treatments. Because CV-N is a large protein, it may not be able to penetrate well into the respiratory tract, making i.n. (or aerosol) treatments obligatory. In contrast, the ability of CV-N to reduce the severity of Ebola virus infection in mice may be attributed to the fact that this virus is found in the blood stream where CV-N can interact with it (Barrientos et al., 2003).
The positive control ribavirin was more protective in the first (Table 1, 70% survival) than in the second experiment (Table 2, 30% survival), although the same experimental conditions were employed. The delay in the time to death was long in the second experiment, but the treatment was not quite sufficient to keep them alive. The placebo-treated animals died 1 day sooner than in the first experiment, indicating that the group of mice were more quickly overcome by the infection. These results suggest that ribavirin at this dose and regimen is borderline in efficacy. Four days of i.n. treatment with ribavirin was not as effective as seven days of s.c. treatment that afforded 100% protection from death (paragraph 3.3).
Ferrets are susceptible to un-adapted human influenza viruses and develop signs of illness similar to those in humans (Potter et al., 1975; Sweet et al., 1991; Sidwell and Smee, 2000). Several important features (e.g., similarity of the receptor structure of the ferret and the human respiratory epithelial cell) make a ferret a viable preclinical model for testing of drug and vaccine candidates (Fenton et al., 1977; Mendel et al., 1998; Mishin et al., 2005a). The usefulness of the ferret model for the assessment of the influenza virus susceptibility to drug candidates is well recognized. The results of our studies provide proof-of-principal for anti-influenza potential of CV-N in this animal model.
The observed antiviral effect of CV-N was superior to that achieved by the treatment of ferrets infected with influenza A (H3N2) virus and treated with oseltamivir, the potent oral NA inhibitor (less than a 3-fold reduction in the nasal wash virus titers), when given at 5 mg/kg twice daily starting 2 hours prior to infection (Sweet et al., 2002). In another study, oseltamivir-treatment of ferrets with the higher dose (25/mg/kg) was accompanied by a 8-fold reduction in the nasal wash virus titers (Mendel et al., 1998). Side-by-side assessment of antiviral potencies of CV-N and NA inhibitors would be desirable. CV-N exerted a potent inhibitory effect on the human influenza virus replication in the ferret upper respiratory tract. The i.n. delivery of CV-N (and other drugs) has certain limitations such as insufficient and uneven drug distribution within the ferret respiratory tract. Therefore, we anticipate that alternative routes of delivery of the compound could potentially produce a more potent antiviral effect in the ferret model.
A single passage in the ferret did not result in loss of ability of CV-N to neutralize virus recovered from nasal washes. Virus is not generally passaged from ferret to ferret to enhance its virulence as is done with mice, probably due to expense of ferrets. It took multiple passages in mice to develop virus that was resistant to CV-N, and the same may apply to ferrets.
In summary, CV-N demonstrated antiviral activity in mice and ferrets by i.n. delivery and early initiation of treatment, causing reductions in mortality and pneumonitis in mice, and nasal virus titer reductions in ferrets. CV-N lacked activity by the s.c. treatment route in the mouse model, suggesting poor ability to reach the lungs. CV-N may have potential through the inhalation route for the prophylaxis or early treatment of influenza virus infections in humans.
Acknowledgments
This work was supported in part by contract NO1-AI-15435 from the Virology Branch, NIAID, NIH, by the Intramural Research Program of the National Cancer Institute, NIH, and by the Japanese Society for the Promotion of Science. The contents of this article do not necessarily reflect the position or policy of the government and no official endorsement should be inferred. The animal experiments were conducted in animal care facilities accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, International.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balzarini J. Carbohydrate-binding agents: a potential future cornerstone for the chemotherapy of enveloped viruses? Antivir Chem Chemother. 2007;18:1–11. doi: 10.1177/095632020701800101. [DOI] [PubMed] [Google Scholar]
- Barrientos LG, O’Keefe BR, Bray M, Sanchez A, Gronenborn AM, Boyd MR. Cyanovirin-N binds to the viral surface glycoprotein, GP1,2 and inhibits infectivity of Ebola virus. Antivir Res. 2003;58:47–56. doi: 10.1016/s0166-3542(02)00183-3. [DOI] [PubMed] [Google Scholar]
- Boyd MR, Gustafson KR, McMahon JB, Shoemaker RH, O’Keefe BR, Mori T, Gulakowski RJ, Wu L, Rivera MI, Laurencot CM, Currens MJ, Cardellina JH, II, Buckheit RW, Jr, Nara PL, Pannell LK, Sowder RC, II, Henderson LE. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob Agents Chemother. 1997;41:1521–1530. doi: 10.1128/aac.41.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichelberger MC. The cotton rat as a model to study influenza pathogenesis and immunity. Viral Immunol. 2007;20:243–249. doi: 10.1089/vim.2007.0017. [DOI] [PubMed] [Google Scholar]
- Fenton RJ, Bessell C, Spilling CR, Potter CW. The effects of peroral or local aerosol administration of 1-aminoadamantane hydrochloride (amantadine hydrochloride) on influenza infections of the ferret. J Antimicrob Chemother. 1977;3:463–472. doi: 10.1093/jac/3.5.463. [DOI] [PubMed] [Google Scholar]
- Hoffmann E, Neumann G, Kawaoka Y, Hobom G, Webster RG. A DNA transfection system for generation of influenza A virus from eight plasmids. PNAS. 2000;97:6108–6113. doi: 10.1073/pnas.100133697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendel DB, Tai CY, Escarpe PA, Li W, Sidwell RW, Huffman JH, Sweet C, Jakeman KJ, Merson J, Lacy SA, Lew W, Williams MA, Zhang L, Chen MS, Bischofberger N, Kim CU. Oral administration of a prodrug of the influenza virus neuraminidase inhibitor GS 4071 protects mice and ferrets against influenza infection. Antimicrob Agents Chemother. 1998;42:640–646. doi: 10.1128/aac.42.3.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishin VP, Nedyalkova MS, Hayden FG, Gubareva LV. Protection afforded by intranasal immunization with the neuraminidase-lacking mutant of influenza A virus in a ferret model. Vaccine. 2005a;23:2922–2927. doi: 10.1016/j.vaccine.2004.11.058. [DOI] [PubMed] [Google Scholar]
- Mishin VP, Novikov D, Hayden FG, Gubareva LV. Effect of hemagglutinin glycosylation on influenza virus susceptibility to neuraminidase inhibitors. J Virol. 2005b;79:12416–24. doi: 10.1128/JVI.79.19.12416-12424.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Gustafson KR, Pannell LK, Shoemaker RH, Wu L, McMahon JB, Boyd MR. Recombinant production of cyanovirin-N, a potent human immunodeficiency virus-inactivating protein derived from a cultured cyanobacterium. Protein Expr Purif. 1998;12:151–158. doi: 10.1006/prep.1997.0838. [DOI] [PubMed] [Google Scholar]
- O’Keefe BR, Smee DF, Turpin JA, Saucedo CJ, Gustafson KR, Mori T, Blakeslee D, Buckheit R, Boyd MR. Potent anti-influenza activity of cyanovirin-N and interactions with viral hemagglutinin. Antimicrob Agents Chemother. 2003;47:2518–2525. doi: 10.1128/AAC.47.8.2518-2525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter CW, McLaren C, Jennings R. Assessment of resistance to influenza virus infection in animal models. Dev Biol Stand. 1975;28:307–318. [PubMed] [Google Scholar]
- Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- Shenoy SR, O’Keefe BR, Bolmstedt AJ, Cartner LK, Boyd MR. Selective interactions of the human immunodeficiency virus-inactivating protein cyanovirin-N with high-mannose oligosaccharides on gp120 and other glycoproteins. J Pharmacol Exp Ther. 2001;297:704–710. [PubMed] [Google Scholar]
- Sidwell RW, Smee DF. In vitro and in vivo assay systems for study of influenza virus inhibitors. Antivir Res. 2000;48:1–16. doi: 10.1016/s0166-3542(00)00125-x. [DOI] [PubMed] [Google Scholar]
- Sidwell RW, Smee DF, Huffman JH, Barnard DL, Bailey KW, Morrey JD, Babu YS. In vivo virus-inhibitory effects of the cyclopentane neuraminidase inhibitor RWJ-201201. Antimicrob Agents Chemother. 2001;45:749–757. doi: 10.1128/AAC.45.3.749-757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidwell RW, Bailey KW, Wong MH, Barnard DL, Smee DF. In vitro and in vivo influenza virus-inhibitory effects of viramidine. Antivir Res. 2005;68:10–17. doi: 10.1016/j.antiviral.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Smee DF, Wandersee MK, Wong MH, Bailey KW, Sidwell RW. Treatment of mannan-enhanced influenza B virus infections in mice with oseltamivir, ribavirin, and viramidine. Antivir Chem Chemother. 2004;15:261–268. doi: 10.1177/095632020401500505. [DOI] [PubMed] [Google Scholar]
- Smee DF, Wandersee MK, Checketts MB, O’Keefe BR, Saucedo C, Boyd MR, Mishin VP, Gubareva LV. Influenza A (H1N1) virus resistance to cyanovirin-N arises naturally during adaptation to mice and by cell culturing in the presence of the inhibitor. Antivir Chem Chemother. 2007;18:317–327. doi: 10.1177/095632020701800604. [DOI] [PubMed] [Google Scholar]
- Sweet C, Hayden FG, Jakeman KJ, Grambas S, Hay AJ. Virulence of rimantadine-resistant human influenza A (H3N2) viruses in ferrets. J Infect Dis. 1991;164:969–972. doi: 10.1093/infdis/164.5.969. [DOI] [PubMed] [Google Scholar]
- Sweet C, Jakeman KJ, Bush K, Wagaman PC, McKown LA, Streeter AJ, Desai-Krieger D, Chand P, Babu YS. Oral administration of cyclopentane neuraminidase inhibitors protects ferrets against influenza virus infection. Antimicrob Agents Chemother. 2002;46:996–1004. doi: 10.1128/AAC.46.4.996-1004.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CC, Emau P, Jiang Y, Tian B, Morton WR, Gustafson KR, Boyd MR. Cyanovirin-N gel as a topical microbicide prevents rectal transmission of SHIV89.6P in macaques. AIDS Res Hum Retroviruses. 2003;19:535–541. doi: 10.1089/088922203322230897. [DOI] [PubMed] [Google Scholar]
- Tsai CC, Emau P, Jiang Y, Agy MB, Shattock RJ, Schmidt A, Morton WR, Gustafson KR, Boyd MR. Cyanovirin-N inhibits AIDS virus infections in vaginal transmission models. AIDS Res Hum Retroviruses. 2004;20:11–18. doi: 10.1089/088922204322749459. [DOI] [PubMed] [Google Scholar]
- Tumpey TM, Maines TR, Van Hoeven N, Glaser L, Solorzano A, Pappas C, Cox NJ, Swayne DE, Palese P, Katz JM, Garcia-Sastre A. A two-amino acid change in the hemagglutinin of the 1918 influenza virus abolishes transmission. Science. 2007;315:655–659. doi: 10.1126/science.1136212. [DOI] [PubMed] [Google Scholar]