Abstract
The fat-derived hormone adiponectin has been shown to have a protective role in macrovascular disorders. However, nothing is known about the function of adiponectin in retinal microvessel disease. Here, we investigated the causal role of adiponectin in retinal vessel formation and inflammation under conditions of hypoxia. When neonatal mice were subjected to ischemia-induced retinopathy, pathological retinal neovascularization during ischemia was exacerbated in adiponectin-knockout (APN-KO) mice compared with wild-type mice (neovascular area: 17.0±1.0% versus 11.7±0.6%, respectively). APN-KO mice also exhibited increased leukocyte adhesion (2.3±0.4-fold) and tumor necrosis factor (TNF)-α expression (2.6±0.2-fold) in hypoxic retina. Adenovirus-mediated overexpression of adiponectin in wild-type and APN-KO mice attenuated hypoxia-induced pathological retinal neovascularization by 35% in wild-type mice and by 40% in APN-KO mice and leukostasis by 64% in wild-type mice and by 75% in APN-KO mice, which were associated with reduced TNF-α production. TNF-α blockade diminished the enhanced pathological neovascularization in APN-KO mice by 34%, and the inhibitory effects of adiponectin overexpression on retinal neovascularization and leukocyte adhesion were abolished in mice lacking TNF-α. These data provide evidence that adiponectin protects against retinal vessel injury following pathological stimuli through modulation of TNF-α inflammatory responses.
Keywords: adiponectin, neovascularization, ischemia, inflammation, angiogenesis
Pathological retinal neovascularization is a common feature of blinding diseases, in particular, ischemic retinopathies such as retinopathy of prematurity and diabetic retinopathy.1,2 In premature infants receiving high oxygen, the retinal vascularization does not reach to the periphery, and the peripheral retinas are rendered hypoxic, ultimately resulting in stimulation of excessive abnormal neovascularization.2 In the early phase of diabetic retinopathy, hyperglycemia initiates endothelial cell injury, retinal vessel loss, and ischemia, as well as changes in leukocyte adhesion to the vascular endothelium.1,3 These conditions subsequently lead to the overproduction of various proangiogenic factors and proinflammatory cytokines, which, in turn, promotes abnormal neovascular changes.2 The primary goal for treatment of ischemic retinopathy is to preserve vision through the inhibition of abnormal neovascularization and vascular damage.
Adiponectin/ACRP30 is a circulating adipose-derived cytokine with antiinflammatory properties.4,5 In animal models, adiponectin deficiency is associated with the increased inflammatory responses under conditions of stresses including overnutrition and ischemic insult.6,7 In addition, adiponectin has been shown to protect against the development of various diseases including detrimental cardiac and vascular remodeling, ischemic stroke and increased albuminuria.6,8–11 In human populations, circulating adiponectin levels inversely correlate with the inflammatory marker C-reactive protein levels in blood stream.4,12,13 Low plasma adiponectin levels are associated with the increased prevalence of type 2 diabetes and its macrovascular complications including ischemic heart disease.4,14,15 However, the epidemiology of adiponectin is complex, and high levels of adiponectin are associated with the presence of chronic inflammatory diseases including rheumatoid arthritis and type 1 diabetes, as well as advanced heart failure.16,17
Clinical studies regarding the relationship between plasma adiponectin level and retinopathy in diabetes have been inconclusive.18–20 In this regard, the consequences of adiponectin on retinal vessel disease have not been examined previously in any experimental model. Thus, we investigated whether adiponectin affects the retinal vascularization and inflammation in a mouse model of ischemia-induced retinopathy. This model involves the exposure of neonatal mice to 75% oxygen, followed by a return to normoxic conditions.21 This model is routinely used for the screening of physiological mediators of retinal vascularization, and it was used to show that therapies that inhibit vascular endothelial growth factor (VEGF) signaling may have potential for human ischemic retinopathy.22–24
Materials and Methods
Materials
Mouse tumor necrosis factor (TNF)-α ELISA kit was purchased from R&D systems. Recombinant mouse adiponectin was prepared as described previously.6,25 Adenovirus vectors containing the gene for β-galactosidase (Ad-βgal) and full-length mouse adiponectin (Ad-APN) were prepared as described previously.7
Mouse Model of Ischemia-Induced Retinopathy
Adiponectin-knockout (APN-KO)7 and wild-type (WT) mice in a C57BL/6 background and TNF-α-KO and WT mice in a C57BL/6/129 background (The Jackson Laboratory) were used (n=6 to 20 for each experiment). Both genders were used. All animal studies were performed in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research, and were approved by the Institutional Animal Care and Use Committee in Boston University. To produce a clinically relevant ischemic retinopathy model, postnatal mice were subjected to oxygen-induced retinopathy.21 Postnatal day (P)7 mice with their nursing mothers were exposed to in 75±2% oxygen (hyperoxia) for 5 days (P7 to P12) to produce retinal vasoobliteration. At P12, mice were returned to room air (normoxia), in which the nonvascular regions in retina are rendered hypoxic, and this condition promotes the development of pathological neovascular tufts within the vitreous body. P17 is the time when the maximal neovascularization occurs in this model. In some experiments, etanercept (Wyeth) (500 μg per mouse)26 (n=10 to 12) or vehicle (n=10) was intraperitoneally injected into mice at P12, P14, and P16.
Measurement of Neovascularization and Vascular Obliteration
Neovascularization and vascular obliteration were measured with well-established methods.26–28 Eyes were removed from mice at P17, and fixed in 4% paraformaldehyde for 1 hour at 4°C. The retinas were dissected and stained with fluoresceinated Griffonia (Bandeiraea) simplicifolia isolectin B4 (Alexa Fluor 594, Molecular Probes) for 16 hours at 4°C to detect vascular endothelial cells.26–28 The retinas were washed with PBS for 2 hours, radially cut from the edge of retina in the 4 quadrants, and mounted in mounting media (Vector Laboratories). Pictures of whole mounts of retinas were taken at ×5 magnification by fluorescence microscopy. Retinal segments were merged to generate a whole retinal image using Adobe Photoshop. We traced central nonperfused regions without capillaries and optic disc, and defined these areas as vascular “obliteration.” The areas in which the artifacts of dissection and mounting were present were excluded. Vascular obliteration and neovascular tuft formation were quantified by comparing the number of pixels in the affected areas with the total number of pixels in the retina. The physiological normal vascularization was calculated by subtracting the neovascular and vascular obliteration areas from total retinal areas.29 Investigators were blinded to the mouse treatment.
Adenovirus-Mediated Gene Transfer
The 4×107 plaque-forming units (pfu) of Ad-APN (n=6 to 20) or Ad-βgal (n=6 to 20) were injected into the jugular vein of mice at P10. Mouse adiponectin levels were determined by ELISA kit (Otsuka Pharmaceutical Co Ltd).
Administration of Recombinant Adiponectin Protein
Recombinant mouse adiponectin protein (3.0 μg/g body weight) (n=9 to 10) or vehicle (n=9) was intraperitoneally injected into WT and APN-KO mice at the day of ischemic induction (P12) or P13 and daily until P17.
Quantification of Retinal Leukostasis
The retinal vasculature and adherent leukocytes were labeled with FITC-conjugated concanavalin A (Con A) lectin (Vector laboratory).30,31 In brief, mice were perfused with PBS to remove the erythrocytes and nonadherent leukocytes in the retinal vasculature and then perfused with FITC–Con A lectin, followed by perfusion with PBS to remove unbound Con A lectin (n=12 to 20 for each experiment). The retinas were flat-mounted and imaged with fluorescence microscopy. The number of adherent leukocytes in the retinal vasculature was counted.
Determination of mRNA Levels
Total RNA was isolated from retinas of WT mice (n=6 to 8) and APN-KO mice (n=6 to 8) using a Qiagen kit, and cDNA was produced using ThermoScript RT-PCR Systems (Invitrogen). Quantitative real-time PCR was performed on iCycler iQ Real-Time PCR Detection System (Bio-Rad) using SYBR Green I as a double-stranded DNA–specific dye as described previously.6,32 Primers were: 5′-CATCTTCTCAAAATTCGAGTGACAA-3′ and 5′-TGGGAGTAGACAAGGTACAACCC-3′ for mouse TNF-α; 5′-CTGTAACGATGAAGCCCTGGAG-3′ and 5′-TGGTGAGGTTTGATCCGCAT-3′ for mouse VEGF; 5′-CATTCTTCGCTGCCATTCTG-3′ and 5′-GCACATTGCCCATGTTGAATC-3′ for mouse angiopoietin (Ang) 1; 5′-TTAGCACAAAGGATTCGGACAAT-3′ and 5′-TTTTGTGGGTAGTACTGTCCATTCA-3′ for mouse Ang 2; 5′-TGTCTGCGGCGATGTCACT-3′ and 5′-CATGCCGCCCTCTGTTG-3′ for mouse endothelial nitric oxide synthase (eNOS); 5′-CACCTGCCAGGCCTGCAA-3′ and 5′-GCTTGGTGCAGGCGCCTA-3′ for mouse VEGF receptor 2; 5′-AGAGGGAAATCGTGCGTGAC-3′ and 5′-CAATAGTGATGACCTGGCCGT-3′ for mouse β-actin.
Statistical Analysis
All data are expressed as means±SEM. Differences were analyzed by Student’s unpaired t test for 2 groups or ANOVA for multiple comparisons. A level of P<0.05 was accepted as statistically significant.
Results
Exacerbated Pathological Retinal Neovascularization During Ischemia in APN-KO Mice
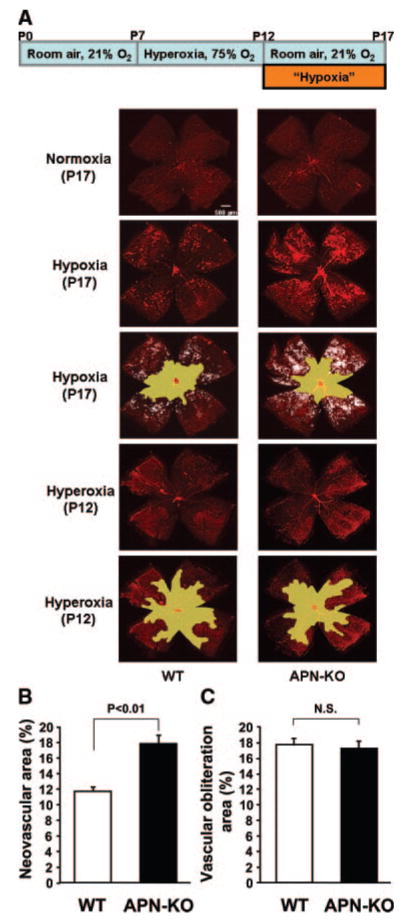
To test the role of adiponectin in regulating retinal vessel growth, APN-KO and WT mice in a C57BL/6 background were subjected to a model of ischemia-driven retinopathy. Neonatal mice were exposed to hyperoxia (75% O2) for 5 days from P7 to P12 and returned to room air at P12 to induce pathological neovascular tufts.21 Retinas were stained with fluoresceinated isolectin B4 to detect ischemia-induced vascularization at P17 in the 2 experimental groups of mice. Representative photographs of retinal whole mounts, stained with fluorescein-labeled isolectin B4, are shown in Figure 1A. Quantitative analysis of neovascular tufts/total retinal area revealed that APN-KO mice display exacerbated pathological retinal neovascular formation compared with WT mice (Figure 1B). In contrast, no significant difference was observed in vascular obliteration/total retinal area between 2 groups (Figure 1C). APN-KO mice exhibited reduced areas of physiological normal vascularization in ischemic retina (65.8±1.3%) compared with WT mice (70.6±1.0%). Thus, endogenous adiponectin is protective in the context of pathological retinal neovascularization under ischemic conditions. No differences in these parameters were observed between male and female mice. In contrast, under normoxic conditions, both APN-KO and WT mice had no central avascular area and no peripheral pathological vascular tuft formation (Figure 1A). At P12, before the return to room air, there was no neovascular tuft formation in both mouse strains (Figure 1A), and no significant differences were seen in vascular obliteration areas between WT (39.0±0.7%) and APN-KO (38.2±0.9%) mice.
Figure 1.
Enhanced pathological retinal neovascularization in response to ischemia in APN-KO mice. A, Mouse model of oxygen-induced retinopathy (top). Representative photographs of retinas stained with isolectin in WT (left) and APN-KO mice (right) at P17 (normoxia and hypoxia) or P12 (hyperoxia) are shown. Mice at P7 were reared in 75% oxygen (hyperoxia) for 5 days, followed by room air for 5 days. Retinas at P17 or P12 were isolated and stained with fluoresceinated isolectin B4 to detect vascularization. Vascular obliteration area is highlighted in yellow. Neovascular tuft area is highlighted in white. Magnification, ×5. B and C, Quantification of retinal neovascularization (B) and vascular obliteration (C) in WT (n=16) and APN-KO (n=20) mice at P17. Neovascularization and vascular obliteration were quantified by comparing the number of pixels in the neovascular tuft and vascular obliteration areas, respectively, with the total number of pixels in the retina. Data are presented as means±SEM.
Delivery of Adiponectin Suppresses Pathological Neovascularization in Ischemic Retina
To confirm that the enhanced pathological neovascularization in response to ischemia was attributable to adiponectin deficiency, we systemically delivered adenoviral vectors expressing either murine adiponectin (Ad-APN) or β-galactosidase (Ad-βgal) as a control into APN-KO and WT mice at P10. At P17, adiponectin levels were 11.0±1.0 μg/mL in WT/control, 20.6±1.5 μg/mL in WT/Ad-APN, <0.05 μg/mL in APN-KO/control and 10.8±2.2 μg/mL in APN-KO/Ad-APN. Treatment with Ad-APN significantly reduced retinal neovascular areas in APN-KO at P17 to the similar levels that were observed in WT mice (Figure 2A). Ad-APN–treated WT mice showed a significant reduction of neovascular areas in retina compared with Ad-βgal–treated WT mice. Thus, supplementation of exogenous adiponectin rescues the increase in pathological retinal neovascularization in APN-KO mice, and overproduction of adiponectin attenuates ischemia-induced abnormal vessel formation in retina in WT mice.
Figure 2.
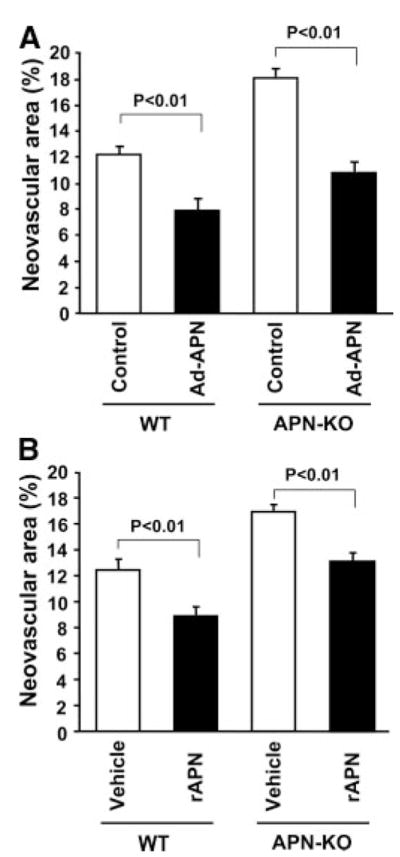
Exogenous adiponectin reduces pathological vessel formation in ischemic retina. A, Adenovirus-mediated supplementation of adiponectin attenuates pathological retinal neovascularization in APN-KO and WT mice. Adenoviral vectors expressing adiponectin (Ad-APN) (4×107 pfu total, n=10) or β-galactosidase (Ad-βgal) (control, n=10) were delivered intravenously via the jugular vein at P10. B, Administration of adiponectin protein reduces retinal neovascularization in APN-KO and WT mice. Recombinant APN protein (rAPN) (3.0 μg/g body weight) (n=9 to 10) or vehicle (n=9) was intraperitoneally injected into APN-KO and WT mice daily from P12 to P 17. Data are presented as means±SEM.
To test whether the administration of adiponectin protein affects the vascular response during ischemic retinopathy, we intraperitoneally administered recombinant mouse adiponectin proteins (3.0 μg/g mouse) to APN-KO and WT mice daily from P12 (at the induction of hypoxia) to P17. Circulating adiponectin levels transiently increased to 8.4±0.8 μg/mL in APN-KO mice at 3 hours after intraperitoneal injection of recombinant adiponectin protein. Treatment with adiponectin protein significantly reduced the increase in retinal neovascular areas in APN-KO mice at P17 (Figure 2B). At 3 hours after intraperitoneal injection of recombinant adiponectin protein into WT mice, circulating adiponectin levels transiently increased by a factor of 1.8±0.3 compared with vehicle-treated mice. As shown in Figure 2B, adiponectin-treated WT mice had significantly diminished neovascular formation in ischemic retina by 29% at P17 compared with vehicle-treated mice. Daily injection of adiponectin protein from P13, 1 day after the induction of ischemia, also resulted in a reduction of retinal neovascular areas of WT mice (22%) at P17 (neovascular area: 12.4±0.9% in vehicle-treated mice and 9.7±0.7% in adiponectin-treated mice). Thus, adiponectin supplementation, either before or after the ischemic insult, is effective at attenuating ischemia-induced retinopathy.
Adiponectin Deficiency Contributes to Enhanced Leukocyte Adhesion to Retinal Vessels
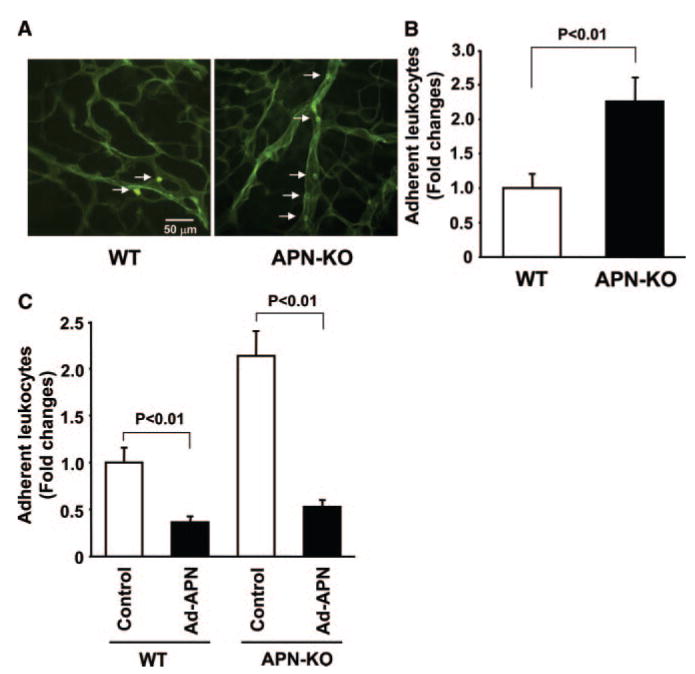
Because inflammation plays an important role in the progression of retinal pathological angiogenesis,29,30,33 the inflammatory responses in retina were assessed by measuring adherent leukocytes labeled with fluorescein-conjugated Con A lectin.30,31 Figure 3A shows representative photographs of retinal vasculature and adherent leukocytes labeled with FITC–Con A lectin in WT and APN-KO mice. Quantitative analysis revealed that APN-KO mice had a higher frequency of adherent leukocytes to vasculature at P15 compared with WT mice (Figure 3B). Conversely, Ad-APN treatment decreased the number of adherent leukocytes in the retinal vasculature in both APN-KO and WT mice (Figure 3C).
Figure 3.
Exacerbated retinal leukostasis in APN-KO mice. A, Representative photographs of retinal vasculature and adherent leukocytes (arrows) labeled with FITC-conjugated Con A lectin in WT (left) and APN-KO (right) mice at P15. B, Quantitative analysis of leukocyte adhesion in ischemic retina in WT (n=18) and APN-KO (n=20) mice. Leukocyte adhesion was assessed by measurement of the number of adherent leukocytes in retina at P15. Results are presented as means±SEM. C, Adenovirus-mediated delivery of adiponectin attenuates leukocyte adhesion in APN-KO and WT mice. Ad-APN or Ad-βgal (control) at 4×107 pfu was intravenously injected into mice at P10. Results are presented as means±SEM (n=12 to 20).
Increased TNF-α Production in Ischemic Retina in APN-KO Mice
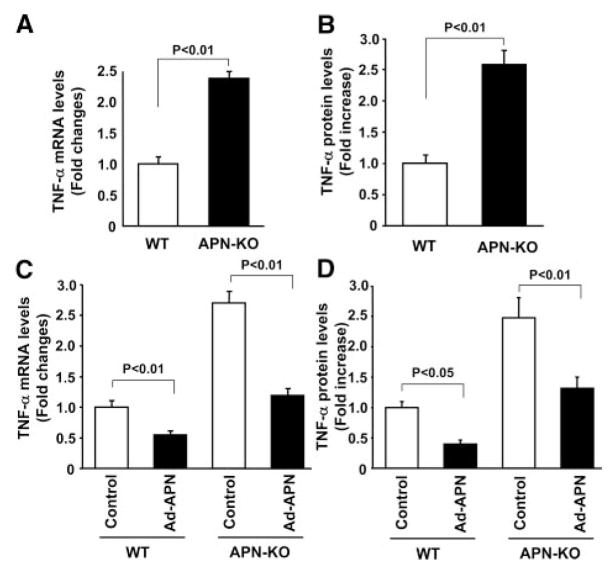
To elucidate the mechanism of the protective action of adiponectin on abnormal neovascularization during ischemic retinopathy, we analyzed the expression of angiogenesis-related and proinflammatory factors in ischemic retina in WT and APN-KO mice at P13, 1 day after return to normoxia, by quantitative real-time PCR methods. Expression of VEGF, Ang 1, Ang 2, eNOS, and VEGF receptor 2 did not differ between WT and APN-KO mice (fold changes in transcripts in APN-KO mice versus WT mice: VEGF, 1.20±0.14; Ang 1, 0.88±0.09; Ang 2, 1.09±0.10; eNOS, 0.95±0.09; VEGF receptor 2, 0.94±0.07). In contrast, APN-KO mice showed a marked increase in retinal TNF-α mRNA levels compared with WT mice (Figure 4A). TNF-α protein levels in ischemic retina were also increased in APN-KO mice at P14 compared with WT mice (Figure 4B).
Figure 4.
Elevated TNF-α expression in ischemic retina in APN-KO mice. A, Gene expression levels of TNF-α in retina at P13. Retinas were harvested from eyes of WT (n=7) or APN-KO (n=8) mice at P13. TNF-α mRNA levels were determined by quantitative real-time PCR methods and expressed relative to β-actin mRNA levels. Data are presented as means±SEM. B, TNF-α protein expression in ischemic retina in WT and APN-KO mice at P14. TNF-α protein levels were determined by ELISA. Data are presented as means±SEM (n=8). C and D, Adiponectin supplementation reduces retinal TNF-α mRNA (C) and protein (D) levels at P14 in WT and APN-KO mice. Ad-APN (4×107 pfu total) or Ad-βgal (control) was delivered intravenously at P10. TNF-α mRNA levels were measured by quantitative real-time PCR methods and expressed relative to β-actin mRNA levels. TNF-αprotein levels were determined by ELISA. Data are presented as means±SEM (n=6).
To test whether increased adiponectin expression could decrease TNF-α production in retina, retinal levels of TNF-α mRNA and protein were determined at P14 in WT and APN-KO mice treated with Ad-APN or Ad-beta; gal. Ad-APN treatment markedly decreased TNF-α transcript levels in the retina of both WT and APN-KO mice (Figure 4C). Similarly, Ad-APN treatment reduced TNF-α protein levels in ischemic retina in WT and APN-KO mice (Figure 4D).
Role of TNF-α in Adiponectin-Mediated Suppression of Pathological Neovascularization
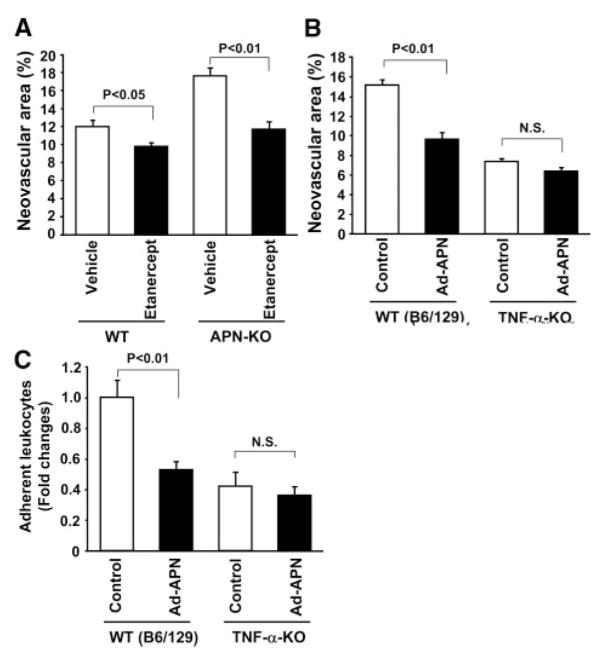
To assess whether inhibition of TNF-α signaling can rescue the retinal vascular phenotype of the APN-KO mice, we examined the impact of a TNF receptor fusion protein (etanercept) on retinal neovascularization in WT and APN-KO mice. Treatment with etanercept reduced the retinal neovascular area of WT mice and attenuated the increase in retinal neovascular formation of APN-KO mice to the levels similar to WT mice (Figure 5A).
Figure 5.
Contribution of TNF-α signaling to adiponectin-mediated retinal protection. A, TNF receptor fusion protein etanercept inhibits retinal neovascularization at P17 in WT and APN-KO mice. Etanercept (500 μg/mouse) or vehicle was intraperitoneally injected into mice at P12, P14, and P16. Data are presented as means±SEM (n=10 to 12). B and C, Effect of adiponectin on retinal neovascularization (B) and leukocyte adhesion (C) in TNF-α-KO mice. TNF-α-KO and WT mice were subjected to ischemia-induced retinopathy. Retinal neovascular areas were quantified at P17. Adherent leukocytes to retinal vasculature were measured by Con A lectin staining at P15. Mice were intravenously treated with Ad-APN or Ad-βgal (control) (4×107 pfu each) at P10. Data are presented as means±SEM (n=12 to 20).
To further examine the involvement of TNF-α in retinal protection by adiponectin, we assessed the consequences of adiponectin overexpression on retinal neovascularization under conditions of TNF-α deficiency. TNF-α-KO and WT mice in a background of C57BL/6/129 were subjected to oxygen-induced retinopathy, and Ad-APN or Ad-βgal (control) was systemically delivered at P10. At P17, adiponectin levels were 8.1±0.4 μg/mL in WT (C57BL/6/129)/control, 17.8±4.2 μg/mL in WT (C57BL/6/129)/Ad-APN, 6.2±0.6 μg/mL in TNF-α-KO/control, and 16.2±2.5 μg/mL in TNF-α-KO/Ad-APN. Compared with WT mice, TNF-α-KO mice showed reduced retinal neovascular area (Figure 5B), consistent with a previous report.26 Ad-APN treatment significantly attenuated TNF-α protein levels in ischemic retina by 55% in WT (C57BL/6/129) mice that is similar to the effect of Ad-APN treatment on WT (C57BL/6) mice (60%) (Figure 4D). Although treatment with Ad-APN attenuated the retinal neovascular areas of WT mice at P17 (36%), the adiponectin-mediated decrease in neovascularization was diminished in TNF-α-KO mice (14%) (Figure 5B). Similar results were obtained when recombinant adiponectin proteins were intra-peritoneally injected at P12 and daily until P17 (percentage decrease in neovascular areas: 28% in WT mice and 15% in TNF-α-KO mice). We also assessed leukocyte adhesion to retinal vessels in TNF-α-KO and WT mice following treatment with adiponectin. TNF-α-KO mice had a smaller proportion of adherent leukocytes at P15 compared with WT mice (Figure 5C). In contrast to the marked inhibition of leukostasis by Ad-APN in WT mice (47%), administration of Ad-APN did not result in significant reduction of adherent leukocytes in TNF-α-KO mice (13%) (Figure 5C). Thus, the effect of adiponectin on ischemic retinopathy is markedly reduced under the conditions of TNF-α ablation, suggesting a functionally significant role of TNF-α suppression in retinal protection by adiponectin.
Discussion
The present study for the first time provides in vivo evidence that the plasma protein adiponectin protects against ischemia-driven pathological retinal microvessel formation and that this protection involves, at least in part, the downregulation of a TNF-α-mediated inflammatory response. Adiponectin deficiency contributed to severe pathological retinal neovascularization and enhanced leukocyte adherence in the retinal vessels during ischemia, which was associated with elevated TNF-α production. Conversely, adiponectin delivery led to decrease in retinal neovascularization, leukostasis, and TNF-α production. Thus, these observations indicate that adiponectin acts as an endogenous modulator of microvascular function and inflammation.
We have previously demonstrated that adiponectin suppresses lipopolysaccharide (LPS)-stimulated TNF-α production in cultured cardiac myocytes and macrophages.6,34,35 Adiponectin deficiency also leads to an increase in circulating TNF-α in mouse models.7,36 However, the functional significance of TNF-α suppression by adiponectin has never been addressed experimentally in vivo. Inhibition of TNF-α signaling by etanercept normalized the exacerbated neovascular response in APN-KO mice. Furthermore, the inhibitory effects of adiponectin overexpression on retinal neovascularization and inflammatory responses were abolished in TNF-α-KO mice. Thus, the beneficial actions of adiponectin on retinal injury are likely to be mediated by its ability to reduce TNF-α expression. It has been shown that macrophages and microglia are major sources of TNF-α in retina and that inhibition of TNF-α signaling confers resistance to pathological neovascularization and inflammation in a model of ischemic retinopathy.26,29,33,37 Furthermore, TNF-α is a key proinflammatory cytokine that strongly associates with the pathology of diabetic retinopathy.2,38,39 Taken together, these data suggest that adiponectin-mediated suppression of the TNF-α inflammatory responses can represent a common pathway contributing to the salutary effects of adiponectin on microvascular complications in the retina.
The present study provides the first in vivo documentation that adiponectin-mediated suppression of TNF-α inflammatory responses can functionally contribute to the beneficial actions of adiponectin on the vasculature. Adiponectin regulates TNF-α production by a number of mechanisms. Adiponectin has been shown to attenuate LPS-stimulated TNF-α production in macrophages by suppression of nuclear factor κB and early growth response protein 1 activation.40–42 It has also been shown that interleukin-10 induction by adiponectin is required for adiponectin-mediated reduction of LPS-induced TNF-α.43 Furthermore, adiponectin promotes removal of early apoptotic cells by macrophages, which is associated with the downregulation of TNF-α.36 In cardiac cells, adiponectin negatively regulates LPS-induced TNF-α production partly through its ability to modulate cyclooxygenase-2/prostaglandin E2– dependent pathway.6 Collectively, these in vitro studies demonstrate that adiponectin suppresses TNF-α production through the modulation of multiple cellular mechanisms.
Our data also show that loss of adiponectin causes increased leukocyte adhesion to retinal vasculature in response to ischemia. In addition, APN-KO mice exhibit elevated mRNA expression of intercellular adhesion molecule (ICAM)-1 in ischemic retina by a factor of 1.8±0.4 compared with WT mice (Higuchi A et al, unpublished data). Consistent with these findings, it has been reported that disruption of adiponectin is responsible for an increase in leukocyte adhesion and rolling in periintestinal venules in mice, which is associated with enhanced expression of endothelial adhesion molecules.44 We have previously shown that adiponectin protein attenuates TNF-α–stimulated expression of adhesion molecules including ICAM-1 in human aortic endothelial cells.15 ICAM-1 is a crucial mediator of leukocyte recruitment to injured vascular endothelium.45 Therefore, in addition to the inhibitory effect of adiponectin on TNF-α production, adiponectin may directly act on surface adhesion molecule expression on vascular endothelial cells, leading to the resolution of an inflammatory response in the vasculature.
Whereas it has been shown that low levels of circulating adiponectin are observed in preterm infants compared with full-term infants, presumably because of decreased body weight,46 nothing is known about adiponectin levels in infants with retinopathy of prematurity. Furthermore, the epidemiological data regarding the role of adiponectin in diabetic retinal complications do not provide straightforward conclusions. One clinical study has shown that patients with type 2 diabetes have lower adiponectin concentrations that are associated with the severity of diabetic retinopathy.18 In contrast, another study indicated that adiponectin level was not associated with the presence of diabetic retinopathy.19 Furthermore, a recent study demonstrated that adiponectin levels are positively correlated with the severity of retinopathy in patients with type 2 diabetes,20 perhaps because of secondary effects resulting from kidney complications, leading to impaired clearance of adiponectin. Thus, the complex pathology of diabetic retinopathy may confound the results of epidemiological studies that attempt to correlate the incidence of diabetic retinopathy with hypoadiponectinemia.
In summary, we used a widely used mouse model of oxygen-induced retinopathy that produces abnormal retinal vessel growth similar to that observed in the human ischemic retinopathies including retinopathy of prematurity and proliferative diabetic retinopathy.21 Experiments with genetic loss-of- and gain-of-function manipulations showed that adiponectin functions as a negative regulator of pathological microvessel formation in the retina. Furthermore, it was shown that the suppression of TNF-α expression by adiponectin is causally linked to the protective actions of this adipokine in the retina.
Acknowledgments
Sources of Funding
This study was funded by NIH grants HL77774, HL86785, AG15052, and HL81587 (to K.W.). N.O. was supported by an American Heart Association grant. A.H. was supported by a grant from the Uehara Memorial Foundation.
Footnotes
Disclosures
None.
References
- 1.Sheetz MJ, King GL. Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. JAMA. 2002;288:2579–2588. doi: 10.1001/jama.288.20.2579. [DOI] [PubMed] [Google Scholar]
- 2.Gariano RF, Gardner TW. Retinal angiogenesis in development and disease. Nature. 2005;438:960–966. doi: 10.1038/nature04482. [DOI] [PubMed] [Google Scholar]
- 3.Spranger J, Pfeiffer AF. New concepts in pathogenesis and treatment of diabetic retinopathy. Exp Clin Endocrinol Diabetes. 2001;109(suppl 2):S438–S450. doi: 10.1055/s-2001-18601. [DOI] [PubMed] [Google Scholar]
- 4.Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Walsh K. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol. 2003;14:561–566. doi: 10.1097/00041433-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 6.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 8.Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, Eto K, Yamashita T, Kamon J, Satoh H, Yano W, Froguel P, Nagai R, Kimura S, Kadowaki T, Noda T. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 2002;277:25863–25866. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- 9.Sharma K, Ramachandrarao S, Qiu G, Usui HK, Zhu Y, Dunn SR, Ouedraogo R, Hough K, McCue P, Chan L, Falkner B, Goldstein BJ. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest. 2008;118:1645–1656. doi: 10.1172/JCI32691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishimura M, Izumiya Y, Higuchi A, Shibata R, Qiu J, Kudo C, Shin HK, Moskowitz MA, Ouchi N. Adiponectin prevents cerebral ischemic injury through endothelial nitric oxide synthase dependent mechanisms. Circulation. 2008;117:216 –223. doi: 10.1161/CIRCULATIONAHA.107.725044. [DOI] [PubMed] [Google Scholar]
- 11.Shibata R, Ouchi N, Kihara S, Sato K, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J Biol Chem. 2004;279:28670–28674. doi: 10.1074/jbc.M402558200. [DOI] [PubMed] [Google Scholar]
- 12.Matsushita K, Yatsuya H, Tamakoshi K, Wada K, Otsuka R, Zhang H, Sugiura K, Kondo T, Murohara T, Toyoshima H. Inverse association between adiponectin and C-reactive protein in substantially healthy Japanese men. Atherosclerosis. 2006;188:184–189. doi: 10.1016/j.atherosclerosis.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 13.Engeli S, Feldpausch M, Gorzelniak K, Hartwig F, Heintze U, Janke J, Mohlig M, Pfeiffer AF, Luft FC, Sharma AM. Association between adiponectin and mediators of inflammation in obese women. Diabetes. 2003;52:942–947. doi: 10.2337/diabetes.52.4.942. [DOI] [PubMed] [Google Scholar]
- 14.Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, Arita Y, Okamoto Y, Shimomura I, Hiraoka H, Nakamura T, Funahashi T, Matsuzawa Y. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 15.Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 16.Fantuzzi G. Adiponectin and inflammation: consensus and controversy. J Allergy Clin Immunol. 2008;121:326–330. doi: 10.1016/j.jaci.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Kistorp C, Faber J, Galatius S, Gustafsson F, Frystyk J, Flyvbjerg A, Hildebrandt P. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112:1756–1762. doi: 10.1161/CIRCULATIONAHA.104.530972. [DOI] [PubMed] [Google Scholar]
- 18.Yilmaz MI, Sonmez A, Acikel C, Celik T, Bingol N, Pinar M, Bayraktar Z, Ozata M. Adiponectin may play a part in the pathogenesis of diabetic retinopathy. Eur J Endocrinol. 2004;151:135–140. doi: 10.1530/eje.0.1510135. [DOI] [PubMed] [Google Scholar]
- 19.Matsuda M, Kawasaki F, Yamada K, Kanda Y, Saito M, Eto M, Matsuki M, Kaku K. Impact of adiposity and plasma adipocytokines on diabetic angiopathies in Japanese Type 2 diabetic subjects. Diabet Med. 2004;21:881–888. doi: 10.1111/j.1464-5491.2004.01261.x. [DOI] [PubMed] [Google Scholar]
- 20.Kato K, Osawa H, Ochi M, Kusunoki Y, Ebisui O, Ohno K, Ohashi J, Shimizu I, Fujii Y, Tanimoto M, Makino H. Serum total and high molecular weight adiponectin levels are correlated with the severity of diabetic retinopathy and nephropathy. Clin Endocrinol (Oxf) 2008;68:442–449. doi: 10.1111/j.1365-2265.2007.03063.x. [DOI] [PubMed] [Google Scholar]
- 21.Smith LE, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan R, D’Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 22.Ozaki H, Seo MS, Ozaki K, Yamada H, Yamada E, Okamoto N, Hofmann F, Wood JM, Campochiaro PA. Blockade of vascular endothelial cell growth factor receptor signaling is sufficient to completely prevent retinal neovascularization. Am J Pathol. 2000;156:697–707. doi: 10.1016/S0002-9440(10)64773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aiello LP, Pierce EA, Foley ED, Takagi H, Chen H, Riddle L, Ferrara N, King GL, Smith LE. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci U S A. 1995;92:10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sone H, Kawakami Y, Segawa T, Okuda Y, Sekine Y, Honmura S, Suzuki H, Yamashita K, Yamada N. Effects of intraocular or systemic administration of neutralizing antibody against vascular endothelial growth factor on the murine experimental model of retinopathy. Life Sci. 1999;65:2573–2580. doi: 10.1016/s0024-3205(99)00526-3. [DOI] [PubMed] [Google Scholar]
- 25.Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004;279:1304–1309. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, Hong S, Pravda EA, Majchrzak S, Carper D, Hellstrom A, Kang JX, Chew EY, Salem N, Jr, Serhan CN, Smith LE. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banin E, Dorrell MI, Aguilar E, Ritter MR, Aderman CM, Smith AC, Friedlander J, Friedlander M. T2-TrpRS inhibits preretinal neovascularization and enhances physiological vascular regrowth in OIR as assessed by a new method of quantification. Invest Ophthalmol Vis Sci. 2006;47:2125–2134. doi: 10.1167/iovs.05-1096. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Connor KM, Aderman CM, Smith LE. Erythropoietin deficiency decreases vascular stability in mice. J Clin Invest. 2008;118:526–533. doi: 10.1172/JCI33813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gardiner TA, Gibson DS, de Gooyer TE, de la Cruz VF, McDonald DM, Stitt AW. Inhibition of tumor necrosis factor-alpha improves physiological angiogenesis and reduces pathological neovascularization in ischemic retinopathy. Am J Pathol. 2005;166:637–644. doi: 10.1016/s0002-9440(10)62284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishida S, Yamashiro K, Usui T, Kaji Y, Ogura Y, Hida T, Honda Y, Oguchi Y, Adamis AP. Leukocytes mediate retinal vascular remodeling during development and vaso-obliteration in disease. Nat Med. 2003;9:781–788. doi: 10.1038/nm877. [DOI] [PubMed] [Google Scholar]
- 31.Kaji Y, Usui T, Ishida S, Yamashiro K, Moore TC, Moore J, Yamamoto Y, Yamamoto H, Adamis AP. Inhibition of diabetic leukostasis and blood-retinal barrier breakdown with a soluble form of a receptor for advanced glycation end products. Invest Ophthalmol Vis Sci. 2007;48:858–865. doi: 10.1167/iovs.06-0495. [DOI] [PubMed] [Google Scholar]
- 32.Ouchi N, Shibata R, Walsh K. AMP-activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circ Res. 2005;96:838–846. doi: 10.1161/01.RES.0000163633.10240.3b. [DOI] [PubMed] [Google Scholar]
- 33.Kociok N, Radetzky S, Krohne TU, Gavranic C, Joussen AM. Pathological but not physiological retinal neovascularization is altered in TNF-Rp55-receptor-deficient mice. Invest Ophthalmol Vis Sci. 2006;47:5057–5065. doi: 10.1167/iovs.06-0407. [DOI] [PubMed] [Google Scholar]
- 34.Summer R, Little FF, Ouchi N, Takemura Y, Aprahamian T, Dwyer D, Fitzsimmons K, Suki B, Parameswaran H, Fine A, Walsh K. Alveolar macrophage activation and an emphysema-like phenotype in adiponectin-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1035–L1042. doi: 10.1152/ajplung.00397.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, Kihara S, Funahashi T, Tenner AJ, Tomiyama Y, Matsuzawa Y. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723–1732. [PubMed] [Google Scholar]
- 36.Takemura Y, Ouchi N, Shibata R, Aprahamian T, Kirber MT, Summer RS, Kihara S, Walsh K. Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J Clin Invest. 2007;117:375–386. doi: 10.1172/JCI29709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vinores SA, Xiao WH, Shen J, Campochiaro PA. TNF-alpha is critical for ischemia-induced leukostasis, but not retinal neovascularization nor VEGF-induced leakage. J Neuroimmunol. 2007;182:73–79. doi: 10.1016/j.jneuroim.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joussen AM, Poulaki V, Mitsiades N, Kirchhof B, Koizumi K, Dohmen S, Adamis AP. Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-alpha suppression. FASEB J. 2002;16:438–440. doi: 10.1096/fj.01-0707fje. [DOI] [PubMed] [Google Scholar]
- 39.Limb GA, Chignell AH, Green W, LeRoy F, Dumonde DC. Distribution of TNF alpha and its reactive vascular adhesion molecules in fibrovascular membranes of proliferative diabetic retinopathy. Br J Ophthalmol. 1996;80:168–173. doi: 10.1136/bjo.80.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME. Adiponectin differentially regulates cytokines in porcine macrophages. Biochem Biophys Res Commun. 2004;316:924–929. doi: 10.1016/j.bbrc.2004.02.130. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi N, Argueta JG, Masuhiro Y, Kagishita M, Nonaka K, Saito T, Hanazawa S, Yamashita Y. Adiponectin inhibits Toll-like receptor family-induced signaling. FEBS Lett. 2005;579:6821–6826. doi: 10.1016/j.febslet.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 42.Park PH, Huang H, McMullen MR, Mandal P, Sun L, Nagy LE. Suppression of lipopolysaccharide-stimulated tumor necrosis factor-alpha production by adiponectin is mediated by transcriptional and post-transcriptional mechanisms. J Biol Chem. 2008;283:26850–26858. doi: 10.1074/jbc.M802787200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park PH, McMullen MR, Huang H, Thakur V, Nagy LE. Short-term treatment of RAW264.7 macrophages with adiponectin increases tumor necrosis factor-alpha (TNF-alpha) expression via ERK1/2 activation and Egr-1 expression: role of TNF-alpha in adiponectin-stimulated interleukin-10 production. J Biol Chem. 2007;282:21695–21703. doi: 10.1074/jbc.M701419200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ouedraogo R, Gong Y, Berzins B, Wu X, Mahadev K, Hough K, Chan L, Goldstein BJ, Scalia R. Adiponectin deficiency increases leukocyte-endothelium interactions via upregulation of endothelial cell adhesion molecules in vivo. J Clin Invest. 2007;117:1718–1726. doi: 10.1172/JCI29623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith CW, Marlin SD, Rothlein R, Toman C, Anderson DC. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989;83:2008–2017. doi: 10.1172/JCI114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siahanidou T, Mandyla H, Papassotiriou GP, Papassotiriou I, Chrousos G. Circulating levels of adiponectin in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2007;92:F286–F290. doi: 10.1136/adc.2006.106112. [DOI] [PMC free article] [PubMed] [Google Scholar]